Immune checkpoint blockade with pembrolizumab after high-dose cytarabine salvage chemotherapy is tolerable and leads to encouraging clinical outcomes in relapsed/refractory AML patients.
Abstract
Immune suppression, exhaustion, and senescence are frequently seen throughout disease progression in acute myeloid leukemia (AML). We conducted a phase II study of high-dose cytarabine followed by pembrolizumab 200 mg i.v. on day 14 to examine whether PD-1 inhibition improves clinical responses in relapsed/refractory (R/R) AML. Overall responders could receive pembrolizumab maintenance up to 2 years. Among 37 patients enrolled, the overall response rate, composite complete remission (CRc) rate (primary endpoint), and median overall survival (OS) were 46%, 38%, and 11.1 months, respectively. Patients with refractory/early relapse and those receiving treatment as first salvage had encouraging outcomes (median OS, 13.2 and 11.3 months, respectively). Grade ≥3 immune-related adverse events were rare (14%) and self-limiting. Patients who achieved CRc had a higher frequency of progenitor exhausted CD8+ T cells expressing TCF-1 in the bone marrow prior to treatment. A multifaceted correlative approach of genomic, transcriptomic, and immunophenotypic profiling offers insights on molecular correlates of response and resistance to pembrolizumab.
Significance:
Immune-checkpoint blockade with pembrolizumab was tolerable and feasible after high-dose cytarabine in R/R AML, with encouraging clinical activity, particularly in refractory AML and those receiving treatment as first salvage regimen. Further study of pembrolizumab and other immune-checkpoint blockade strategies after cytotoxic chemotherapy is warranted in AML.
See related commentary by Wei et al., p. 551.
This article is highlighted in the In This Issue feature, p. 549
Introduction
Despite therapeutic advancements in the management of patients with acute myeloid leukemia (AML) over the last several years, outcomes remain dismal for those with relapsed/refractory (R/R) disease. The advent of targeted therapeutic approaches has changed the treatment landscape, providing salvage options for a subset of patients. Nonetheless, complete remission (CR) rates of approximately 20% to 30% and median overall survival (OS) of 8 to 9 months in patients with R/R AML with FLT3, IDH1, and IDH2 mutations treated with gilteritinib (1), ivosidenib (2), and enasidenib (3), respectively, underscore the poor outcomes in this patient population even with targeted therapies.
The majority of R/R AML patients do not have targeted treatment options. Intensive salvage chemotherapy regimens including high-dose cytarabine (HiDAC) are generally used for younger, fit patients, though there is currently no standard-of-care or salvage chemotherapy regimen that has consistently been shown to improve outcomes in R/R AML (4,5,6). Multiple prognostic factors such as duration of CR, age, cytogenetics, and previous history of allogeneic stem cell transplantation (alloSCT) can discriminate 1-year OS rates from 16% to 70% based on overall risk status at the time of AML relapse (7). However, in a pooled analysis of patients with R/R AML treated with first salvage chemotherapy, CR and median OS rates were approximately 14% and 6.3 months, respectively, and outcomes are worse in those receiving second or third salvage chemotherapy (8). Thus, there is a large unmet need to develop novel therapeutic approaches in this patient population.
Patients with AML carry innate and adaptive immune aberrations at diagnosis that lead to immune suppression, exhaustion, and senescence (9,10,11). Multiple studies have shown that upregulation of inhibitory receptors (IR), such as the programmed-death 1 (PD-1)/PD-L1 axis, plays a role in immune evasion by leukemic cells (11,12,13,14,15,16,17). PD-1 is expressed on the surface of activated T cells, B cells, and natural killer cells. When bound by its ligands, PD-1 stimulation leads to suppression of T-cell activation and inhibition of T-cell responses. Preclinical models have shown that PD-1–expressing CD8+ T cells and regulatory T cells (Treg) accumulate during AML progression, leading to T-cell exhaustion, which can be restored by Treg depletion followed by PD-1/PD-L1 blockade (12). Further, PD-1 knockout mice have less leukemia burden and improved OS (12). Upregulation of PD-L1 on leukemic blasts is more frequently observed at relapse than at diagnosis and is associated with poor prognosis (13, 18). Similarly, the frequency of T cells coexpressing multiple IRs increases with disease progression (11, 17). Reversibility of the phenotypic and transcriptional signatures of CD8+ T cells in patients with AML who achieve CR suggests that T-cell exhaustion, an important feature of R/R AML, may be susceptible to therapeutic intervention such as PD-1/PD-L1 axis blockade (11).
Monoclonal antibody blockade of IRs, most specifically PD-1, has led to a paradigm shift in the management of cancer with a multitude of FDA approvals and breakthrough therapies. However, there is a dearth of clinical data with these agents in combination with cytotoxic chemotherapy in R/R AML. We designed a phase II study of HiDAC followed by pembrolizumab, a human IgG4 monoclonal antibody targeting PD-1, in R/R AML, the first study investigating the combination of immune-checkpoint blockade (ICB) with cytotoxic salvage chemotherapy. Pembrolizumab was administered on day 14 after completion of HiDAC during a time period of expected heightened inflammation and early onset of lymphocyte recovery (10). This clinical–translational study was designed with the hypothesis that pembrolizumab would augment antileukemia T-cell responses and improve the clinical activity of HiDAC in R/R AML. Additionally, we used flow cytometry and genomic approaches to evaluate mechanisms of immune dysfunction in patients with R/R AML, with the goal of identifying biomarkers that predict for response to HiDAC/pembrolizumab, and novel pathways that lead to resistance to HiDAC/pembrolizumab in AML.
Results
Patient Characteristics
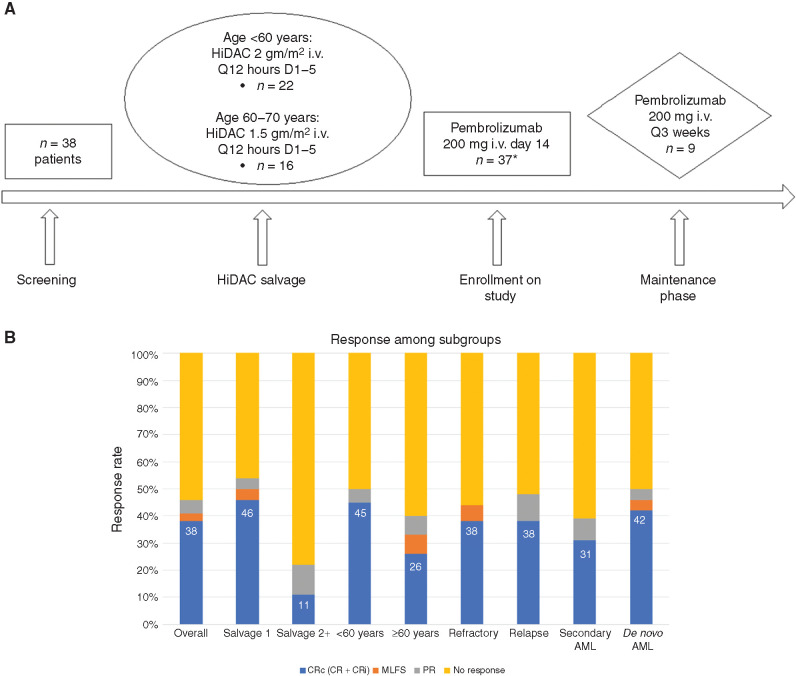
Between October 2016 and April 2019, 38 patients were enrolled, and 37 were treated with HiDAC followed by pembrolizumab (Table 1; Fig. 1A). One patient received HiDAC but did not receive pembrolizumab due to infection, cellulitis, and grade 3 diarrhea and was thus replaced with another subject as per protocol criteria. The median age was 54 years with 41% of enrolled patients ≥60 years. The majority had either refractory (43%) or relapsed disease with CR duration <1 year (43%). All patients received intensive induction chemotherapy as first-line treatment, with 76% receiving treatment on study as their first salvage therapy. Favorable, intermediate, and adverse-risk disease by European LeukemiaNet classification was seen in 19%, 24%, and 56% of patients, respectively. The most common genomic classification (19) of patients on study was AML with NPM1 mutation (24%); AML with mutated chromatin, RNA-splicing genes, or both (22%); and AML with MLL fusion genes (22%).
