Abstract
Background
Preterm infants born earlier than 32 weeks of gestational age (GA) often need red blood cell (RBC) transfusions, which have been associated with an increased incidence of complications of prematurity, due to changes in tissue oxygenation. Transfusion of umbilical cord blood (UCB) could be beneficial for this group. The aims of this study were: (i) to determine the RBC transfusion needs in infants <32 weeks in Hospital Clinic of Barcelona; (ii) to identify the target GA group that would benefit most from UCB transfusion; and (iii) to assess the current availability of UCB as a potential source of RBC transfusion for these premature infants in our tertiary referral blood bank.
Material and methods
A retrospective observational study was performed on infants born at <32 weeks GA, divided into two groups: (i) extremely low gestational age neonates (ELGAN) (from 230 to 276 weeks) and (ii) very preterm neonates (VPN) (from 280 to 316 weeks). Their complications and transfusion rates were compared. Processing and availability of UCB samples in the reference blood bank were assessed.
Results
Overall, 1,651 infants <32 weeks GA were admitted in the study period. While 12.5% of VPN received at least one RBC transfusion, the percentage increased to 60% among the ELGAN. Retinopathy of prematurity and bronchopulmonary dysplasia were diagnosed more frequently in the ELGAN group (p<0.001) than in the VPN group. The annual average volume of RBC transfusion in our study group was 1.35 L (95% CI: 1.07–1.64). The reference blood bank was able to produce 16 L (95% CI: 14–18) of UCB-RBC per year.
Conclusion
Considering the data obtained about RBC transfusion needs and morbidities, the ELGAN group has been identified as the target group that would benefit most from UCB-RBC transfusions. We have demonstrated that our blood bank is able to produce enough RBC from UCB. Randomised control trials are warranted to study the potential benefits of UCB compared to adult blood for RBC transfusions.
Keywords: neonatal infant, extremely premature, transfusion, foetal haemoglobin
INTRODUCTION
Anaemia of prematurity is a well-known entity. Strategies such as delayed cord clamping or cord milking have been implemented in the last decades to decrease the incidence of anaemia by increasing the haemoglobin concentration1. Despite this, red blood cell (RBC) transfusions are often needed within the first weeks of life in very preterm neonates (VPN) and extremely low gestational age neonates (ELGAN). The causes of anaemia in these groups are shorter RBC half-life, lower iron reserves and repeated blood sampling2–5.
RBC transfusions in preterm infants born earlier than 32 weeks of gestational age (GA) have been associated with an increased incidence of complications such as retinopathy of prematurity (ROP)6,7 and bronchopulmonary dysplasia (BPD)8,9 due to changes in tissue oxygenation. The role of oxygen in the pathogenesis of ROP is well known10. The use of adult donor blood for transfusions in neonates causes a change in the concentration of foetal haemoglobin (HbF) in neonates’ bloodstream. HbF has a greater affinity for oxygen compared to adult haemoglobin (HbA) so this change ultimately affects tissue oxygenation11. Stutchfiel et al. found an association between the replacement of HbF by HbA and a higher incidence of ROP after transfusion of blood from an adult donor12. Thus, the presence of a higher concentration of HbF in umbilical cord blood (UCB) could act as a protective factor for ROP.
Several studies have evaluated the safety of UCB transfusions in preterm infants looking at different ranges of GA and birthweight13,14. None of them found a higher risk of complications compared with that occurring with adult donor blood transfusions. However, the processing of UCB samples to obtain RBC is more complex and expensive than processing adult blood. The transfusion of autologous UCB carries a lower risk of cross-reactions, but it does not seem feasible in preterm infants since the volume of UCB obtained from each of them is too small13. A recent, prospective study demonstrated the feasibility of using allogeneic UCB, obtained from infants born at term, for transfusion to preterm infants, although the benefits of this strategy are still unknown14.
