Abstract
The Pb99 gene is specifically expressed in pre-B cells and thymocytes and not in mature B and T cells or nonlymphoid tissues, implying that it may function in early lymphoid development. We have previously described the cloning of an incomplete cDNA for Pb99. Here we report the isolation of full-length cDNAs and genomic clones for the murine Pb99 gene and the mapping of its location to mouse chromosome 8. Sequence analyses of different Pb99 cDNA clones suggest that there may be at least three forms of the Pb99 protein generated by differential processing of the Pb99 transcript. The cDNA with the longest open reading frame encodes a putative protein that has seven hydrophobic domains similar to those of seven membrane-spanning proteins, such as the classical G protein-coupled receptors. To directly address the role of the Pb99 protein in lymphoid development, Pb99-deficient mice were generated by gene targeting, and lymphocyte development in these mice was analyzed.
Lymphocyte development is an ordered process that depends on the assembly and expression of antigen receptor genes and the coordinate expression of nonreceptor genes that encode proteins required for cell growth and differentiation (reviewed in reference 34). The isolation of genes expressed during B- and T-cell development has yielded valuable information about the molecular basis of lymphocyte development. These genes include those that encode transcription factors, signaling proteins, members of antigen receptor complexes, and proteins involved in antigen receptor gene recombination (reviewed in references 1, 7, and 33). For example, recombination-activating genes 1 and 2 are expressed in early B and T cells and encode proteins that introduce double-stranded DNA breaks at the junction of variable gene segment coding and signal sequences and, as such, are critical for V(D)J recombination and lymphocyte development (16, 19, 26, 27, 32).
We have previously described the use of subtractive hybridization to isolate cDNA clones for genes that are expressed during early B-cell development (36). Several of the clones isolated represent sequences that encode proteins of known function, such as CD2, CD19, TCRγ, λ5, and Vpre-B (36). However, several sequences were not identified when the clones were compared to genes reported in databases, and we have focused our attention on defining a role for these genes in lymphocyte development. In this regard, we have previously demonstrated that one of these genes, PLRLC, which is homologous to regulatory myosin light-chain genes, is expressed in pre-T cells and in pre-B cells derived from the bone marrow but not in those derived from fetal liver (20). In addition, PLRLC expression is inducible by interleukin-7 (20).
Here we report the analysis of another early lymphocyte-specific gene, Pb99, identified in this screen (36). Pb99 is expressed in early B-cell lines up to the pre-B-cell stage and in pre-T cells in the thymus but not in mature lymphoid or nonlymphoid tissues (15, 36). The restricted expression pattern suggests that the Pb99 protein functions in early B- and T-cell development. We have obtained full-length cDNA and genomic clones for Pb99 and have determined the intron-exon structure and chromosomal localization of the Pb99 gene. In order to help define the role of the Pb99 protein in lymphocyte development, we analyzed Pb99-deficient mice generated by gene targeting.
MATERIALS AND METHODS
Isolation of Pb99 cDNA and genomic clones.
The incomplete Pb99 cDNA fragment (36) was used as a probe to screen a λGT10 cDNA library derived from the Abelson murine leukemia virus (A-MuLV)-transformed pre-B-cell line 22D6. Positive clones (n = 45) were isolated from 0.6 × 106 clones screened and were grouped into two types based on restriction endonuclease mapping. The Pb99.3 and Pb99.5 clones represented the two types. The Pb99.2 clone was isolated by screening an oligo(dT)-primed BALB/c mouse intestine cDNA library constructed in the UniZAP XR vector (Stratagene, La Jolla, Calif.) with the Pb99.3 cDNA.
Genomic lambda phage clones were isolated from a 129sv genomic library (Stratagene) using the Pb99.3 cDNA as a probe. Two partially overlapping clones were identified and their restriction map was determined by partial restriction digestion and hybridization with T3 and T7 oligonucleotide probes as previously described (28). Fragments of these genomic clones were subcloned into pBluescript, and oligonucleotides synthesized on the basis of cDNA sequences were used as hybridization probes in PCRs and as sequencing primers to determine the intron/exon structure.
Interspecific mouse backcross mapping.