Table 1.
Patient characteristics
| Patient characteristics | N = 37 |
|---|---|
| Age, median (range) | 54 (24–70) |
| ≥55 years | 17 (46%) |
| ≥60 years | 15 (41%) |
| Male | 20 (54%) |
| Female | 17 (46%) |
| BM blast % prior to treatment, median (range) | 28% (6%–94%) |
| Refractory AMLa | 16 (43%) |
| Relapsed AML | 21 (57%) |
| CR duration ≤6 months | 9/21 (43%) |
| CR duration ≤1 year | 16/21 (76%) |
| Salvage therapy 1 | 28 (76%) |
| Salvage therapy 2 | 8 (22%) |
| Salvage therapy 3 | 1 (3%) |
| Secondary AML | 13 (35%) |
| ELN risk | |
| Favorable | 7 (19%) |
| Intermediate | 9 (24%) |
| Adverse | 21 (56%) |
| Genomic classificationb | |
| AML with NPM1 mutation | 9 (24%) |
| AML with mutated chromatin, RNA-splicing genes, or both | 8 (22%) |
| AML with MLL fusion genes | 8 (22%) |
| AML with TP53 mutations, chromosomal aneuploidy, or both | 6 (16%) |
| AML with Inv(3); GATA2, MECOM | 4 (11%) |
| AML with Inv(16); CBFB–MYH11 | 1 (3%) |
| AML with t(6;9); DEK–NUP214 | 1 (3%) |
| Most common mutations | |
| NPM1 | 9 (24%) |
| DNMT3A | 8 (22%) |
| ASXL1 | 6 (16%) |
| IDH2 | 6 (16%) |
| TP53 | 5 (14%) |
| CEBPA | 4 (11%) |
| NRAS | 4 (11%) |
| WT1 | 4 (11%) |
| Site | |
| University of North Carolina | 30 (81%) |
| Johns Hopkins | 7 (19%) |
Abbreviations: BM, bone marrow; ELN, European LeukemiaNet.
aRefractory AML defined as no response to one or two cycles of induction chemotherapy (>28 days after induction chemotherapy) or no response to salvage treatment after subsequent relapse.
bGenomic classification determined by Papaemmanuil et al. (19).
Figure 1.
A, Treatment schema. Q, every. *One patient received HiDAC but did not receive pembrolizumab due to ongoing grade 3 diarrhea and was thus replaced on this study. B, ORR among different patient subsets. CRi, CR with incomplete platelet recovery; MLFS, morphologic leukemia-free state; PR, partial remission.
Toxicity
Pembrolizumab was administered on day 14 in 32/37 (86%) patients. Three (8%) patients received pembrolizumab on day 15, and two (5%) patients received pembrolizumab on day 19 (Supplementary Table S1). The most common pembrolizumab-related toxicities were febrile neutropenia (62%), alanine aminotransferase (ALT) elevation (41%), hypocalcemia (30%), alkaline phosphatase elevation (30%), aspartate aminotransferase (AST) elevation (30%), hyperbilirubinemia (30%), lung infection (26%), and hypokalemia (24%). Most of these adverse events were grade 1/2 (Supplementary Table S2). Nonhematologic pembrolizumab-related grade ≥3 toxicities are shown in Table 2. Rare grade ≥3 immune-related adverse events (iRAE) after pembrolizumab administration included maculopapular rash (n = 2; 5%), aminotransferase elevation (n = 2; 5%), and lymphocytic infiltration on liver biopsy (n = 1; 3%). Systemic steroids were administered to five (14%) patients for suspected grade ≥2 iRAEs (except for maculopapular rash) during induction phase (suspected grade 3 hepatitis: n = 2, suspected grade 3 pneumonitis: n = 1, grade 3 hemolytic anemia: n = 1, grade 2 hyperbilirubinemia: n = 1). Median time to administration of systemic steroids after pembrolizumab and total duration of steroids was 15 (range, 5–23) and 14 (range, 1–35) days, respectively. Steroids were rapidly tapered in three of five patients after diagnostic work-up revealed alternative etiologies or no evidence of an iRAE. Overall, iRAEs were self-limiting and fully resolved after administration of systemic steroids.
Table 2.
Treatment-related grade ≥3 nonhematologic adverse events after pembrolizumab
| Toxicity | Number (total %) |
|---|---|
| Electrolyte abnormalities | |
| Hypokalemia | 1 (3%) |
| Hepatic | |
| ALT increase | 2 (5%) |
| AST increase | 2 (5%) |
| Alkaline phosphatase increase | 2 (5%) |
| Lymphocytic infiltration of liver | 1 (3%) |
| Infections | |
| Catheter-related infection | 3 (8%) |
| Clostridium difficile colitis | 1 (3%) |
| Febrile neutropenia | 23 (62%) |
| Hepatic infection | 1 (3%) |
| Lung infection | 10 (26%) |
| Typhlitis | 1 (3%) |
| Pulmonary | |
| Pulmonary edema | 1 (3%) |
| Skin | |
| Maculopapular rash | 2 (5%) |
NOTE: The proportion of grade ≥3 nonhematologic adverse events related to pembrolizumab is shown in this table.
There was no treatment-related death on study. Overall, 30-day and 60-day mortality was 0 and 3% (one patient died on day 56 due to progressive disease), respectively. In those achieving a response, median time to full neutrophil (≥1 × 109/L) and platelet (≥100 × 109/L) recovery was 32 (range, 22–49) and 31 (range, 20–55) days, respectively.
Clinical Activity
The overall response rates (ORR) and composite CR [CRc: CR + CR with incomplete platelet recovery (CRi)] rates were 46% and 38%, respectively (Fig. 1B). Of the 14 CRc patients, 13 achieved full hematologic recovery and 1 patient had CRi. Seven (50%) of the 14 CRc patients had no evidence of measurable residual disease (MRD; Supplementary Table S3). CRc rates were encouraging in those receiving treatment as first salvage therapy (13/28 = 46%), <60 years (10/22 = 45%), and refractory AML (6/16 = 38%). Further, 9/25 (36%) patients with refractory or early relapse (CR1 duration ≤6 months) achieved CRc. Notably, 2/8 (25%) patients who were previously refractory to salvage chemotherapy with HiDAC prior to enrolling on study (n = 1) or relapsed within 6 months of HiDAC consolidation (n = 1) achieved CRc on this study, one of whom also had no evidence of MRD. In HiDAC-naïve patients, the CRc rate and median OS were 47% and 13.6 months, respectively (n = 17; Supplementary Table S4).
Maintenance Phase
Nine (24%) patients received pembrolizumab maintenance (median number of cycles = 3; range, 1–14) after achieving CR (n = 8) or partial remission (PR; n = 1). Two patients subsequently received an alloSCT after two and three cycles of pembrolizumab maintenance, respectively. All nine patients who received maintenance pembrolizumab developed relapse or progression (median time to relapse/progression = 5.8 months; range, 1.1–16.0 months). One patient achieved PR after HiDAC + pembrolizumab [17% blasts in bone marrow (BM)] and remained with stable PR for 12 cycles of pembrolizumab maintenance. One patient achieved CR without MRD after HiDAC + pembrolizumab and developed flow-cytometric MRD after cycle 12, before ultimately relapsing after cycle 14 of pembrolizumab maintenance.
No iRAEs or treatment-related grade ≥3 adverse events were observed during pembrolizumab maintenance (Supplementary Table S5). One patient developed grade 3 aseptic meningitis with a negative diagnostic workup, and symptoms improved with empiric antibiotics and supportive care without systemic steroids. One patient received empiric systemic steroids after developing acute-onset grade 3 systolic heart failure, but after diagnostic workup including catheterization, cardiac MRI, and transmyocardial biopsy revealed no evidence of myocarditis, steroids were stopped. Both patients continued on pembrolizumab maintenance without difficulty. Thus, no patient discontinued maintenance due to toxicity.