The potential benefits of the use of UCB-RBC transfusions in preterm neonates can be predicted. Significant amounts of heavy metals such as mercury and lead have been found in RBC concentrates obtained from adult donor blood15. There are no data on the presence of heavy metals in UCB but it seems likely that the risk could be lower than that with adult blood.
Clinical trials are needed to elucidate the potential benefit of UCB transfusion in preterm infants. Accordingly, the aims of this study were: (i) to determine the RBC transfusion needs of infants born at <32 weeks of GA; (ii) to identify the target GA group that would benefit most from UCB transfusion; and (iii) to assess the current availability of UCB as a potential source of RBC transfusion in these infants in our tertiary referral blood bank.
MATERIALS AND METHODS
Study design and data collection
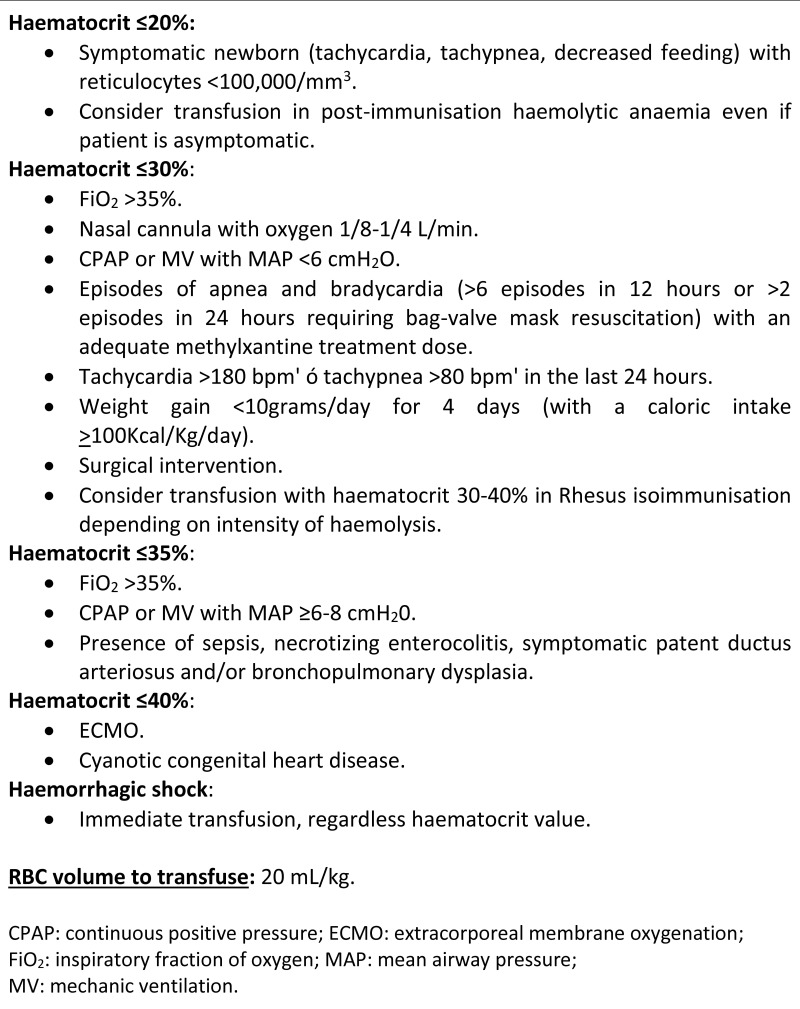
This retrospective descriptive study was carried out with data from the Neonatal Intensive Care Unit (NICU) of Hospital Clinic of Barcelona. Neonates born earlier than 32 weeks’ GA between January 2005 and December 2018 were included in the study and their clinical records were reviewed. The Hospital Clinic Ethics Committee approved the study (CIF-G-08431173). The most up-to-date guidelines for transfusion at our hospital were applied during the study period (Figure 1). To facilitate the data analysis, patients were divided into two groups: (i) ELGAN (from 230 to 276 weeks of GA) and (ii) VPN (from 280 to 316 weeks).
