Abstract
The impressive breath-hold capabilities of marine mammals are facilitated by both enhanced O2 stores and reductions in the rate of O2 consumption via peripheral vasoconstriction and bradycardia, called the dive response. Many studies have focused on the extreme role of the dive response in maximizing dive duration in marine mammals, but few have addressed how these adjustments may compromise the capability to hunt, digest and thermoregulate during routine dives. Here, we use DTAGs, which record heart rate together with foraging and movement behaviour, to investigate how O2 management is balanced between the need to dive and forage in five wild harbour porpoises that hunt thousands of small prey daily during continuous shallow diving. Dive heart rates were moderate (median minimum 47–69 bpm) and relatively stable across dive types, dive duration (0.5–3.3 min) and activity. A moderate dive response, allowing for some perfusion of peripheral tissues, may be essential for fuelling the high field metabolic rates required to maintain body temperature and support digestion during diving in these small, continuously feeding cetaceans. Thus, despite having the capacity to prolong dives via a strong dive response, for these shallow-diving cetaceans, it appears to be more efficient to maintain circulation while diving: extreme heart rate gymnastics are for deep dives and emergencies, not everyday use.
Keywords: dive response, exercise modulated, ECG, foraging, biologging, bradycardia
1. Background
Marine mammals face a physiological trade-off in that they must hold their breath while expending O2 to hunt and consume prey underwater. The remarkable breath-hold capability of marine mammals is enabled by large O2 stores and physiological adjustments including peripheral vasoconstriction and bradycardia during diving that minimize O2 consumption. This so-called dive response regulates the magnitude and distribution of peripheral blood flow, ultimately conserving blood O2 for critical tissues such as the heart and brain [1–3], and is influenced by a variety of factors including dive duration, depth, exercise, age and volition [4–11]. Due to the challenge of studying blood flow and cardiac output in wild marine mammals, heart rate (ƒH) is often measured as a proxy for evaluating dive response and O2 management [12]. Heart rate is an excellent proxy because to maintain stable blood pressure, there is a strong relationship between peripheral vasoconstriction and cardiac output, and endotherms are finely tuned to maintain a relatively stable blood pressure through baroreceptors, chemoreceptors and the autonomic nervous system [12–15]. Until recently, it was not possible to even measure ƒH in wild cetaceans, but with new tagging technology, ƒH has now been measured in two species: a single foraging blue whale (8.5 h, Balaenoptera musculus, the largest baleen whale, approximately 70 000 kg) [16], and five narwhals (approx. five dives each, Monodon monoceros, mid-size toothed whale, approximately 1000–1600 kg) released from prolonged net entanglement and stranding [17]. Both species exhibited extreme bradycardia, with ƒH dropping to 10–20% of surface values, but also exhibited exercise-induced increases in diving ƒH [16,17]. While these recent studies have improved our understanding of O2 management in larger cetaceans, it is still unknown how small wild cetaceans living in thermally challenging environments regulate ƒH while diving and exercising to maximize foraging returns. Here, we investigate the dive heart rate response in the second smallest cetacean, the harbour porpoise (Phocoena phocoena), in shallow inner Danish waters.
Harbour porpoises have high metabolic rates to combat heat loss in their high-latitude habitats [18,19]. Porpoises meet these metabolic demands, at least in inner Danish waters (the so-called Belt Sea population [20]), by high-rate shallow foraging on small prey [21,22]. Understanding how they manage O2 in active foraging dives is critical to assessing how physiological capacity may limit their ability to deal with natural environmental variation and anthropogenic disturbances. Trained porpoises are capable of extreme bradycardia as exhibited by a ƒH below 15 beats per minute (bpm) (approx. 10% of surface ƒH) in long dives for this species of 4 min [8]. However, dive responses in captivity are typically moderate (30–45% of surface ƒH), and mildly influenced by dive duration, exercise and volition during shorter dives [7,8,23,24]. As wild porpoises in the Belt Sea population, in contrast with captive conspecifics, attempt to capture 100 prey per hour and hence must spend a higher proportion of their time engaged in active hunting dives, we hypothesized (i) that wild foraging porpoises will exhibit graded, and occasionally extreme, fluctuations between high surface and low dive ƒH, potentially decreasing ƒH to <10% of surface values in longer dives, and (ii) that they will display a stronger exercise response than the porpoises in captivity due to a greater range of effort between non-foraging dives and active foraging dives. Here, we test these hypotheses by quantifying ƒH in relation to fine-scale movement and foraging behaviour in five wild porpoises using suction cup-attached biologging tags that can uniquely measure heart rate, breathing, exercise and foraging simultaneously in these small wild cetaceans.
2. Methods
(a) . Animal handling and instrumentation
Between September 2016 and September 2018, five harbour porpoises (1 adult female, 1 adult male and 3 juvenile males; see table 1 for detail) that were incidentally trapped in pound nets in the inner Danish waters of the Kattegat and the Belt seas were instrumented with ECG-DTAG3 multi-sensor data loggers (15.5 × 8.5 × 3 cm with two 20–40 cm wires for suction cup electrodes, 265 g in air and slightly buoyant in seawater) [21,30]. Porpoises were instrumented within 24 h of being observed by the fisherman. Porpoises were free to swim within the compass of the net (10–30 m diameter and 5–10 m deep) until tagged and released. For instrumentation, porpoises were lifted onto a fishing boat and placed on a stretcher and soft pad. Standard body length and girth were measured, body condition evaluated, and sex and age class determined [25]. Mass was estimated from body length using sex-specific equations [19]. Only porpoises that appeared healthy were instrumented. Porpoises were released immediately after instrumentation, with all procedures lasting less than 17 min to minimize stress. The porpoises resumed feeding within 2 h of release.
Table 1.
