Abstract
Background
Basilar artery perforator aneurysms (BAPAs) are rare. Traditional surgical clipping and endovascular coiling have proven to be challenging. We present three patients treated with endovascular electrothrombosis and describe the characteristics of this new approach.
Methods
Three patients presented with subarachnoid hemorrhages (SAHs). Cerebral angiography identified BAPAs. Endovascular electrothrombosis was performed after obtaining informed consent. We placed the microwire into the sac of the aneurysms through the microcatheter and connected its proximal tip to the Solitaire stent detachment system. Electrothrombosis was conducted using 1.0 mA current.
Results
Two aneurysms were successfully occluded without treatment-related complication. The third one failed and converted to endovascular coiling using a 1.3-F microcatheter. The patient suffered brainstem infarction and finally died of severe SAH. At follow-up, the two patients were neurologically intact and angiography showed total occlusion of both aneurysms.
Conclusion
Endovascular electrothrombosis might be a potential alternative to traditional treatment for BAPAs. Close follow-up with caution should be mandatory. More research is needed to confirm its safety and efficacy.
Keywords: Basilar artery, microaneurysm, subarachnoid hemorrhage, endovascular procedures
Introduction
Basilar artery perforator aneurysm (BAPA) is a rare subtype of intracranial aneurysm, first described by Ghogawala et al. in 1996. 1 Fewer than 60 cases have been reported in the English literature. The natural history of the disease is unknown. Traditional treatment for aneurysms such as clipping or coiling has proven challenging. 2 Recently, endovascular electrothrombosis was suggested to be a potential alternative to BAPAs. 3 In this study, we reported three consecutive BAPA cases of endovascular electrothrombosis.
Case description
Case 1
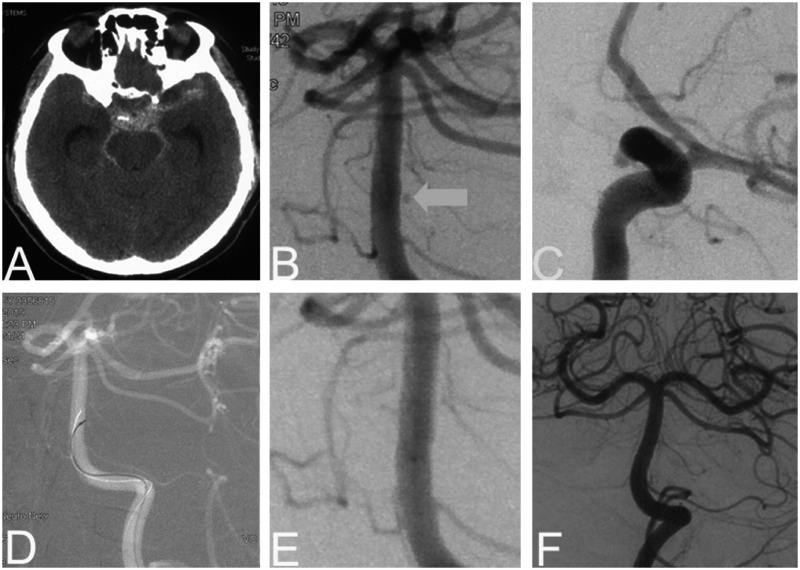
A 48-year-old man presented with a sudden headache accompanied by nausea and vomiting. On admission, the Glasgow score (GCS) was 13 and the WFNS grade was I. Cranial computed tomography (CT) revealed SAH of Fisher grade 2 (Figure 1). Under general anesthesia, emergent cerebral angiography was performed and two aneurysms were detected. The first was a BAPA (0.8 mm) arising from the lateral dorsal wall of the basilar trunk (Figure 1). The second (2.9 mm) was a left paraclinoid wide-necked aneurysm. We believed the BAPA was ruptured and decided to embolize it immediately. We first tried to catheterize with an Echelon 10 microcatheter (ev3, Endovascular, Plymouth, MN, USA) into the aneurysm but failed because of its very narrow neck. After obtaining informed consent, we performed endovascular electrothrombosis. We placed a Traxcess-14 micro guidewire (MicroVention, Tustin, CA, USA) into the sac of the aneurysm through the microcatheter, and connected its proximal tip to the positive electric probe of the Solitaire stent detachment system (ev3) (Figure 1). The negative electrode was connected to the groin of the patient using a needle. Electrothrombosis was conducted at 4.0 V and 1.0 mA current three times for 30 s each. Immediate control cerebral angiography revealed total occlusion (Figure 1). Upon awakening, the patient showed no novel neurological deficits. One-week follow-up cerebral angiography revealed complete obliteration. Two months later, the patient was free of symptoms and we treated the other aneurysm with stent-assisted coiling. At a six-month follow-up, the patient was neurologically intact with antiplatelet medication. There was no recurrence at 18-month follow-up.
