Abstract
Background
Leukoreduction to eliminate mononuclear cells within blood products is necessary to prevent graft-versus-host disease after transfusion. Published reports document low concentrations of mononuclear cells leftover in fresh-frozen plasma products, however the phenotype and the proliferative potential of these cells has not been tested.
Materials and methods
We investigated residual cellular components contained within fresh and fresh-frozen plasma products and characterised their proliferative potential in co-cultures with unrelated allogeneic cells. We designed a flow-based assay to phenotype cells and quantify cell division by measuring the dilution of fluorescently labeled protein as cells divide. Leukocytes from consenting donors were purified from fresh liquid or fresh-frozen plasma units and cultured for three to seven days with unrelated irradiated allogeneic targets.
Results
We discovered a median of 1.6×107 viable lymphocytes were detectable in fresh plasma units after collection (n=8), comprised of a mixture of CD3+ CD8+ and CD3+ CD4+ cells. Furthermore, we identified a median of 8.4% of live CD3+ plasma lymphocytes divided as early as Day 4 when co-cultured with unrelated allogeneic cells, expanding to a median 88.8% by Day 7 (n=3). Although freezing the plasma product reduced the total number of viable leukocyte cells down to 2.3×105 (n=10), residual naive CD3+ cells were viable and demonstrated division through Day 7 of co-culture.
Discussion
The evidence of viable proliferative lymphocytes in fresh and fresh-frozen plasma products derived from centrifugation suggests that additional leukoreduction measures should be investigated to fully eradicate reactive lymphocytes from centrifuged plasma products.
Keywords: TA-GVHD, fresh-frozen plasma, lymphocytes, T cells
INTRODUCTION
Transfusion-associated graft versus host disease (TA-GVHD) is a rare but almost universally fatal complication arising from unanticipated recognition of host tissue by residual lymphocytes present within transfused blood products1,2. The pathophysiology of TA-GVHD arises from a HLA mismatch between recipient (host) and residual donor lymphocytes within transfused blood products, where host lymphocytes see donor lymphocytes as self1, while donor lymphocytes recognize the host as foreign and initiate the disease response2,3. TA-GVHD is considered preventable if the proliferative potential of donor lymphocytes is eradicated3. TA-GVHD and other leukocyte-associated transfusion reactions have thus spurred the implementation of practices aimed at inhibiting the activity of viable lymphocytes within blood products, which include leukoreduction, irradiation, and pathogen reduction, as well as processes such as freezing and thawing1,3–6.
Preparation of liquid plasma products involves manual collection from centrifuged whole blood which may or may not have passed through a leukoreduction filter, or automated collection within apheresis machines using centrifugation programs specific for plasma isolation. During manual fractionation of whole blood, centrifugation renders the top plasma layer distinct from the intermediate buffy coat layer (containing leukocytes and platelets) and the bottom packed red blood cell pellet. Published reports suggest plasma unit production derived from whole blood donation may contain a greater contaminating white blood cell fraction (up to 30×106 cells per unit) as compared with plasma derived from apheresis collection (less than 5×106 cells per unit)7–9. Recommendations for leukocyte counts in liquid plasma are published to be less than 100 white blood cells per microliter before initiating transfusion2,10,11. Addition of further treatment steps to eradicate residual cellular proliferation within the collected plasma, such as irradiation or pathogen inactivation, is also institution specific. However, viable leukocyte counts within fresh plasma are not routinely measured before initiating irradiation or freezing, nor are they routinely measured after these treatments and before use in patients due to cost and inherent inefficiencies of quantification of low cellular numbers in large volumes.
Guidelines for the preparation and use of fresh, frozen, or irradiated products in patients continue to evolve as new filtration products and centrifugation equipment become available12–16. A detailed analysis of the phenotype and activity of viable leukocytes remaining in transfused blood products is required as more clinical therapies utilize blood and cellular products for treatment of disease5,17–19. Importantly, published clinical reports have already identified TA-GVHD in patients receiving irradiated blood products1,20,21, yet data is sparse regarding the ability of previously frozen lymphocytes to divide and proliferate22,23. Given that many patients are ill at the time of plasma transfusion and the lack of standardised reporting, TA-GVHD secondary to fresh-frozen plasma transfusions is likely underdiagnosed and underreported. Therefore, our goal was to define the viability, phenotype and proliferative potential of lymphocytes present in fresh and fresh frozen plasma products. For this, we utilised flow cytometry to phenotypically characterize residual lymphocytes within plasma units from healthy donors and measured their viability and capacity for division in mixed lymphocyte cultures with unrelated allogeneic cells.
MATERIALS AND METHODS
Blood collection
For plasma effectors, de-identified peripheral blood was collected from healthy consenting donors according to IRB-approved protocol (American Red Cross IRB n. 2004-033). Whole blood was collected in Fenwal triple collection sets (Fresenius Kabi, Lake Zurich, IL, USA) and centrifuged in a Sorvall HBB6 rotor (Thermofisher Scientific, Waltham MA, USA) at 4,500 rpm with an accumulated centrifugal effect (ACE) setting of 1.0×108 and a brake setting of 7 to isolate liquid plasma from cellular pellet. ACE centrifugal settings analyse the effect differing rotor loading has upon acceleration and deceleration times to apply the same amount of total centrifugal time for samples. Residual leukocytes in liquid plasma were measured on the ADAM-wRBC (NanoEnTek, Waltham MA, USA). Liquid plasma was rapidly frozen at <−32°C in a blast freezer (Harris BPF-99E, Thermofisher Scientific) for at least one hour prior to transport on dry ice to the study site. Liquid plasma and leukocyte replete red blood cells (RBCs) were transported to the study site on wet ice. Transport time was less than 1 h, and samples were immediately processed upon receipt.
For targets, peripheral leukocyte cells were collected from de-identified TRIMA Accel platelet transfusion filters (Terumo Medical, Somerset NJ, USA). Donors for target peripheral cells were consented according to IRB-approved protocol (Children’s National Hospital IRB n. 4033). Residual cellular fractions were processed as peripheral blood as subsequently noted.
