Abstract
Objectives
There is limited data on upfront middle meningeal artery (MMA) embolization in the context of significant midline shift (MLS) (greater than 5mm) for the treatment of chronic subdural hematomas (cSDH). This study reports the temporal changes following MMA embolization as an upfront treatment of cSDH in patients with or without MLS and either mild, no symptoms or mild and stable neurological deficits.
Methods
A retrospective series of patients with a cSDH from a single institution in the United States between 2018-2020 was conducted. Eligible patients were treated with upfront MMA embolization.
Results
27 upfront MMA embolization procedures in 23 patients were included. Twelve patients had MLS of 5 millimeters or more (52%). The median maximal thickness at diagnosis was 18 mm [11-22]. The mean distance of MLS was 5 mm ±4. There were no procedural complications. The overall rescue surgery rate was 15%. A single rescue surgery secondary to an increase in hematoma thickness was required (4%). The temporal changes for both hematoma and MLS showed gradual improvement between 2 weeks and 4 weeks post-procedure. The average time-to-resolution of MLS was 46 days in patients with less than 5 mm MLS and 51 days in those with 5 mm or more.
Conclusion
Upfront MMA embolization for cSDH with a thickness up to 25 mm provides adequate symptom relief, stabilization and/or progressive resorption of the cSDH during follow-up in carefully selected asymptomatic or mildly symptomatic patients even in the presence of a MLS greater than 5 mm.
Keywords: Embolization, subdural hematoma, cerebrovascular, middle meningeal artery embolization, midline shift
Introduction
Background and objectives
Chronic subdural hematomas (cSDH) are one the most common yet challenging pathologies encountered in the field of neurosurgery, due to the relatively high recurrence rate following treatment with traditional surgical evacuation (2–37%).1–8 There is a rising incidence of subdural hematomas, especially among older populations due to aging, medical co-morbidities and the wide spread necessity of anticoagulation. It has been estimated that by 2030 there will be approximately 60,000 cases of cSDHs occurring each year in the United States. 9 Currently, the effectiveness of performing a transarterial embolization of the middle meningeal artery (MMA) as an upfront or adjunctive treatment for new or recurrent cSDH is being evaluated.10,11 However, unlike the immediate decompressive effect accomplished by surgical intervention, the course of radiological and neurological symptom resolution following MMA embolization remains to be elucidated.
While a specific amount of MLS in cSDH in which surgical evacuation is mandated has not been defined, many surgeons use the brain trauma guidelines for the management of acute SDH as a surrogate, specifically, when the amount of MLS is greater than 5mm. 12 Currently, there is little data available on MMA embolization in the context of significant MLS (greater than 5mm). The aim of the present study is two-fold; to provide data on the temporal changes observed in both radiologic and clinical findings following MMA embolization as an upfront treatment of cSDH, and to evaluate the effectiveness of this procedure in patients that present without symptoms or with mild and stable neurological deficits with 5 mm or more of midline shift.
Methods
Study design, setting and participants
We performed a retrospective cohort study of patients diagnosed with a cSDH from a single major academic institution in the United States between 2018–2020. Eligible patients were those treated with upfront middle meningeal artery embolization. Patients were excluded if they had a prior or an adjunctive treatment for the cSDH. Focal, non-convexity cSDHs’ were also excluded. Underlying conditions such as vascular malformations, tumors or hematomas outside the arterial supply of the middle meningeal artery were considered ineligible. Patients were included regardless of their functional status or the degree of midline shift. For this type of study formal consent is not required. This study received approval by the Institutional Review Board prior to its commencement.
MMA embolization, variables, data source and measurements
This study focused on patients with a chronic subdural hematoma treated with upfront MMA embolization. This procedure was performed under general anesthesia in all instances. A single heparin dose was administered in every case. Intraarterial access was accomplished through the femoral or the radial artery. After angiographic guidance into the middle meningeal artery, a combination technique is used for distal penetration with polyvinyl alcohol (PVA) particles (150–250 microns) followed by coil-embolization for permanent proximal trunk occlusion of the MMA. The endovascular procedure was considered successful when every targeted MMA branch was embolized without the presence of any intra-procedural related complications. The variables collected for this study included demographic characteristics from each patient, as well as lesion specific characteristics and procedural-related data-points. The data-source was the patients’ medical record, which contained the self-reported questionnaire, neurosurgery notes from the outpatient clinic, and radiologic reports of any imaging studies. The recorded variables included; patient age, sex, past medical history, medications, baseline functional status, clinical presentation, maximal thickness and volume of subdural hematoma and midline shift in millimeters, as reported in the radiologic computed tomography (CT) report.
