Abstract
We herein report a 48-year-old man with a history of chronic atrial fibrillation (AF) and repeated hemoptysis after radiofrequency ablation. Contrast tomography showed soft tissue thickening of the left hilar region and left pulmonary vein stenosis. We performed bronchial artery embolization, but the hemoptysis did not disappear, and AF was not controlled. We performed left lung lobectomy and maze procedures since we considered surgical removal necessary as radical treatment. After the surgery, hemoptysis and atrial fibrillation did not recur. Refractory hemoptysis after catheter ablation is rare, but occasionally occurs in patients with severe pulmonary vein stenosis.
Keywords: hemoptysis, bronchial artery embolization, atrial fibrillation, pulmonary vein stenosis, maze procedure
Introduction
Hemoptysis is a common symptom of many respiratory diseases. The most common causes are lung cancer, tuberculosis, aspergillosis, nontuberculous mycobacteriosis, and bronchiectasis (1). However, the etiology cannot be identified in some cases of hemoptysis, and approximately 20% of cases are diagnosed as cryptogenic (2). Pulmonary vein stenosis (PVS) is a late complication of catheter ablation in patients with atrial fibrillation (AF). Its incidence is reported to range from 1% to 42% depending on the operator's technique (3-8) and has decreased with advancements in medical technology. The major symptoms of PVS are cough, hemoptysis, dyspnea, and fatigue. Hemoptysis due to PVS is rare, but severe PVS has been reported to present with hemoptysis in 27% of cases (3). Refractory hemoptysis due to severe PVS after catheter ablation is relatively rare.
We herein report a case of refractory hemoptysis caused by severe PVS due to multiple catheter ablations.
Case Report
A 48-year-old man with a history of radiofrequency ablation for chronic AF consulted our department for repeated hemoptysis. His previous medical conditions included hypertension, dyslipidemia, sick sinus syndrome, and chronic heart failure. He was an ex-smoker (23 pack-years) with no remarkable family history. He worked as a taxi driver and was allergic to cedar and cypress. He had no dyspnea and his blood gas analysis showed no hypoxemia, as shown in the Table. The patient did not require supplemental O2 administration.
Table.
Arterial Blood Gas Data at the First Visit to Our Hospital.
| Arterial blood gas (room air) | ||
|---|---|---|
| pH | 7.421 | |
| PaO2 | 100.7 | mmHg |
| PaCO2 | 39.4 | mmHg |
| HCO3- | 25.6 | mEq/L |
| BE | 1.8 | mEq/L |
PaO2: partial pressure of arterial oxygen, PaCO2: partial pressure of arterial carbon dioxide, BE: base excess
He had received his first, second, and third catheter ablation procedures 13, 10, and 5 years previously, respectively, and he had received his most recent fourth ablation 3 years previously. All of them were radiofrequency ablations. His AF was poorly controlled, although multiple catheter ablations were performed. He developed hemoptysis approximately a month prior to presentation. He was referred to our department to avoid surgical treatment because he had failed to receive bronchial artery embolization (BAE) treatment at the previous hospital.
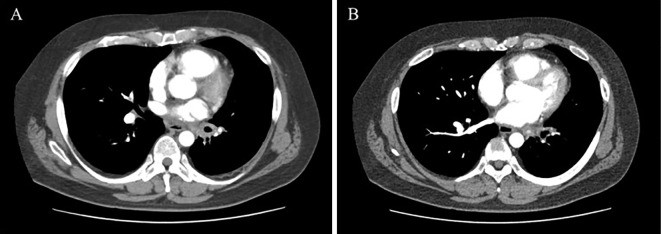
His physical examination findings were unremarkable, and the patient was normotensive. His medication was warfarin and carvedilol for AF, but he had stopped taking them at the time of his visit because of hemoptysis. Warfarin was discontinued due to hemoptysis and carvedilol was discontinued due to severe bradycardia and heart failure. Contrast computed tomography (CT) showed soft tissue thickening of the left hilar region and left inferior PVS (Fig. 1, 2).