Cell isolation and labeling
Fresh-frozen plasma units were thawed in 37°C water baths until liquid. Newly thawed fresh-frozen plasma units and fresh plasma was divided into 50 mL collection tubes and spun at 600 ×g for 10 min to concentrate leukocyte cells. Pellet fractions were re-suspended in 5 mL of 1× PBS, combined into one 50 mL collection tube, and spun at 600 ×g for 10 min. The resulting pellet fraction was re-suspended in 5 mL of 1× PBS and cell counts were enumerated by automated count using a Luna-FL system (Logos Biosystems, Annandale, VA, USA). Plasma cells were re-suspended to a final concentration of 1×106 cells per mL in 1× PBS. 5×106 cells were added to 5 μL of 5 μM of Cell Trace Violet (Thermofisher Scientific) for a final concentration of 5 μM in 15 mL collection tubes. Plasma cells were incubated at 37°C for 20 min, after which 10 mL of complete media was added to the cell suspension and subsequently incubated at 37°C for additional 5 min. Plasma cells were spun down and washed 1× with 10 mL of complete media. Plasma cells were re-suspended in 5 mL of complete media for a final concentration of 1×106 cells per mL for subsequent co-culture. 100 μL cell suspension was added to individual wells of a 96 well U-bottom plate for a final concentration of 1×105 effectors per well. Complete medium contained 150 mL RPMI (Hyclone, Thermofisher Scientific), 100 mL Click’s Medium (Irvine Scientific, Santa Ana CA, USA), 2.5 mL Glutamax (Gibco, Thermofisher Scientific), and 25 mL of inactivated Human Serum (Valley Biomedical, Winchester VA, USA) per 250 mL of media which was sterile filtered.
Peripheral blood was processed to isolate peripheral blood mononuclear cell (PBMC) for use as targets or effectors. For effectors, PBMCs were separated from blood by Ficoll-Paque® (Sigma-Aldrich, St. Louis, MI, USA) and spun at 800 ×g for 25 min to purify the leukocyte fraction. The leukocyte fraction was washed once with 1× PBS and spun at 450 ×g for 5 min to pellet lymphocytes. For effectors, PBMCs were processed as described previously for labeling with Cell Trace Violet (Thermofisher Scientific). For targets, PBMCs from three unrelated donors were collected by harvesting cells from TRIMA Accel platelet transfusion filters (Terumo Medical). Target cells from the three filters were mixed in an equal ratio of 1: 1: 1 and 5×106 of the mixed cells were used for labeling. 5×106 of mixed targets were added to 5 μL of 5 μM of Cell Trace Far Red (Thermofisher Scientific) for a final concentration of 1 μM in 15 mL collection tubes. Mixed target cells were incubated at 37°C for 20 min, after which 10 mL of complete media was added to the cell suspension and incubated at 37°C for an additional 5 min. Mixed target cells were spun down and washed 1× with 10 mL of complete media. Labeled mixed target cells were re-suspended in 5 mL of complete media for a final concentration of 1×106 of the mixed cells per mL. Target cells were irradiated with 25 Gy, after which targets were added to wells for co-culture.
For mixed lymphocyte cultures (test wells), 100 μL effector cell suspension (1×105 effectors) and 100 μL of target cell suspension (1×105 targets) was added to individual wells of a 96 well U-bottom plate for a 1: 1 ratio of effectors to targets. Control wells consisted of 100 μL effector cell suspension with 100 μL of media alone, 100 μL effector cell suspension with 100 μL of media plus PHA or IL2, and 100 μL target cell suspension with 100 μL of media alone. Cells were cultured at 37°C for three to seven days.
Antibody staining
Cells were spun down at 400 ×g for 5 min and washed once in 100 μL 1× PBS. For viability staining with Live Dead Aqua (Thermofisher Scientific), cells were re-suspended in 50 μL of 1× PBS containing Live Dead Aqua at a dilution of 1: 500 and then stained for 20 min at 4°C, and washed with 100 μL of 1× PBS containing 2% fetal bovine serum (FBS). Cells were re-suspended in 50 μL of staining buffer containing either 1× PBS/2% FBS or 1× PBS/2% FBS plus Brilliant Violet staining solution for panels which contained Brilliant Violet dyes (Biolegend, San Diego, CA, USA). Cells were stained for at least 30 min at 4°C then washed twice with 100 μL 1× PBS/2% FBS. Cells were re-suspended in 50 μL of 1× PBS/2% FBS for collection. Markers for staining included CD3 PerCP Cy5.5 (Biolegend; clone SK7), CD3 APC Vio770 (Miltenyi Biotec [Bergisch Gladbach, Germany], clone Bw264/56), CD4 BV570 (Biolegend; clone RPA-T4), CD4 PE Vio770 (Miltenyi Biotec, clone REA623), CD4 PE Cy7 (BD Biosciences, clone SK3), CD8 APC Vio770 (Miltenyi Biotec, clone REA734), CD8 VioGreen (Miltenyi Biotec, clone REA734), CD8 Brilliant Violet 421 (Biolegend, clone SK1), CD8 BV711 (Biolegend, clone SK1), CD127 PE Dazzle594 (Biolegend, clone A019D5), CD56 PE (Miltenyi Biotec, clone REA196), CD14 VioBlue (Miltenyi Biotec, clone REA599), CCR7 FITC (Biolegend, clone G043H7), CCR7 PE Vio770 (Miltenyi Biotec, clone REA546), CD19 PE (Miltenyi Biotech, clone REA675), CD45RO BV605 (Biolegend, clone UCHL1), CD45RO AlexaFluor488 (Biolegend, clone UCHL1), CD25 BV650 (Biolegend, clone BC96), CD83 APC (Miltenyi Biotec, clone HB15), TCRα/β PerCP Vio700 (Miltenyi Biotech, clone REA652), Live Dead Aqua (Thermofisher), and 7AAD (Miltenyi Biotec). Cells were loaded onto a Cytoflex S (Beckman Coulter, Atlanta, GA) or a Sartorius IQue Screener Plus (Sartorius, Göttingen, Germany) for collection of samples. Data was analysed in Flowjo software (FlowJo, Ashland, OR, USA), and statistics were analysed in Graphpad Prism software (GraphPad, San Diego, CA, USA).
