Abstract
Intracranial high-resolution vessel wall MRI (VW-MRI) is an imaging paradigm that is useful in site-of-rupture identification in patients presenting with spontaneous subarachnoid hemorrhage and multiple intracranial aneurysms. Only a handful of case reports describe its potential utility in the evaluation of more complex brain vascular malformations. We report for the first time three patients with ruptured cranial dural arteriovenous fistulas (dAVFs) that were evaluated with high-resolution VW-MRI. The presumed site-of-rupture was identified based on contiguity of a venous ectasia with adjacent blood products and thick, concentric wall enhancement. This preliminary experience suggests a role for high-resolution VW-MRI in the evaluation of ruptured cranial dAVFs, in particular, site-of-rupture identification. It also supports an emerging hypothesis that all spontaneously ruptured, macrovascular lesions demonstrate avid vessel wall enhancement.
Keywords: Dural arteriovenous fistula, vessel wall MRI, magnetic resonance
Introduction
Ruptured cranial dural arteriovenous fistulas (dAVFs) are always associated with retrograde leptomeningeal venous drainage, more commonly referred to as cortical venous reflux (CVR), and resultant venous congestion. 1 Treatment is mandated to eliminate venous congestion and prevent re-hemorrhage. This is typically accomplished by endovascular or surgical disconnection of CVR with induced-thrombosis of the site-of-rupture. 2
Intracranial high-resolution vessel wall MRI (VW-MRI) is useful in site-of-rupture identification in patients presenting with spontaneous subarachnoid hemorrhage (SAH) and multiple intracranial aneurysms.3–6 Only a handful of case reports describe its potential utility in the evaluation of more complex, ruptured brain arteriovenous malformations (AVMs).7,8 Herein, we present three cases of ruptured cranial dAVFs studied using high-resolution VW-MRI.
Methods
High-resolution VW-MRI protocol
Details of the intracranial VW-MRI protocol have been described elsewhere. 3 MR imaging was performed on a Verio 3.0 T scanner with a 32-channel head coil (Siemens Healthcare, Erlangen Germany). The VW-MRI protocol included TOF MRA and T1WI (TSE with FOV = 16×16 cm; acquired matrix 512×512; slice thickness = 2 mm; total slab thickness = 4–6 cm; repetition time/echo time 590/10 ms) before and after IV administration of gadolinium (with constant scan parameters) in axial, coronal, and sagittal planes.
Case report
Case 1
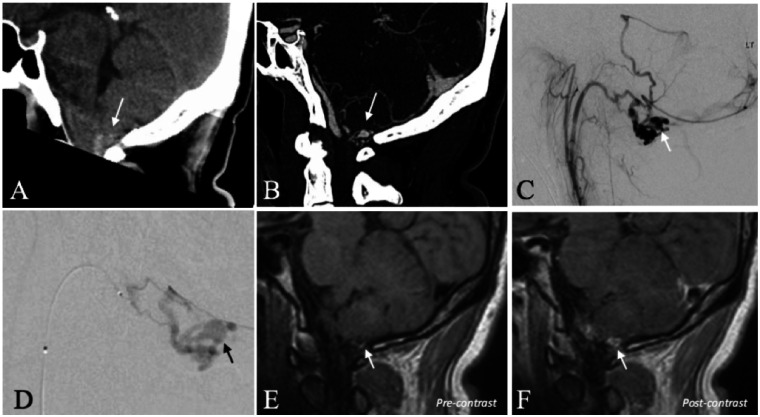
A 39-year-old man presented five days after a sudden-onset, severe headache. A CT head demonstrated acute SAH with a preponderance of blood at the level of the foramen magnum (Figure 1). The accompanying CTA was suspicious for a shunting lesion of the cranio-cervical junction. DSA confirmed a dAVF of the hypoglossal canal (Borden Type III) with a conspicuous venous ectasia. On high-resolution black blood T1WI, the venous ectasia was contiguous with hyperintense blood products suggestive of the presumed site-of-rupture. The venous varix also selectively demonstrated thick, concentric wall enhancement. Trans-arterial glue embolization with penetration of n-BCA into the proximal vein resulted in a durable cure (1-year DSA and 5-year clinical follow-up).