Metadata and summary heart rate (ƒH) variables for five wild porpoises instrumented with ECG-DTAG3. Median and range provided for dive duration and maximum dive depth. Median and 2.5–97.5 quantiles provided for minimum dive, median bottom-of-dive, maximum surface and dive cycle ƒH.
| ID | deployment IDa | sex (age classb) | length (cm) massc (kg) | deploy. dur. (h) | total dives | dives analysedd (>0.5 min) | dive dur. (min) | ind. cADLe/ (cADLf) (min) | maximum depth (m) | minimum dive ƒH (bpm) | median bottom-of-dive ƒH (bpm) | maximum surface ƒH (bpm) | dive cycle ƒgH (bpm) | calculated restingh/maxi ƒH (bpm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF | hp16_264a | female (adult) | 163 67 | 12 | 298 | 214 | 0.9 (0.5–2.2) | 5.0 (3.5–6.9) | 6.3 (3.5–29.7) | 60 (48–71) | 75 (67–88) | 191 (167–218) | 104 (83–132) | 84/240 |
| AM | hp18_095a | male (adult) | 143 44 | 16.5 | 382 | 306 | 1.5 (0.5–3.3) | 4.2 (3.5–6.9) | 6.7 (2.2–19.9) | 57 (43–68) | 76 (66–97) | 204 (174–224) | 106 (84–127) | 93/257 |
| JM1 | hp18_120a | male (juvenile) | 120 30 | 10.5 | 210 | 183 | 1.0 (0.5–2.4) | 3.0 (3.3–6.5) | 6.4 (2.9–25.3) | 59 (40–73) | 72 (66–87) | 219 (199–250) | 113 (95–136) | 103/274 |
| JM2 | hp18_254a | male (juvenile) | 116 28 | 11 | 572 | 138 | 0.9 (0.5–2.3) | 3.6 (3.3–6.5) | 5.9 (3.3–12.9) | 47 (36–56) | 60 (50–70) | 188 (163–213) | 87 (67–114) | 105/278 |
| JM3 | hp16_316a | male (juvenile) | 113 26 | 40 | 1252 | 918 | 0.8 (0.5–1.7) | 2.2 (3.3–6.5) | 9.7 (4.4–38.2) | 69 (56–86) | 113 (80–162) | 208 (192–224) | 141 (118–171) | 107/280 |
aDeployment ID is a unique identification code created from species and date deployed (f.ex. hp = harbour porpoise, 16 = deployed in 2016, 264 – Julian day, a = first tag deployed on that day).
bAge class determined by length [25].
cMass estimated using equations from [19].
dOnly dives more than 2 h after release and longer than 30 s are included in analyses.
eCalculated aerobic dive limit (cADL) using porpoise mass and calculated metabolic rate [26].
fcADL estimated from average age class total body oxygen stores and two and four times Kleibers estimated metabolic rate [27].
gDive cycle ƒH is the total number of heartbeats during the dive and the following surface interval divided by the dive cycle duration (dive duration + post-dive interval duration).
hResting ƒH = 241 × body mass−0.25 [28].
iMaximum ƒH = 477 × body mass−0.163 [29].
The ECG-DTAG3 was placed approximately 5 cm behind the blow hole via four silicone suction cups and a silver chloride electrode embedded in a 5 cm suction cup was placed on each side of the porpoise, with the left electrode caudal and right electrode rostral to the heart to maximize the ECG signal. The ECG-DTAG3 recorded the differential potential between the electrodes relative to a ground in water with a sampling rate of 5 kHz (16-bit resolution and a 2-pole, 200 Hz anti-alias filter). The tag also recorded pressure at 50 Hz, 3-axis acceleration at 625 Hz and stereo sound at 500 kHz (16 bit, 0.5 to 150 kHz bandwidth), allowing determination of activity, ventilations and echolocation behaviour with precise synchrony to the ƒH data (figure 1). The dataloggers detached by a pre-programmed release of air into the suction cup or passively after 10.5 to 40 h (table 1).
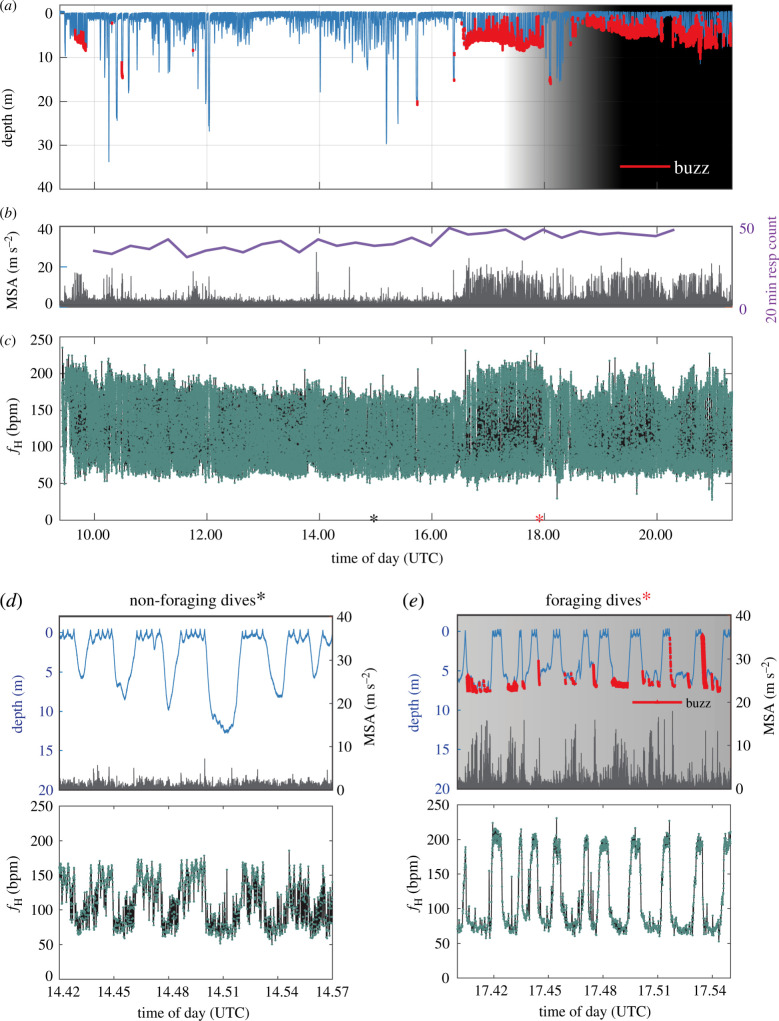
Figure 1.
Diving behaviour and heart rate (ƒH) of the wild adult female harbour porpoise instrumented with an ECG-DTAG. (a) Diving behaviour in relation to daylight (background shading). Red dots signify buzzes likely related to foraging. (b) MSA and 20 min respiration count [26] indicating that the porpoise was more active and had a higher metabolic rate when foraging. (c) Instantaneous ƒH profile. Surface ƒH was elevated after release and during foraging bouts. Excerpts are example dive, MSA and ƒH profiles from non-foraging (d) and foraging dives (e). (Online version in colour.)
(b) . Data processing
Data processing was performed using custom scripts in MATLAB (MathWorks, Inc.). Raw voltages recorded by pressure sensors and accelerometers were converted to depth (m) and acceleration (m s−2) using calibration values. Sound files were examined aurally and visually in 5 s windows using a spectrogram display (Hamming window, 512 point fast Fourier transform, 75% overlap) (MATLAB code available at www.animaltags.org) to identify ventilations and feeding buzzes [21,31]. Dives were identified from a combination of depth and respiration data [21]. Respirations defined the start and end of each inter-breath interval. Inter-breath intervals were only considered a dive if the maximum dive depth was greater than two times the body length of the porpoise (i.e. 2.5–3.5 m). Dives with feeding buzzes were classified as foraging dives.