RESULTS
Fresh plasma retains a substantial number of leukocytes after centrifugation
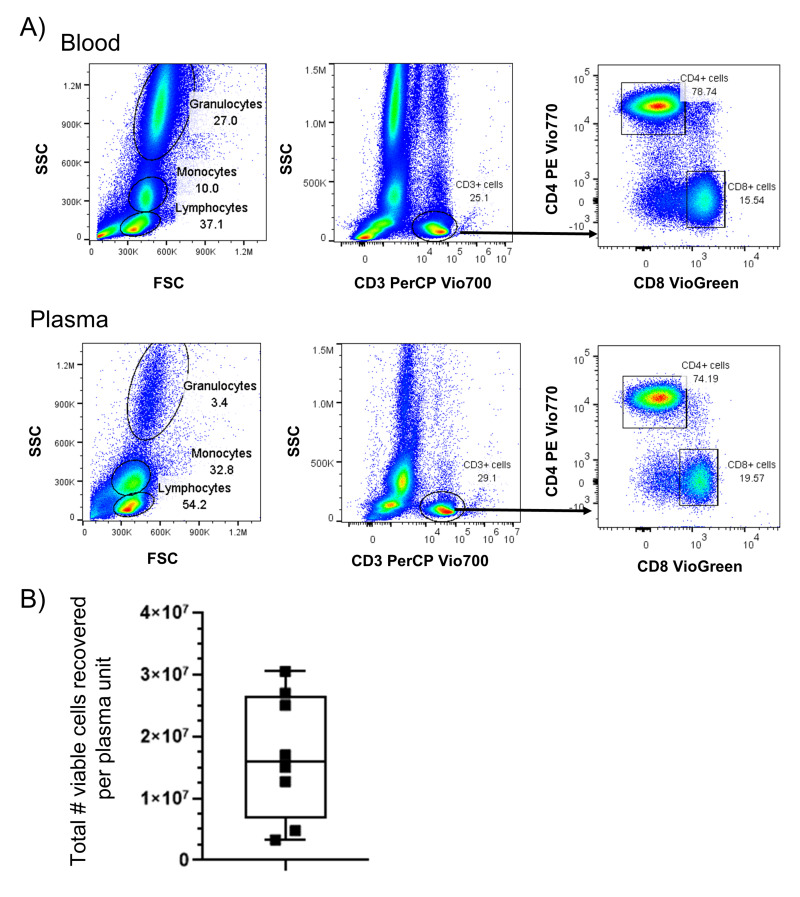
We first characterised the frequency and phenotype of leukocytes within fresh plasma products in comparison with peripheral blood drawn from the same donors. We identified that fresh plasma contains leukocytes consistent in size and granularity with lymphocyte, monocyte, and granulocyte subpopulations (Figure 1A). These cells were similar in size and granularity to cells within fresh blood from which the plasma was purified. We further evaluated the lymphocyte subpopulation for lineage staining using flow cytometry and identified CD3+ T cells, including both CD4+ and CD8+ T cells, were present within lymphocyte gated populations. Overall, we measured a median of 1.6×107 leukocytes per unit from eight fresh plasma products analysed (Figure 1B), which equates to 64 leukocytes per microliter in a volume of 250 mL for plasma units, which represented an approximate 60-fold to 150-fold reduction as compared with whole blood, which has a published mean of 2.1 to 5.2×109 leukocytes per unit (4,000–10,000 leukocytes per microliter; 520 mL per unit24).
Figure 1. Viable leukocytes persist after fractionation to produce fresh plasma.
A) Representative sample of blood and fresh plasma from the same donor were analysed by flow cytometry for the presence of lymphocytes, monocytes, and granulocytes by size (FSC) and granularity (SSC). Fresh plasma was stained with antibodies against CD3, CD4, and CD8 to identify the frequency of subpopulations of lymphocytes by flow cytometry. B) Viable cell counts were measured in whole units of fresh plasma from n=8 independent donors.
Leukocytes from fresh plasma divide in response to mixed HLA stimulation
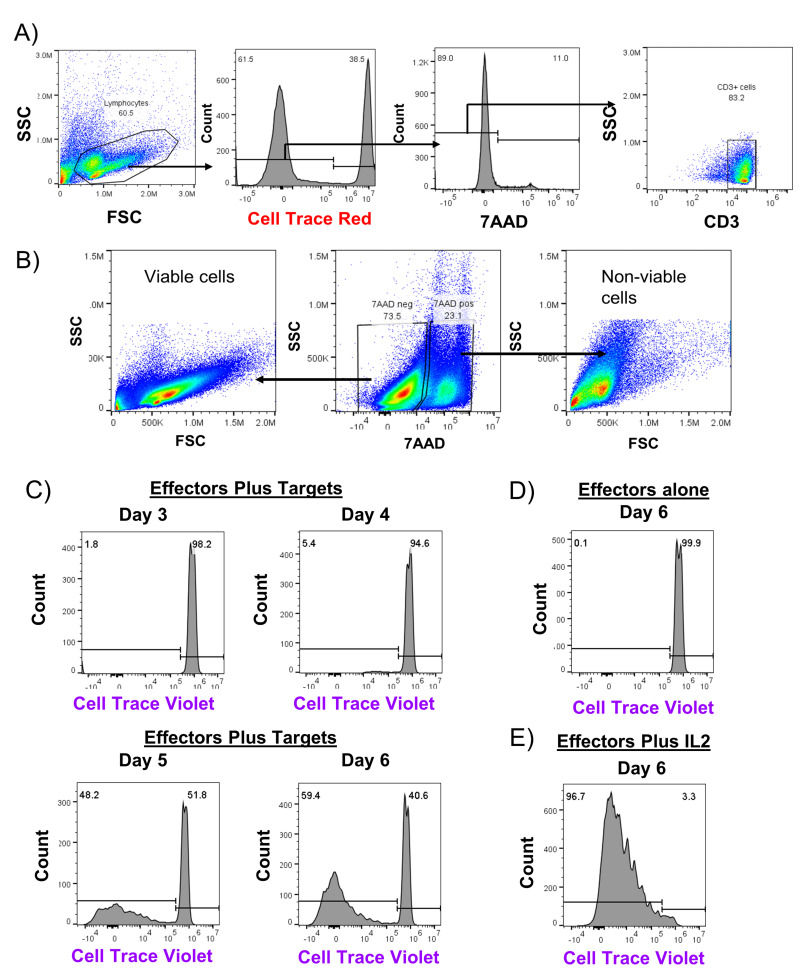
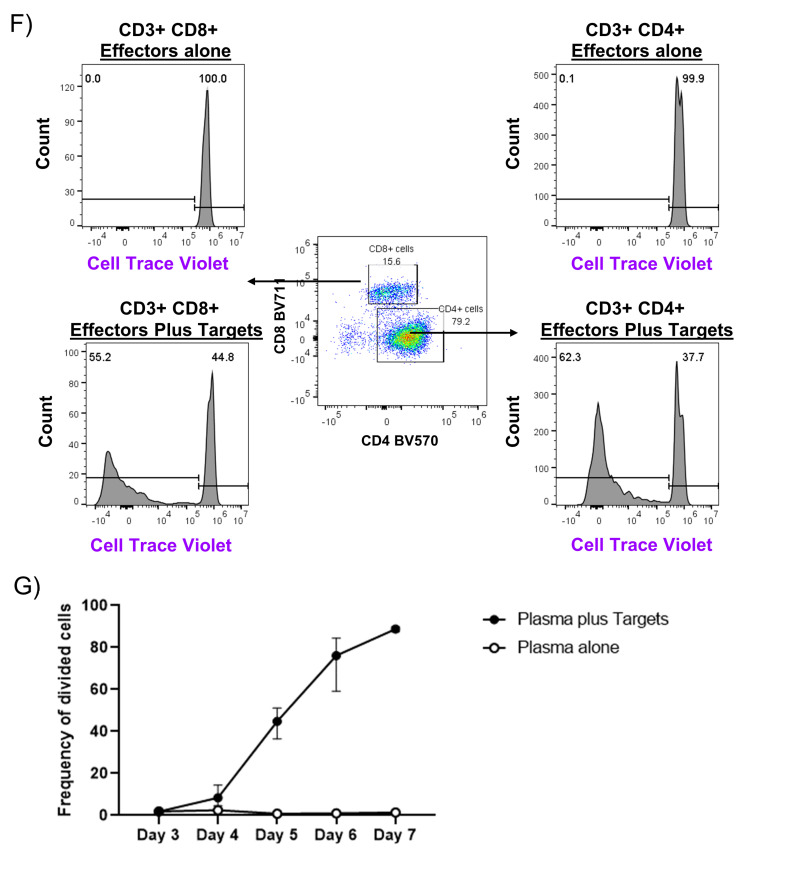
We next investigated whether cells present within fresh plasma were capable of division after co-culture with allogeneic cells using a flow-based mixed lymphocyte reaction (MLR) assay to identify division of effector cells. Leukocytes isolated from fresh plasma were labeled with Cell Trace Violet tracking dye and co-cultured with irradiated target cells from unrelated donors which were labeled with Cell Trace Red (Figure 2A). To enumerate division, we measured the frequency of viable CD3+ cells (Figure 2B) which had diluted out the tracking dye as a fraction of total CD3+ cells in the co-culture well. By Day 4, 5.5% of plasma-derived leukocytes co-cultured with allogeneic target cells showed cell division, eventually reaching 59.5% on Day 6 (Figure 2C). In contrast, as shown in Figure 2D, these same plasma-derived leukocytes remained quiescent and showed no division out through Day 6. Cells also divided rapidly in response to exogenous IL-2 (Figure 2E). For this sample, we additionally performed staining for CD4 and CD8 subpopulations, and identified both groups of T cells divided in response in co-cultures (Figure 2F). Overall, we identified division of lymphocytes isolated from fresh plasma as early as Day 4 when cultured with HLA mismatched cells, with a median of 8.4% of live CD3+ cells showing division (Figure 2G). By Day 7, the majority of plasma cells had divided, with a median of 88.8% of live CD3+ cells diluting out the viable dye.