Interspecific backcross progeny were generated by mating (C57BL/6J × Mus spretus)F1 females and C57BL/6J males as previously described (5). A total of 205 N2 mice were used to map the Pb99 locus (see below for details). DNA isolation, restriction enzyme digestion, agarose gel electrophoresis, Southern blot transfer, and hybridization were performed essentially as described previously (10). All blots were prepared with Hybond-N+ nylon membranes (Amersham). The probe, a 400-bp EcoRI/PstI fragment of the Pb99.3 cDNA, was labeled with [α-32P]dCTP using a nick translation labeling kit (Boehringer Mannheim); washing was done to a final stringency of 0.6 M sodium chloride–7.5 mM sodium citrate–5 mM potassium phosphate–0.1% sodium dodecyl sulfate at 65°C. A fragment of 4.1 kb was detected in BglI-digested C57BL/6J DNA, and a fragment of 5.8 kb was detected in BglI-digested M. spretus DNA. The presence or absence of the 5.8-kb BglI M. spretus-specific fragment was monitored in backcross mice.
The probes and restriction fragment length polymorphisms (RFLPs) for the loci linked to Pb99, including Lyl1, Gnao, and Cbfb, have been described previously (24). Recombination distances were calculated using Map Manager, version 2.6.5. The gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns.
Cell culture.
A-MuLV-transformed pre-B-cell lines were established as previously described (23). Bone marrow cells from −/−, +/+, and +/− mice were infected with A-MuLV and plated in soft agar; individual colonies were picked, transferred to liquid medium, and expanded. The −/−, +/+, and +/− genotypes of all cell lines were confirmed by Southern blot analysis (data not shown).
Generation of Pb99-deficient mice.
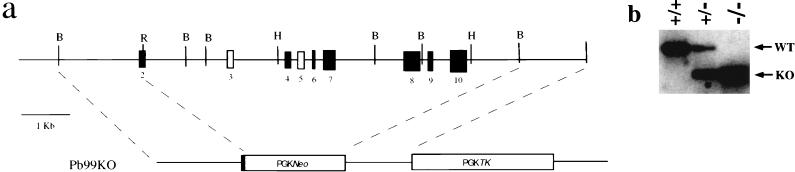
The Pb99-targeting construct (Pb99KO) was made by subcloning a cDNA containing the neomycin resistance gene driven by the PGK promoter (PGKNeo) between a 1.8-kb BamHI/EcoRI 5′ Pb99 genomic DNA fragment (5′ homology region) and a 2.5-kb HindIII/SalI 3′ Pb99 genomic DNA fragment (3′ homology region) (see Fig. 2a). The SalI site was derived from the phage cloning arm. A cDNA containing the herpes simplex virus thymidine kinase gene driven by the PGK promoter (PGKTk) was then subcloned 3′ of the Pb99 3′ homology region to complete the construct (see Fig. 2a). J1 ES cells were electroporated with 15 μg of PvuI-linearized Pb99KO DNA and selected in media containing G418 and ganciclovir as previously described (12). +/− J1 embryonic stem (ES) cells were identified by Southern blot analysis as described below. These cells were injected into C57BL/6 blastocysts, and chimeric mice capable of transmitting the Pb99 targeted allele to offspring were identified.
FIG. 2.
Structure of the murine Pb99 gene and targeting construct. (a) Intron-exon structure of the nine exons (numbered 2 through 10) contained in the Pb99 genomic clones isolated. The unfilled boxes are exons that are not present in the Pb99.2 (exon 5) and Pb99.5 (exons 3 and 5) clones. Also shown is the Pb99-targeting construct (Pb99KO) described in the text. BamHI (B), EcoRI (R), and HindIII (H) sites are shown. (b) Southern blot of EcoRI-digested genomic DNA from +/+, +/−, and −/− mice probed with the 3′ KO probe, as described in Materials and Methods. Bands generated by the wild-type (WT; 20 kb) and targeted (KO; 16 kb) alleles are indicated.
DNA and RNA analysis.
Genomic DNA was isolated as previously described (14). Whole-cell RNA was prepared using the Trizol reagent (GIBCO BRL) according to the directions of the manufacturer. Southern and Northern blotting were carried out as previously described (35) using Zetaprobe membranes (Bio-Rad) and probes generated by random hexamer priming (Boehringer Mannheim) using [α-32P]dCTP. A 250-bp genomic DNA fragment extending from a NotI site in a polylinker to a BglII site in the Pb99 gene was used as a probe (5′ KO) to screen for appropriate 5′ integration of the Pb99KO construct. A 300-bp EcoRI/PstI fragment from the 3′ portion of the Pb99.3 cDNA was used as a probe (3′ KO) to detect appropriate 3′ integration of the Pb99KO construct.