Figure 1.
Contrast computed tomography showing soft tissue thickening of the left hilar region (A) and left inferior pulmonary vein stenosis (B).
Figure 2.
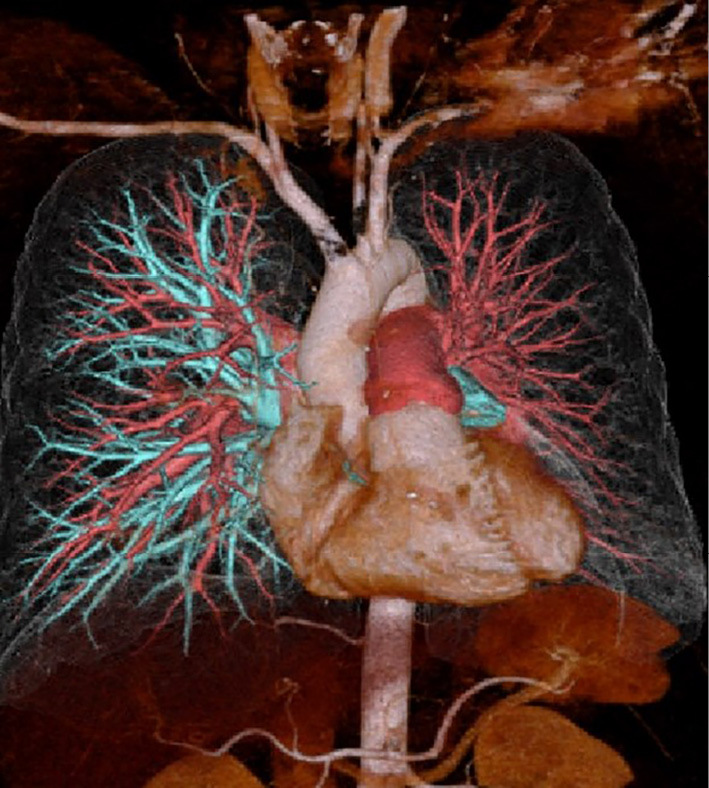
Pulmonary arteriography showing complete disruption of blood flow in the left pulmonary vein.
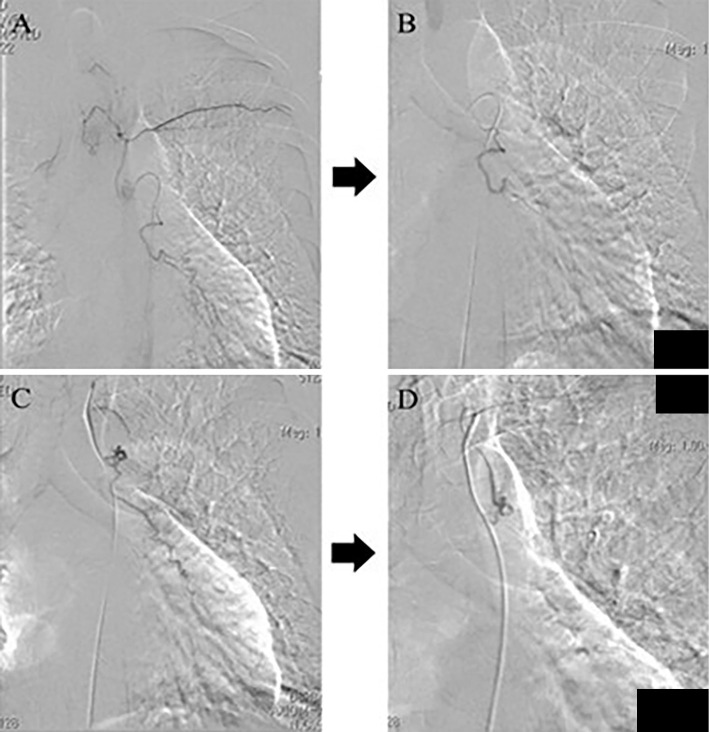
First, we performed BAE to control the hemoptysis. Two left bronchial arteries were embolized using gelatin sponge (Fig. 3). After treatment, the hemoptysis disappeared temporarily.