Figure 2. Leukocytes from fresh plasma divide during co-culture with unrelated PBMCs.
A) Flow gating strategy for representative experiment co-culturing cells from fractionated fresh plasma (effectors) labeled with Cell Trace Violet with irradiated PBMCs from an unrelated donor (targets) which had been labeled with Cell Trace Red for three to seven days. B) Size and granularity profile of ungated viable and non-viable cells from a representative fresh plasma co-culture well. In (C), the frequency of CD3+ and viable effectors which were actively dividing in mixed cultures of effectors and targets was evaluated by dilution of Cell Trace Violet dye as compared to wells with only effectors (D). In ( E), the frequency of effectors dividing in response to addition of exogenous IL-2 stimulatory cytokine was measured. F) Cultures were additionally stained with antibodies against CD4 and CD8 to measure division by subpopulations of T cells. G) The median frequency of cell division by CD3+ cells from plasma for n=3 experiments with error bars indicating range of values.
Fresh-frozen plasma (FFP) samples retain viable lymphocytes which divide in response to mixed HLA stimulation
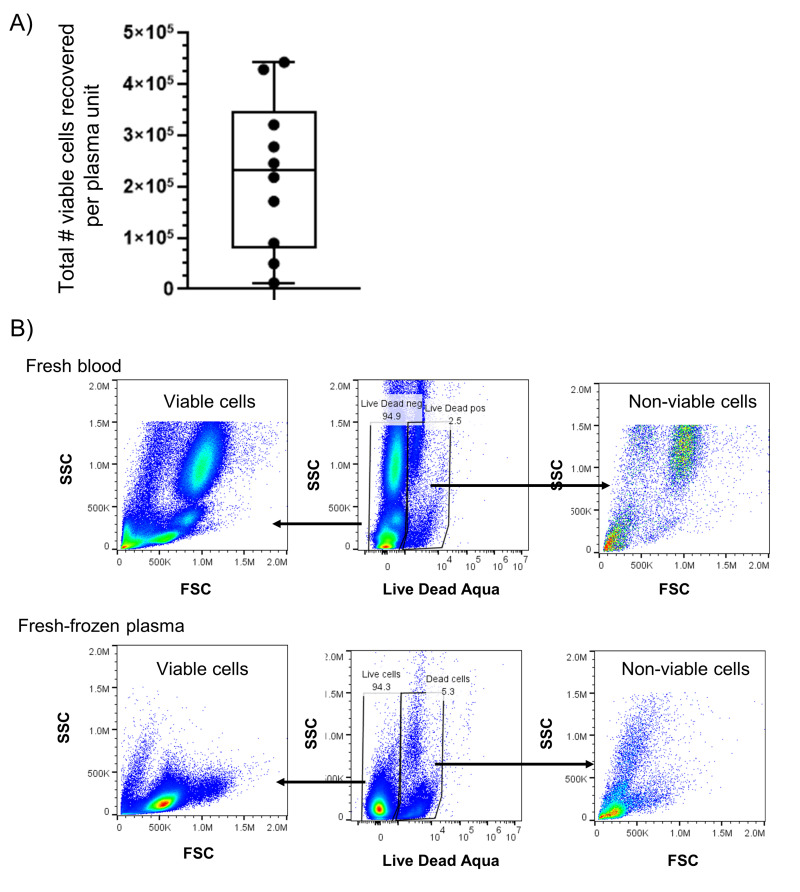
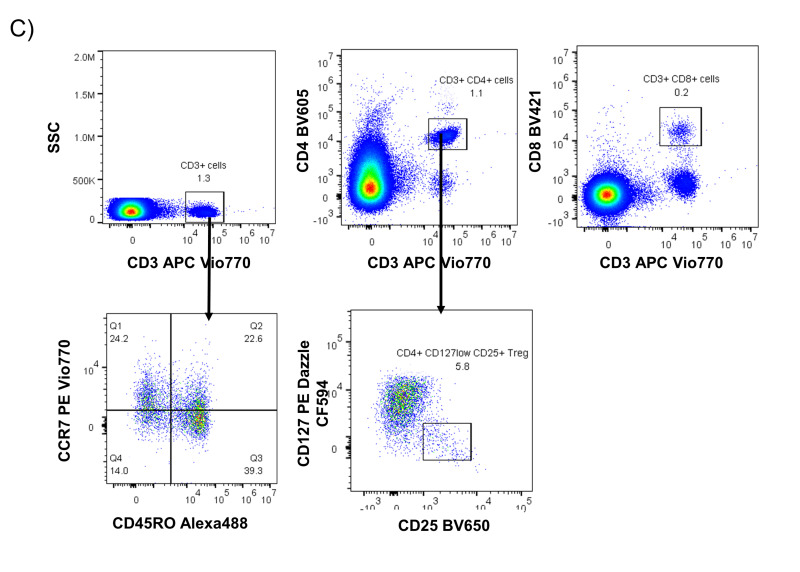
We next sought to identify whether rapid freezing of FFP was sufficient for complete lymphoreduction of T lymphocytes. We measured a median of 2.3×105 residual leukocytes per unit from 10 independent FFP units, which was a 100-fold reduction as compared with fresh plasma (Figure 3A). This equates to 0.9 leukocytes per microliter for a 250 mL volume per unit of frozen plasma. We further characterised the phenotype of two FFP units by flow cytometry and identified viable cells within fresh-frozen plasma samples (Figure 3B), in which CD3+ cells were present at a frequency of 0.7% and 1.3% of total cells (Figure 3C). Within the CD3+ T cell population, we identified several putative subgroups of T cells, including CD3+ CD8+ cells (cytotoxic T cells), CD3+ CD4+ cells (helper T cells), and CD4+ CD25+ CD127low cells (regulatory T cells). The expression of CCR7 and CD45RO markers on T cells were also measured to identify T cell memory subtypes. In two phenotyped samples, we observed that CD3+ cell populations detected in the FFP units comprised both naive (CCR7+ CD45RO−) and effector memory (CCR7− CD45RO+) subtypes.
Figure 3. Viable T cells persist within frozen plasma.