ECG data were decimated to a sampling rate of 250 Hz and filtered to remove excess noise (finite impulse response filter to remove 50-Hz electrical noise generated by sampling of other sensors). R-peaks in the ECG QRS-complex were identified using a peak detector script and records were visually inspected to correct for missing or spurious peaks. Instantaneous ƒH for each heartbeat was determined from the R–R peak intervals (60 s divided by the difference in time between the current and previous R peak).
As an index for swimming effort, we calculated the minimum specific acceleration (MSA) from the three-axis acceleration data decimated to a sampling rate of 25 Hz. MSA is a measure of how much the total acceleration deviates from the gravity acceleration [32] and is an underestimate of the specific acceleration generated by the animal. It is calculated as the absolute value of the norm of acceleration minus the gravity acceleration. In captive porpoises, MSA and overall dynamic body acceleration (a commonly used activity metric [33,34]) were highly correlated (Pearson r = 0.98) [8], and in California sea lions MSA and stroke rate were highly correlated; however, MSA had explained more of the variation in dive ƒH suggesting it was a better indicator of effort [9].
For each dive cycle (dive + post-dive interval), 13 variables were calculated, as follows: (i) dive duration (s); (ii) post-dive interval (s, duration between the respiration ending a dive and the respiration starting the following dive); (iii) dive cycle duration (s); (iv) maximum dive depth (m); (v) mean MSA (m s−2, total dive MSA/number of samples); (vi) upper quartile (UQ) pre-dive instantaneous ƒH (bpm, UQ (75%) in 10 s preceding the dive to avoid bias from decreases in ƒH during surface breath-holds); (vii) lower quartile (LQ) dive ƒH (bpm, 25th quantile—an outlier-robust estimate of how low the ƒH was during the dive); (viii) UQ post-dive interval ƒH (bpm); (ix) median bottom-of-dive ƒH (bpm, bottom-of-dive is defined as the time between when the porpoise initially reached a depth of one body length above maximum depth and when the porpoise last leaves that depth—this is a proxy for dive ƒH, but avoids the influence of the initial decline and final increase in ƒH); (x) minimum dive ƒH (bpm); (xi) post-dive interval (PDI) ƒH (bpm, total beats during post-dive interval/post-dive interval duration); (xii) dive cycle ƒH (bpm, total heart beats during a dive cycle (dive + post-dive interval)/dive cycle duration); and (xiii) per cent of surface ƒH during a dive (as an indicator of degree of bradycardia) was estimated by the ratio of LQ dive ƒH to UQ pre-dive ƒH (LQ dive fH/UQ pre-dive fH). In addition to the above dive variables, we divided the bottom phase of the dive into 5 s intervals and determined the mean MSA (m s−2) and ƒH for each interval to quantify the relationship between behaviour and ƒH within dives.
(c) . Statistical analysis
Statistical analyses were performed in R v. 3.5.3 (R Core Team). After release, porpoises typically swam rapidly away from the site of release primarily performing shallow swimming. During this phase, ƒH was often elevated, both at the surface and submerged. Based on these observations, dives from the first 2 h post-release were excluded from analyses to reduce the influence of capture stress on results [19]. We also excluded dives less than 30 s in duration to exclude brief submergences between breaths.
We used linear mixed-effects models to investigate the effect of dive duration and mean MSA on dive and surface ƒH (package ‘nlme’ [35]). In these models, dive duration and mean MSA were fitted as fixed-effect variables, with individual as a random effect to account for the lack of independence of dives from the same individual. Linear mixed-effects models were also used to examine the relationship between behaviour and ƒH during the bottom phase of the dive. The response variable was mean dive interval heart rate. Mean interval MSA and dive duration were fitted as fixed-effect variables, with the individual as a random effect. A correlation structure (AR1) was used to account for temporal correlation in the data and the VarIdent variance function structure was used to account for the difference in variance between individuals [36]. Covariance and random effect structures of the full models were evaluated using Akaike's information criterion (AIC) and examination of residual plots [36]. Once the covariance and random effect structure was determined, the best model was selected by removing a single variable and comparing the full and reduced model using a log-likelihood chi-squared test. If the reduced model was better, another variable was removed until removing variables did not improve the model. The models with lowest AIC are reported in electronic supplementary material, tables S1 and S2. Additionally, we used linear mixed-effects ANOVAs to examine differences in ƒH between foraging and non-foraging dives. Foraging state was the fixed-effect variable, and individual was fitted as a random intercept (electronic supplementary material, tables S3 and S4). All models were run with and without Juvenile Male 3 (JM3). JM3 was an outlier. His ƒH was much higher and more variable than the other four porpoises, resulting in improved normality and homoscedasticity of residuals in models without JM3.
3. Results and discussion
The dive response was first discovered during forced submergence of seals and porpoises, and based on observations of deep bradycardias in such highly stressed animals, it was presented as a dramatic on–off master switch of life [2]. Subsequently, it has become clear from studies of trained animals that the dive response in marine mammals is a much more graded physiological phenomenon that is tailored to a suite of intrinsic and extrinsic conditions while diving [5–10]. Despite this deep understanding we still know very little about how the dive response is implemented during hunting in the wild where the diving animal must balance the opposing forces of physiological mechanisms enabling prolonged breath-holds and those that facilitate thermoregulation, digestion, and body maintenance as a function of a dynamic environment. Here, we use one of the smallest marine mammals, the harbour porpoise, as a model organism to address the implementation of the dive response during natural hunting behaviour in the wild. Specifically, we sought to test the hypotheses that (i) wild porpoises would exhibit a graded and occasionally extreme bradycardia tailored to the dive duration and (ii) porpoises would exhibit an exercise-modulated dive ƒH response driven by hunting activity. To test these hypotheses, we obtained 10.5–40 h of DTAG data from five porpoises in Danish waters (table 1) from which we analysed 138–918 dives per individual that were longer than 30 s after excluding the 2 h following release (table 1). As typical for harbour porpoises in the Belt Sea population, dives tended to be short and shallow (median duration: 43–76 s, median maximum depth: 6–10 m) [19,21], and well within the calculated aerobic dive limit (cADL, table 1) [26,27].