Clinical Outcomes
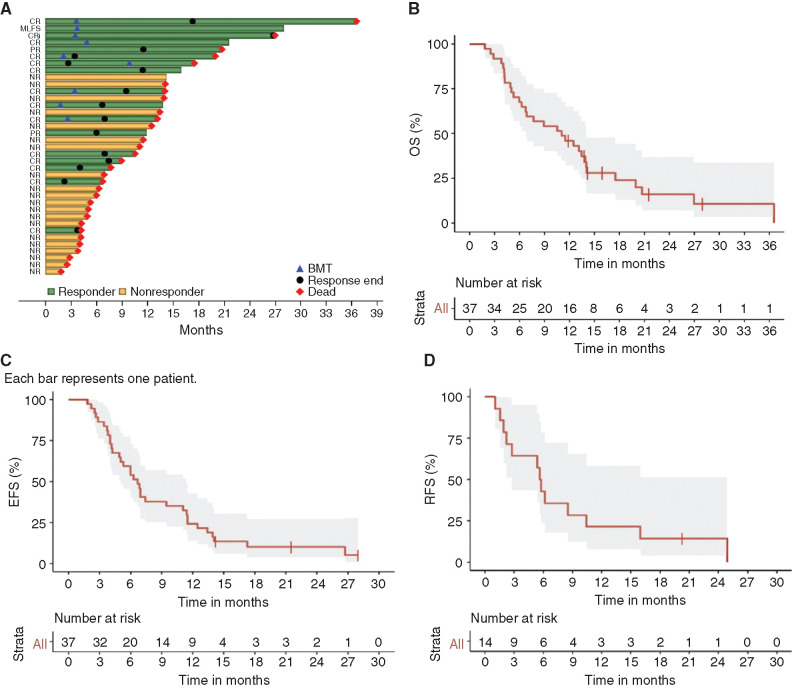
A swimmer plot of the 37 patients enrolled on study is illustrated in Fig. 2A. Thirty-one of 37 (84%) expired at data cutoff due to progressive AML (n = 26) or infection-related complications (n = 5). Of the six patients alive at data cutoff, three received an alloSCT. Nine (24%) patients received an alloSCT after achieving CR (n = 7), CRi (n = 1), or morphologic leukemia-free state (MLFS: n = 1). One patient who achieved CR without MRD relapsed and received an alloSCT after subsequent therapy. Six (67%) patients relapsed post-alloSCT (median time to relapse: 5.5 months; range, 1.3–23.6 months), including two who relapsed with extramedullary disease (Supplementary Table S6). Two patients died of infectious complications 8.3 and 35.9 months post-alloSCT, respectively. There were no instances of sinusoidal obstruction syndrome or grade ≥3 acute graft-versus-host disease in patients receiving pembrolizumab prior to alloSCT.
Figure 2.
Clinical outcomes. A, Swimmer plot of best treatment response and survival for all 37 patients. The swim lanes (rows) represent patients in the study and their survival until date of last follow-up or death. End of response = relapse (after CR/CRi) or progression (after PR). BMT, allogeneic bone marrow transplant. B, Kaplan–Meier estimates of OS measured from day 1 of treatment until death or date of last follow-up. C, Kaplan–Meier estimates of EFS measured from day 1 of treatment until no response, relapse, or death. D, Kaplan–Meier estimates of RFS measured from date of CR/CRi until relapse, death, or date of last follow-up.
With a median follow-up of 15.1 months, the median OS was 11.1 months [95% confidence interval (CI), 6.3–13.9 months; Fig. 2B]. Median event-free survival (EFS) and relapse-free survival (RFS) were 6.7 months (95% CI, 4.9–11.1 months) and 5.8 months (95% CI, 2.2–10.4 months), respectively (Fig. 2C and D). Additionally, median progression-free survival (PFS) was 5.7 months (95% CI, 1.9–10.4 months). Median OS was 14.4 months (95% CI, 13.2–N/A) among overall responders versus 5.0 months (95% CI, 4.0–11.5 months) in those without a response to HiDAC plus pembrolizumab. Further, median OS was 11.3 months (95% CI, 5.3–20 months) versus 5.0 months (95% CI, 2.9–N/A) in patients receiving no prior salvage therapy versus those receiving ≥1 prior salvage therapy, respectively. In patients with refractory disease or early relapse (CR1 duration ≤6 months), median OS was 13.2 months (95% CI, 5.2–17.5 months) compared with 7.0 months (95% CI, 4.0–N/A) in patients with late relapse (CR1 duration >6 months).
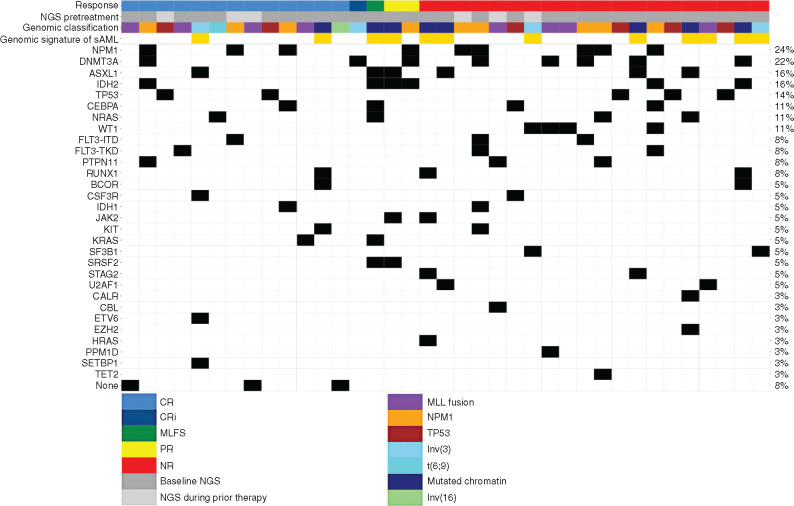
Genomic Characteristics Associated with Response
A plot of genomic signatures and mutations associated with response is shown in Fig. 3. The CRc rate was 50% in those with AML with MLL fusion genes (4/8) and Inv(3)/t(3;3) (2/4), respectively (Supplementary Table S7). Notably, two of five (40%) patients with TP53 mutations achieved CR and three of six (50%) with ASXL1 mutations achieved an overall response (CR = 17%). In those with IDH1/2 mutations, ORR and CRc were 63% (5/8) and 25% (2/8), respectively. None of the four patients with WT1 mutations had a response to treatment. Interestingly, all three patients without a detectable mutation achieved CR. ORR and CRc were 33% and 25%, respectively, among 12 patients having genomic signature of secondary AML (20).
Figure 3.
Genomic signatures and response matrix. Gene matrix representing mutations identified by next-generation sequencing (NGS) prior to treatment. Each individual patient is listed as a column on the x-axis. Mutations identified as present prior to treatment are colored in black. Thirty-one patients had NGS performed as baseline prior to treatment on study (dark gray), whereas six patients (light gray) had NGS performed during prior lines of therapy. Genomic classification was determined based on Papaemmanuil et al. (19). Genomic signature of secondary AML (sAML) was determined based on Lindsley et al. (ref. 20; beige color denotes patients with secondary AML based on genomic signature). Mutations are listed in order of prevalence on the y-axis. The percentage listed in the last column represents prevalence of the gene mutation in the overall cohort.