Figure 3.
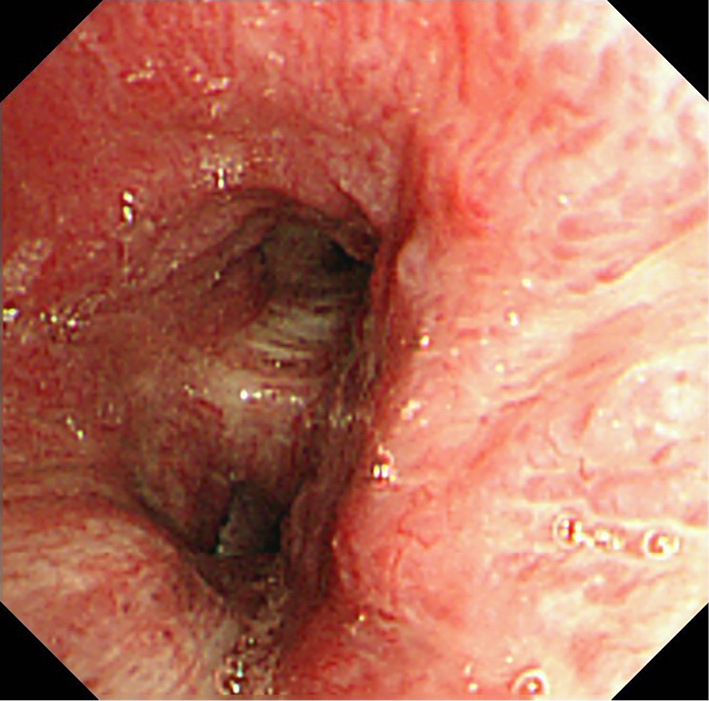
Bronchoscopy findings: Cobblestone appearance over the wall of the left bronchus.
Subsequently, we performed bronchoscopy to observe the bronchial lumen, suspecting that the lesion might be malignant. We noticed a cobblestone-like swelling over the wall of the left bronchus (Fig. 4). An endobronchial transbronchial biopsy was performed; the pathological findings of the specimen indicated invasion of nonspecific inflammatory cells, thereby ruling out malignancy.
Figure 4.
Digital subtraction angiography (DSA) images of the patient. A: Selective image of the left bronchial artery branched from the aorta. B: DSA image of the artery after embolization. C: Selective image of the left bronchial artery branched from the left subclavian artery. D: DSA image of the artery after embolization.
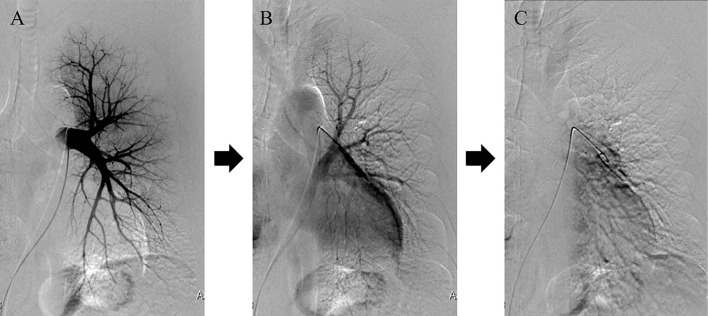
However, approximately eight months later, his hemoptysis recurred, and he was re-admitted to our hospital. We performed BAE a second time, but did not achieve complete hemostasis. On pulmonary arteriography, the image of the left pulmonary vein was delayed and showed dead branch findings (Fig. 5). Based on this observation, we concluded that the patient had severe PVS which could not be treated endoscopically.
Figure 5.
Pulmonary angiography (PAG) images of the patient. The left pulmonary vein was slowly imaged by PAG. A: Nine seconds after the administration of contrast medium. B: After 23 seconds. C: After 42 seconds.
