Abstract
Introduction
Somatic KRAS mutations have been identified in the majority of brain arteriovenous malformations (AVM) specimens. The aim of our study was to evaluate the prevalence of Kirsten rat sarcoma (KRAS)/murine sarcoma viral oncogene homolog B1 (BRAF) mutations in brain AVM.
Methods
A systematic literature review was performed in November 2019. We reviewed MEDLINE/PubMed, Cochrane Library, and ClinicalTrials.gov for citation or ongoing trials from January 2010 to March 2020.
Results
6 studies were identified as meeting the inclusion criteria of this review. The total frequency of KRAS mutations in 1726 patients with AVM was 55%. The prevalence of BRAF mutation was 7.5%. The prevalence of AVMs with grade 2 was the most (39%). Frontal and parietal lobes were the commonest sites of AVMs (21%). the most prevalent presentation of patients with AVM was hemorrhage (62%).
Conclusion
Our findings support a high prevalence of somatic activating mutations in KRAS and less commonly, BRAF in the overwhelming majority of brain AVMs. Practically and importantly, this pathway homogeneity in CNS arteriovenous malformations also supports the development of targeted therapies with RAS/RAF pathway inhibitors. However, more studies are needed to confirm this hypothesis.
Keywords: KRAS, BRAF, brain arteriovenous malformation
Introduction
Brain arteriovenous malformations (BAVMs) are high flow vascular abnormalities that occur in the brain in 15 cases per 100,000 people and cause hemorrhagic stroke in children.1–4 These malformations are abnormal tortuous and morphological vascular channels between the arteries and veins and lack a mediating capillary network, which causes high-pressure of arterial blood to enter the veins directly from the feeding arteries. The underlying cause of cerebral arteriovenous malformations is not known, but similar malformations are found in rare genetic syndromes such as hereditary hemorrhagic telangiectasia (a set of disorders caused by inactivation of germinal mutations in β-SMAD-regulating growth factor regulator)5–7 and also in capillary malformation-arteriovenous malformation syndrome (CM-AVM) (RASopathy due to inactivation of RASA1 or EPHB4 mutations).8,9 Based on this pattern, genetics may be the cause of cerebral arteriovenous malformations, but most of these malformations occur as sporadic malformations in people without a family history of the disease 2 Hemorrhagic stroke, or intracerebral hemorrhage, refers to the rupture of an artery in the brain. Rare cerebral arteriovenous malformations with a family history may occur in connection with terminal mutations as hereditary hemorrhagic telangiectasia (caused by a mutation in ENG, ACVRL1, and SMAD4) or capillary malformation-arteriovenous malformation (caused by RASA1 mutation as well as EPHB4).3,8,10–12 However, majority of intracranial arteriovenous malformations are non-hereditary and sporadic. Somatic mutations in KRAS have been reported in most cases of cerebral arteriovenous malformations.13–15 The pathogenetic mechanisms that happens as a result of KRAS-initiated activation of the MAPK–ERK pathway are mediated by angiogenic genes (e.g., VEGF A/C) and proteins in the notch signaling pathway. 16 This study aimed to investigate the prevalence of KRAS/BRAF mutation in cerebral arteriovenous malformations.
Materials and methods
Data sources and search strategy
A systematic literature review was performed in November 2019. We reviewed MEDLINE/PubMed, Cochrane Library, and ClinicalTrials.gov for citation or ongoing trials from January 2010 to March 2020. The search criteria were limited to human studies published in English language. The Medical Subject Heading terms used for the search in PubMed were ‘KRAS’, ‘BRAF’, ‘arteriovenous malformations’ or ‘AVM’, ‘brain’ and ‘somatic mutation’.
Selection criteria
To be included in this review, an article had to fulfill the following inclusion criteria: performed in patients with AVM; report results of genetic tests for KRAS mutation status (containing BRAF mutational status or not) from resected specimens. We included studies published after 2010 in order to reflect more updated results regarding recent surgical techniques. Duplicate articles or articles that were on the basis of the same patient cohorts were not included: if inclusion of another published cohort was specified, we included the article with the higher number of patients, if not specified, we included the older article.