Flow cytometry.
Single-cell suspensions were prepared from thymus, spleen, and lymph nodes as previously described (4). Cells were stained with Cy-chrome (Cyc)-, fluorescein isothiocyanate (FITC)-, or phycoerythrin (PE)-conjugated antibodies and were analyzed by a FACScan (Becton Dickinson). The following antibodies from Pharmingen were used in these studies: PE-conjugated anti-CD4 (RM4-5), FITC-conjugated anti-CD8 (53-6.7), Cyc- and FITC-conjugated anti-B220 (RA3-6B2), PE-conjugated anti-immunoglobulin M (IgM) (R6-60.2), FITC-conjugated anti-CD43 (S7), and PE-conjugated anti-Thy 1.2 (53-2.1).
Nucleotide sequence accession number.
The Pb99 cDNA sequence can be retrieved from GenBank, accession number AF249738.
RESULTS
Isolation and sequence analysis of murine Pb99 cDNA clones.
To obtain full-length Pb99 cDNA clones, the previously obtained (36) incomplete cDNA clone was used to screen cDNA libraries as described in Materials and Methods. Full-length cDNA clones of three types, represented by Pb99.2, Pb99.3, and Pb99.5, were isolated and sequenced (Fig. 1a and data not shown). Sequence analysis revealed that the difference between these three clones is likely due to differential processing of the Pb99 transcript (Fig. 1a and 2a). The Pb99.2 clone, which lacks exon 5, contains the longest open reading frame, encoding a putative 542-amino-acid protein (Fig. 1a). Pb99.3 contains exons 2 through 8, whereas Pb99.5 is missing exons 3 and 5. In addition, the 5′ regions of the Pb99.2, Pb99.3, and Pb99.5 cDNAs differ, possibly due to differential splicing of exons that are 5′ of exon 2 (data not shown).
FIG. 1.
(a) cDNA sequence and amino acid translation of the longest open reading frame of Pb99.2. The nucleotides in italics represent the sequence of exon 5, which is included only in the Pb99.3 clone. (b) Hydrophilicity plot of the longest open reading frame of the Pb99.2 cDNA according to the method of Kyte and Doolittle (13).
Analysis of the predicted amino acid sequence of the longest open reading frame of the Pb99.2 clone reveals that it contains a short N-terminal hydrophobic region that may function as a signal peptide (Fig. 1b). This is followed by a relatively hydrophilic region of approximately 200 amino acids (Fig. 1b). The C terminus encoded by Pb99.2 contains seven hydrophobic regions, suggesting that the putative protein encoded by Pb99.2 may be a seven-membrane-spanning protein similar to G protein-coupled receptors (Fig. 1b) (2).
Structure of the murine Pb99 gene.
Genomic Pb99 clones were isolated from the 129sv lambda phage genomic library by screening with the Pb99.3 cDNA. Two partially overlapping clones were identified and mapped by restriction digestion. Portions of these genomic clones were subcloned into pBluescript, and the intron-exon structure was determined by PCR and sequence analysis (Fig. 2a and Table 1). There are a total of nine exons contained within a 9-kb region (Fig. 2a, 2 through 10). Southern blot analysis revealed that the 5′-most and 3′-most exons are not included in the genomic clones isolated (Fig. 1 and 2a, Table 1, and data not shown). Accordingly, we have designated the 5′-most exon in the genomic clones described here exon 2 (Fig. 2a). As determined by Southern blot analysis, the 5′-most exon(s) is more than 20 kb upstream of exon 2 (Fig. 2a and data not shown). This exon(s) contains the start site of translation and encodes the first 67 amino acids of the putative protein encoded by Pb99.2. The exon(s) that encodes the 36 most C-terminal amino acids of this protein is also not included in the genomic clones isolated.
TABLE 1.