The volumetric assessment was performed with the ABC/2 formula (A = maximal diameter in axial CT; B = maximal diameter perpendicular to the axial plane; C = number of CT slices multiplied by its thickness in mm). We also recorded clinical and radiological follow-up details such as the modified Rankin Score and radiologic improvement/worsening of the cSDH. Any procedural complications, rescue surgery or patient death was recorded if present.
Quantitative variables and statistical methods
Continuous data-points are reported with the mean and standard deviation or their median and interquartile range, depending on data-normality characteristics. Categorical variables are reported as proportions. Midline shift was recorded preoperatively, at 24 hours after the procedure, 2 weeks, 6 weeks and at 90 days post-procedure. Additionally, a figure was constructed summarizing the average values of maximal thickness, maximal volume and midline shift at each specific follow-up time, regardless of the degree of midline shift. These representative figures were constructed based on data-points available at each specific follow-up time, summarized as the median value of all the available data-points as well as the respective standard errors.
Results
Patient characteristics and indications for imaging
This study included 27 upfront MMA embolization procedures performed in 23 patients. There were 13 female patients (56%). The median age at presentation [IQR] was 74 [56–86]. In accordance with the inclusion criteria, there were no patients with either a prior SDH or a prior treatment of a known cSDH. There were 13 patients with a positive history for chronic antithrombotic therapy (10 patients under long-term antiplatelets, 43%; 3 patients on anticoagulation, 13%; none on both). The median baseline modified Rankin Score was 0 [0–2] and the most common reason for imaging was a fall, documented in 35% of the cases. A total of 12/23 patients had CNS related symptoms (52%; 7 with intracranial hypertension related symptoms such as headaches or nausea and 5 with a mild focal neurologic deficit). A total of 19 patients had a unilateral cSDH (83%). More than half of the patients had 5 millimeters or more of MLS (12 patients, 52%). The patients baseline characteristics can be found in Table 1.
Table 1.
Per-patient baseline characteristics consisting of 23 patients with 27 chronic subdural hematomas treated with upfront middle meningeal artery embolization.
| Variable | Per-patient number (%) (n=23) |
|---|---|
| Age, in years, median [IQR] | 74 [56–86] |
| Female | 13 (56%) |
| Antiplatelets agents use upon presentation | 10 (43%) |
| Anticoagulation use upon presentation | 3 (13%) |
| Baseline modified Rankin score | 0 [0–2] |
| Presentation | – |
| Fall | 8 (35%) |
| Intracranial hypertension related symptoms | 7 (30%) |
| Focal neurologic deficit | 5 (22%) |
| Asymptomatic | 3 (13%) |
| Maximal thickness, in mm; median [IQR] | 18 [11–22] |
| Midline shift | – |
| <5 | 11 (48%) |
| ≥5 | 12 (52%) |
| Midline shift, in mm, mean ± SD | 5 ± 4 |
N indicates the total number of patients; IQR, interquartile range; mm, millimeters.
Lesion characteristics and MMA embolization
The median maximal thickness at diagnosis was 18 mm [11–22] and the median maximal volume at diagnosis was 24 mL [13–47]. The mean distance of midline shift was 5 mm ±4. There were no periprocedural complications, and 3 cSDH in 3 patients required post procedure surgery. The overall rescue surgery rate was 15%. Only a single rescue surgery secondary to an increase in hematoma thickness was required (4%). Two patients, with a stable hematoma thickness at the second week post-embolization follow-up, developed seizures, necessitating surgical intervention. A total of 22 cSDHs’ (81%) involved the entire convexity, 3 were isolated to the frontal region (11%) and 2 were frontoparietal (7%). In terms of laterality, 18 cSDHs were in the left hemisphere (67%). Inflammatory membranes were identified in 21 (78%) cases based on radiographical imaging. The endovascular access of choice was through the femoral artery in 24 procedures (89%) and through the radial artery in 3 (11%).