Data extraction
Data extraction was conducted independently according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses. Two authors selected studies following the previously described inclusion criteria. After this first selection, if needed, another author was consulted to achieve a shared decision. A predefined protocol for data extraction was used to retrieve data of each study, including first author name, year of publication, journal of publication, study period, sample size, study design, demographic profile, KRAS mutational status, BRAF mutational status, surgical interventions, other procedures and significant variable at univariate and multivariate analyses.
Statistical analysis
Meta-analysis was carried out using the inverse variance method, in which the specific weight of each study among the pool is calculated as the reciprocal of the squared standard error. The heterogeneity among the studies was evaluated using the Cochran Q and of the I2 statistics. Fixed and random effects were reported.
Results
Literature search results
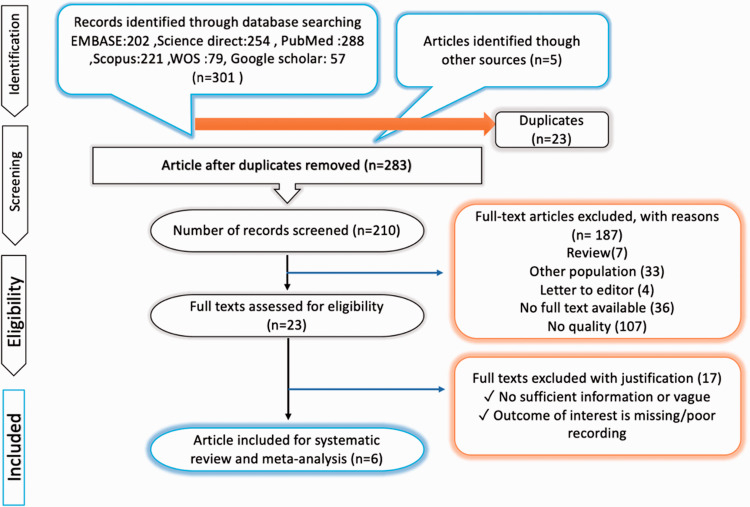
The electronic search provided a total of 306 results. After screening, 23 were eliminated because they were duplicates and 73 were removed because they were not in English language; 187 were excluded because they were reviews, editorial letters, case reports, no full text available or no quality. At the end of the review process, 6 studies were identified as meeting the inclusion criteria of this review. These articles constitute the study population (Figure 1). Study characteristics are shown in Table 1 and Figure 1. The Cochrane database of systematic review was then cross-checked to ensure that no similar systematic reviews had been undertaken.
Figure 1.
PRISMA flow diagram.
Table 1.
Characeristics of included studies.
| Author/year | Mean age | M/F | N | MKRAS | MBRAF |
Location |
presentation |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | O | F | C | seizure | hemorrhage | headache | ||||||
| Hong et al. 14 | 28.6 | 0.4 | 21 | 76.2 | 4.8% | 28.5% | 28.5% | 14.2% | 14.2% | 14.2% | N/A | N/A | N/A |
| Nikolaev et al. 13 | N/A | N/A | 72 | 62.5% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Goss et al. 17 | 19 | 1.4 | 22 | 40.9% | 9% | 13.6% | 13.6% | 13.6% | 9% | N/A | 3 | 5 | 3 |
| Goss et al. 18 | 15.5 | 0.7 | 16 | 50% | 12.5% | 31.2% | 25% | 25% | 18.7% | 0.0 | 5 | 0 | 10 |
| Oka et al. 19 | 28.5 | 1.1 | 38 | 65.7% | N/A | 13.1% | 23% | 16.4% | 42.6% | 4.9% | 14 | 32 | N/A |
| Priemer et al. 15 | 32 | 0.7 | 21 | 28.5% | N/A | 19% | 23.8% | 9.5% | 38% | 4.7% | 4 | 10 | 5 |
MBRAF: prevalence of mutant BRAF; MKRAS: prevalence of mutant KRAS; M/F: male to female ratio; N: sample size; T: temporal lobe; P: parietal lobe; O: occipital lobe; Frontal lobe; C: cerebellum; N/A: not available.