Immune Biomarker Correlates
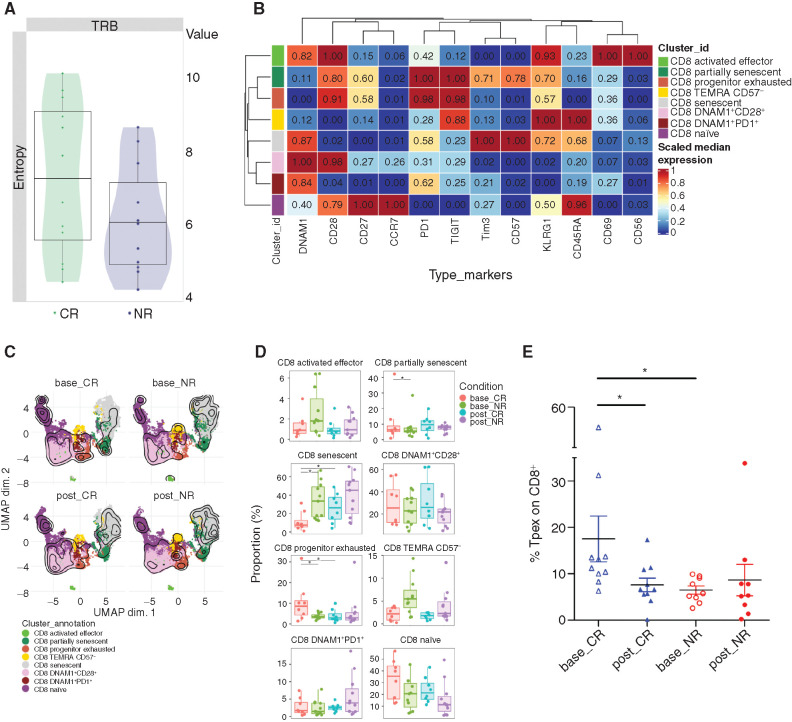
Previous work has shown that anti–PD-1 therapy may be most effective in patients with a diverse baseline T-cell receptor (TCR) repertoire (21). We performed high-throughput antigen receptor sequencing of the TCR Vβ CDR3 region on sorted peripheral blood (PB) CD3+ T cells at diagnosis and analyzed them based on the response [CR, n = 12; no response (NR), n = 11]. Although greater than the prespecified significance level of <0.05, TCR Vβ sequencing data revealed that patients who subsequently achieved CR had a trend toward higher TCR diversity at baseline compared with NR patients as assessed by Shannon entropy (P = 0.15; Fig. 4A).
Figure 4.
Immune biomarkers associated with response. A, Shannon entropy of CR (n = 12) and NR (n = 11) pretreatment PB TCR samples. Uncorrected P = 0.15 comparing CR versus NR patients. TRB, TCR Vβ. B, Heatmap showing the 0 to 1 scaled mean fluorescence intensity values of 12 markers over the eight CD8+ BM subsets from all samples (NR: n = 11; CR: n = 8). The median marker expression identifies the markers that characterize each cell subset. Each CD8+ subpopulation is colored according to the cluster identified using the FlowSOM algorithm:
CD8 activated effector: DNAM1+CD28+KLRG1+CD69+CD56+; CD8 partially senescent: CD28+CD27+KRLG1+CD57+; CD8 progenitor exhausted: CD28+PD1+TIGIT+; CD8 TEMRA CD57−: CD45RA+KLRG1+CD57−; CD8 senescent: CD45RA+KLRG1+CD57+; CD8 DNAM1+CD28+: DNAM1+CD28+; CD8 DNAM1+PD1+: DNAM1+PD1+; and CD8 naïve: CCR7+CD45RA+CD27+CD28+. C, UMAP visualization overlaid with contour plots (kernel density estimation) of the eight CD8+ BM subpopulations in nonresponders (NR, n = 11) and those achieving complete remission (CR, n = 8), at baseline (base_CR, base_NR) or after therapy (post_CR, post_NR). Each CD8+ subpopulation is colored according to the cluster identified using the FlowSOM algorithm. D, Boxplots showing the relative abundance of BM CD8+ subpopulations in NR and CR patients at baseline and posttreatment. Horizontal bars indicate median values. Asterisks indicate adjusted P values (*, Padj < 0.05). E, Frequency of BM CD8+CD45RA−CD27+/intCD28+PD1+TCF1+ T cells in patients who achieved CR compared with NRs at baseline and at response assessment (*, P < 0.05). Tpex, precursor exhausted.
As the clonal repertoire determined by TCR sequencing of CD3+ T cells does not inform on functional differences between different T-cell subpopulations, we next studied T-cell dynamics by flow cytometry to probe cellular immune signatures that may correlate with response to HiDAC plus pembrolizumab treatment in paired BM and PB samples. Recognizing heterogeneity of dysfunctional T cells in AML that cannot be reliably assessed with a single phenotypic marker, we performed unsupervised clustering for cell subpopulation identification and Uniform Manifold Approximation and Projection (UMAP) for dimensionality reduction (Fig. 4B and C). The advantage of this analysis lies in its integration of markers at a single-cell level, providing an improved understanding of their high-dimensional relationship. At baseline, we detected a significantly higher frequency of senescent T cells (CD45RA+KLRG1+CD57+; ref. 11) in the BM and PB and terminally differentiated effector T cells (TEMRA) cells in the PB in NRs as compared with those who achieved CR (Fig. 4D; Supplementary Fig. S1). Interestingly, patients who achieved CR had increased frequency of CD8+ T cells expressing CD28, PD-1, and TIGIT, and lacking expression of Tim-3 and CD57 in the BM at baseline, a phenotype suggestive of progenitor exhausted T cells (22, 23). Additional analysis using the manual gating strategy (Supplementary Fig. S2) confirmed that there is a statistically significant increase in pretreatment CD8+CD45RA−CD27+/intCD28+PD1+TCF1+ T cells in patients who achieved CR compared with NRs (Fig. 4E). TCF-1 is a transcription factor essential for the stem-like properties of intratumoral CD8+ T cells. The presence of CD8+ T cells coexpressing TCF-1 and PD-1 appears to be critical for immunotherapy response (24, 25). There were no significant differences observed in CD4+ T-cell subpopulations at baseline and after treatment between CR and NR patients. However, there was a significant increase in Tregs in NR patients after treatment compared with baseline (Supplementary Fig. S3). Sequencing data have been deposited in Gene Expression Omnibus (GEO) under accession number GSE183415.
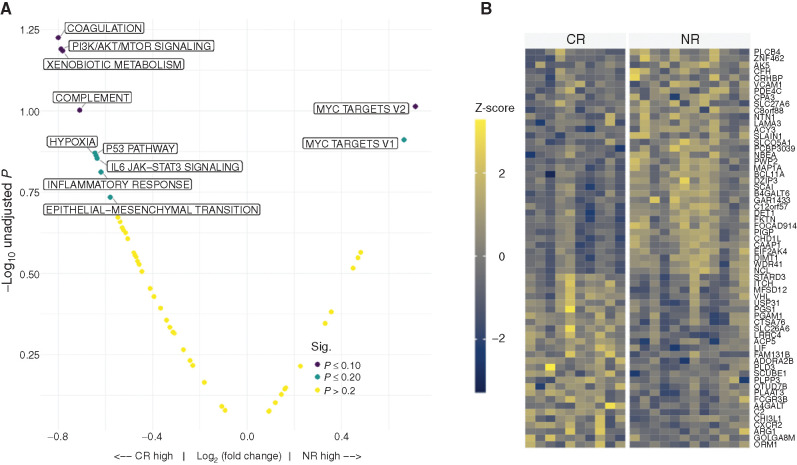
We next sought to examine transcriptional changes in enriched AML blasts using bulk RNA sequencing (RNA-seq). Probing the Molecular Signature Database (MSigDB) and published gene sets revealed that at baseline, upregulation of the PI3K/AKT/mTOR signaling pathways in BM blasts was significantly associated with CR compared with NR patients by gene set enrichment analysis (GSEA; Fig. 5A). Notably, the P53 pathway and inflammatory response pathways also showed upregulation in CR compared with NR patients at baseline. On the other hand, MYC targets were significantly upregulated in NR patients compared with CR. Genes with increased expression in CR included ARG1, which could regulate T-cell metabolism by diminishing local concentrations of L-arginine (Fig. 5B). Expression of multiple gene sets associated with major histocompatibility complex (MHC I/II) antigen presentation on pretreatment leukemia blasts was significantly associated with CR (Supplementary Fig. S4). There was no significant difference in PD-L1 or PD-L2 expression on blasts and nonblast fraction in CR versus NR patients, respectively (Supplementary Fig. S5). Finally, there was no significant association with total mutational burden and CR versus NR (Supplementary Fig. S6).