A) Viable cell counts were measured in whole units of fresh-frozen plasma after thaw and centrifugation from n=10 independent donors. B) Size and granularity profile of ungated viable and non-viable cells from a representative blood and fresh-frozen plasma co-culture wells. C) Representative sample of leukocyte cells from fresh-frozen plasma was investigated for lymphocyte subpopulations using flow cytometry to evaluate size ( FSC), granularity ( SSC), and expression of lineage surface markers including CD3, CD4, CD8, CD45RO, CCR7, CD127 and CD25.
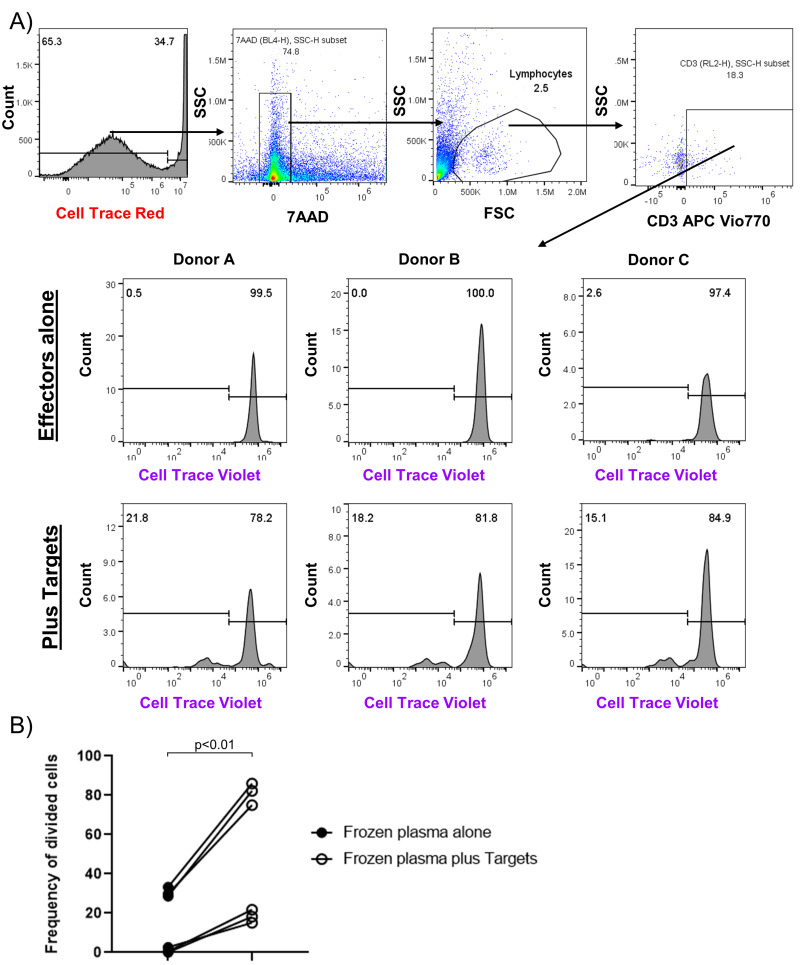
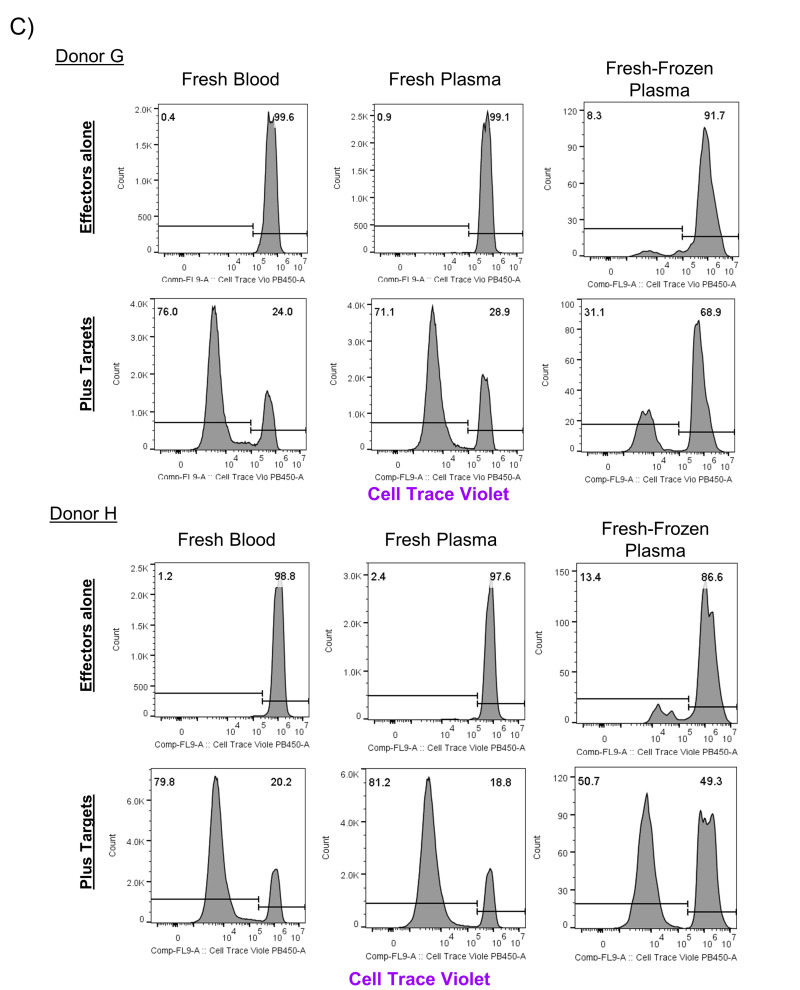
We also co-cultured cells isolated from FFP units with a mixed population of irradiated cells from unrelated donors and measured proliferation after five days of culture (three independent donors, n=2 experiments). After five days of culture, we identified that between 15 to 22% of viable CD3+ cells in co-cultures had divided in response to co-culture with unrelated donor PBMCs (Figure 4A). In contrast, in the absence of co-culture with allogeneic PBMC, the FFP derived CD3+ T cells showed minimal cell division for the first set of samples tested (3% or less). We identified a higher background division for a second set of samples tested, however all samples from both groups of FFP donation expanded after co-culture with allogeneic targets (Figure 4B). To further validate these findings, we collected samples of fresh blood, fresh plasma, and fresh-frozen plasma derived from one single donation and measured cell division after co-culture for seven days. Two independent experiments identified division in fresh blood, fresh plasma, and fresh-frozen plasma (Figure 4C). In co-culture assays with allogeneic targets, a median of 76.6%±1.1% CD3+ T cells derived from fresh blood, 75.9%±8.6% CD3+ T cells from fresh plasma, and 35.3%±15.2% CD3+ T cells from frozen plasma divided after seven days. Without co-culture with unrelated target cells, a median of 1%±1.4% CD3+ T cells from fresh blood, 1.9%±2% CD3+ T cells from fresh plasma, and 10.5%±6.9% CD3+ T cells from fresh-frozen plasma had divided after seven days. These examples show that viable T cells can survive the flash freezing process in sufficient numbers to divide and expand after exposure to unrelated donor cells, although the extent of division appears to be less than that of lymphocytes derived from peripheral blood or fresh plasma.
Figure 4. Viable CD3+ T cells from frozen plasma divide after co-culture with unrelated PBMCs.
A) CD3+ cells from three independent fresh-frozen plasma units (effectors) were assayed for division after culture for five days with irradiated PBMCs from unrelated donors in duplicate wells (targets; n=2 experiments). B) The frequency of division of viable CD3+ T cells was measured in wells with frozen plasma alone or co-cultured with targets from 2 experiments (Donors A through F) was analysed by paired T test. C) CD3+ cells isolated from whole blood, fresh plasma, and fresh-frozen plasma ( effectors) were assayed for division after culture for seven days in duplicate wells with irradiated PBMCs from unrelated donors ( n=2 experiments; Donors G, H).