Figure 1.
A 39-year-old man with a ruptured dAVF of the hypoglossal canal. (a) CT scan (sagittal reconstruction) demonstrates acute SAH in the cerebello-medullary cistern (arrow). (b) CTA (sagittal reconstruction) demonstrates regional abnormal vascularity with a conspicuous, aneurysmal vascular out-pouching (arrow). (c) Selective injection of the ascending pharyngeal artery (DSA, lateral projection) confirms a dAVF of the hypoglossal canal. The conspicuous aneurysmal vascular structure corresponds to a venous ectasia (arrow). (d) Superselective, microcatheter injection of the hypoglossal branch of the ascending pharyngeal artery (DSA, lateral projection) better demonstrates the venous ectasia (arrow). (e) High-resolution black blood T1WI before the administration of gadolinium demonstrates hyperintense blood product contiguous with the inferior wall of the venous ectasia (arrow) consistent with the presumed site-of-rupture. (f) High-resolution black blood T1WI after the administration of gadolinium demonstrates selective, thick, concentric enhancement of the venous ectasia wall (arrow).
Case 2
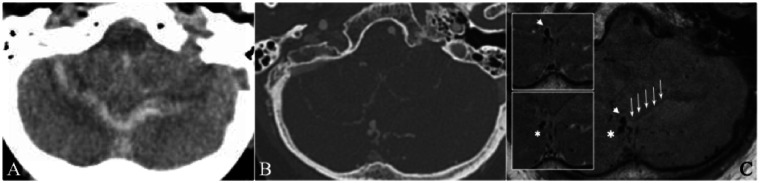
An 87-year-old female presented with a sudden-onset, severe headache accompanied by nausea and vomiting, and a decreasing level of consciousness. A CT head demonstrated a midline cerebellar hemorrhage with extension into the fourth ventricle (Figure 2). The accompanying CTA demonstrated abnormal vascularity in the cerebellum with a conspicuous, aneurysmal out-pouching at the base of the cerebellar hematoma. DSA confirmed a posterior fossa dAVF (Borden Type III). The aneurysmal out-pouching on CTA correlated with a bi-lobed venous varix. On high-resolution black blood T1WI, only the anteriorly-directed lobule was contiguous with the base of the hematoma cavity, suggestive of the presumed site-of-rupture. Additionally, only this lobule demonstrated thick, concentric enhancement. Trans-arterial glue embolization failed to penetrate onto the venous side of the fistula. The family declined further interventions and the patient expired on the same hospital admission.
Figure 2.
An 87-year-old woman with a ruptured cerebellar dAVF. (a) CT head demonstrates a midline cerebellar hematoma with associated SAH. (b) CTA demonstrates a conspicuous, bi-lobed venous varix adjacent to the midline cerebellar hematoma. (c) High-resolution black blood T1WI before the administration of gadolinium demonstrates contiguity of the anteriorly-projecting lobe of the venous varix (short arrow) with the cerebellar hematoma (long arrows), but not the posteriorly-projecting lobe (asterisk). Post-contrast images (insets) demonstrate circumferential enhancement of the anterior, but not posterior, lobe of the ruptured venous varix.