Exon borders and sizea
| Exon | 5′ border | 3′ border | Size (nucleotides) |
|---|---|---|---|
| 2 | AGATACTGGT | ATGCTTTCCC | 136 |
| 3 | AGATCCCGAG | AATGTCTTTA | 147 |
| 4 | AGGGCCCCAA | TCAACCACCT | 137 |
| 5 | GCAATGATCC | ATCTCCCCAT | 122 |
| 6 | AACGTGATCC | TGGCTAAAGG | 40 |
| 7 | AGACTGGGAT | ATGTTGCCTT | 201 |
| 8 | CAGGTTCTCT | TGGCCTGGGG | 285 |
| 9 | CCTGCCTGTT | TGGAGTTGTG | 96 |
| 10 | CTGGTTCCAG | CTCTACAAGG | 273 |
The sizes of the exons and the sequences of the 5′ and 3′ borders of the nine exons in Fig. 2a are shown.
Chromosomal mapping of the Pb99 gene.
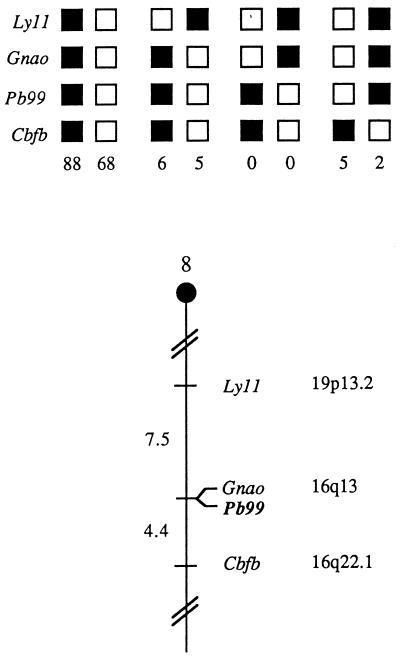
The mouse chromosomal location of Pb99 was determined by interspecific backcross analysis using progeny derived from matings of [(C57BL/6J × M. spretus)F1 × C57BL/6J] mice. This interspecific backcross mapping panel has been typed for over 2,500 loci that are well distributed among all the autosomes as well as the X chromosome (10). C57BL/6J and M. spretus DNAs were digested with several enzymes and analyzed by Southern blot hybridization for informative RFLPs using a fragment of Pb99.3. The 5.8-kb BglI M. spretus RFLP (see Materials and Methods) was used to monitor the segregation of the Pb99 locus in backcross mice. The mapping results indicate that Pb99 is located in the central region of mouse chromosome 8 linked to Lyl1, Gnao, and Cbfb (Fig. 3). Although 174 mice were analyzed for every marker and are shown in the segregation analysis (Fig. 3), up to 187 mice were typed for some pairs of markers. Each locus was analyzed in pairwise combinations for recombination frequencies using additional data. The most likely gene order is centromere, Lyl1, Gnao, Pb99, and Cbfb. The ratios of the total number of mice exhibiting recombinant chromosomes to the total number of mice analyzed for each pair of loci are as follows: 14/187 for Lyl1 and Gnao, 0/186 for Gnao and Pb99, and 8/181 for Pb99 and Cbfb. The recombination frequencies (expressed as mean genetic distances, in centimorgans, ± the standard error) are as follows: 7.5 ± 1.9 for Lyl1 and the Gnao and Pb99 loci and 4.4 ± 1.5 for the Gnao and Pb99 loci and Cbfb. No recombinants were detected between Gnao and Pb99 in 186 animals typed in common, suggesting that the two loci are within 1.6 centimorgans of each other (upper 95% confidence limit).
FIG. 3.
Pb99 maps in the central region of mouse chromosome 8. Pb99 was placed on mouse chromosome 8 by interspecific backcross analysis. The segregation patterns of Pb99 and flanking genes in 174 backcross animals that were typed for all loci are shown at the top. For individual pairs of loci, more than 174 animals were typed (see text). Each column represents the chromosome identified in the backcross progeny that was inherited from the (C57BL/6J × M. spretus)F1 parent. The black boxes represent the presence of a C57BL/6J allele, and the white boxes represent the presence of an M. spretus allele. The number of offspring inheriting each type of chromosome is listed at the bottom of each column. A partial chromosome 8 linkage map showing the location of Pb99 in relation to linked genes is shown at the bottom. Recombination distances between loci, in centimorgans, are shown to the left of the chromosome, and the positions of the loci in human chromosomes, where known, are shown to the right. References for the human map positions of loci cited in this study can be obtained from the GDB (Genome Data Base), a computerized database of human linkage information maintained by The William H. Welch Medical Library of The Johns Hopkins University (Baltimore, Md.).