Figure 1.
Most up-to-date guidelines on red blood cell transfusion at the Hospital Clinic of Barcelona during the study period
Total volume and number of RBC transfusions were recorded. Perinatal data and outcomes were collected from the local hospital database and morbidities were defined according to the international Vermont Oxford Network database16. We considered the diagnosis of sepsis when there was a positive blood culture or clinical symptoms plus raised serial C-reactive protein concentration (≥15 mg/L) warranting a minimum of 5 days of antibiotic treatment. Respiratory distress syndrome was defined as PaO2 <50 mmHg in room air, a requirement of supplemental oxygen to maintain PaO2 >50 mmHg or a requirement of supplemental oxygen to maintain a pulse oximeter saturation over 85% within the first 24 hours of life and a chest radiograph consistent with respiratory distress syndrome within the first 24 hours of life16. BPD was defined as oxygen dependency at 28 days of life and its severity was classified based on the situation at 36 weeks of post-menstrual age17. Necrotising enterocolitis (NEC) was taken into account when it was graded as higher than stage 2 of Bell’s classification18. ROP was classified following the International Committee for Classification of ROP19. Intraventricular haemorrhage was graded according to the Papile classification20 and patent ductus arteriosus was defined by the presence of a left-to-right or bidirectional ductal shunt on Doppler echocardiography or a systolic or continuous murmur and at least two of the following clinical signs: (i) hyperdynamic precordium; (ii) bounding pulses; (iii) wide pulse pressure; or (iv) pulmonary vascular congestion, cardiomegaly or both16.
Collection, storage and processing of umbilical cord blood
UCB was obtained following non-complicated vaginal or Caesarean deliveries as part of the public programme for cord blood donations of the blood bank (Programa Concordia)21. Sixty-six maternity units from different regions of Spain are part of this programme whose aims are to work for rapid and efficient donations, to facilitate transplants and to promote clinical research and exchange of knowledge. “Programa Concordia Banc de Sang i Teixits” is a FACT-Netcord accredited public cord blood bank and has a long experience in collecting CB units for transplantation. International quality certifications are guaranteed and accredited by the standards of the Foundation for the Accreditation of Cellular Therapy (FACT)-Netcord. Written informed parental consent was signed before collecting the UCB. After umbilical cord clamping had been performed as per standard protocol, cord blood was collected by gravity using multiple puncturing of the umbilical cord with a sterile technique. The ratio of blood volume to the volume of anticoagulant/preservative solution is important to maintain RBC quality. Collection bags contained 25 mL anticoagulant solution for a recommended volume of 150 mL of blood. The collected UCB was stored at 2–8°C until it was shipped to the reference blood bank to be processed. Samples of whole UCB that arrived at the blood bank within 44 hours after collection were assessed for quality parameters. First, nucleated cells were counted and samples with a total nucleated cell count >1.5×109 were selected for hematopoietic stem cell (HPSC) transplantation. Microbiological analyses (bacterial and fungal cultures) and serological and molecular testing (for hepatitis B and C viruses, cytomegalovirus, human immunodeficiency virus and syphilis) were carried out in this subgroup of samples and any results suspicious of infection led to that sample being discarded.
The remaining UCB samples were considered suitable for different additional applications or were used for research purposes.
Availability of cord blood for transfusion
UCB donations from January 2018 to December 2019 were reviewed to assess the current availability of UCB as a potential source of RBC transfusion in infants <32 weeks of GA in our tertiary referral blood bank.
The potential number of suitable UCB-RBC bags was calculated taking into consideration the following information. In accordance with Bianchi et al.14 only bags with a blood volume greater than 60 mL were considered suitable for RBC production. Microbiological results of the HPSC samples were extrapolated and non-contaminated samples available for RBC production were calculated. The blood group distribution of the donated UCB samples and the distribution in our study population were also considered in order to evaluate the potential of the blood bank to supply enough matched samples.