The embolization materials used were particles (embosphere gold particles, EmboGold® Microspheres, MeritMedical) and coils (Target® Nano™ Detachable Coils, Stryker) in every case, with the position of the catheter prior to embolization at the origin of the MMA in 26 cases. A single case had the catheter positioned at the frontal branch prior to embolization. We carefully evaluated every case for potential collaterals to the ophthalmic segment and did not identify any. The procedure was technically successful in all 27 instances and there were no complications. The procedure related outcomes can be seen in Table 2.
Table 2.
Procedure and outcome related characteristics encountered in 27 chronic subdural hematomas treated with upfront middle meningeal artery embolization.
| Variable | Lesions with a midline shift less than 5 mm | Lesions with a midline shift more than 5 mm |
|---|---|---|
| Number (%) (n=15) | Number (%) (n=12) | |
| Procedure related complications | 0 (0%) | 0 (0%) |
| Rescue surgery | 2 (13%) | 1 (8%) |
| Length of stay post-procedurea | 2 [2–9] | 6 [2–8] |
| First improvement in thickness of hematoma, in days, median [IQR] | 23 [13–46] | 6 [3–11] |
| Hematoma thickness Improvement within 2 weeksb | 8 (53%) | 9 (75%) |
| First follow-up, in days, median [IQR] | 14 [11–23] | 9 [6–21] |
| Time-to 50% decrease in thickness, in days, median [IQR] | 50 [46–67] | 43 [28–51] |
| Complete resolution at last follow-up | 11 (73%) | 6 (50%) |
| Hematoma thickness at last follow-up, in mm, median [IQR] | 8 [3–12] | 4 [0–15] |
| Increased hematoma thickness during follow-up | 2 (13%) | 1 (8%) |
| Increased midline shift during follow-upa | 1 (9%) | 1 (8%) |
| Time-to-resolution of midline shift, in days, median [IQR]a | 46 [9–58] | 51 [43–70] |
| Midline shift at last follow-up, in mm, median [range]a | 0.13 ± 0.5 | 1.75 ± 3.2 |
| Modified Rankin score at last follow-up, median, [IQR] | 0 [0–1] | 0 [0–1] |
| Favorable modified Rankin score at last follow-upc | 13 (87%) | 10 (83%) |
| Follow-up, in months, median [IQR] | 5 [2–6] | 2 [1–4] |
Patients were grouped based on the degree of midline shift prior to intervention.
aPer-patient variable
b Missing data in 1 patient from each group.
cA favorable mRS status was considered in patients with mRS from 0–2.
Follow-up characteristics: hematoma thickness, midline shift and functional outcomes
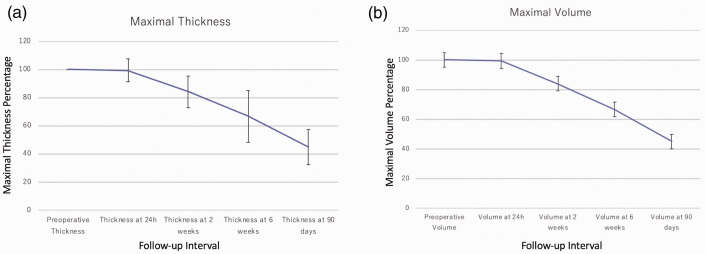
There were 15 cSDHs with less than 5 mm of MLS and 12 cases with 5 mm of MLS or more. The follow-up was observed at an interval of 2 weeks, 6 weeks and 12 weeks. Specifically, the median follow-up was 3 months [2–5]. In cSDHs with a MLS of less than 5 mm, the first follow-up image showing improvement in thickness was observed at a median of 23 days [13–46]. In those hematomas with a MLS of 5 mm or more, an initial improvement in maximal thickness was observed at a median of 6 days [3–11]. There were only 4 cSDHs that had an increase in their maximal thickness during follow-up. Three of these cases had subsequent reduction of the hematoma thickness (1 cSDH at the 6-week follow-up and 2 at 90 days) while one underwent retreatment. This translates into a rescue surgery in 3/27 patients, including two patients that did not experienced an increased thickness during follow-up. The average time course and its evolution in both maximal thickness and midline shift can be seen in Figure 1 and Figure 2. Greater than 50% improvement was seen in 92% of the patients and complete resolution was seen in 17 patients (63%). An illustrative case with complete resolution at 3 months can be seen in Figure 3. The average time-to a 50% reduction in the hematoma thickness was 50 days in cSDHs with 5 mm of MLS or less and 43 days in cSDHs with more than 5 mm of MLS. The median maximal hematoma thickness at last follow-up was 8 mm in the group with <5 mm MLS, and patients with a MLS of 5 mm or more had a median maximal hematoma thickness at last follow-up of 4 mm. From the 12 patients with CNS related symptoms (52%), nine had complete resolution of their symptoms at last follow-up.