Figure 1.
Patient 1. A 48-year-old man presented with acute SAH (a). Preoperative angiography revealing a BAPA (<1 mm, b) and a paraclinoid wide-necked aneurysm (3 mm, c). We placed a Traxcess-14 microwire into the sac of the perforator aneurysm and started electrothrombosis (d). Immediate control DSA showed occlusion of the aneurysm (e). The paraclinoid aneurysm was treated two month later. At six-month follow-up, cerebral angiography showed no signs of recurrence (f).
Case 2
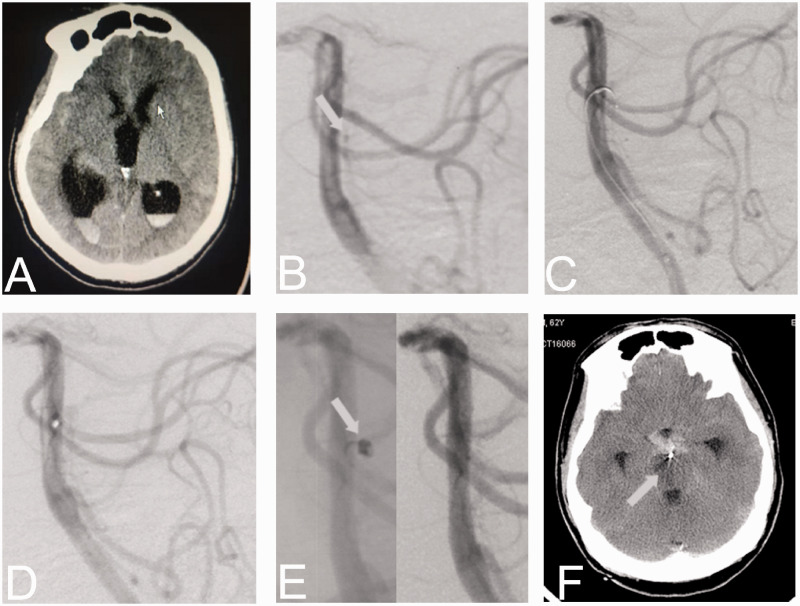
A 65-year-old man presented with a coma after a sudden-onset headache. The GCS was 6 and WFNS grade was grade IV. Cerebral CT scan revealed diffuse SAH with intraventricular hemorrhage in bilateral ventricles. The Fisher grade was 4 (Figure 2). Cerebral DSA showed ruptured BAPAs in the middle 1/3 of the basilar artery (Figure 2). We initially failed to pass an Echelon 10 microcatheter into the aneurysm and converted to electrothrombosis. After electrothrombosis three times (total of 3 min) at 4 V and 1 mA current, there remained persistent contrast filling of the aneurysm. We retried the catheterization of the microcatheter and finally succeeded with a Headway DUO 1.3-F microcatheter (MicroVention). A Target NANO 1/2 coil (Stryker Neurovascular, Fremont, CA, USA) was inserted into the aneurysm cavity. Final angiography suggested total occlusion (Figure 2). After the operation, the patient still suffered severe intracranial hypertension; therefore, the external ventricular drainage was performed. Post-operational CT revealed a lacunar infarction of the right brainstem which was thought to be due to the occlusion of perforator artery. We performed lumbar cistern drainage to alleviate the severe subarachnoid hemorrhage. The patient’s condition remained stable in the following days. On the morning of the fourth day, the patient suffered acute hyperthermia (39°C) with worsening of his neurological deficits. In the afternoon, his oxygen saturation and the blood pressure continued to decrease, and he was placed on mechanical ventilation. All physiological reflexes disappeared in the next day. The patient finally died of many factors including severe subarachnoid hemorrhage, obstructive hydrocephalus, hyperpyrexia induced by intracranial infection, and respiratory failure.