DISCUSSION
In this study, we utilised flow cytometry to uncover the presence of viable lymphocytes within samples of fresh and fresh-frozen plasma sample derived from multiple independent donors and characterised their phenotype by surface marker staining. The proliferative potential of these lymphocytes was also evaluated in flow-based MLR assays. We identified that CD3+ T cells identified in both fresh and fresh-frozen plasma were fully capable of division when cultured with unrelated PBMCs in our flow-based MLR assay. These findings suggest that the isolation of plasma from fresh blood by centrifugation and subsequent flash-freezing process is not sufficient to completely eliminate allo-reactive CD3+ T cells within fresh and fresh-frozen plasma products.
Currently, plasma is transfused for a variety of indications including in settings of coagulation factor deficiencies, thrombotic disorders and in massive transfusions and other trauma surgery25, and, while rare, the fatal nature of TA-GVHD necessitates risk mitigation to prevent the disease process1,2. Though historically, immunocompromised patients are considered to be at highest risk for TA-GVHD20,26, 65.2% of these published cases would not have been predicted to be at risk for TA-GVHD under current guidelines within the United States and did not meet criteria to receive an irradiated product1,3. Fresh plasma was identified as the transfused product in one of these cases, but 28.7% of cases either received multiple products or the culprit was not identified1. Additionally, a survey of 89 institutions in the United States evaluating 3,387 total plasma transfusions identified 4.7% of institutions documented a serious non-fatal transfusion reaction and 5.9% of institutions reported a fatal plasma-related transfusion reaction within 5 years prior to study27. Reviews further identify a 2.1% increased risk of multi-organ failure independently associated with transfusion of fresh-frozen plasma units28 concomitant with red cell infusion which is not associated with infusion of red cell products alone. It is unclear whether the addition of red cell products in this study may have induced an immunosuppressive environment favorable for expansion of viable cells from the frozen plasma units29,30. Diagnosis of donor lymphocyte infusion as a contributing factor in GVHD is challenging as a pathologically confirmed diagnosis requires detection of persistent donor lymphocytes derived from the transfused component in affected tissue biopsy or peripheral blood31. Cases are therefore likely under recognised secondary to other competing diagnoses. Furthermore, products potentially containing proliferative lymphocytes may also contribute to other transfusion reactions.