(a) . Wild porpoises exhibit consistent moderate dive responses
Contrary to our hypothesis that wild porpoises would exhibit a graded and occasionally extreme bradycardia, tagged porpoises displayed moderate and consistent dive ƒH in dives up to 3.5 min (figures 2, 3b, 4 and 5). Dive heart rate only declined to 23–55% of surface values and minimum ƒH was greater than 45 bpm in 95% of dives (electronic supplementary material, figure S1A). Despite a large range in estimated mass (26–67 kg), when the smallest porpoise was excluded, porpoises exhibited remarkably similar dive ƒH (figures 2, 3b and 4, i.e. 95% of all LQ ƒHs fell between 55 and 84 bpm). This is unexpected because among mammals, small young animals generally have higher ƒH than larger adults (table 1) [28]. That two juveniles (JM1 and JM2) exhibited similar dive ƒH to the two adults (figures 2, 3b, 4 and 5 and electronic supplementary material, figures S1 and S2) suggest that the juveniles are employing a relatively more intense bradycardia, allowing them to dive similarly to adults despite smaller absolute oxygen stores due to their smaller size. These results are consistent with a previous study that determined juvenile and adult porpoises have similar cADLs [27]. For one juvenile porpoise (JM3, 26 kg), dive ƒH was highly variable and higher than the other porpoises (figure 3b, 4, 5; electronic supplementary material, figures S1 and S2). This animal exhibited the highest respiration rate in a previous study [19]. JM3's high and variable dive ƒH may be an example of the natural variability between individuals, seasonal differences due to changes in temperature or prey availability (JM3 was instrumented later in the year than other porpoises), or a result of a potential respiratory disease (common in the Belt Sea population) [37]. Because JM3 data were so distinct and strongly influenced model results, ƒH was analysed with and without this porpoise (electronic supplementary material, tables S1–S3). While the porpoises only exhibited a moderate dive response, all animals displayed a true bradycardia in over 99% of the analysed dives (figure 3b), with ƒH decreasing to below their estimated resting ƒH (estimated from mass of each porpoise, table 1) [28].
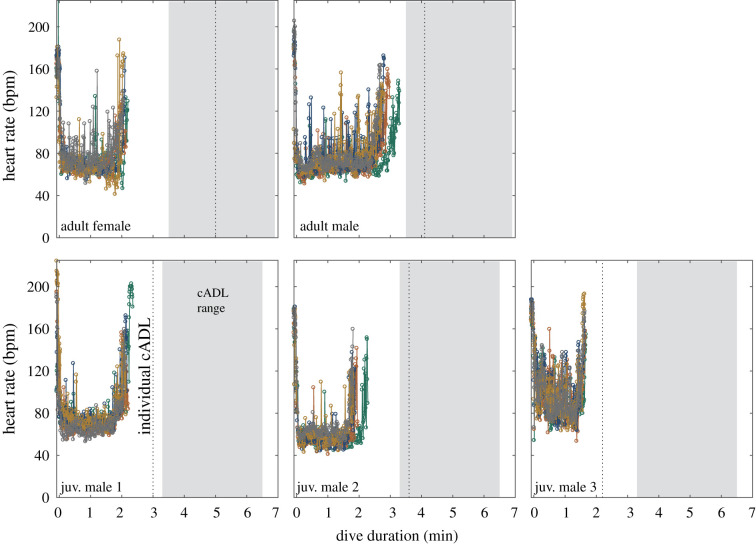
Figure 2.
Dive ƒH profiles from the five longest dives of each porpoise. No dives exceeded the Individual cADL or the minimum cADL estimated by age class [26,27]. While ƒH profiles differed slightly between individuals, this did not appear to be due to age. Porpoises exhibited relatively consistent, moderate ƒH across the range of dive durations observed. See table 1 footnotes for information about cADL calculations. (Online version in colour.)
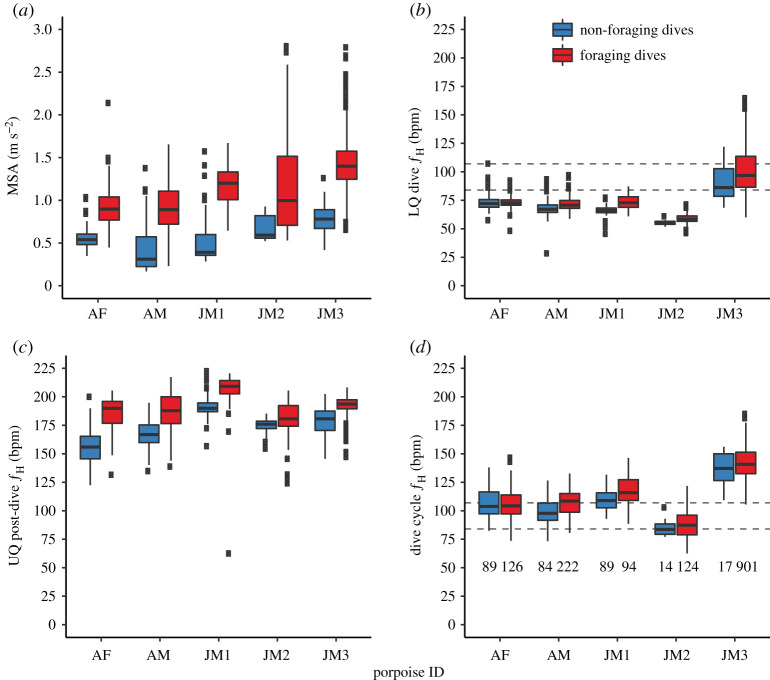
Figure 3.
(a) Harbour porpoises exhibited higher mean MSA (activity proxy) during foraging dives compared to non-foraging dives; however, (b) there were likely no biologically relevant differences in LQ dive heart rate (ƒH). (c) Foraging dives had higher post-dive ƒH for most porpoises, (d) but there were no biologically relevant differences in ƒH between foraging and non-foraging dives when averaged over a dive cycle (electronic supplementary material, table S3). Grey lines indicate estimated resting ƒH from scaling [28] for a 26 kg (upper) and 67 kg (lower) mammal. Sample size provided in (d). The bottom and top of the box correspond to the 25th and 75th percentiles. The lower and upper whisker extend to the smallest and largest value that is no further than 1.5 × the inter-quartile range. Data beyond the end of the whiskers are outliers and are plotted individually. (Online version in colour.)