According to the guidelines for transfusion at Hospital Clinic (Figure 1), the policy is to transfuse a RBC volume of 20 mL/kg per infant. Considering the weight range of the patients, we calculated a minimum volume needed of 20 mL with a haematocrit of about 60% of the final UCB-RBC bags obtained from the blood bank.
Statistical analysis
Categorical variables are presented as numbers (%), whereas continuous variables are described by the mean and standard deviation or median and interquartile range (IQR).
Appropriate statistical tests were performed for comparisons between GA groups: A Student’s t test or Mann-Whitney test was used for continuous variables and a chi-square test or Fisher’s exact test for categorical variables. Multivariate regression analysis was performed to evaluate the association between complications of prematurity and RBC transfusion, adjusting for possible confounders. All hypothesis tests were two-sided and p values less than 0.05 were considered statistically significant. The statistical analysis was performed using IBM SPSS version 22.0 software (IBM Corporation, Armonk, NY, USA).
RESULTS
Perinatal data
A total of 1,651 preterm infants born earlier than 32 weeks’ GA were admitted to the NICU at Hospital Clinic of Barcelona between January 2005 and December 2018. Table I presents the perinatal data collected for the two groups divided according to GA. The incidence of the main complications of prematurity was found to be higher in the ELGAN group (p<0.001).
Table I.
Perinatal data and outcomes according to gestational age
| Gestational age groups | p value | ||
|---|---|---|---|
| 230–276 | 28–316 | ||
| N=530 | N=1,121 | ||
| Birth weight (grams), median (IQR) | 810 (680–960) | 1,324 (1,100–1,560) | <0.001 |
| Male, n. (%) | 279 (52.6) | 603 (53.8) | 0.662 |
| Apgar 1 minute, median (IQR) | 5 (3–7) | 8 (6–9) | <0.001 |
| Apgar 5 minute, median (IQR) | 8 (6–9) | 9 (8–10) | <0.001 |
| IUGR, n. (%) | 81 (15.8) | 171 (15.3) | 0.799 |
| Early or late onset sepsis, n. (%) | 178 (33.6) | 85 (7.6) | <0.001 |
| RDS, n. (%) | 369 (69.6) | 359 (32) | <0.001 |
| PDA, n. (%) | 262 (49.4) | 201 (17.9) | <0.001 |
| NEC, n. (%) | 42 (7.9) | 26 (2.3) | <0.001 |
| ROP, n. (%) | 208 (57.6) | 125 (11.7) | <0.001 |
| ROP >2, n. (%) | 132 (36.6) | 46 (4.3) | <0.001 |
| BPD (O 2 28 days), n. (%) | 110 (30.5) | 36 (3.4) | <0.001 |
| BPD (O2 >36 weeks PMA), n. (%) | 31 (8.6) | 23 (2.2) | <0.001 |
| IVH, n. (%) | 196 (37) | 134 (12) | <0.001 |
| IVH ≥2, n. (%) | 81 (15.3) | 26 (2.3) | <0.001 |
| Neonatal death, n. (%) | 169 (31.9) | 54 (4.8) | <0.001 |
BPD: broncopulmonary dysplasia; IUGR: intrauterine growth restriction; IVH: intraventricular haemorrhage; NEC: necrotising enterocolitis; PDA: patent ductus arteriosus; PMA: postmenopausal age; RDS: respiratory distress syndrome; ROP: retinopathy of prematurity.