Figure 5.
A, GSEA of pretreatment BM blast samples using the hallmark gene sets from the MSigDB (http://www.gsea-msigdb.org/gsea/msigdb/collections.jsp; ref. 53). B, Heatmap displaying the differential expression of genes from pretreatment BM blast RNA samples comparing CR versus NR patients. FDR P ≤ 0.20 shown.
Discussion
This study is the first to explore the use of ICB after cytotoxic salvage chemotherapy for patients with R/R AML. The study was designed as a phase II study with continuous monitoring for toxicity and early stopping rules for unacceptable toxicity given the uncertainty of administering ICB after salvage chemotherapy. Unacceptable toxicity, as defined in “Methods” (“Safety Assessment”), was not seen in any patient treated with pembrolizumab on this study. Despite being administered during the time of nadir after HiDAC salvage, pembrolizumab administration was associated with rare iRAEs, no severe toxicity or delay in hematologic recovery, and an impressive 30-day mortality rate of 0%. In fact, only five (14%) patients experienced a grade ≥3 iRAE, all of which resolved with steroids and/or supportive care. In contrast to data in solid malignancies where pneumonitis occurs in up to 10% of patients treated with PD-1 inhibitors (26), there were no incidences of pneumonitis after pembrolizumab administration on this study, both during induction and maintenance. Rigorous diagnostic workup of suspected iRAEs led to alternative etiologies in several patients, reinforcing the importance of ruling out more likely alternative causes in patients with AML treated with ICB.
The overall CRc rate of HiDAC plus pembrolizumab was 38%, meeting the primary endpoint of this study, and the median OS was 11.1 months, thus substantiating clinical activity of this regimen and comparing favorably to other salvage regimens. This study was designed based on a historical control CR rate of 20% with HiDAC alone (4, 5). Reported clinical studies of salvage chemotherapy regimens in R/R AML are fraught with heterogeneity of patient populations, disease settings, chemotherapy regimens used, and outcomes. Although response rates as high as 58% (27, 28) have been reported in single-arm phase II studies, randomized phase II/III studies with multiagent cytotoxic chemotherapy have consistently shown CR rates of ≤35% and median OS <8 months in patients with R/R AML (1,4,5,6). Further, in HiDAC-naïve patients, CRc and median OS were 47% and 13.6 months, respectively, which compare favorably to HiDAC-naïve treated patients on three randomized phase III studies (CRc = 12%–32%; median OS = 5–8 months; Supplementary Table S4; refs. 5, 29, 30). We chose to use HiDAC as a single agent in this study to attenuate lymphocyte depletion and T-cell suppression that can be seen after purine analogues such as fludarabine, clofarabine, and cladribine (31), which are commonly used in salvage chemotherapy regimens for AML. Whether ICB would be clinically active after more conventional multiagent cytotoxic salvage chemotherapy regimens is unclear and worthy of further investigation.
R/R AML encompasses a heterogeneous group of patients with variably poor clinical outcomes. Primary refractory AML and early relapse (typically defined as CR duration ≤6 months) represent a particularly dismal subgroup of R/R AML, with CR rates and median OS of approximately 14% and 3 to 4 months, respectively (32,33,34). Only five (14%) patients on this study had a CR1 duration >1 year, whereas 68% of patients had either refractory AML or early relapse (CR1 duration ≤6 months). The clinical outcomes of this highest-risk subgroup of patients with refractory/early relapse AML was particularly encouraging, with CRc of 36% and median OS of 13.2 months. This patient population is inherently chemoresistant and represents one of the highest unmet needs in AML. Higher levels of expression of IFNγ-related genes and an immune infiltrative tumor microenvironment with increased expression of immune checkpoints are hallmarks of refractory AML and may define a subset of patients who respond best to immunotherapy (35). Recent data suggest that patients with primary refractory/early relapse AML may have improved responses to immune-based therapies, such as flotetuzumab, compared with late relapse patients due to higher BM immune infiltration, inflammatory chemokine, expression of IFNγ-related genes, and tumor inflammation signature scores (35, 36). Thus, immune intervention with pembrolizumab may have reactivated T-cell responses in this refractory patient population and contributed to antileukemia activity of this regimen.
Encouraging clinical outcomes were also seen in patients receiving HiDAC plus pembrolizumab as their first salvage therapy with ORR and median OS of 54% and 11.3 months, respectively. Daver and colleagues also reported improved outcomes in patients with R/R AML receiving azacitidine plus nivolumab as their first salvage therapy when compared with historical controls receiving azacitidine alone (median OS = 10.6 vs. 5.3 months, respectively; ref. 37). These data, therefore, suggest that PD-1 inhibitors should be incorporated into combination therapies during earlier lines of therapy in R/R AML.
Higher CD3+ T cells in PB/BM have been associated with responses to azacitidine and nivolumab (37). In our study, we did not observe correlation between baseline BM and PB CD3+ T cells and response possibly due to small sample size. However, we observed higher frequency of highly differentiated and senescent T cells in nonresponders to therapy. We previously reported that this T-cell subpopulation is less proliferative and ineffective in killing leukemia cells (11). Further, TCR repertoires of patients who achieved CR were more diverse than those who did not respond to therapy, suggesting that pretreatment TCR repertoire diversity may imply a greater likelihood of response. Similar results were seen with ipilimumab in melanoma and PD-1 blockade in classic Hodgkin lymphoma (21, 38). Further probing of immune signatures revealed enrichment of progenitor exhausted TCF1+ CD8+ T-cell subpopulation in patients who achieved CR. This population had been described to give rise to effector cells after PD-1 inhibition. Higher percentages of progenitor exhausted CD8+ T cells in the tumor microenvironment pretreatment have also been reported in patients with melanoma whom experienced durable responses to ICB (25, 39). This finding suggests that T-cell differentiation state rather than the sole number of T cells may be predictive of response to PD-1 inhibition in AML.
Interestingly, CR in this study was associated with increased expression of the PI3K/AKT/mTOR pathway in BM blasts. Previous evaluations have predominantly focused on the role of mutations in PI3K in the immune response in cancer, with little data on the effects of increased expression of this pathway. However, mutations of PTEN lead to activation of PI3K and in murine lung cancer models, led to upregulation of PD-L1 (40). Treatment with combined ICB and an mTOR inhibitor led to increased CD8+ T cells, decreased Tregs, and improved tumor control (41). Notably, none of the four patients with WT1 mutations achieved a response. Becker and colleagues (42) revealed that WT1 mutations were associated with a downregulation in genes involving the PI3K pathway, purporting a potential mechanism of resistance to PD-1 inhibition. Future studies assessing the combination of ICB and mTOR inhibition are warranted in AML.
In terms of genomic predictors of response, small numbers of patients on this study precluded a robust analysis, but a few observations are noteworthy. We observed an ORR of 50% in patients with ASXL1 mutations in our study. ASXL1 mutations were associated with improved ORR and OS in a phase II study of azacitidine plus nivolumab in R/R AML (37). Two of four patients with inv(3)/t(3;3) cytogenetics, which has been shown to be the most adverse-risk abnormality in AML (22), achieved CRc on study and subsequently received an alloSCT. Patients with inv(3)/t(3;3) frequently harbor mutations in RAS/receptor tyrosine kinase pathways and notably have higher mutational burden than other AML subtypes (43). Lastly, two of five patients enrolled with TP53 mutations achieved CRc. TP53-mutated AML has particularly poor outcomes with conventional chemotherapy agents, yet novel immunotherapy agents have shown promising clinical activity in this patient subset (44, 45). Recent data suggest that TP53 mutations as well as myelodysplastic syndrome–like subtypes are associated with high cytolytic scores and PD-L1 expression (46), suggesting that future studies of novel immune-based combinations deserve exploration in TP53 mutant AML patients.