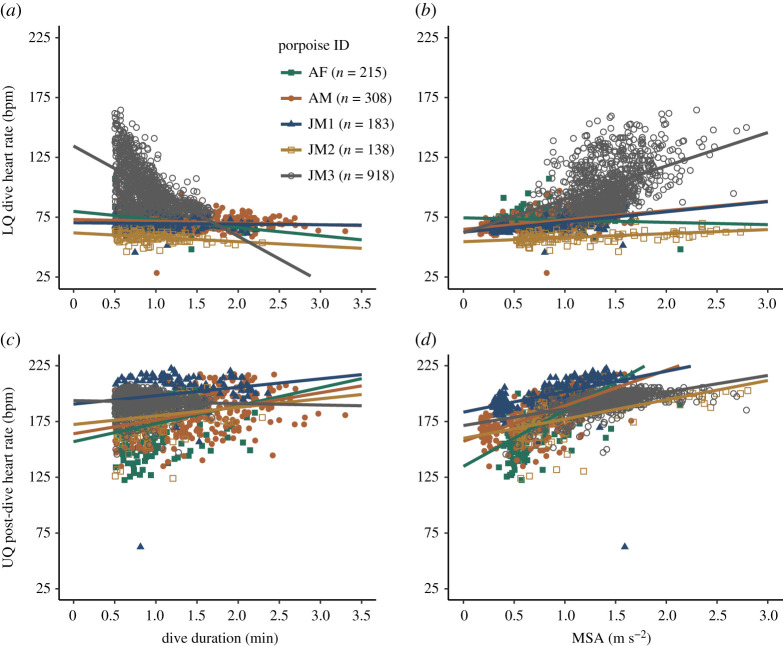
Figure 4.
Influence of dive duration and mean MSA on (a,b) lower quartile dive fH and (c,d) upper quartile post-dive surface fH. See electronic supplementary material, table S1 for statistics. (Online version in colour.)
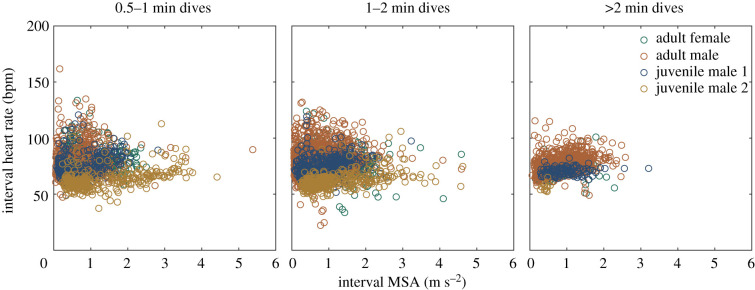
Figure 5.
Interval MSA (m s−2) did not influence interval heart rate (ƒH) when JM3 was excluded. To facilitate visualization of the consistency of ƒH across the range of dive durations observed, data are separated by dive duration category (0.5–1, 1–2 and greater than 2 min dives). Interval ƒH was similar across the range of dive durations observed, but less variable in the longer dives. See electronic supplementary material, table S2 for statistics and figure S4 for JM3 data. (Online version in colour.)
Surprisingly, dive duration did not influence LQ, bottom-of-dive, minimum dive nor dive interval ƒH (figures 4 and 5; electronic supplementary material, figure S1 and tables S1 and S2). Although the associations between dive duration and bottom-of-dive and LQ ƒH were statistically significant when JM3 was excluded, the slope coefficient was not biologically relevant (i.e. a 1 min increase in dive duration, which is a doubling of the median dive duration, only resulted in a 3 bpm decrease in LQ and bottom-of-dive ƒH and dive duration explained less than 3% of the variation in ƒH; electronic supplementary material, table S1) [38,39]. While Weddell seals do not appear to modify ƒH with increased dive duration in aerobic dives (dives shorter than ADL) [40], ƒH typically decreases with increasing dive duration during aerobic dives in other free-ranging marine mammals [4,16,41], and a decrease in ƒH with increased dive duration was documented in captive porpoises performing short dives [8,23]. Low ƒH in long dives is thought to be key to maximizing dive duration by minimizing blood flow to exercising muscle and other organs. Even in the longest dives performed by each porpoise of up to 3.3 min, ƒH was moderate and not different from shorter dives (figure 2; electronic supplementary material, figure S2). The generally high ƒH is probably required to support the high field metabolic rates documented in porpoises [19].
However, as these porpoises performed short dives that rarely approached their cADL (table 1 and figure 2), there may be no need to modify ƒH to maximize dive duration. Instead, the consistent moderate bradycardia implies some blood flow to organs such as the intestine and stomach throughout the dive. The ability to digest prey while continuing to acquire more prey may be essential for small cetaceans with high metabolic demands that feed at high rates for approximately 70% of the day, but with small effective digestion systems where more food per body mass must be processed per day compared to larger cetaceans [42,43]. Larger species, or even porpoise populations that feed on larger prey, may be able to defer digestion and its metabolic cost until after foraging bouts [44–46]; captive grey seals almost completely deferred the metabolic costs of digestion until after simulated deep foraging dives, exhibiting extended surface intervals with elevated metabolic rate following foraging bouts. However, during simulated shallow dives, grey seals exhibited shorter dive durations and increased digestive costs during dives [45]. Similarly, Steller sea lions exhibit a partial deferment of digestion when actively foraging at depth, but Rosen et al. [46] suggest, based on differences in ƒH between shallow and deep dives [47], that the degree of deferment is specific to diving conditions. For harbour porpoises in the inner Danish waters, where continuous foraging takes place at night in depths primarily less than 25 m [21,30], energy gain may be maximized by performing shorter dives with a moderate ƒH allowing continued foraging while digesting. However, we predict that porpoises targeting deep-water/mesopelagic prey, such as in Greenlandic waters where porpoises dive down to 410 m [48,49], will exhibit a graded and occasionally extreme diving bradycardia with extended post-dive intervals after the longest dives.
(b) . Wild porpoises exhibit an exercise-modulated surface ƒH response
We hypothesized that wild porpoises would display an exercise-modulated dive ƒH response and that the exercise response would be stronger than documented in captive harbour porpoises [8,23,50] due to the greater diversity of behaviours exhibited in the wild. The few studies that have investigated the relationship between an activity index and dive ƒH in pinnipeds and captive cetaceans found a positive relationship, at least in shorter aerobic dives [6,8,9,11,47], indicative of increased blood flow to exercising muscles. In wild narwhals, a positive relationship between stroke rate and ƒH was observed once animals started to recover from prolonged capture stress [17]. Similarly, a blue whale exhibited a transient doubling of instantaneous ƒH during costly feeding lunges [16]. By contrast, we found little support for exercise modulation of dive ƒH in wild foraging porpoises. We used mean MSA as an index of activity [8,9,32] and, as predicted, mean MSA was higher during foraging dives compared to dives without foraging, (figure 3a; electronic supplementary material, table S3; mixed-effects ANOVA: 0.48 (coefficient) ± 0.02 (standard error, s.e.), p < 0.001). We hypothesized that this increased activity would lead to higher dive ƒH, but there was no biologically relevant difference in LQ, median bottom-of-dive or minimum dive ƒH between foraging and non-foraging dives (figure 3b; electronic supplementary material, tables S3 and S4). For example, LQ ƒH was only 3.4 bpm higher in foraging dives, and foraging explained < 1% of the variation in ƒH. Similarly, there was no biologically meaningful relationship between MSA and LQ ƒH or interval MSA and interval ƒH in the four largest porpoises (figures 4b and 5; electronic supplementary material, table S1 and S2): while the association between MSA and LQ ƒH was statistically significant at the level of the dive (electronic supplementary material, table S1), an increase in mean MSA of 1 m s−2 only increased ƒH by 1.8 bpm (mean dive MSA ranged between approximately 0.2–2.8 m s−2). At a finer scale, there was no statistically significant relationship between interval MSA and interval ƒH during the bottom of the dive (electronic supplementary material, table S2). The lack of relationship between activity and dive ƒH in most of the porpoises could be a by-product of a consistent moderate dive response that allows for digestion during diving. The moderate ƒH would be accompanied by a widespread decrease in sympathetic vasoconstriction with redistribution of blood to multiple tissues, including muscle [51]. If muscle is already moderately perfused during dives, porpoises may not increase ƒH to provide more oxygenated blood to O2-depleted muscle in moments of activity, at least while operating below a certain dive duration and activity threshold. In contrast with the four larger porpoises, JM3 exhibited an increase in dive ƒH with increased activity as predicted (figure 4b; electronic supplementary material, figures S1 and S4).