Meta-analysis of frequency of KRAS and BRAF mutations
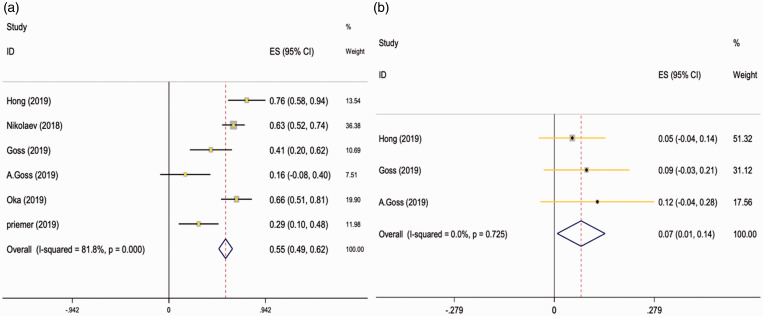
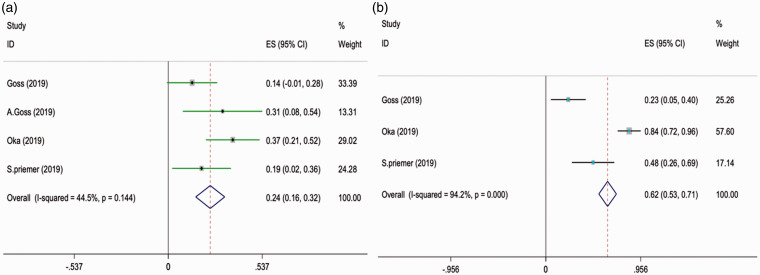
Based on the random effect model, the total frequency of KRAS mutations in 1726 patients with AVM was 55% (95% confidence interval [CI]:48%–62%, I2 = 81.8%) (Table 2, Figure 2(a)). The prevalence of BRAF mutation was 7.5% (95% confidence interval [CI]:0.8%-14.1%) (Table 2, Figure 2(b)).
Table 2.
Meta-analysis results of the prevalence of KRAS and BRAF mutations in BAVMs.
| Somatic mutations and patient characteristics |
95% Conf. interval |
||||
|---|---|---|---|---|---|
| Low | Up | ES | Weight | ||
| KRAS and BRAF mutations prevalence | KRAS | 0.48 | 0.62 | 0.55 | 100 |
| BRAF | 0.008 | 0.141 | 0.075 | 100 | |
| Spetzler-Martin grade | 1 | 0.14 | 0.36 | 0.25 | 100 |
| 2 | 0.26 | 0.51 | 0.39 | 100 | |
| 3 | 0.18 | 0.41 | 0.30 | 100 | |
| 4 | 0.016 | 0.057 | 0.021 | 100 | |
| Location of AVM | T | 0.126 | 0.137 | 0.131 | 100 |
| P | 0.143 | 0.291 | 0.217 | 100 | |
| O | 0.082 | 0.208 | 0.145 | 100 | |
| F | 0.143 | 0.283 | 0.213 | 100 | |
| C | 0.008 | 0.112 | 0.060 | 100 | |
| Presentation | Seizure | 0.157 | 0.322 | 0.240 | 100 |
| Hemorrhage | 0.53 | 0.71 | 0.62 | 100 | |
| Headache | 0.19 | 0.25 | 0.210 | 100 | |
Figure 2.
Meta-analysis of KRAS (a)/BRAF (b) mutations in BAVMs.