Generation of Pb99-deficient mice.
To address the role of Pb99 in lymphoid development, Pb99-deficient mice were generated by gene targeting. The Pb99-targeting vector (Pb99KO) was constructed as described in Materials and Methods (Fig. 2a). Gene targeting by homologous recombination using Pb99KO resulted in deletion of 9 kb of the Pb99 gene, including eight of the coding exons (Fig. 2a). J1 ES cells were transfected with the Pb99KO vector, and the resulting clones were screened for targeted deletions of the Pb99 gene by Southern blot analysis of EcoRI-digested genomic DNA using the 3′ KO probe as described in Materials and Methods (data not shown). Confirmation of appropriate 5′ integration of Pb99KO was carried out on HindIII-digested genomic DNA using the 5′ KO probe (data not shown).
+/− J1 ES cells were used to generate chimeric mice, and those capable of transmitting the targeted allele were identified. These mice were bred with 129sv mice to generate +/− mice which were in turn bred to generate +/+, +/−, and −/− mice (Fig. 2b). Since the J1 ES cell is of 129sv origin, the resulting mice have a homogenous 129sv genetic background. Breeding of +/− mice resulted in the expected number of −/− offspring, given Mendelian inheritance of the Pb99 targeted allele. In addition, the −/− mice appeared grossly normal in size and morphology, and both males and females were fertile.
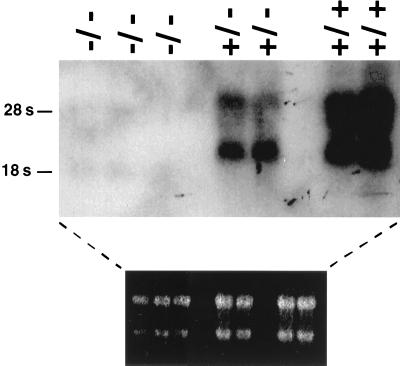
To assay for Pb99 expression, A-MuLV-transformed pre-B-cell lines were derived from bone marrow of +/+, +/−, and −/− mice. Pb99 transcripts could be detected in +/+ and +/− cells but were lacking in −/− A-MuLV-transformed B cells (Fig. 4).
FIG. 4.
Pb99 expression in +/+, +/−, and −/− A-MuLV-transformed pre-B-cell lines. Total cell RNA isolated from three independent −/− and two independent +/− and +/+ A-MuLV-transformed pre-B-cell lines was subjected to Northern blot analysis using the Pb99.3 cDNA as a probe. The ethidium stain of the gel is shown to demonstrate RNA loading.
Lymphocyte development in Pb99-deficient mice.
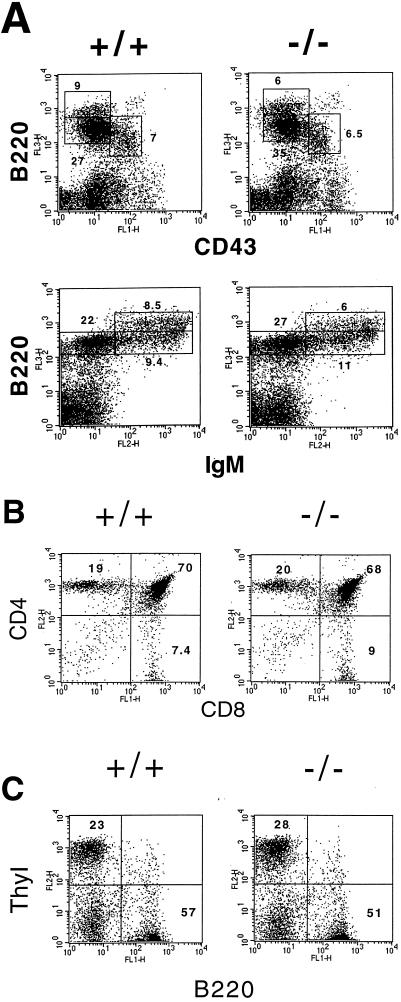
Lymphocyte development in +/+, +/−, and −/− mice was assayed in mice ranging from 3 to 8 weeks of age. Flow cytometric analysis of thymocyte development in these mice revealed essentially normal numbers of CD4− CD8− (double negative), CD4+ CD8+ (double positive), and CD4+ CD8− or CD4− CD8+ (single positive) thymocytes in +/+, +/−, and −/− mice (Fig. 5b and data not shown). Analysis of peripheral T cells in the spleen and lymph nodes revealed essentially normal numbers of Thy 1+ T cells (Fig. 5c and data not shown). These results demonstrate that Pb99 is not absolutely required for T-cell development.