Figure 2.
Patient 2. A 65-year-old man with brain CT scan revealing diffuse SAH (a). Cerebral DSA revealing a BAPA at the middle 1/3 of the basilar artery (b). After electrothrombosis for three times, there remained persistent contrast filling of the aneurysm (c). Catheterization was retried and a Target NANO 1/2 coil was successfully inserted into the aneurysm (d and e). Post-operational CT revealed a lacunar infarction of the right brainstem (f).
Case 3
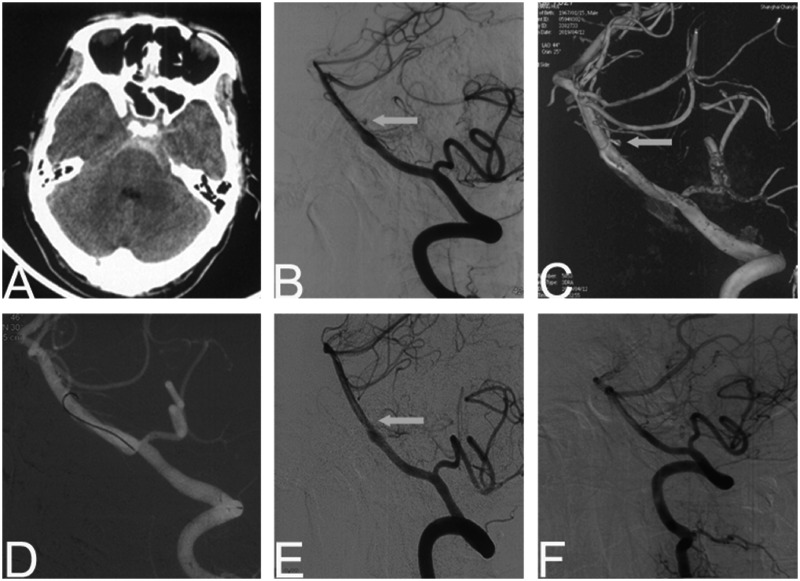
A 52-year-old man presented with vomiting after a sudden headache. Brain CT revealed SAH of Fisher grade of 2 (Figure 3). On admission, the GCS was 12, and WFNS grade was I. Cerebral angiography revealed a fenestration at the proximal segment of the basilar artery. A tiny perforator aneurysm with a maximal diameter of 1.3 mm was detected near the fenestration (Figure 3). We tried to use a Headway DUO 1.3 F microcatheter (MicroVention) to catheterize the aneurysm but failed. Thereafter, a 1.5-min electrothrombosis succeeded uneventfully (Figure 3). Cranial CT performed one day later showed no novel infarction. After one week, FU cerebral angiography showed complete disappearance of the aneurysm. At his 6-month follow-up, the patient was neurologically intact, and FU DSA showed no signs of aneurysm recurrence (Figure 3). The patient was normal at 18-month follow-up.
Figure 3.
Patient 3. A 52-year-old man presented with sudden headache. Cranial CT revealing diffuse SAH (a). Cerebral angiography and three-dimensional representation revealing a perforator aneurysm (1.3 mm) near the fenestration of basilar artery (b and c). The electrothrombosis was performed for 1.5 min (d) and the aneurysm disappeared (e). At six-month follow-up, DSA showed no signs of aneurysm recurrence (f).
Discussion
Basilar artery perforator aneurysms are rare; however, they may lead to severe SAH. These lesions are often small and partially thrombosed and located at the dorsally basilar location and tiny diameter of the parent artery.4,5
Conservative treatment was considered as a viable treatment option for tiny BAPAs. 6 However, a review showed an 11.1% re-hemorrhage rate after a mean of 15 days post-ictus and 27.8% of the conservatively-treated patients developed perforator infarction with clinical consequences. The risk of aneurysm rupture remained. 7 For non-conservative management, microsurgical management is extremely difficult. 8 The anatomic location and small diameter of perforator arteries make the aneurysms hard to find and clip. The possible cranial nerve injuries and damage of normal brain tissues are concerned. 9 Clipping the proximal end of parent arteries is the major operative maneuver, because the necks of these aneurysms are too small to clip. Endovascular treatment such as coil embolization, overlapping stents, and flow diverter has also been used to treat BAPAs. Coil embolization seldom succeeds because the perforator artery and aneurysm neck are both too narrow for catheterization. Buell et al. 10 reported that endovascular coil embolization failed in three patients, resulting in severe clinical complications in two patients (brainstem infarct and death). Overlapping stents and flow diverter are also prospective alternatives to BAPAs. 11 Their curative value is evident for huge aneurysms; however, for tiny aneurysms, its value remains to be demonstrated. Bhogal et al. reported the application of flow diverter in six patents with BAPAs, five of whom had a good outcome (mRS < 2). Nevertheless, the latency period to obliteration of the aneurysm requires weeks or longer, during which time the aneurysm remains at risk for hemorrhage. 2 The complication of flow diverter in basilar artery is concerned. In-stent thrombosis occurred in two of the three cases reported by Peschillo et al. 12 The other patient suffered a hemorrhage.