While the diving ƒH was moderate and not influenced by exercise, UQ instantaneous ƒH in PDIs and PDI ƒH (total beats during PDI/PDI duration) were high and influenced by activity in all porpoises (UQ ƒH is used as a proxy for surface ƒH to avoid the influence of temporary decreases in ƒH associated with short submergences). Post-dive UQ and PDI ƒH were 11% and 23% higher, respectively, after foraging dives as compared to non-foraging dives (figure 3c; electronic supplementary material, table S3), and showed a positive relationship with mean MSA of the preceding dive (figure 4d; electronic supplementary material, figure S1 and table S1; ex. mixed effect model UQ surface ƒH: 13.1 ± 4.0, p = 0.001, i.e. an increase in mean MSA of 1 m s−2 increased ƒH by 13 bpm). The high post-dive UQ ƒH after all dives facilitates rapid offloading of CO2 and onloading of O2, allowing the porpoise to maximize time submerged. The even higher post-dive UQ ƒH during foraging bouts may serve to minimize time at the surface so the porpoise can quickly return to the prey patch. Although these elevated surface ƒH-values after active foraging dives (median maximum ƒH ranged from 188 to 219 bpm) are the highest documented for a marine mammal, they are nonetheless rarely close to the maximum ƒH predicted from body mass of mammals (257 bpm for AF, the largest porpoise, and 280 bpm for JM3, the smallest porpoise, figure 2 and table 1) [29]. However, the porpoises occasionally exhibited ƒH approaching the predicted maximum ƒH, indicating they can increase ƒH more if required.
4. Conclusion
These first longer term, continuous heart rate data from multiple, unstressed wild cetaceans demonstrate how these consummate divers juggle the conflicting demands of a deep bradycardia to prolong dives in search of prey while still delivering the O2 needed for active hunting and digestion. We find that porpoises in shallow waters forgo deep bradycardia and instead maintain a relatively stable diving heart rate that is not influenced by dive duration or activity, and possibly size. This moderate bradycardia may be essential for providing cardiac output to support the high metabolic rate and upholding digestion during near-continuous feeding. Thus, for porpoises in this environment, extreme dive responses enabling extended dive durations, that may be critical for efficient foraging in marine mammals elsewhere, are not needed; extreme heart rate gymnastics are for deep/long dives and emergencies. Instead, shallow-feeding short duration diving porpoises have moderate dive heart rates combined with high surface heart rates that may help to balance the demands of prey capture, digestion and thermoregulation. This new understanding of how one of the smallest marine mammals manages O2 during active foraging dives provides insight into how physiological responses are not necessarily taken to their extremes in the wild.
Supplementary Material
Acknowledgements
We thank colleagues from Aarhus University from the Section for Marine Mammal Research and the Marine Bioacoustics Lab, including S. Sveegaard, L. Mikkelsen, M. V. Jensen, R. Dietz, P. Sørensen, A. Bøttcher, L. Havmøller, L. Bach, J. Balle, E. Iglesias, I. Amirali, F. Larsen, M. Ladegaard, K. Sprogis, S. Videsen, J. Tougaard, P. Tønnesen and L. Kyhn, as well as all the helpful fishermen and pilot U. Gosewinkel involved in tag deployments and recoveries.
Ethics
Handling and instrumentation of porpoises was carried out under permission issued to J.T. from the Environmental Protection Agency (Ministry of Environment and Food of Denmark, NST-3446-0016) and the Animal Experiments Inspectorate (Ministry of Environment and Food of Denmark, 2015-15-0201-00549) during 2015–2018.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.z612jm6cs [52]. Code used to process data is available at www.animaltags.org.
Authors' contributions
B.I.M.: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, visualization, writing-original draft, writing-review and editing; S.L.E.: conceptualization, data curation, formal analysis, investigation, methodology, writing-original draft, writing-review and editing; M.J.: conceptualization, data curation, funding acquisition, methodology, resources, software, writing-original draft, writing-review and editing; D.M.W.: data curation, formal analysis, investigation, visualization, writing-review and editing; L.R.-D.: data curation, formal analysis, investigation, writing-review and editing; A.G.: investigation, writing-review and editing; U.S.: funding acquisition, writing-review and editing; J.T.: funding acquisition, investigation, methodology, project administration, Supervision, writing-review and editing; P.T.M.: conceptualization, funding acquisition, methodology, project administration, resources, supervision, writing-original draft, writing-review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by grants from the Office of Naval Research (N000141612852 awarded to B.I.M., M.J. and P.T.M.), the Carlsbergfondet (awarded to P.T.M.) and a National Science Foundation International Research Postdoctoral Fellowship (OISE – 1159123 awarded to B.I.M.). This study was also funded by the German Federal Agency for Nature Conservation via a grant to U.S., J.T. and M.J. (‘Effects of underwater noise on marine vertebrates', Cluster 7, Z1.2-53302/2010/14) and ‘Under Water Noise Effects– UWE’ (Project no. FKZ 3515822000). D.M.W. was partially funded under a Marie Skłodowska-Curie Individual Fellowship (grant agreement no. 748026).