Figure 1.
Line graph: hematoma maximal thickness and volume improvement during follow-up of a cSDH after treatment with upfront middle meningeal artery embolization. This line graph corresponds to the average thickness and volume among patients treated with an upfront middle meningeal artery embolization for a chronic subdural hematoma. Panel A, demonstrates the temporal changes in maximal thickness. Panel B, a volumetric assessment with the ABC/2 formula, demonstrates similar/parallel trending lines to that of the maximal thickness/width of the cSDH. The gray bars represent standard errors. The y axis represents the thickness percent relative to the x axis, the follow-up interval.
Figure 2.
Line graph: midline shift improvement during follow-up of a csDH after treatment with upfront middle meningeal artery embolization. This line graph corresponds to the average midline shift among 23 patients treated with an upfront middle meningeal artery embolization for a chronic subdural hematoma. The gray bars represent standard errors. The y axis represents the midline shift percent relative to the x axis, the follow-up interval.
Figure 3.
Illustrative case: a left convexity chronic subdural hematoma found in an 89-year-old male with a mild headache and tachycardia. Panel A demonstrates a supra-selective lateral view angiography of the middle meningeal artery (MMA) as seen prior to embolization. Panel B shows the supra-selective angiography of the MMA after particle embolization and coil-embolization for permanent proximal trunk occlusion. Panel C, an axial computed tomography scan prior to intervention showing a left-sided mainly-hypodense collection of fluid, compatible with a cSDH, with minimal displacement of midline structures and a side-by-side comparison of an axial CT scan including follow-up images at 1 and 3 month post-intervention.
Although there were 2 patients with a transient increase in MLS, all 23 patients in this series had midline shift resolution observed at last follow-up, with an average time-to-resolution of 46 days in patients with 5 mm of MLS or less, and 51 days in cSDH with 5 mm or more. Additional details on radiologic-related outcomes can be found in Table 2.
Discussion
In this study, we presented the temporal changes in radiologic and clinical parameters of patients treated with an upfront MMA embolization for cSDH. A total of 27 lesions were identified in 23 patients with one (4%) symptomatic hematoma enlargement post treatment. The hematoma thickness was significantly reduced at 2 weeks. Midline shift was significantly improved at 4 weeks when compared to baseline. A surgical rescue treatment was performed in one patient for hematoma enlargement and in two patients for seizure control but without hematoma enlargement at a median of 8 days [5–11] after MMA embolization.
Consistent with previous studies, the temporal changes observed herein serve as a guide that shows the changes in cSDHs one could expect after treatment with an upfront MMAE.10,11 Although MMA embolization has been reported as an efficient alternative to surgery for new or recurrent cSDH, or as a prophylactic measure to prevent recurrence after surgical evacuation,4,10,11 clear indications for this procedure remain unclear due to, in part, lack of data demonstrating the temporal effect of MMA embolization. Comparisons on both angiographic outcomes and safety parameters between treatment techniques have not yet been tested in a randomized trial, and these are key areas of ongoing efforts that will ultimately provide clearer indications for adequate patient selection. In the present study, the majority (89%) of patients were neurologically intact or with a mild and stable deficit at presentation. Upfront MMA embolization was performed in patients with different severity of midline shift, ranging from 0 to 11 mm, and almost half of our series consisted of patients with a MLS of 5 mm or more. Our findings suggest that MMA embolization as upfront treatment was beneficial (i.e. provides stabilization of the lesion, resolution of mass effect and thickness or resolution) in these carefully selected patients with MLS greater than 5 mm. Therefore, 5mm or more of MLS on imaging should not preclude a patient as a potential MMA embolization candidate.