We decided that radical surgery was necessary because his hemoptysis and AF did not improve despite multiple BAE procedures and catheter ablations. The patient consented to the surgery. A team of cardiovascular and thoracic surgeons at our hospital performed left lung lobectomy, left atrial maze procedure, left lower pulmonary vein closure, and left atrial appendectomy. The postoperative course was uneventful, and hemoptysis and AF did not recur for one year after the surgery.
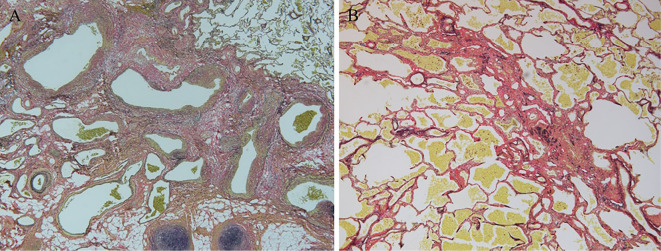
The pathological findings of the resected lung specimen indicated fibrous hypertrophy of the alveolar septum, dilation of the capillaries, congestion, and bleeding into the alveolar space (Fig. 6).
Figure 6.
Histopathological findings. A: The left hilar region. There are numerous small blood vessels around the bronchus and bronchial arteries and veins that irregularly expand with a mixture of fibrosis and arteries and veins (Elastica van Gieson staining; magnification, ×40). B: There is a fibrotic enlargement of the alveolar septum and dilation of capillaries, congestion, and bleeding into the alveolar space (Elastica van Gieson staining; magnification, ×100).
Discussion
Hemoptysis can be caused by various diseases, and refractory hemoptysis after catheter ablation has also been reported (9). Several studies have reported that this can occur due to severe PVS with both radiofrequency and cryoballoon ablation (10,11). PVS can occur following catheter ablation of the pulmonary vein. This process may be related to thermal injury to the tissues from radiofrequency ablation, which induces fibrosis and scarring. Although the incidence of PVS after catheter ablation varies widely, it is dependent on the skill of the operator. An early study found that 42.4% of ablated pulmonary veins had stenosis (3). With improved experience and changes in procedural techniques, the incidence has decreased. The current frequency is reported to be around 1-3% (12). The occurrence of PVS requiring treatment was reported to be 0.29% (13), and 0.4% of the cases who underwent catheter ablation for AF presented PVS >50% on imaging (14). However, over 60% of the cases are considered asymptomatic, and those with massive hemoptysis, like the one reported here, are relatively rare. In their series of 359 cryoballoon ablations with a complete registry of complications, Bhagwandien et al. found clinically significant hemoptysis requiring readmission in 2 patients. In both cases, hemoptysis improved upon anticoagulant withdrawal. Four more patients had reported hemoptysis at the three-month follow-up visit, but these cases were mild and resolved within a day of withholding anticoagulation (15).
Watanabe et al. reported that hemoptysis after cryoballoon ablation can be classified into two groups according to the time of the onset (16). In the acute phase, hemoptysis occurs soon after ablation due to direct injury of the bronchus by the balloon, and most of these cases improve spontaneously or by stopping the anticoagulant. Hemoptysis in the chronic phase after ablation is caused by stagnation of the pulmonary circulation following PVS and abnormal development of the bronchial artery (15,17). Accordingly, our patient had late hemoptysis, and evidence of PVS was also observed on CT. Furthermore, the PVS, in this case, showed repeated hemoptysis, and the PAG results showed severe stenosis.
The pathological findings of the lung specimen, in the present case, indicated fibrous hypertrophy of the alveolar septum, dilation of the capillaries, congestion, and bleeding into the alveolar space. Katzenstein et al. reported the pathological findings of pulmonary vein obstruction, including alveolar wall thickening and the presence of hemosiderin phagocytic macrophages in the alveoli (18). The pathological findings in the present case were similar to those of pulmonary vein occlusion and thought to be due to severe PVS, which is comparable to pulmonary vein occlusion. We hypothesize that the slow narrowing of the pulmonary vein and inadequate perfusion due to congestion following catheter ablation induced the abnormal development of the bronchial artery and led to refractory hemoptysis.