FIG. 5.
Flow cytometric analysis of B and T cells in −/− mice. (a) Bone marrow from +/+ and −/− mice was stained with Cyc-conjugated anti-B220 and FITC-conjugated anti-CD43 or with PE-conjugated anti-IgM and Cyc-conjugated anti-B220. (b) Thymocytes from +/+ and −/− mice were stained with PE-conjugated anti-CD4 and FITC-conjugated anti-CD8. (c) Splenocytes from +/+ and −/− mice were stained with PE-conjugated anti-Thy 1.2 and FITC-conjugated anti-B220. Shown are representative analyses of at least eight mice of each genotype.
To investigate whether an absence of Pb99 expression affects B-lymphocyte development, we performed flow cytometric analyses on bone marrow from +/+, +/−, and −/− mice. We observed normal numbers of pro-B (IgM−, B220dull, CD43+, CD2−, and CD25−), pre-B (IgM−, B220+, CD43−, CD2+, and CD25+), and mature B (IgM+, B220+ CD43−, CD2+, and CD22+) cells in the bone marrow of −/− mice (Fig. 5a and data not shown). The number of B-lineage cells in spleen and lymph nodes also appeared to be normal (Fig. 5c and data not shown). Therefore, Pb99 is also not required for B-cell development.
DISCUSSION
We have previously described the cloning of several genes that are expressed in immature B cells by subtractive hybridization and have been interested in further defining the role of these genes in regulating lymphocyte development (20, 36). Here we expanded the analysis to include the Pb99 gene, which is expressed exclusively in early B and T cells and, therefore, likely functions during B- and T-cell development. In the present study we describe the molecular cloning of full-length Pb99 cDNAs and Pb99 genomic clones. These analyses reveal that the murine Pb99 gene spans a distance of more than 30 kb and that at least three different forms of the protein may exist based on differential processing of transcripts.
The Pb99 gene was mapped to mouse chromosome 8. We have compared our interspecific map of chromosome 8 with a composite mouse linkage map that reports the map location of many uncloned mouse mutations (provided from the Mouse Genome Database, a computerized database maintained at The Jackson Laboratory, Bar Harbor, Maine). Pb99 mapped in a region of the composite map that lacks mouse mutations with a phenotype that might be expected for an alteration in this locus (data not shown). The central region of mouse chromosome 8 shares regions of homology with human 19p and 16q (summarized in Fig. 3). In particular, Gnao has been mapped to 16q13. The close linkage between Gnao and Pb99 in mouse suggests that the human homolog of Pb99 will map to 16q13 as well.
Analysis of the predicted amino acid sequence of Pb99.2 shows that it contains a hydrophobic signal peptide and seven distinct hydrophobic domains, suggesting that it may be an integral membrane protein that spans the membrane seven times, similar to the classical G protein-coupled receptors (Fig. 1b) (2). Comparison with sequences reported in the database revealed that Pb99 shares homology with several putative seven-membrane-spanning proteins (3, 6, 8, 17, 21). Most notably, Pb99 shares some homology with members of the recently described EGF-TM7 subfamily of cell surface receptors, which are expressed primarily on white blood cells (reviewed in reference 18). These proteins include the mouse F4/80 (17), human EMR1 (3), and human CD97 (8) proteins. The F4/80 protein is expressed on macrophages early in development, and CD97 is expressed by resting B and T cells and is upregulated upon activation (reviewed in reference 18). Given the homology to hormone receptors and pattern of expression, it has been proposed that the EGF-TM7 proteins are receptors which function in the immune response (reviewed in reference 18). However, a clear role for the EGF-TM7 receptors in the immune response has yet to be elucidated. Pb99 is also highly homologous to a 391-bp expressed sequence tag, p8C12, isolated from a caffeine-stimulated pre-B-cell line (9; GenBank accession number U14114).