Our results identified a residual level of contaminating leukocytes within fresh plasma units and is in agreement with several prior publications quantifying leukocytes when investigating protocols for plasma creation11,17,19,22,23. Rieske et al. suggest inherent variability of human samples during fractionation can lead to a more diffuse buffy coat layer, increasing the likelihood of human error during plasma collection. Although the proposed addition of a leukoreduction filter step before centrifugation eliminated cellularity from fresh blood components in their investigation19, a different review still identified cases of TA-GVHD from leukoreduced fresh blood components which were not irradiated3. The persistent presence of contaminating white blood cells within plasma products collected from manual fractionation of whole blood suggest additional processing steps are necessary to achieve cell free plasma products17,19.
Our experiments demonstrate that flash freezing alone is an incomplete method for leuko-reduction, as freezing only reduced the viable cellular numbers of fresh plasma by 100-fold. This is far less than is typical for reduction of plasma components with 25 Gy of gamma irradiation (100,000-fold) or with amotosalen and UVA light treatment for pathogen inactivation (100,000,000-fold; reviewed in 3). Flash freezing is also less effective at reducing viable leukocytes compared to the use of leukocyte reduction filters (>1,000-fold)3. Importantly, these leukocyte destructive processes may still produce plasma units which promote allergic reactions due to release of the products of cell lysis, since recent reports suggest debris from plasma cellularity as low as 1×106 is sufficient to induce inflammatory IL-6 production in recipients17. Such inflammatory cytokines are produced through sensing of danger signals from lysis of leukocytes, red cells, and platelets, and can exacerbate allograft rejection through inhibition of regulatory T cell activity and initiation of Th17 development32,33. These compounding interactions between host cells and residual cellular debris highlight the need to carefully evaluate and standardize protocols for eliminating all cellularity within fractionated fresh plasma components, and to incorporate more sensitive methods for analysis of cellular division, such as flow cytometry. Furthermore, frozen components should not be considered acellular, and at least one additional treatment step for leukocyte reduction should be investigated prior to freezing, such as irradiation, pathogen inactivation, or leukocyte filtration.
In this study, the complexity of preparation and subsequent processing of individual samples of fresh blood, fresh plasma, and fresh-frozen plasma units at the same time created logistical challenges which limited our ability to perform both phenotypic analysis and mixed lymphocyte co-cultures on individual units. Although we could identify putative granulocytes and monocytes based on size and granularity within plasma fractionated from whole blood, we focused on tracking the expansion of viable CD3+ T cells in cultures as they are the causative agents of TA-GVHD. We also used peripheral blood from donors to confirm our staining was accurate and the CD3+ effectors from the donor were reactive to the targets. Our assay of polyclonal cell division in co-cultures identified reactive cells as a single peak which increased in number over time rather than a series of distinct division peaks, as is common with stimulation of monoclonal T cells or stimulation with exogenous cytokine stimuli. Thus, without multiple division peaks, traditional proliferation modeling was not able to definitively identify the peak number of the divided cells. However, our assay clearly identified a qualitative CD3+ response present within fresh and frozen plasma units. Additionally, although these experiments measured individual reactions to mismatched HLA antigens on target cells, the amount of HLA mismatch was not measured as typing was not performed on effector or target cells. In anticipation of this, we utilised a suspension of three different donor PBMCs to maximize the potential for HLA mismatch, and verified this suspension induced robust stimulation of donor cells from peripheral blood. Finally, several factors may have influenced the variation we have identified within fresh and fresh-frozen plasma samples, including inherent heterogeneity between samples, human error during separation of plasma from diffuse buffy coats, and variation in leukocyte survival during the freezing and thawing process in the absence of cryopreservative. Our observations would suggest that, inclusive of the variation, manual fractionation of whole blood still produces a lingering population of contaminating cells within plasma products.
CONCLUSIONS
In summary, we have demonstrated that viable lymphocytes with proliferative potential exist in plasma products obtained from fractionation of whole blood, and while the current standard freezing process may reduce the lymphocyte burden, it is not sufficient to completely eradicate lymphocytes within both fresh and fresh-frozen plasma products. For these reasons, further leukoreduction strategies should be considered when administering plasma products, especially to high-risk patients.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (K23-HL136783-01) award to MK and a Board of Visitors grant to CB. The Authors would like to acknowledge our blood, plasma, and platelet filter donors, staff at Holland Laboratory Research Blood Program, staff at Edward J. Miller Blood Donor Center, and A. Turgeon for processing whole blood units.
Footnotes
AUTORSHIP CONTRIBUTIONS
Conception, design, methodology: CL, SW, PH; Data collection and analysis: CL, KT, PY, SW. Writing, review, and/or manuscript revision: CL, KT, SW, PH, MK, CB, NL, PY. Study supervision: PH, NL, SW, CB.
CONFLICT OF INTEREST DISCLOSURES
CB is a co-founder and SAB member of Mana Therapeutics and Catamaran Bio, owns stock in Repertoire Immune Medicine and Neximmune, and serves on the board of directors for Cabaletta Bio. PH is a co-founder and serves on the board of directors for Mana Therapeutics and on the Scientific Advisory Board of Cellevolve. MK serves on an advisor board for Gilead Sciences. CL, KT, PY, NL, SW declare no conflicts of interest.
Commented by editorial 10.2450/2021.0186-21 (BLT-19-445)
SUPPORT
This work was supported by the National Institutes of Health (K23-HL136783-01) award to MK and a Board of Visitors grant to CB.