Meta-analysis of Spetzler-Martin grade
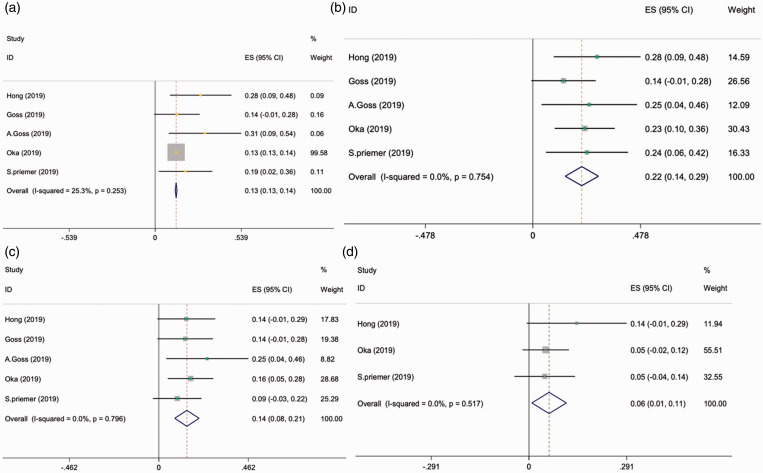
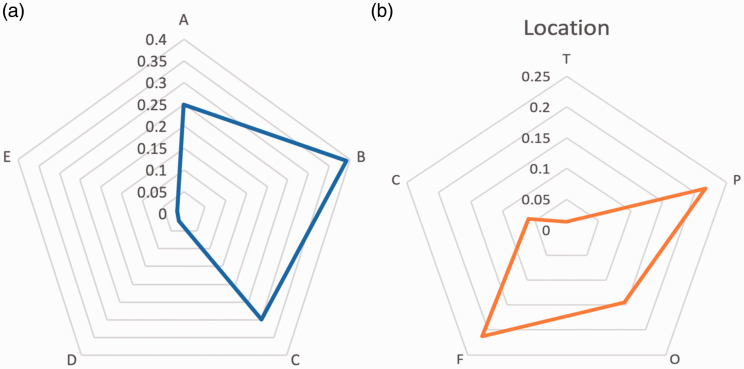
Based on the random effect model, the prevalence of grade 2 Spetzler-Martin AVMs was the most(with the prevalence of 39% (95% confidence interval [CI]:026%-51%)).followed by grade3 (with the prevalence of 30% (95% confidence interval [CI]:18%-41%)), grade1(with the prevalence of 25% (95% confidence interval [CI]:14%-36%)) and grade 4 (with the prevalence of 2.1%(95% confidence interval [CI]:1.6%-5.7%)) (Table 2, Figures 3 and 4(a)).
Figure 3.
Meta-analysis of Spetzler-Martin grade 1(a), 2(b), 3(c) and 4(d).
Figure 4.
Radar chart of Spetzler-Martin grade (a) and location (b) in BAVMs.
Meta-analysis of location of AVM
Based on the random effect model, Frontal and parietal lobes were the commonest sites of AVMs (with the prevalence of 21%) followed by Occipital (with the prevalence of 14.5%), Temporal (with the prevalence of 13.1%) and cerebellum (with the prevalence of 6%) (Table 2, Figure 4(b)).
Meta-analysis of presentation of AVM
Based on the random effect model, the most prevalent presentation of patients with AVM was hemorrhage (with the prevalence of 62%) followed by seizure (with the prevalence of 24%) (Table 2, Figure 5).
Figure 5.
Meta-analysis of presentation of AVM (hemorrhage (a) and seizure (b)).
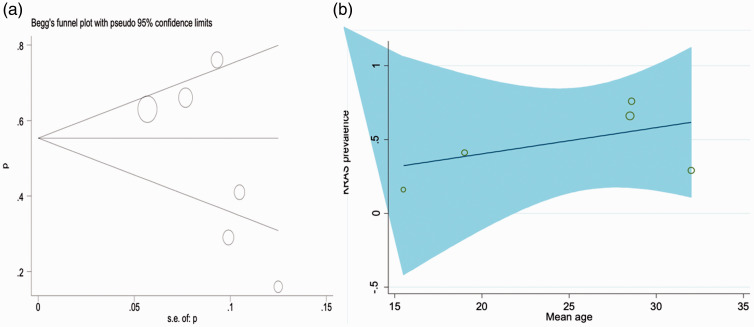
Publication bias
The funnel plot resembled symmetrically which means that there is no publication bias. The size of each circle in the figure is an indicator of the sample size of the corresponding study (bigger circles shows more sample and smaller circles shows fewer sample size).
Meta-regression finding based on mean age of participants and frequency of KRAS mutations
The studies’ meta-regression was according to the association between frequency of KRAS mutations and the mean age of the participants, and the overall rate of KRAS mutations. There was no statistically significant linear trend in univariate meta-regression to explain effect size variation by mean of age of study with coefficient = 0.14 (95% CI –2.17, 2.46), P = 0.88 (Figure 6(b)).
Figure 6.
Begg’s funnel plot for publication bias (a). Meta-regression finding based on the mean of age and frequency of KRAS mutations (b).