The most frequent symptoms in patients with severe PVS include dyspnea (67%), cough (45%), fatigue (45%), decreased exercise tolerance (45%), and exertional chest pain (38%). Hemoptysis occurred infrequently (27%) (3). Severe stenosis requires urgent intervention owing to the severity of the symptoms, and the risk of progression to complete occlusion, with subsequent pulmonary venous congestion and infarction (11). Patients who develop severe venous occlusion have severe chest pain and hemoptysis, and medical treatment is often inefficient (11).
The standard treatment for PVS includes balloon angioplasty and stent placement (3). However, the restenosis rate is as high as 33-61%, and repeated treatment is required (19). To our knowledge, no previous study has examined the BAE success rate for hemoptysis with PVS. However, BAE is typically the first choice for the treatment of hemoptysis. Therefore, we preferred performing BAE in the present case. Furthermore, we suspected that his PVS had a long onset, so it was too difficult to treat with a catheter for PVS and could not be improved by catheter treatment in our hospital. In addition, he had undergone repeated catheter ablations and had no hopes of undergoing any other surgical procedures.
In cases of total PV occlusion or intractable cases, medical treatment is difficult and often requires surgery. There have been several reports of cases requiring lung resection, similar to the present case (20-22). In one of these reports, hemoptysis and atrial fibrillation improved after surgery (21), but in another case, hemoptysis improved but AF persisted (22). There have been case reports in which surgical lobectomy was used to successfully treat PVS after AF ablation. In the present case, we considered that this would result in blood flow stagnation in the left hilar region, which might lead to the abnormal development of the bronchial artery as a compensatory response to severe PVS. Owing to the repeated failure of medical treatment, we suspected that the abnormal development of the bronchial artery could not be suppressed without surgical intervention for the left PVS. Consequently, hemoptysis was not controlled by BAE; therefore, left lower lobectomy was performed to resolve the left PVS.
Since this patient also had AF, a maze procedure was performed in conjunction with lung resection. The maze procedure was developed by Cox et al. in 1991 and is currently considered the most effective nonpharmacological treatment for AF (23,24). The left atrial maze procedure is an improved operative method developed by Sueda et al. in 1996 (25). In a Japanese survey conducted in 2000, AF had disappeared in more than 70% of cases at >1 following the procedure (23). To our knowledge, there are no case reports of left lung resection and maze surgery being performed together, to resolve hemoptysis and chronic AF. Hemoptysis and AF in the present patient did not recur for at least one year after the surgery. We successfully performed both of these procedures simultaneously to treat hemoptysis and AF, thereby relieving the patient's refractory symptoms and improving his quality of life.
Conclusion
We reported a case of massive hemoptysis due to abnormal development of the bronchial artery due to severe PVS following catheter ablation. Although severe PVS is a rare complication, it should be considered when hemoptysis occurs after catheter ablation for AF. We also report that pulmonary resection is required for cases of refractory hemoptysis due to severe PVS.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors would like to thank Keigo Sekihara M.D., Satoshi Nagasaka M.D., and Satsuki Kina M.D. for performing surgical lobectomy. We also thank Tomoki Tamura M.D. and Tetsuya Horai M.D. for performing the maze procedure. Finally, we would like to thank the Department of Thoracic and Cardiovascular Surgery in our hospital for their support in the treatment strategy of this case.