Comparison of the deduced amino acid sequences of the human and mouse Pb99 cDNAs has revealed a 79% homology (J. E. Berman and W. Xu, unpublished data). This level of conservation suggests that Pb99 has a functional role; however, analysis of B- and T-cell development in Pb99-deficient mice revealed no significant quantitative or qualitative defects (Fig. 5). These findings do not exclude a role for the Pb99 protein in B- and T-cell development, as in its absence, other proteins may carry out its function. This type of functional redundancy is exemplified by the MyoD and Myf-5 proteins, which play important roles in myogenesis. Mice deficient in either of these proteins exhibit normal muscle development; however, mice deficient in both of these proteins fail to develop due to a block in myogenesis (25). Therefore, it is possible that proteins which are functionally redundant with Pb99 affect normal B- and T-cell development in Pb99-deficient mice.
Alternatively, Pb99 function, although important, may not be absolutely required for normal B- and T-cell development. This was observed with the CD2 and CD5 proteins, which are expressed early in development and which function in lymphocyte signaling. Mice deficient in the CD2 or CD5 protein exhibit normal B- and T-cell development (11, 29). However, more detailed analyses of these mice have subsequently revealed defects in thymocyte positive selection (30, 31). Future detailed analyses of the Pb99-deficient mice will further elucidate the role of the Pb99 protein in lymphocyte function and development (22).
ACKNOWLEDGMENTS
We thank Debra J. Gilbert for excellent technical assistance. Ganciclovir was a gift of the Syntex Corporation.
This work is supported by the Howard Hughes Medical Institute, by National Institutes of Health grants A.I.20047 (F.W.A.), CA61009 (B.A.M.), A.I.01297-01 (B.P.S.), and A.I.32590 (J.E.B.), and by the National Cancer Institute, DHHS, under contract with ABL (N.A.J.). B.P.S. is a recipient of a Career Development Award in the Biomedical Sciences from the Burroughs Wellcome Fund. C.G.B. is a Howard Hughes Medical Institute Medical Student Fellow.
Barry P. Sleckman and Wasif N. Khan contributed equally to this study.
REFERENCES
- 1.Alt F W, Oltz E M, Young F, Gorman J, Taccioli G, Chen J. VDJ recombination. Immunol Today. 1992;13:306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin J M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993;12:1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baud V, Chissoe S L, Viegas-Pequignot E, Diriong S, N'Guyen V C, Roe B A, Lipinski M. EMR1, an unusual member in the family of hormone receptors with seven transmembrane segments. Genomics. 1995;26:334–344. doi: 10.1016/0888-7543(95)80218-b. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Lansford R, Stewart V, Young F, Alt F W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copeland N G, Jenkins N A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991;7:113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- 6.Davletov B A, Shamotienko O G, Lelianova V G, Grishin E V, Ushkaryov Y A. Isolation and biochemical characterization of a Ca2+-independent alpha-latrotoxin-binding protein. J Biol Chem. 1996;271:23239–23245. doi: 10.1074/jbc.271.38.23239. [DOI] [PubMed] [Google Scholar]
- 7.Georgopoulos K. Transcription factors required for lymphoid lineage commitment. Curr Opin Immunol. 1997;9:222–227. doi: 10.1016/s0952-7915(97)80139-2. [DOI] [PubMed] [Google Scholar]
- 8.Hamann J, Eichler W, Hamann D, Kerstens H M J, Poddighe P J, Hoovers J M N, Hartmann E, Strauss M, van Lier R A. Expression cloning and chromosomal mapping of the leukocyte activation antigen CD97, a new seven-span transmembrane molecule of the secretin receptor superfamily with an unusual extracellular domain. J Immunol. 1995;155:1942–1950. [PubMed] [Google Scholar]