Case 3
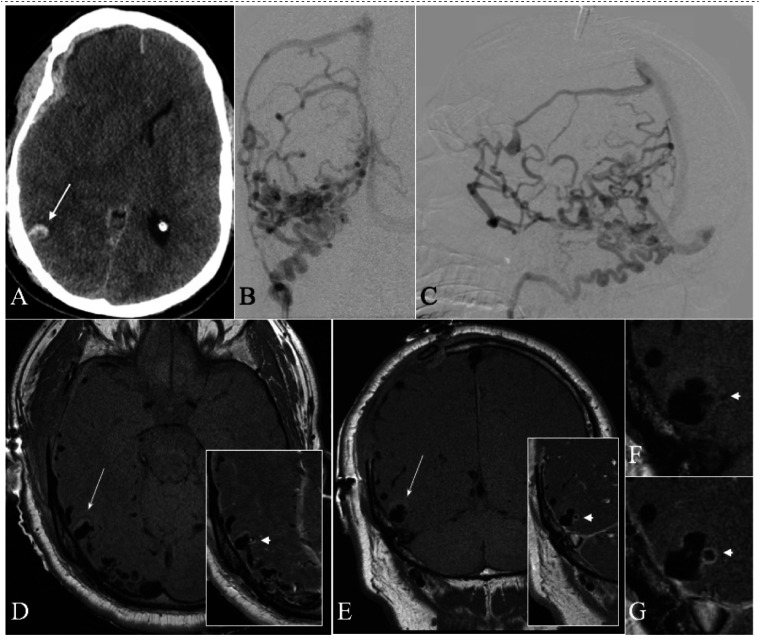
A 54-year-old man presented with a clinical herniation syndrome. A CT head demonstrated a spontaneous, acute right subdural hematoma with midline shift (Figure 3). A preoperative CTA demonstrated abnormal vascularity throughout the right cerebral hemisphere with many dilated vessels subjacent to the subdural hematoma. Notably, an aneurysmal out-pouching was associated with a focal intraparenchymal hemorrhage. The patient underwent an emergent decompressive craniectomy and made a rapid clinical recovery. A postoperative DSA confirmed an aggressive dAVF (Borden Type III). The aneurysmal out-pouching on CTA correlated with a bi-lobed venous varix. On high-resolution black blood T1WI, the venous varix was contiguous with the base of the intraparenchymal hematoma in keeping with the presumed site-of-rupture. Only the medially-directed lobule selectively demonstrated thick, concentric enhancement. Trans-arterial glue embolization significantly slowed flow through the fistula with thrombosis of the site-of-rupture. Gamma Knife radiosurgery provided a durable cure (3-year DSA and 8-year clinical follow-up).
Figure 3.
A 54-year-old man with a ruptured cranial dAVF. (a) Admission CT head demonstrates an acute subdural hematoma (SDH) with associated midline shift. Note was made of a small, contiguous intraparenchymal hemorrhage (long arrow) with central ghosting. (b, c) DSA was performed post-craniectomy for evacuation of the SDH. Selective injection of the occipital artery ((b) anteroposterior projection; (c) lateral-oblique projection) demonstrates a Borden type III cranial dAVF with significant cortical venous reflux subjacent to the evacuated SDH. (d, e) High-resolution black blood T1WI before the administration of gadolinium ((d) axial; (e) coronal) demonstrates a prominent bi-lobed venous varix at the base of the intraparenchymal hematoma (long arrow). Note the surrounding hyperintense blood product contiguous with the ruptured venous varix. Post-contrast images (insets) demonstrate selective, thick, concentric enhancement of the medially-projecting bleb (arrowhead). (f, g) Magnified views of the ruptured venous varix before (f) and after (g) the administration of gadolinium (corresponding to panel (e)).
Discussion
In this report, we present our preliminary experience using high-resolution VW-MRI in the evaluation of ruptured cranial dAVFs and suggest its utility in the determination of the presumed site-of-rupture. Although surgical confirmation is lacking, we take it on principle that a ruptured macrovascular substructure must, as a minimum criterion, be contiguous with adjacent blood products on neuroimaging. The true “added value” of high-resolution VW-MRI is the ability to resolve the precise spatial relationship between acute blood products, e.g., a parenchymal hematoma, and a specific, macrovascular substructure. In all 3 of our cases, the application of this criterion alone suggested only a single, presumed site-of-rupture.