References
- 1.Scholander P, Irving L, Grinnell S. 1942. Aerobic and anaerobic changes in seal muscles during diving. Int. J. Biol. Chem. 142, 431-440. ( 10.1016/S0021-9258(18)72738-5) [DOI] [Google Scholar]
- 2.Scholander PF. 1940. Experimental investigations on the respiratory function in diving mammals and birds. Hvalradets Skrifter 22, 1-131. [Google Scholar]
- 3.Zapol WM, Liggins GC, Schneider RC, Qvist J, Snider MT, Creasy RK, Hochachka PW. 1979. Regional blood flow during simulated diving in the conscious Weddell seal. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 968-973. [DOI] [PubMed] [Google Scholar]
- 4.Thompson D, Fedak MA. 1993. Cardiac responses of grey seals during diving at sea. J. Exp. Biol. 174, 139-154. ( 10.1242/jeb.174.1.139) [DOI] [PubMed] [Google Scholar]
- 5.Davis RW, Williams TM. 2012. The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J. Comp. Physiol. A 198, 583-591. ( 10.1007/s00359-012-0731-4) [DOI] [PubMed] [Google Scholar]
- 6.Williams TM, et al. 2015. Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep-diving marine mammals. Nat. Commun. 6, 6055. [DOI] [PubMed] [Google Scholar]
- 7.Elmegaard SL, Johnson M, Madsen PT, McDonald BI. 2016. Cognitive control of heart rate in diving harbor porpoises. Curr. Biol. 26, R1175-R1176. ( 10.1016/j.cub.2016.10.020) [DOI] [PubMed] [Google Scholar]
- 8.McDonald BI, Johnson M, Madsen PT. 2018. Dive heart rate in harbour porpoises is influenced by exercise and expectations. J. Exp. Biol. 221, jeb168740. [DOI] [PubMed] [Google Scholar]
- 9.McDonald BI, Tift MS, Hückstädt LA, Jeffko M, Ponganis PJ. 2020. Stroke effort and relative lung volume influence heart rate in diving sea lions. J. Exp. Biol. 223, jeb214163. [DOI] [PubMed] [Google Scholar]
- 10.Noren SR, Cuccurullo V, Williams TM. 2004. The development of diving bradycardia in bottlenose dolphins (Tursiops truncatus). J. Comp. Physiol. B 174, 139-147. ( 10.1007/s00360-003-0398-9) [DOI] [PubMed] [Google Scholar]
- 11.Noren SR, Kendall T, Cuccurullo V, Williams TM. 2012. The dive response redefined: underwater behavior influences cardiac variability in freely diving dolphins. J. Exp. Biol. 215, 2735-2741. ( 10.1242/jeb.069583) [DOI] [PubMed] [Google Scholar]
- 12.Ponganis PJ. 2015. Diving physiology of marine mammals and seabirds. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Grinnell S, Irving L, Scholander P. 1942. Experiments on the relation between blood flow and heart rate in the diving seal. J. Cell. Comp. Physiol. 19, 341-350. ( 10.1002/jcp.1030190309) [DOI] [Google Scholar]
- 14.Jobsis PD, Ponganis PJ, Kooyman GL. 2001. Effects of training on forced submersion responses in harbor seals. J. Exp. Biol. 204, 3877-3885. ( 10.1242/jeb.204.22.3877) [DOI] [PubMed] [Google Scholar]
- 15.Butler PJ, Brown JA, Stephenson DG, Speakman JR. 2020. Animal physiology: an environmental perspective. Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Goldbogen J, et al. 2019. Extreme bradycardia and tachycardia in the world's largest animal. Proc. Natl Acad. Sci. USA 116, 25 329-25 332. ( 10.1073/pnas.1914273116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams TM, Blackwell SB, Richter B, Sinding MH.S, Heide-Jørgensen MP. 2017. Paradoxical escape responses by narwhals (Monodon monoceros). Science 358, 1328-1331. ( 10.1126/science.aao2740) [DOI] [PubMed] [Google Scholar]
- 18.Williams TM, Maresh JL. 2015. Exercise energetics. In Marine mammal physiology: requisites for ocean living (eds Castellini MA, Mellish JA), pp. 47-68. Boca Raton, FL: CRC Press. [Google Scholar]
- 19.Rojano-Doñate L, McDonald BI, Wisniewska DM, Johnson M, Teilmann J, Wahlberg M, Højer-Kristensen J, Madsen PT. 2018. High field metabolic rates of wild harbour porpoises. J. Exp. Biol. 221, jeb.185827. ( 10.1242/jeb.185827) [DOI] [PubMed] [Google Scholar]
- 20.Sveegaard S, et al. 2015. Defining management units for cetaceans by combining genetics, morphology, acoustics and satellite tracking. Glob. Ecol. Conserv. 3, 839-850. ( 10.1016/j.gecco.2015.04.002) [DOI] [Google Scholar]
- 21.Wisniewska DM, Johnson M, Teilmann J, Rojano-Doñate L, Shearer J, Sveegaard S, Miller LA, Siebert U, Madsen PT. 2016. Ultra-high foraging rates of harbor porpoises make them vulnerable to anthropogenic disturbance. Curr. Biol. 26, 1441-1446. ( 10.1016/j.cub.2016.03.069) [DOI] [PubMed] [Google Scholar]
- 22.Wisniewska DM, Johnson M, Teilmann J, Rojano-Doñate L, Shearer J, Sveegaard S, Miller LA, Siebert U, Madsen PT. 2018. Response to ‘Resilience of harbor porpoises to anthropogenic disturbance: must they really feed continuously?’ Mar. Mammal. Sci. 34, 265-270. ( 10.1111/mms.12463) [DOI] [Google Scholar]
- 23.Reed J, Chambers C, Hunter C, Lockyer C, Kastelein R, Fedak M, Boutilier R. 2000. Gas exchange and heart rate in the harbour porpoise, Phocoena phocoena. J. Comp. Physiol. B 170, 1-10. ( 10.1007/s003600050001) [DOI] [PubMed] [Google Scholar]
- 24.Teilmann J, Tougaard J, Miller LA, Kirketerp T, Hansen K, Brando S. 2006. Reactions of captive harbor porpoises (Phocoena phocoena) to pinger-like sounds. Mar. Mammal. Sci. 22, 240-260. ( 10.1111/j.1748-7692.2006.00031.x) [DOI] [Google Scholar]
- 25.Lockyer C, Kinze C. 2003. Status, ecology and life history of harbour porpoise (Phocoena phocoena), in Danish waters. NAMMCO Sci. Publ. 5, 143-175. ( 10.7557/3.2745) [DOI] [Google Scholar]
- 26.Rojano-Doñate L. 2020. Acoustics and energetics of echolocators in a noisy world. Aarhus, Denmark: Aarhus Universitet. [Google Scholar]
- 27.Noren SR, Noren DP, Gaydos JK. 2014. Living in the fast lane: rapid development of the locomotor muscle in immature harbor porpoises (Phocoena phocoena). J. Comp. Physiol. B 184, 1065-1076. ( 10.1007/s00360-014-0854-8) [DOI] [PubMed] [Google Scholar]