Discussion
BAVMs are of important causes of intracerebral hemorrhage. In addition to the clinical signs associated with hemorrhage, BAVMs() have symptoms such as seizure or headache, and the rest of these patients (at least 15%) are asymptomatic. 3 In this study, in line with previous studies, the most common symptoms of BAVMs were hemorrhage (62%), seizure (24%), and headache (21%). These findings were consistent with a study by Schaller et al who reported hemorrhage as the most common symptom of BAVMs with a prevalence of 50%, as well. 20 In most cases, BAVMs are sporadic, but familial cases are rare, and BAVMs have been reported in several cases of congenital syndromes.21,22 For example, the prevalence of BAVMs has been reported in 4 to 13% of patients with hereditary hemorrhagic telangiectasia.23,24 In this systematic review and meta-analysis, the prevalence of mutant KRAS and BRAF genes in patients with BAVMs were assessed. Based on the results of preliminary studies, the total prevalence of mutant KRAS gene in this study was 55% in patients with BAVMs.Out of 6 studies included in the present meta-analysis 4 of them reported the presence of KRAS mutations in more than half of their cases which may indicate the pathogenic role of KRAS mutations. KRAS-activated mutations play a pathogenic role in development of some tumors, and the presence of KRAS mutant genes is an independent prognostic factor in a number of tumors.25–27 KRAS gene mutation is mostly associated with tumor formation and cancer development. These mutant genes activate growth factor signal transduction pathways, thereby resulting in cell change and genome instability.16,28–30 The BRAF mutations are of other gene mutations that are considered as prognostic factors in some types of cancer. In their systematic review and meta-analysis, Ny et al. concluded that malignant melanoma patients with mutant type of BRAF genes had a poorer prognosis and were more at risk compared to patients with wild type BRAF. 31 In contrast, Vuong et al in their systematic review and meta-analysis reported that BRAF mutations had a favorable prognostic impact in gliomas and their prognostic value might be dependent on patient’s age and tumor grade. 32 In another study, Xing et al. reported that the prevalence of mutant BRAF genes in patients with papillary thyroid carcinoma was 45%. 33 The prevalence of BRAF in this study was 7% in patients with BAVMs. All of the mutations found in AVM specimens including BRAF and KRAS mutations has been reported to be oncogenic activators and cancer growth stimulators which is in contrast with the presence of these mutations in BAVM specimens, because AVMs are not malignant vascular growth. This indicates the context-dependent and tissue-specific role of the RAS/RAF pathway in vascular tissue or it might demonstrate that there is a requirement for multiple additional genetic hits in order to develop cancer which is opposed to a monogenetic nature of arteriovenous malformations.16,34The results of frequent and common KRAS mutations in BAVMs, the pathogenic nature of those mutations, the lack of KRAS mutations in cerebrovascular malformations other than BAVMs 13 and the direct development of arteriovenous malformations by induction of mutation in an animal model 35 suggest that KRAS mutations occur in arteriovenous malformations due to endothelial proliferation, angiogenic signaling, or vascular remodeling process. Also, the lack of relationship between the frequency of mutations with age in this study is inconsistent with the hypothesis of passenger mutations, in which KRAS/BRAF mutations increase randomly over time. In human specimens of Brain AVMs, activated form of NF-kB can be found in some types of endothelial and inflammatory cells. 36 On the other hand, studies conducted about VEGF illustrated that it plays a great role in different pathogenic sets such as causing age-related macular degeneration by angiogenesis stimulation and raising the permeability of vessels as well as activating intracellular signaling in a RAS-dependent manner.VEGF-C and VEGF-A both are recognizable using immunohistochemistry in specimens of patients with brain AVM. 37 There are also other studies indicating the high prevalence of VEGF expression in patients with brain AVM compared with the normal cerebral cortex.38,39 It has been reported that the induction of KRAS mutations in endothelial cells has performed as an exacerbating factor of brain AVM which can be explained by the fact that KRAS mutation can increase the endothelial cell migratory activity and It can also induces other genes which promote angiogenesis. These results are also in line with the results of other studies demonstrating a correlation between the presence of KRAS mutations and Spetzler-Martin grade of brain AVMs. 19 Realizing that a high proportion of BAVMs have KRAS or BRAF mutations, in potential clinical trials, the treatment can be initiated without prior tissue sampling and genetic confirmation, which is not possible without surgery in BAVMs.In a recent case report, the therapeutic response to trametinib which is an anti-cancer drug was evaluated in a patient with mutant KRAS AVM, and showed a significant decrease in blood flow to the AVM, with a 75% reduction in arterial inflow after 6 months of trametinib therapy. 15 these results suggests that KRAS function may contribute to the pathobiology of BAVM and can become a therapeutic target.