Endovascular electrothrombosis therapy is seldom reported; however, it might be a potential alternative approach to this rare small lesion. In 1953, Sawyer and Pate 13 stated that the positively charged intravascular metallic electrode could precipitate a thrombus inside the artery by attracting the negatively charged blood particles including blood cells, platelets and fibrinogen. Mullan et al. 14 performed electrothrombosis in large aneurysms by puncturing the electrode into aneurysm sac during open surgery. The operation proved to be strenuous, risky, and ineffective. In recent decades, interventional techniques have advanced; electrodes can be navigated into the aneurysm through a micro guidewire, making electrothrombosis more accurate and safer. Jiang and Li 15 reported five patients with ruptured tiny intracranial aneurysms treated by electrocoagulation. All patients had good outcomes at 10.4 months mean follow-up.
In our case series, electrothrombosis technique was performed because these ruptured BAPAs were too small to catheterized by a microcatheter. The conductive micro guidewire Traxcess-14 was selected for electrothrombosis. The thinner and softer distal tip of this micro guidewire was suitable for super selection of the tiny aneurysm. We carefully shaped the distal tip as a bended hook to provide better support (Figure 2(d)) After the micro guidewire super selected into the tiny aneurysm sac, we advanced the microcatheter as close as possible to the neck of the aneurysm to prevent thrombosis induced by the naked electric microwire in the basilar artery. The electric current was maintained at 1.0 mA and electrothrombosis was performed for 30 to 60 s. These settings were based on the normal working condition of solitaire detachment system; we believe it was safe for electrothrombosis. A control angiogram was performed after each electrothrombosis to determine whether the aneurysm had disappeared. The same treatment procedure would be repeated several times if necessary. Final angiography was performed 15 min later if the aneurysm was not visualized after electrothrombosis. According to the literature, it is safe when the current is maintained at 1 mA and electrothrombolysis is performed within 5 min. 15 Because of the rare incidence of this disease, optimum electric current and duration of electrothrombosis needs to be further studied.
There are two potential advantages of endovascular electrothrombosis. First, the effect of instant hemostasis is significant. The micro guidewire is placed into the aneurysm sac with the microcatheter tip covering the neck. In so doing, the electrothrombosis is accurately limited in the aneurysm sac. The concentrated electronic current makes it cost less time than coil embolization. In addition, in contrast to FD placement, which takes one week or longer to occlude the aneurysm, endovascular electrothrombosis may alleviate the hemorrhage symptoms earlier. Second, the overall hemorrhage risk decreased. Compared to catheterization and coiling, advancing the micro guidewire tip into the aneurysm is much safer. Endovascular electrothrombosis avoids rebleeding complications induced by the anti-platelet regime after stenting or FD placement.
Nevertheless, the safety of this technique remains a concern. The aneurysm of the second patient failed to be embolized by electrothrombosis and he suffered brainstem infarction. This technique is suitable for BAPAs with symptoms because recurrent bleeding in this area is dangerous. For tiny unruptured asymptomatic BAPAs, conservative therapy may be preferred. In addition, the aneurysm sac should be small because the immediate thrombosis induced by the current might not be able to totally embolize a large aneurysm sac. The patients available for this treatment should be seriously selected. Finally, the durability of occlusion and the risk of recurrence remain concerns.
Conclusion
Selective endovascular electrothrombosis may be an alternative approach for BAPAs. The findings in this report also provide a potential treatment option for other tiny intracranial aneurysms that are inaccessible to catheterization with microcatheters. More research is needed to further demonstrate its safety and efficacy.