REFERENCES
- 1.Kopolovic I, Ostro J, Tsubota H, et al. A systematic review of transfusion-associated graft-versus-host disease. Blood. 2015;126:406–14. doi: 10.1182/blood-2015-01-620872. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder ML. Transfusion-associated graft-versus-host disease. Br J Haematol. 2002;117:275–87. doi: 10.1046/j.1365-2141.2002.03450.x. [DOI] [PubMed] [Google Scholar]
- 3.Kleinman S, Stassinopoulos A. Transfusion-associated graft-versus-host disease reexamined: potential for improved prevention using a universally applied intervention. Transfusion. 2018;58:2545–63. doi: 10.1111/trf.14930. [DOI] [PubMed] [Google Scholar]
- 4.Chun S, Phan MT, Hong S, et al. Double-filtered leukoreduction as a method for risk reduction of transfusion-associated graft-versus-host disease. PloS one. 2020;15:e0229724. doi: 10.1371/journal.pone.0229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. 2019;133:1831–9. doi: 10.1182/blood-2018-10-833988. [DOI] [PubMed] [Google Scholar]
- 6.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(Suppl 1):65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valbonesi M, Bruni R, Florio G, et al. Cellular contamination of plasma collected with various apheresis systems. Transfus Apher Sci. 2001;24:91–4. doi: 10.1016/s0955-3886(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 8.Vrielink H, van der Meer PF. Collection of white blood cell-reduced plasma by apheresis. Transfusion. 2004;44:917–23. doi: 10.1111/j.1537-2995.2004.03314.x. [DOI] [PubMed] [Google Scholar]
- 9.Riggert J, Schwartz DW, Wieding JU, et al. Prestorage inline filtration of whole blood for obtaining white cell-reduced blood components. Transfusion. 1997;37:1039–44. doi: 10.1046/j.1537-2995.1997.371098016442.x. [DOI] [PubMed] [Google Scholar]
- 10.Europe Co. Guide to the preparation, use and quality assurance of blood components : recommendation No R (95) 15. 20th ed. Strasbourg: Council of Europe Pub; 2020. [Google Scholar]
- 11.Willis JI, Lown JA, Simpson MC, Erber WN. White cells in fresh-frozen plasma: evaluation of a new white cell-reduction filter. Transfusion. 1998;38:645–9. doi: 10.1046/j.1537-2995.1998.38798346632.x. [DOI] [PubMed] [Google Scholar]
- 12.Storch EK, Custer BS, Jacobs MR, et al. Review of current transfusion therapy and blood banking practices. Blood Rev. 2019;38:100593. doi: 10.1016/j.blre.2019.100593. [DOI] [PubMed] [Google Scholar]
- 13.Treleaven J, Gennery A, Marsh J, et al. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology blood transfusion task force. Br J Haematol. 2011;152:35–51. doi: 10.1111/j.1365-2141.2010.08444.x. [DOI] [PubMed] [Google Scholar]
- 14.Asai T, Inaba S, Ohto H, et al. Guidelines for irradiation of blood and blood components to prevent post-transfusion graft-vs.-host disease in Japan. Transfus Med. 2000;10:315–20. doi: 10.1046/j.1365-3148.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 15.Moroff G, Leitman SF, Luban NL. Principles of blood irradiation, dose validation, and quality control. Transfusion. 1997;37:1084–92. doi: 10.1046/j.1537-2995.1997.371098016450.x. [DOI] [PubMed] [Google Scholar]
- 16.Moroff G, Luban NL. The irradiation of blood and blood components to prevent graft-versus-host disease: technical issues and guidelines. Transfus Med Rev. 1997;11:15–26. doi: 10.1016/s0887-7963(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 17.Tan YB, Rieske RR, Audia JP, et al. Plasma transfusion products and contamination with cellular and associated pro-inflammatory debris. J Am Coll Surg. 2019;229:252–8. doi: 10.1016/j.jamcollsurg.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loi MM, Kelher M, Dzieciatkowska M, et al. A comparison of different methods of red blood cell leukoreduction and additive solutions on the accumulation of neutrophil-priming activity during storage. Transfusion. 2018;58:2003–12. doi: 10.1111/trf.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieske RR, Kutcher ME, Audia JP, et al. Analysis of plasma products for cellular contaminants: comparing standard preparation methods. J Am Coll Surg. 2020;230:596–602. doi: 10.1016/j.jamcollsurg.2019.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otori K, Masuda C, Wanibuchi H, et al. An autopsy report of graft-versus-host disease (GVHD) following blood transfusion. Osaka City Med J. 1999;45:37–43. [PubMed] [Google Scholar]
- 21.Sproul AM, Chalmers EA, Mills KI, et al. Third party mediated graft rejection despite irradiation of blood products. Br J Haematol. 1992;80:251–2. doi: 10.1111/j.1365-2141.1992.tb08908.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernvil SS, Abdulatiff M, al-Sedairy S, et al. Fresh frozen plasma contains viable progenitor cells--should we irradiate? Vox Sang. 1994;67:405. doi: 10.1111/j.1423-0410.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 23.Wieding JU, Vehmeyer K, Dittman J, et al. Contamination of fresh-frozen plasma with viable white cells and proliferable stem cells. Transfusion. 1994;34:185–6. doi: 10.1046/j.1537-2995.1994.34294143956.x. [DOI] [PubMed] [Google Scholar]
- 24.Blumenreich MS. The White Blood Cell and Differential Count. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd edition. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 25.Roback JD, Caldwell S, Carson J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion. 2010;50:1227–39. doi: 10.1111/j.1537-2995.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 26.Wintergerst U, Meyer U, Remberger K, Belohradsky BH. [Graft versus host reaction in an infant with DiGeorge syndrome]. Monatsschr Kinderheilkd. 1989;137:345–7. [In German.] [PubMed] [Google Scholar]
- 27.Alcorn K, Ramsey G, Souers R, Lehman CM. Appropriateness of plasma transfusion: a College of American Pathologists Q-Probes study of guidelines, waste, and serious adverse events. Arch Pathol Lab Med. 2017;141:396–401. doi: 10.5858/ARPA.2016-0047-CP. [DOI] [PubMed] [Google Scholar]
- 28.Watson GA, Sperry JL, Rosengart MR, et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221–7. doi: 10.1097/TA.0b013e3181ad5957. discussion 228–30. [DOI] [PubMed] [Google Scholar]
- 29.Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol. 1994;1:457–61. [PubMed] [Google Scholar]
- 30.Bordin JO, Blajchman MA. Immunosuppressive effects of allogeneic blood transfusions: implications for the patient with a malignancy. Hematol Oncol Clin North Am. 1995;9:205–18. [PubMed] [Google Scholar]
- 31.Ni Loingsigh S, Flegel WA, Hendrickson JE, Tormey CA. Preventing transfusion-associated graft-versus-host disease with blood component irradiation: indispensable guidance for a deadly disorder. Br J Haematol. 2020;191:653–7. doi: 10.1111/bjh.17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen H, Goldstein DR. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. JASN. 2009;20:1032–40. doi: 10.1681/ASN.2008070778. [DOI] [PMC free article] [PubMed] [Google Scholar]