Limitations
The meta-analysis does not show possible differences between the different types of KRAS and BRAF mutations because KRAS mutations were not distinct in these studies and therefore no information was extracted for analysis. Therefore, due to the lack of information in the studies, it was not possible to compare the rate of BAVMs and grading between the mutant group and the wild type.
Conclusion
The findings of this study show the high prevalence of somatic activating mutations in KRAS and BRAF in most cases of brain arteriovenous malformations. Practically and more importantly, the pathway homogeneity in the central nervous system (CNS) arteriovenous malformations indicates the development of targeted therapies with Ras/Raf pathway inhibitors. However, more studies are needed to confirm this hypothesis.
Footnotes
Availability of data and supporting materials section: Please contact author for data requests
Author contribution: GB participated in Conception and design of the study, library searches and assembling relevant literature, critical review of the paper, supervising writing of the paper, Database management. MS and FP participated in Data collection, library searches and assembling relevant literature, writing the paper, and critical review of the paper.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fateme Parooie https://orcid.org/0000-0002-2367-3780
References
- 1.Brown RD, Jr, Wiebers DO, Torner JC, et al. Incidence and prevalence of intracranial vascular malformations in Olmsted county, Minnesota, 1965 to 1992. Neurology 1996; 46: 949–952. [DOI] [PubMed] [Google Scholar]
- 2.Berman MF, Sciacca RR, Pile-Spellman J, et al. The epidemiology of brain arteriovenous malformations. Neurosurgery 2000; 47: 389–397. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shahi R, Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain 2001; 124: 1900–1926. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shahi R, Fang JS, Lewis SC, et al. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry 2002; 73: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasia type 1. Nat Genet 1994; 8: 345–351. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 1996; 13: 189–195. [DOI] [PubMed] [Google Scholar]
- 7.Gallione CJ, Repetto GM, Legius E, et al. A combined syndrome of juvenile pol- yposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 2004; 363: 852–859. [DOI] [PubMed] [Google Scholar]
- 8.Revencu N, Boon LM, Mulliken JB, et al. Parkes weber syndrome, vein of galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat 2008; 29: 959–965. [DOI] [PubMed] [Google Scholar]
- 9.Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation 2017; 136: 1037–1048. [DOI] [PubMed] [Google Scholar]
- 10.McDonald J, Bayrak-Toydemir P, Pyeritz RE. Hereditary hemorrhagic telangiectasia: an overview of diagnosis, management, and pathogenesis. Genet Med 2011; 13: 607–616. [DOI] [PubMed] [Google Scholar]
- 11.Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation–arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet 2003; 73: 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiex R, Mulliken JB, Revencu N, et al. A novel association between RASA1 mutations and spinal arteriovenous anomalies. AJNR Am J Neuroradiol 2010; 31: 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med 2018; 378: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong T, Yan Y, Li J, et al. High prevalence of KRAS/BRAF somatic mutations in brain and spinal cord arteriovenous malformations. Brain 2019; 142: 23–34. [DOI] [PubMed] [Google Scholar]
- 15.Priemer DS, Vortmeyer AO, Zhang S, et al. Activating KRAS mutations in arteriovenous malformations of the brain: frequency and clinicopathologic correlation. Hum Pathol 2019; 89: 33–39. [DOI] [PubMed] [Google Scholar]
- 16.Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell 2017. ; 170: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goss JA, Smits PJ, Greene AK. Genotype-phenotype analysis of somatic mutations in intracranial arteriovenous malformations. J Am Coll Surg 2019. ; 229: S190. 1 [Google Scholar]
- 18.Goss JA, Huang AY, Smith E, et al. Somatic mutations in intracranial arteriovenous malformations. PloS One 2019; 14: e0226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka M, Kushamae M, Aoki T, et al. KRAS G12D or G12V mutation in human brain arteriovenous malformations. World Neurosurg 2019; 126: e1365-73–e1373. [DOI] [PubMed] [Google Scholar]
- 20.Schaller K. AVM Presentation. In: Beneš V., Bradáč O. (eds) Brain Arteriovenous Malformations. Springer, Cham, 2017. DOI: 10.1007/978-3-319-63964-2_6.