Footnotes
Author contributor statement: Rui Zhao, Qinghai Huang and Jianmin Liu have given substantial contributions to the conception or the design of the manuscript; Yibin Fang and Qiang Li to acquisition, analysis and interpretation of the patient material; Hongyu Ma, Pengfei Yang and Qinghai Huang to preparation of the figures. Hongyu Ma and Rui Zhao have participated to drafting the manuscript. Qinghai Huang, Pengfei Yang Yi Xu, Bo Hong and Jian-min Liu provided critical review of the manuscript. All authors read and approved the final version of the manuscript.
Ethical approval statement: The publication of this case report was approved by the Ethics Committee of Changhai Hospital and the informed consent was waived because it is a retrospective study.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Qinghai Huang https://orcid.org/0000-0001-9068-5194
Jian-Min Liu https://orcid.org/0000-0002-3599-8267
References
- 1.Ghogawala Z, Shumacher JM, Ogilvy CS. Distal basilar perforator artery aneurysm: case report. Neurosurgery 1996; 39: 393–396. [DOI] [PubMed] [Google Scholar]
- 2.Bhogal P, AlMatter M, Hellstern V, et al. Basilar artery perforator aneurysms: report of 9 cases and review of the literature. J Clin Neurosci 2019; 63: 122–129. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Luo J, Zheng J, et al. Endovascular pure electrocoagulation of intracranial perforator blister-like aneurysm not accessible to microcatheter – new approach to treat small vessel hemorrhage disease. Int J Stroke 2016; 11: NP60–1. [DOI] [PubMed] [Google Scholar]
- 4.Chalouhi N, Jabbour P, Starke RM, et al. Treatment of a basilar trunk perforator aneurysm with the pipeline embolization device: case report. Neurosurgery 2014; 74: E697–701. [DOI] [PubMed] [Google Scholar]
- 5.Marinković SV, Gibo H. The surgical anatomy of the perforating branches of the basilar artery. Neurosurgery 1993; 33: 80–87. [DOI] [PubMed] [Google Scholar]
- 6.Forbrig R, Eckert B, Ertl L, et al. Ruptured basilar artery perforator aneurysms–treatment regimen and long-term follow-up in eight cases. Neuroradiology 2016; 58: 285–291. [DOI] [PubMed] [Google Scholar]
- 7.Finitsis S, Derelle AL, Tonnelet R, et al. Basilar perforator aneurysms: presentation of 4 cases and review of the literature. World Neurosurg 2017; 97: 366–373. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Mejia RO, Lawton MT. Distal aneurysms of basilar perforating and circumferential arteries. Report of three cases. J Neurosurg 2007; 107: 654–659. [DOI] [PubMed] [Google Scholar]
- 9.Gross BA, Puri AS, Du R. Basilar trunk perforator artery aneurysms. Case report and literature review. Neurosurg Rev 2013; 36: 163–168. [DOI] [PubMed] [Google Scholar]
- 10.Buell TJ, Ding D, Raper D, et al. Posterior circulation perforator aneurysms: a proposed management algorithm. J Neurointerv Surg 2018; 10: 55–59. [DOI] [PubMed] [Google Scholar]
- 11.Chau Y, Sachet M, Sédat J. Super-selective coil embolization of a basilar perforator artery aneurysm previously treated by the stent-in-stent technique, using an extremely soft bare coil delivered through a one-marker microcatheter. Interv Neuroradiol 2017; 23: 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peschillo S, Caporlingua A, Cannizzaro D, et al. Flow diverter stent treatment for ruptured basilar trunk perforator aneurysms. J Neurointerv Surg 2016; 8: 190–196. [DOI] [PubMed] [Google Scholar]
- 13.Sawyer PN, Pate JW. Bio-electric phenomena as an etio-logic factor in intravascular thrombosis. Am J Physiol 1953; 175: 103–107. [DOI] [PubMed] [Google Scholar]
- 14.Mullan S, Raimondi AJ, Dobben G, et al. Electrically induced thrombosis in intracranial aneurysms. J Neurosurg 1965; 22: 539–547. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Li Y. Treatment of tiny intracranial aneurysms with guidewire manipulation. Chin Neurosurg J 2017; 3: 39. [Google Scholar]