There were several limitations of this study that will require further investigation in future study designs. First, the majority of patients (76%) who enrolled on this study received only a single dose of pembrolizumab. Incorporation of serial doses of PD-1 inhibitors, with or without other ICB agents or immune therapies, and in those without an initial response to therapy may be required given the potential for delayed onset of clinical activity and to overcome an immunosuppressive BM microenvironment. Ravandi and colleagues (47) reported encouraging clinical outcomes and rare iRAEs despite serial doses of ICB in a phase II study of nivolumab after induction chemotherapy in newly diagnosed AML and high-risk myelodysplastic syndrome. Second, a primary endpoint of CR after one cycle of therapy may underestimate the clinical activity of ICB in AML, and attentive study designs will be necessary to allow adequate time for immune modulation. As a case in point, prolonged response has been observed in patients who achieved PR and MLFS on this study and was comparable to those who achieved CRc (median RFS and PFS, 5.8 vs. 5.7 months, respectively). In fact, recent data suggest that ICB may sensitize patients with Hodgkin lymphoma to subsequent therapies after progression; thus, the impact of ICB may last beyond initial disease evaluations and response (48). Third, uniform MRD monitoring was not performed on this study, in part, due to lack of standardized assessment of MRD in AML. Nonetheless, achievement of CR without MRD is associated with improved clinical outcomes (49), and it would be of interest to examine the effect of pembrolizumab and other ICB therapies on the depth of response over time, as is being done in two randomized clinical trials (NCT04214249 and NCT04284787). Lastly, correlative immunologic studies, although informative in identifying the relevance of distinct subpopulation of progenitor exhausted T cells in relation to response, are limited by the small number of available patients for analysis and relatively few sampling time points. Prospective assessment of natural killer cell function was not performed in this study and is warranted for future exploration.
In conclusion, the addition of pembrolizumab to HiDAC salvage chemotherapy in R/R AML led to an acceptable safety profile, met the primary endpoint of achieving clinical activity with CRc rate of 38%, and led to encouraging clinical outcomes with a median OS of 11.1 months. Patients with refractory/early relapse AML and those receiving HiDAC plus pembrolizumab as their first salvage regimen appeared to have the greatest benefit. Based on these findings, a randomized phase II study of salvage chemotherapy plus pembrolizumab versus salvage chemotherapy alone in refractory/early relapse AML is warranted, including prospective examination of the progenitor exhausted TCF1+ CD8+ BM T-cell subpopulation as a potential predictor of response.
Methods
Study Design and Population
This study was an open-label, single-arm, phase II study of HiDAC followed by pembrolizumab in patients with R/R AML conducted at two institutions: University of North Carolina, Lineberger Comprehensive Cancer Center, and Johns Hopkins Hospital, Sidney Kimmel Comprehensive Cancer Center (ClinicalTrials.gov identifier NCT02768792). Eligible patients included those 18 to 70 years with pathologically confirmed refractory or relapsed AML, defined by ≥5% myeloblasts in BM aspirate and/or biopsy. Patients must have received first-line treatment with ≥1 cycle of intensive induction chemotherapy or ≥4 cycles of hypomethylating agents prior to enrollment. Patients with acute promyelocytic leukemia and those who have received an alloSCT prior to enrollment were excluded. Detailed eligibility criteria are outlined in Supplementary Methods. All laboratory criteria for eligibility must have been met prior to enrollment and before pembrolizumab administration (see “Treatment Plan” below). The study was conducted in accordance with Declaration of Helsinki after approval by the ethics committee of each participating center. Written informed consent was obtained on all subjects prior to participating.
Treatment Plan
Induction Phase.
All patients received HiDAC at the following dose levels: 18 to 59 years: HiDAC 2 gm/m2 i.v. every 12 hours days 1 to 5; 60 to 70 years: HiDAC 1.5 gm/m2 i.v. every 12 hours days 1 to 5 (Fig. 1). HiDAC was permitted to be dose reduced and/or discontinued due to organ dysfunction (i.e., renal or hepatic abnormalities) per institutional standards. Patients must have received >50% planned doses of HiDAC to remain eligible for pembrolizumab administration. Pembrolizumab 200 mg i.v. was administered on day 14 of treatment. Prior to initiation of pembrolizumab, patients were required to meet laboratory eligibility (see Supplementary Methods). If patients did not meet laboratory eligibility criteria and/or had uncontrolled intercurrent illness deemed by the investigator to be unsafe to administer pembrolizumab, pembrolizumab administration was permitted to be delayed up until day 21. Subjects ineligible to receive pembrolizumab by day 21 of therapy were removed from study protocol and replaced by another subject. All patients were hospitalized for treatment and discharged once early hematologic recovery was achieved.
Maintenance Phase.
Patients who achieved a PR, CR, or CRi (see “Assessment of Response”) were eligible to receive maintenance-phase pembrolizumab 200 mg i.v. every 3 weeks for up to 2 years until disease relapse, subsequent therapy (including alloSCT), or death. Prior to initiation of maintenance phase, all patients were required to meet laboratory-based eligibility criteria (see Supplementary Methods), and all treatment-related toxicities must have resolved to ≤ grade 1. Subjects with unacceptable toxicity (see “Safety Assessment”) during induction phase were not eligible to receive maintenance phase. Maintenance phase was permitted 10 to 60 days after full hematologic recovery from induction. Patients could undergo alloSCT after salvage treatment or during maintenance phase, but stem cell infusion day 0 was required to be ≥21 days after the last dose of pembrolizumab.
Safety Assessment
All patients who received pembrolizumab were evaluable for safety. Toxicity was assessed by NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Continuous monitoring for toxicity was performed throughout the study after pembrolizumab administration. Sequential boundaries were used to monitor unacceptable toxicity rates secondary to pembrolizumab. An unacceptable toxicity was defined as any drug-related grade 3 nonhematologic toxicity (exceptions include infusion reactions, rash, fever, infection, nausea, fatigue, and anorexia) persisting for >7 days despite supportive care, or any drug-related grade 4/5 nonhematologic toxicity (excluding infection). The accrual was halted if excessive numbers of unacceptable toxicities were equal to or exceeded boundary (bn) out of n patients with full follow-up (Supplementary Table S8). A Pocock-type stopping boundary was used and yields the probability of crossing the boundary at most 0.05 when the rate of unacceptable toxicity is equal to the acceptable rate of 0.2. The stopping boundary guided enrollment as well as suspension of accrual (i.e., when to stop the trial if necessary). Initially, three patients were enrolled, and accrual was then held until at least one of the first three patients completed follow-up (i.e., full hematologic recovery or no response to treatment) and was confirmed not to have unacceptable toxicity.
Dose modifications and management guidelines of iRAEs secondary to pembrolizumab are outlined in Supplementary Table S9. Drug-related grade ≥2 iRAEs (except for rash, infusion reactions, and thyroid dysfunction) were treated with systemic corticosteroids until resolution to grade ≤1. When possible, pathologic confirmation of iRAEs were recommended prior to initiation of systemic corticosteroids.
Assessment of Response
BM aspirate and biopsy were performed at the time of full hematologic recovery (i.e., absolute neutrophil count ≥1 × 109/L and platelets ≥100 × 109/L) or by day 45 of induction phase. Response criteria were consistent with standardized guidelines by European LeukemiaNet (50). MRD testing was done by institutional standards at the time of each response assessment. BM aspirate and biopsy were performed after every four cycles (i.e., every 3 months) of pembrolizumab for the first year, after every six cycles (i.e., every 4.5 months) for the second year of the maintenance phase, and at any point of clinical suspicion of relapse.