In terms of the temporal effect obtained after performing an MMA embolization, hematoma reduction and improvement of midline shift started to manifest between 2 weeks and 4 weeks post procedure. This delay in effect suggests that this procedure may be less appropriate in patients with either moderate-to-severe symptoms, as the treatment may take up to 4 weeks to have a significant effect. On the other hand, neurological deterioration was observed in three (11%) patients, of which only a single patient (4%) had an associated hematoma enlargement, necessitating rescue treatment. The single patient who presented with an enlarged and retreated cSDH presented with a sudden onset left hemiparesis due to an ischemic stroke at the ipsilateral middle cerebral artery territory 11 days after the MMA embolization procedure.
The other 2 patients that had rescue surgery presented as paroxysmal disturbance in consciousness and therefore, underwent surgery to exclude any mass effect. Although the mechanism of these symptoms remains unclear, it is likely the patient suffered a seizure in the context of a stable cSDH. 13 Alternatively, mechanical compression by the hematoma could have also resulted in paroxysmal transient ischemic attacks. However, our experience suggest that rapid surgical intervention is effective in a small portion of patients who may require further treatment, as seen in these two patients in which there were no procedural complications after the rescue surgery and the symptoms resolved after salvage surgery.
Conclusion
Upfront MMA embolization for cSDH with a thickness up to 25 mm provides adequate stabilization and/or progressive reabsorption of the cSDH during follow-up as well as symptomatic relief in those that present with mild and/or stable neurological deficits. Careful patient selection for MMA embolization procedures should be based on assessing the severity of the presenting neurologic deficit and the imaging findings, specifically the hematoma thickness and degree of MLS. The benefit of an MMA embolization for cSDH is commonly seen after 2 weeks, which suggests that a close follow-up is necessary in these patients. MMA embolization was beneficial in these carefully selected patients with MLS greater than 5 mm. Surgical rescue treatment is a reasonable strategy in the setting of clinical deterioration regardless of the enlargement status of the hematoma.
Footnotes
Authors’ contribution: SG-P, YA and JMM conceived and design the study. SG-P, YA, AE-M and MMS collected the data. SG-P and YA analyzed and interpreted the data under the supervision of JMM. SG-P and YA did the statistical analyses. SG-P performed the literature search, drafted the paper and all authors reviewed and contributed important intellectual content and edited the manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Santiago Gomez-Paz https://orcid.org/0000-0003-2283-3612
Yosuke Akamatsu https://orcid.org/0000-0002-8149-2988
References
- 1.Ducruet AF, Grobelny BT, Zacharia BE, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev 2012; 35: 155–169; discussion 169. [DOI] [PubMed] [Google Scholar]
- 2.Miranda LB, Braxton E, Hobbs J, et al. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg 2011; 114: 72–76. [DOI] [PubMed] [Google Scholar]
- 3.Brodbelt A, Warnke P. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry 2004; 75: 1209–1210; author reply 1210. [PMC free article] [PubMed] [Google Scholar]
- 4.Almenawer SA, Farrokhyar F, Hong C, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg 2014; 259: 449–457. [DOI] [PubMed] [Google Scholar]
- 5.Ivamoto HS Lemos HP JrandAtallah AN.. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg 2016; 86: 399–418. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Bakker NA, Groen RJ. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg 2014; 121: 665–673. rossRef][ 10.3171/2014.5.JNS132715] [DOI] [PubMed] [Google Scholar]
- 7.Xu CS, Lu M, Liu LY, et al. Chronic subdural hematoma management: clarifying the definitions of outcome measures to better understand treatment efficacy – a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 2017; 21: 809–818. [PubMed] [Google Scholar]
- 8.Gernsback J, Kolcun JP, Jagid J. To drain or two drains: recurrences in chronic subdural hematomas. World Neurosurg 2016; 95: 447–450. [DOI] [PubMed] [Google Scholar]
- 9.Balser D, Farooq S, Mehmood T, et al. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg 2015; 123: 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ban SP, Hwang G, Byoun HS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology 2018; 286: 992–999. [DOI] [PubMed] [Google Scholar]
- 11.Link TW, Boddu S, Paine SM, et al. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery 2019; 85: 801–807. [DOI] [PubMed] [Google Scholar]
- 12.Bullock MR, Chesnut R, Ghajar J, et al. Guidelines for the surgical management of traumatic brain injury author group. Neurosurgery 2006; 58: S2-1–S2-3. [Google Scholar]
- 13.Kotwica Z, Brzeinski J. Epilepsy in chronic subdural haematoma. Acta Neurochir (Wien) 1991; 113: 118–120. [DOI] [PubMed] [Google Scholar]