- 21.van Beijnum J, van der Worp HB, Schippers HM, et al. Familial occurrence of brain arteriovenous malformations: a systematic review. J Neurol Neurosurg Psychiatry 2007; 78: 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krings T, Kim H, Power S, et al.; Brain Vascular Malformation Consortium HHT Investigator Group. Neurovascular manifestations in hereditary hemorrhagic telangiectasia: imaging features and genotypephenotype correlations. AJNR Am J Neuroradiol 2015; 36: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porteous ME, Burn J, Proctor SJ. Hereditary haemorrhagic telangiectasia: a clinical analysis. J Med Genet 1992; 29: 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roman G, Fisher M, Perl DP, et al. Neurological manifestations of hereditary hemorrhagic telangiectasia (Rendu-Osler-weber disease): report of 2 cases and review of the literature. Ann Neurol 1978; 4: 130–144. [DOI] [PubMed] [Google Scholar]
- 25.Bournet B, Muscari F, Buscail C, et al. KRAS G12D mutation subtype is a prognostic factor for advanced pancreatic adenocarcinoma. Clin Translat Gastroenterol 2016; 7: e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogura T, Yamao K, Hara K, et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J Gastroenterol 2013; 48: 640–646. [DOI] [PubMed] [Google Scholar]
- 27.Fiala O, Buchler T, Mohelnikova-Duchonova B, et al. G12V and G12A KRAS mutations are associated with poor outcome in patients with metastatic colorectal cancer treated with bevacizumab. Tumor Biol 2016; 37: 6823–6830. [DOI] [PubMed] [Google Scholar]
- 28.Jinesh GG, Sambandam V, Vijayaraghavan S, et al. Molecular genetics and cellular events of K-Ras-driven tumorigenesis. Oncogene 2018; 37: 839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haigis KM. KRAS alleles: the devil is in the detail. Trends Cancer 2017; 3: 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauen KA. The RASopathies. Annu Rev Genomics Hum Genet 2013; 14: 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ny L, Nyakas M, Hernberg M, et al. BRAF mutation as a prognostic marker for survival in malignant melanoma: A systematic review and meta-analysis. [DOI] [PubMed]
- 32.Vuong HG, Altibi AM, Duong UN, et al. BRAF mutation is associated with an improved survival in glioma – a systematic review and meta-analysis. Mol Neurobiol 2018; 55: 3718–3724. [DOI] [PubMed] [Google Scholar]
- 33.Xing MB. BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005. ; 12: 245–262. [DOI] [PubMed] [Google Scholar]
- 34.Prior IA, Lewis PD, Mattos C. A comprehensive survey of ras mutations in cancer. Cancer Res 2012; 72: 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest 2018; 128: 1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aziz MM, Takagi Y, Hashimoto N, et al. Activation of nuclear factor kappaB in cerebral arteriovenous malformations. Neurosurgery 2010; 67: 1669–1679. [DOI] [PubMed] [Google Scholar]
- 37.Koizumi T, Shiraishi T, Hagihara N, et al. Expression of vascular endothelial growth factors and their receptors in and around intracranial arteriovenous malformations. Neurosurgery 2002; 50: 117–124. [DOI] [PubMed] [Google Scholar]
- 38.Wu CY, Wang YH, Meng FG, et al. Development of stereotactic neurosurgery in China. Neurosurgery 2005; 56: 851–860. [DOI] [PubMed] [Google Scholar]
- 39.Edwards EA, Phelps AS, Cooke D, et al. Monitoring arteriovenous malformation response to genotype-targeted therapy. Pediatrics 2020; 146: e20193206. [DOI] [PubMed] [Google Scholar]