Next-Generation Sequencing
DNA was extracted from either the sorted blast populations or unsorted bulk cells, prepared in each case from BM aspirate samples collected prior to treatment on study (n = 31) or during prior lines of therapy (n = 6). Next-generation sequencing (NGS) was then performed using a 34-gene, customized hybridization capture assay (Custom Myeloid Solution, SOPHiA Genetics). Further methodology of the NGS panel and a full list of targeted genes and exons are included in Supplementary Methods and Supplementary Table S10, respectively.
Immune Biomarker Correlates
Mononuclear cells were isolated from PB and BM aspirates by Ficoll–Hypaque gradient centrifugation and cryopreserved. AML blasts were further enriched for RNA-seq analysis (Supplementary Methods). DNA and RNA were extracted from selected AML blast cells with the AllPrep DNA/RNA Mini kit (Qiagen, catalog number 80204). Samples of total RNA were extracted from AML blasts from BM aspirates by Qiagen RNeasy. Illumina TruSeq RNA Access sequencing libraries were created to convert total RNA into template molecules followed by sequence-specific capture of coding RNA. Sequencing was performed on an Illumina HiSeq 4000 platform using the Illumina HiSeq SBS 150 cycles with paired-end 2 × 75 base read pairs.
Samples of total RNA extracted from CD8+ bead selected lymphocytes from PB mononuclear cells (PBMC; Qiagen RNeasy Plus mini catalog number 79134 if >500,000 cells, or RNeasy Plus micro if <500,000 cells catalog number 74034) were used to prepare mRNA stranded sequencing libraries (Illumina Tru-Seq Stranded Library Prep Kit; cat. no. 20020594). Enrichment procedures of CD8+ lymphocytes for adaptive immune receptor repertoire analysis and mRNA stranded sequencing libraries are outlined in Supplementary Methods.
Flow Cytometry
BM mononuclear cells and PBMCs were serially collected from patients with AML (BM, n = 20; PB, n = 21) at baseline and at the time of response assessment (see “Assessment of Response”) after HiDAC plus pembrolizumab. Flow cytometry was performed on a BD-Fortessa (Becton Dickinson) provided with BD FACSDiva software (Becton Dickinson) version 8.0.1. Antibodies used for analysis are listed in Supplementary Table S11. Flow-cytometry data were biexponentially transformed, compensated using single stained controls, and preprocessed (aggregates and dead cell removal) in FlowJo V10 (TreeStar). The percentage of CD4+ and CD8+ T cells and other tertiary markers on each T-cell subpopulation was analyzed on GraphPad Prism software version 7. Pregated CD8+ T cells were then exported in R (version 4.0.2) for further analyses performed with a customized pipeline based on Nowicka and colleagues' (51) workflow. In particular, CD8+ T-cell clusters were obtained using the FlowSOM algorithm and then visualized using the implementation of UMAP available in CATALYST R package. The different frequencies of the T-cell subpopulations in CR and NR at the two time points (baseline and after treatment) were identified using the differential abundance analysis provided by the diffcyt R package (52). Further details are listed in Supplementary Methods.
Statistical Assessment
The primary objective of this single-arm, open-label phase II study was to estimate the CRc (CR + CRi) rate of HiDAC followed by pembrolizumab in R/R AML. The study design was a Simon-like two-stage design with relaxed stopping for futility. Relaxed stopping refers to inclusion of PR in the first stage, as some of these PRs may convert to a CR during the maintenance phase. The null hypothesis that the true CRc rate for HiDAC followed by pembrolizumab is 20% was tested against a one-sided alternative hypothesis. In the first stage, 19 patients were enrolled. If the number of patients who achieved a CRc plus the number of patients with PR was equal to ≤4 in these 19 patients, the study would be stopped for futility. Otherwise, 18 additional patients were enrolled for a total of 37 patients. The null hypothesis will be rejected if ≥12 CRcs are observed in 37 patients. Assuming that the PR rate has a uniform distribution, this design yields a type 1 error rate of at most 5% and power of at least 84% when the true CR rate for HiDAC followed by pembrolizumab is 40%.
Secondary endpoints of this study included rates of unacceptable toxicity as defined in “Safety Assessment”; toxicity of HiDAC plus pembrolizumab induction phase and pembrolizumab maintenance; ORR (CR + CRi + PR + MLFS); OS defined as day 1 of treatment until date of the last known follow-up; RFS defined as time from CRc until relapse or death; PFS defined as time from ORR until relapse, progression, or death; and EFS defined as day 1 of treatment until no response, relapse or death. Survival measurements were summarized by Kaplan–Meier methodology. Database lock was done on May 1, 2020.
Supplementary Material
Acknowledgments
This study was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily reflect those of Merck Sharp & Dohme Corp. The authors thank the research staff and coinvestigators at the University of North Carolina and Johns Hopkins for their invaluable contribution to this study. They also thank the patients and their families who have participated in this study.
Footnotes
Note: Supplementary data for this article are available at Blood Cancer Discovery Online (https://bloodcancerdiscov.aacrjournals.org/).
#J.S. Serody and I. Gojo contributed equally to this article.
Blood Cancer Discov 2021;2:616–29
Authors' Disclosures
J.F. Zeidner reports grants from Merck Sharp & Dohme Corp. during the conduct of the study, as well as personal fees from Agios, Bristol Myers Squibb/Celgene, Daiichi Sankyo, Genentech, Pfizer, Shattuck Labs, Servier, AbbVie, and AsystBio Laboratories, grants and personal fees from Gilead and Takeda, and grants from Arog, Astex, and Sumitomo Dainippon Pharma/Tolero outside the submitted work. B.G. Vincent reports other support from GeneCentric Therapeutics outside the submitted work. M.C. Foster reports grants from Merck during the conduct of the study, as well as grants and personal fees from Macrogenics, grants from Bellicum Pharmaceuticals and Rafael Pharmaceuticals, and personal fees from Agios and Daiichi Sankyo outside the submitted work. C.C. Coombs reports personal fees from LOXO, AstraZeneca, AbbVie, Genentech, Novartis, and Octapharma outside the submitted work. J.A. Webster reports personal fees from Pfizer and Amgen outside the submitted work. A.E. DeZern reports other support from Novartis, Taiho, Takeda, Bristol Myers Squibb, and Geron outside the submitted work. M.J. Levis reports personal fees from AbbVie, Amgen, Daiichi Sankyo, Jazz, and Bristol Myers Squibb, grants from FujiFilm, and grants and personal fees from Astellas outside the submitted work. N.D. Montgomery reports grants from Merck Sharp & Dohme Corp. during the conduct of the study. L. Luznik reports grants from Merck during the conduct of the study, as well as grants from Genentech, personal fees from Gilead Sciences, Rubius Therapeutics, Precision Biosciences, and Talais Therapeutics, and other support from WindMiL Therapeutics outside the submitted work. J.S. Serody reports grants from Merck Sharpe & Dohme Corp. and University Cancer Research Fund during the conduct of the study, as well as other support from PIQUE, and grants from GlaxoSmithKline and Carisma outside the submitted work. I. Gojo reports grants from Merck during the conduct of the study, as well as grants from Merck, Amgen, Amphivena, Gilead, Celgene, and Genentech and personal fees from Bristol Myers Squibb, Amgen, and Jazz outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
J.F. Zeidner: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. B.G. Vincent: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. A. Ivanova: Conceptualization, formal analysis, writing–review and editing. D. Moore: Formal analysis, writing–review and editing. K.P. McKinnon: Formal analysis, investigation, writing–original draft, writing–review and editing. A.D. Wilkinson: Formal analysis, investigation, writing–original draft, writing–review and editing. R. Mukhopadhyay: Formal analysis, investigation, writing–original draft, writing–review and editing. F. Mazziotta: Formal analysis, investigation, writing–original draft, writing–review and editing. H.A. Knaus: Formal analysis, investigation, writing–review and editing. M.C. Foster: Investigation, writing–review and editing. C.C. Coombs: Investigation, writing–review and editing. K. Jamieson: Investigation, writing–review and editing. H. Van Deventer: Investigation, writing–review and editing. J.A. Webster: Investigation, writing–review and editing. G.T. Prince: Investigation, writing–review and editing. A.E. DeZern: Investigation, writing–review and editing. B.D. Smith: Investigation, writing–review and editing. M.J. Levis: Investigation, writing–review and editing. N.D. Montgomery: Formal analysis, writing–review and editing. L. Luznik: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. J.S. Serody: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. I. Gojo: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 2019;381:1728–40. [DOI] [PubMed] [Google Scholar]