- 28.Stahl WR. 1967. Scaling of respiratory variables in mammals. J. Appl. Physiol. 22, 453-460. ( 10.1152/jappl.1967.22.3.453) [DOI] [PubMed] [Google Scholar]
- 29.Bishop CM. 1997. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Phil. Trans. R. Soc. Lond. B 352, 447-456. ( 10.1098/rstb.1997.0032) [DOI] [Google Scholar]
- 30.Sveegaard S, Teilmann J, Tougaard J, Dietz R, Mouritsen KN, Desportes G, Siebert U. 2011. High-density areas for harbor porpoises (Phocoena phocoena) identified by satellite tracking. Mar. Mammal. Sci. 27, 230-246. ( 10.1111/j.1748-7692.2010.00379.x) [DOI] [Google Scholar]
- 31.Wisniewska DM, Johnson M, Teilmann J, Siebert U, Galatius A, Dietz R, Madsen PT. 2018. High rates of vessel noise disrupt foraging in wild harbour porpoises (Phocoena phocoena). Proc. R. Soc. B 285, 20172314. ( 10.1098/rspb.2017.2314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon M, Johnson M, Madsen PT. 2012. Keeping momentum with a mouthful of water: behavior and kinematics of humpback whale lunge feeding. J. Exp. Biol. 215, 3786-3798. ( 10.1242/jeb.071092) [DOI] [PubMed] [Google Scholar]
- 33.Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081-1090. ( 10.1111/j.1365-2656.2006.01127.x) [DOI] [PubMed] [Google Scholar]
- 34.Halsey L, Shepard E, Quintana F, Laich AG, Green J, Wilson RP. 2009. The relationship between oxygen consumption and body acceleration in a range of species. Comp. Biochem. Phys. A 152, 197-202. ( 10.1016/j.cbpa.2008.09.021) [DOI] [PubMed] [Google Scholar]
- 35.Pinheiro J, Bates D, DebRoy S, Sarkar D, Heisterkamp S, Van Willigen B, Maintainer R. 2017. Package ‘nlme’: linear and nonlinear mixed effects models, version 3.1–131. See https://cran.r-project.org/web/packages/nlme.
- 36.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 37.Siebert U, et al. 2020. Health assessment of harbour porpoises (Phocoena phocoena) from Baltic area of Denmark, Germany, Poland and Latvia. Environ. Int. 143, 105904. ( 10.1016/j.envint.2020.105904) [DOI] [PubMed] [Google Scholar]
- 38.Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. 2008. An R2 statistic for fixed effects in the linear mixed model. Stat. Med. 27, 6137-6157. ( 10.1002/sim.3429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wasserstein RL, Lazar NA. 2016. The ASA statement on p-values: context, process, and purpose. Am. Stat. 70, 129-133. ( 10.1080/00031305.2016.1154108) [DOI] [Google Scholar]
- 40.Hill RD, et al. 1987. Heart rate and body temperature during free diving of Weddell seals. Am. J. Physiol. Reg. I 253, R344–R351. [DOI] [PubMed] [Google Scholar]
- 41.McDonald BI, Ponganis PJ. 2014. Deep-diving sea lions exhibit extreme bradycardia in longduration dives. J. Exp. Biol. 217, 1525-1534. ( 10.1242/jeb.098558) [DOI] [PubMed] [Google Scholar]
- 42.Chivers DJ, Hladik CM. 1980. Morphology of the gastrointestinal tract in primates: comparisons with other mammals in relation to diet. J. Morphol. 166, 337-386. ( 10.1002/jmor.1051660306) [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Nielsen K, Knut SN. 1984. Scaling: why is animal size so important? Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Crocker DE, Le Boeuf BJ, Costa DP.. 1997. Drift diving in female northern elephant seals: implications for food processing. Can. J. Zool. 75, 27-39. ( 10.1139/z97-004) [DOI] [Google Scholar]
- 45.Sparling CE, Fedak MA, Thompson D. 2007. Eat now, pay later? Evidence of deferred food-processing costs in diving seals. Biol. Lett. 3, 95-99. ( 10.1098/rsbl.2006.0566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen DA.S., Gerlinsky CD, Trites AW. 2015. Evidence of partial deferment of digestion during diving in Steller sea lions (Eumetopias jubatus). J. Exp. Mar. Biol. Ecol. 469, 93-97. ( 10.1016/j.jembe.2015.04.017) [DOI] [Google Scholar]
- 47.Hindle AG, Young BL, Rosen DA, Haulena M, Trites AW. 2010. Dive response differs between shallow-and deep-diving Steller sea lions (Eumetopias jubatus). J. Exp. Mar. Biol. Ecol. 394, 141-148. ( 10.1016/j.jembe.2010.08.006) [DOI] [Google Scholar]
- 48.Nielsen NH, Teilmann J, Heide-Jørgensen MP. 2019. Indications of mesopelagic foraging by a small odontocete. Mar. Biol. 166, 78. ( 10.1007/s00227-019-3525-1) [DOI] [Google Scholar]
- 49.Nielsen NH, Teilmann J, Sveegaard S, Hansen RG, Sinding M-HS, Dietz R, Heide-Jørgensen MP. 2018. Oceanic movements, site fidelity and deep diving in harbour porpoises from Greenland show limited similarities to animals from the North Sea. Mar. Ecol. Prog. Ser. 597, 259-272. ( 10.3354/meps12588) [DOI] [Google Scholar]
- 50.Elmegaard SL, McDonald BI, Madsen PT. 2019. Drivers of the dive response in trained harbour porpoises (Phocoena phocoena). J. Exp. Biol. 222, jeb208637. ( 10.1242/jeb.208637) [DOI] [PubMed] [Google Scholar]
- 51.Elsner R, Franklin DL, Van Citters RL, Kenney DW.. 1966. Cardiovascular defense against asphyxia. Science 153, 941-949. ( 10.1126/science.153.3739.941) [DOI] [PubMed] [Google Scholar]
- 52.McDonald BI, Elmegaard SL, Johnson M, Wisniewska DM, Rojano-Donate L, Galatius A, Siebert U, Teilmann J, Madsen PT. 2021. Data from: High heart rates in hunting porpoises. Dryad Digital Repository. ( 10.5061/dryad.z612jm6cs) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- McDonald BI, Elmegaard SL, Johnson M, Wisniewska DM, Rojano-Donate L, Galatius A, Siebert U, Teilmann J, Madsen PT. 2021. Data from: High heart rates in hunting porpoises. Dryad Digital Repository. ( 10.5061/dryad.z612jm6cs) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.z612jm6cs [52]. Code used to process data is available at www.animaltags.org.