In addition, the ruptured venous varix selectively demonstrated thick, concentric wall enhancement. This finding is consistent with the observation that vessel wall enhancement aids in site-of-rupture determination in patients presenting with spontaneous SAH and multiple intracranial aneurysms.3–6
The finding that a venous varix can be imaged with high-resolution VW-MRI is also of interest to the research community. Most VW-MRI sequences in clinical use today are not optimized to evaluate the venous vessel wall due to an inability to adequately suppress signal from slower flowing blood. In fact, venous contamination is a well-recognized source of spurious enhancement in traditional applications of VW-MRI.9,10 The ability to evaluate venous vascular enhancement in cranial dAVFs is likely due to the arterialized nature of the draining vein. This is consistent with recent case reports suggesting the utility of high-resolution VW-MRI in the evaluation of ruptured brain AVMs.7,8
Identification of the site-of-rupture in ruptured cranial dAVFs may provide the opportunity to better monitor treatment efficacy, in particular, in cases of partial or failed endovascular disconnection of CVR subsequently referred for open surgery or Gamma Knife radiosurgery.
Conclusion
Intracranial high-resolution VW-MRI is already in routine clinical use for site-of-rupture identification in patients presenting with spontaneous SAH and multiple intracranial aneurysms. In this report, we extend its potential utility to the evaluation of ruptured cranial dAVFs. Our observations also support an emerging hypothesis that all spontaneously ruptured, macrovascular lesions demonstrate avid vessel wall enhancement.
Acknowledgements
An earlier version of this work was presented as a “Turbo Talk” at the 54th Annual Meeting of the American Society of Neuroradiology, May 23–25, 2016.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Branden J Cord https://orcid.org/0000-0002-9120-5989
Nanthiya Sujijantarat https://orcid.org/0000-0002-5857-5718
Joseph Antonios https://orcid.org/0000-0002-5147-1377
Charles C Matouk https://orcid.org/0000-0003-3234-9541
References
- 1.Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg 1996; 85: 830–837. [DOI] [PubMed] [Google Scholar]
- 2.Davies MA, Saleh J, Ter Brugge K, et al. The natural history and management of intracranial dural arteriovenous fistulae. Part 1: benign lesions. Interv Neuroradiol 1997; 3: 295–302. [DOI] [PubMed] [Google Scholar]
- 3.Matouk CC, Mandell DM, Günel M, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 2013; 72: 492–496. [DOI] [PubMed] [Google Scholar]
- 4.Omodaka S, Endo H, Niizuma K, et al. Circumferential wall enhancement on magnetic resonance imaging is useful to identify rupture site in patients with multiple cerebral aneurysms. Neurosurgery 2018; 82: 638–644. [DOI] [PubMed] [Google Scholar]
- 5.Texakalidis P, Hilditch CA, Lehman V, et al. Vessel wall imaging of intracranial aneurysms: systematic review and meta-analysis. World Neurosurg 2018; 117: 453–458.e1. [DOI] [PubMed] [Google Scholar]
- 6.Wang GX, Wen L, Lei S, et al. Wall enhancement ratio and partial wall enhancement on MRI associated with the rupture of intracranial aneurysms. J Neurointerv Surg 2018; 10: 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matouk CC, Cord BJ, Yeung J, Malhotra A, et al. High-resolution vessel wall magnetic resonance imaging in intracranial aneurysms and brain arteriovenous malformations. Top Magn Reson Imaging 2016; 25: 49–55. [DOI] [PubMed] [Google Scholar]
- 8.Bhogal P, Lansley J, Wong K, et al. Vessel wall enhancement of a ruptured intra-nidal aneurysm in a brain arteriovenous malformation. Interv Neuroradiol 2019; 25: 310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017; 38: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santarosa C, Cord B, Koo A, et al. Vessel wall magnetic resonance imaging in intracranial aneurysms: principles and emerging clinical applications. Interv Neuroradiol 2020; 26: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]