- 9.Hubank M, Schatz D G. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 1994;22:5640–5648. doi: 10.1093/nar/22.25.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkins N A, Copeland N G, Taylor B A, Lee B K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Killeen N, Stuart S G, Littman D R. Development and function of T cells in mice with a disrupted CD2 gene. EMBO J. 1992;11:4329–4336. doi: 10.1002/j.1460-2075.1992.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 13.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 14.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma A, Fisher P, Dildrop R, Oltz E, Rathbun G, Achacoso P, Stal A, Alt F W. Surface IgM mediated regulation of RAG gene expression in Eμ-N-myc B cell lines. EMBO J. 1992;11:2727–2734. doi: 10.1002/j.1460-2075.1992.tb05338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBlane J F, van Gent D C, Ramsden D A, Romeo C, Cuomo C, Gellert M, Oettinger M A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 17.McKnight A J, MacFarlane A J, Dri P, Turley L, Willis A C, Gordon S. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271:486–489. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 18.McKnight A J, Gordon S. EGF-TM7: a novel subfamily of seven-transmembrane-region leukocyte cell-surface molecules. Immunol Today. 1996;17:283–287. doi: 10.1016/0167-5699(96)80546-9. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts P, Iacommi J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 20.Oltz E M, Yancopoulos G D, Morrow M A, Rolink A, Lee G, Wong F, Kaplan K, Gillis S, Melchers F, Alt F W. A novel regulatory myosin light chain gene distinguishes pre-B subsets and is IL-7 inducible. EMBO J. 1992;11:2759–2767. doi: 10.1002/j.1460-2075.1992.tb05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterhoff C, Kirchoff C. Cloning of a human epididymis-specific mRNA, HE6, encoding a novel member of a seven transmembrane-domain receptor family. DNA Cell Biol. 1997;16:379–389. doi: 10.1089/dna.1997.16.379. [DOI] [PubMed] [Google Scholar]
- 22.Rajewsky K. A phenotype or not: targeting genes in the immune system. Science. 1992;256:483. doi: 10.1126/science.1570513. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg N, Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976;143:1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi D L, Hardiman G, Copeland N G, Gilbert D J, Jenkins N A, Zlotnick A, Bazan J F. Cloning and characterization of a new type of mouse chemokine. Genomics. 1997;47:163–170. doi: 10.1006/geno.1997.5058. [DOI] [PubMed] [Google Scholar]
- 25.Rudnicki M A, Schnegelsberg P N J, Stead R H, Braun T, Arnold H-H, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 26.Schatz D G, Oettinger M A, Schlissel M S. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–383. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 27.Shinkai Y, Rathbun G, Lam K-P, Oltz E M, Stewart V, Mendelshon M, Charron J, Datta M, Young F, Stall A M, Alt F W. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 28.Sideras P, Muller S, Shiels H, Jin H, Khan W N, Nilsson L, Parkinson E, Thomas J D, Branden L, Larsson I, Paul W E, Rosen F S, Alt F W, Vetrie D, Edvard Smith C I, Xanthopoulos K G. Genomic organization of the mouse and human Bruton's agammaglobulinemia tyrosine kinase (Btk) loci. J Immunol. 1994;153:5607–5617. [PubMed] [Google Scholar]
- 29.Tarakhovsky A, Muller W, Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur J Immunol. 1994;24:1678–1684. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- 30.Tarakhovsky A, Kanner S B, Hombach J, Ledbetter J A, Muller W, Killeen N, Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 31.Teh S-J, Killeen N, Tarakhovsky A, Littman D R, The H-S. CD2 regulates the positive selection and function of antigen-specific CD4−/CD8+ T cells. Blood. 1997;89:1308–1318. [PubMed] [Google Scholar]
- 32.van Gent D, McBlane J F, Ramsden D A, Sadofsky M, Hesse J E, Gellert M. Initiation of V(D)J recombination in a cell-free system. Cell. 1995;81:925–934. doi: 10.1016/0092-8674(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 33.Weis A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 34.Willerford D M, Swat W, Alt F W. Developmental regulation of V(D)J recombination and lymphocyte differentiation. Curr Opin Genet Dev. 1996;6:603–609. doi: 10.1016/s0959-437x(96)80090-6. [DOI] [PubMed] [Google Scholar]
- 35.Yancopoulos G D, Blackwell T K, Suh H, Hood L, Alt F W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986;44:251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- 36.Yancopoulos G D, Oltz E M, Rathbun G, Berman J E, Smith R K, Lansford R D, Rothman P, Okada A, Lee G, Morrow M, Kaplan K, Prockop S, Alt F W. Isolation of coordinately regulated genes that are expressed in discrete stages of B-cell development. Proc Natl Acad Sci USA. 1990;87:5759–5763. doi: 10.1073/pnas.87.15.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]