References
- 1. Ando T, Kawashima M, Masuda K, et al. Clinical and angiographic characteristics of 35 patients with cryptogenic hemoptysis. Chest 152: 1008-1014, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Mal H, Rullon I, Mellot F, et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 115: 996-1001, 1999. [DOI] [PubMed] [Google Scholar]
- 3. Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 100: 1879-1886, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Lin WS, Prakash VS, Tai CT, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation 101: 1274-1281, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Yu WC, Hsu TL, Tai CT, et al. Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 12: 887-892, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Robbins IM, Colvin EV, Doyle TP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 98: 1769-1775, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Yang M, Akbari H, Reddy GP, Higgins CB. Identification of pulmonary vein stenosis after radiofrequency ablation for atrial fibrillation using MRI. J Comput Assist Tomogr 25: 34-35, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Scanavacca MI, Kajita LJ, Vieira M, Sosa EA. Pulmonary vein stenosis complicating catheter ablation of focal atrial fibrillation. J Cardiovasc Electrophysiol 11: 677-681, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Romantowski J, Kuziemski K, Janowicz A, Siemińska A, Szurowska E, Jassem E. Recurrent haemoptysis as a symptom of severe pulmonary vein stenosis - a rare complication of catheter ablation in atrial fibrillation. Respirol Case Rep 5: e00212, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saad EB, Marrouche NF, Saad CP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med 138: 634-638, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Fender EA, Widmer RJ, Hodge DO, et al. Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation: presentation, management, and clinical outcomes. Circulation 134: 1812-1821, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Holmes DR Jr, Monahan KH, Packer D. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv 2: 267-276, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 3: 32-38, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Bertaglia E, Zoppo F, Tondo C, et al. Early complications of pulmonary vein catheter ablation for atrial fibrillation: a multicenter prospective registry on procedural safety. Heart Rhythm 4: 1265-1271, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Bhagwandien R, Van Belle Y, de Groot N, Jordaens L. Hemoptysis after pulmonary vein isolation with a cryoballoon: an analysis of the potential etiology. J Cardiovasc Electrophysiol 22: 1067-1069, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe K, Nitta J, Sato A, Goya M, Isobe M, Hirao K. Hemoptysis after five months of cryoballoon ablation: what is the relationship? HeartRhythm Case Rep 3: 357-359, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kasper Ł, Gross-Sondej I, Machalica B, Soja J, Pawlik W, Sładek K. Hemoptysis and lung disease as a manifestation of pulmonary vein stenosis after cryoballoon catheter ablation for atrial fibrillation. Pol Arch Med Wewn 126: 94-96, 2016. [PubMed] [Google Scholar]
- 18. Katzenstein AL. Katzenstein and Askin's surgical pathology of non-neoplastic lung disease. In: Major Problems in Pathology Series. Saunders, Philadelphia, 2006: 512. [PubMed] [Google Scholar]
- 19. Takahashi A, Kuwahara T, Takahashi Y. Complications in the catheter ablation of atrial fibrillation: incidence and management. Circ J 73: 221-226, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Saad EB, Marrouche NF, Saad CP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation: emergence of a new clinical syndrome. Ann Intern Med 138: 634-638, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Lo CM, Lu HI, Chen YY, Chang JP. Thoracoscopic lobectomy for pulmonary vein occlusion after radiofrequency catheter ablation of atrial fibrillation. J Cardiothorac Surg 11: 12, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steliga MA, Ghouri M, Massumi A, Reul RM. Lobectomy for pulmonary vein occlusion secondary to radiofrequency ablation. J Cardiovasc Electrophysiol 21: 1055-1058, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Cox JL, Schuessler RB, Boineau JP. The surgical treatment of atrial fibrillation. I. Summary of the current concepts of the mechanisms of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 101: 402-405, 1991. [PubMed] [Google Scholar]
- 24. Kosakai Y. Treatment of atrial fibrillation using the Maze procedure: the Japanese experience. Semin Thorac Cardiovasc Surg 12: 44-52, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Sueda T, Nagata H, Shikata H, et al. Simple left atrial procedure for chronic atrial fibrillation associated with mitral valve disease. Ann Thorac Surg 62: 1796-1800, 1996. [DOI] [PubMed] [Google Scholar]