- 2.DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 2018;378:2386–98. [DOI] [PubMed] [Google Scholar]
- 3.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman JK, Montesinos P, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 2014;32:1919–26. [DOI] [PubMed] [Google Scholar]
- 5.Ravandi F, Ritchie EK, Sayar H, Lancet JE, Craig MD, Vey N, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 2015;16:1025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litzow MR, Wang XV, Carroll MP, Karp JE, Ketterling RP, Zhang Y, et al. A randomized trial of three novel regimens for recurrent acute myeloid leukemia demonstrates the continuing challenge of treating this difficult disease. Am J Hematol 2019;94:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 2005;23:1969–78. [DOI] [PubMed] [Google Scholar]
- 8.Ravandi F, Pierce S, Garcia-Manero G, Kadia T, Jabbour E, Borthakur G, et al. Salvage therapy outcomes in a historical cohort of patients with relapsed or refractory acute myeloid leukemia. Clin Lymphoma Myeloma Leuk 2020;20:e871–82. [DOI] [PubMed] [Google Scholar]
- 9.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR.Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood 2011;118:5084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanakry CG, Hess AD, Gocke CD, Thoburn C, Kos F, Meyer C, et al. Early lymphocyte recovery after intensive timed sequential chemotherapy for acute myelogenous leukemia: peripheral oligoclonal expansion of regulatory T cells. Blood 2011;117:608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight 2018;3:e120974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood 2010;116:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N, et al. In acute myeloid leukemia, B7-H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunol Immunother 2010;59:1839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, Shimizu M, et al. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplastic syndromes. Blood 2010;116:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronig H, Kremmler L, Haller B, Englert C, Peschel C, Andreesen R, et al. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol 2014;92:195–203. [DOI] [PubMed] [Google Scholar]
- 16.Dolen Y, Esendagli G.Myeloid leukemia cells with a B7–2(+) subpopulation provoke Th-cell responses and become immuno-suppressive through the modulation of B7 ligands. Eur J Immunol 2013;43:747–57. [DOI] [PubMed] [Google Scholar]
- 17.Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer 2019;125: 1470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Liang C, Wang S, Chio CL, Zhang Y, Zeng C, et al. Expression patterns of immune checkpoints in acute myeloid leukemia. J Hematol Oncol 2020;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016;374:2209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015;125:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cader FZ, Hu X, Goh WL, Wienand K, Ouyang J, Mandato E, et al. A peripheral immune signature of responsiveness to PD-1 blockade in patients with classical Hodgkin lymphoma. Nat Med 2020;26:1468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 2019;576:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galletti G, De Simone G, Mazza EMC, Puccio S, Mezzanotte C, Bi TM, et al. Two subsets of stem-like CD8(+) memory T cell progenitors with distinct fate commitments in humans. Nat Immunol 2020;21:1552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity 2019;50:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 2019;50:195–211. [DOI] [PubMed] [Google Scholar]
- 26.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, Hołowiecki J, et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol 2008;80:115–26. [DOI] [PubMed] [Google Scholar]
- 28.Advani AS, Cooper B, Visconte V, Elson P, Chan R, Carew J, et al. A phase I/II trial of MEC (mitoxantrone, etoposide, cytarabine) in combination with ixazomib for relapsed refractory acute myeloid leukemia. Clin Cancer Res 2019;25:4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol 2012;30:2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karanes C, Kopecky KJ, Head DR, Grever MR, Hynes HE, Kraut EH, et al. A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res 1999;23:787–94. [DOI] [PubMed] [Google Scholar]
- 31.Pettitt AR.Mechanism of action of purine analogues in chronic lymphocytic leukaemia. Br J Haematol 2003;121:692–702. [DOI] [PubMed] [Google Scholar]
- 32.Walter RB, Othus M, Löwenberg B, Ossenkoppele GJ, Petersdorf SH, Pabst T, et al. Empiric definition of eligibility criteria for clinical trials in relapsed/refractory acute myeloid leukemia: analysis of 1,892 patients from HOVON/SAKK and SWOG. Haematologica 2015;100:e409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravandi F, Cortes J, Faderl S, O'Brien S, Garcia-Manero G, Verstovsek S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood 2010;116:5818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M.A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood 1996;88:756. [PubMed] [Google Scholar]
- 35.Vadakekolathu J, Minden MD, Hood T, Church SE, Reeder S, Altmann H, et al. Immune landscapes predict chemotherapy resistance and immunotherapy response in acute myeloid leukemia. Sci Transl Med 2020;12:eaaz0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021;137:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov 2019;9:370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postow MA, Manuel M, Wong P, Yuan J, Dong Z, Liu C, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer 2015;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019; 20:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014;25:590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lastwika KJ, Wilson W, 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res 2016;76:227–38. [DOI] [PubMed] [Google Scholar]
- 42.Becker H, Marcucci G, Maharry K, Radmacher MD, Mrózek K, Margeson D, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood 2010;116:788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gröschel S, Sanders MA, Hoogenboezem R, Zeilemaker A, Havermans M, Erpelinck C, et al. Mutational spectrum of myeloid malignancies with inv(3)/t(3;3) reveals a predominant involvement of RAS/RTK signaling pathways. Blood 2015;125:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vadakekolathu J, Lai C, Reeder S, Church SE, Hood T, Lourdusamy A, et al. TP53 abnormalities correlate with immune infiltration and associate with response to flotetuzumab immunotherapy in AML. Blood Adv 2020;4:5011–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallman DA, Asch AS, Kambhampati S, A Malki MM, Zeidner JF, Donnellan W, et al. The first-in-class anti-CD47 antibody magrolimab combined with azacitidine is well-tolerated and effective in AML patients: phase 1b results [abstract]. In: Proceedings of the 62nd ASH Annual Meeting and Exposition; 2020 Dec 5–8. Washington (DC): American Society of Hematology; 2020. Abstract nr 330. Available from: https://ash.confex.com/ash/2020/webprogram/Paper134728.html. [Google Scholar]
- 46.Dufva O, Pölönen P, Brück O, Keränen MAI, Klievink J, Mehtonen J, et al. Immunogenomic landscape of hematological malignancies. Cancer Cell 2020;38:380–99. [DOI] [PubMed] [Google Scholar]
- 47.Ravandi F, Assi R, Daver N, Benton CB, Kadia T, Thompson PA, et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a single-arm, phase 2 study. Lancet Haematol 2019;6:e480–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carreau NA, Pail O, Armand P, Merryman R, Advani RH, Spinner MA, et al. Checkpoint blockade treatment may sensitize Hodgkin lymphoma to subsequent therapy. Oncologist 2020;25:878–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Short NJ, Zhou S, Fu C, Berry DA, Walter RB, Freeman SD, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: a systematic review and meta-analysis. JAMA Oncol 2020;6:1890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010;115:453–74. [DOI] [PubMed] [Google Scholar]
- 51.Nowicka M, Krieg C, Crowell HL, Weber LM, Hartmann FJ, Guglietta S, et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res 2017;6:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber LM, Nowicka M, Soneson C, Robinson MD.diffcyt: differential discovery in high-dimensional cytometry via high-resolution clustering. Commun Biol 2019;2:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P.The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 2015;1:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.