Red blood cell transfusion rates
Among all infants <32 weeks of GA, 27.5% (n=454) were transfused. While 12.5% (n=140) of VPN received at least one RBC transfusion, the percentage increased to 60% (n=314) in the ELGAN group. The total number of RBC transfusions given to the group of transfused neonates <32 weeks’ GA was 1.541 with a median of 2 (IQR: 1–4) transfusions per infant and an average of 110 (95% confidence interval [95% CI]: 92–128) transfusions per year. The total volume of RBC transfused was 18.92 L (1.35 L/year [95% CI: 1.07–1.64]) and the median was 29 mL (IQR: 17–48) per infant. The most frequent blood groups of the transfused patients were O+, A+ and B+ (69.5% of neonates), with the blood group distribution being the same as that in the general population.
Association between red blood cell transfusion and complications of prematurity
We evaluated the impact of RBC transfusions on complications related to changes in tissue oxygenation. Multivariate logistic regression indicated an association between RBC transfusion and an increased risk of NEC (odds ratio 10.44 [95% CI: 5.54–19.67]; p<0.001) and BPD (odds ratio 1.93 [95% CI: 1.17–3.17]; p<0.05). No statistically significant association was found between RBC transfusion and ROP, nor even ROP >2.
The ability of the blood bank to supply umbilical cord red blood cell transfusions for our population needs
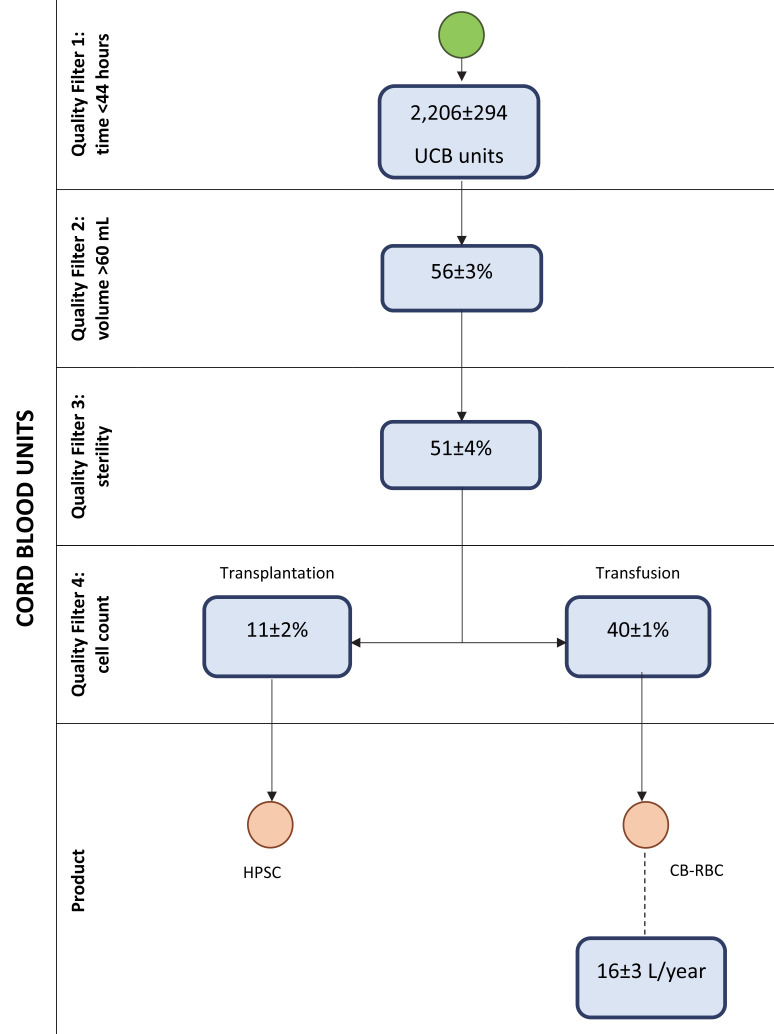
From January 2018 to December 2019 the Programa Concordia collected a total of 6,340 UCB samples. Within the total UCB donations, only samples selected for HPSC transplantation were studied and cell subsets and characteristics expressed as median values were as follows: 127±27 mL/bag; leukocytes 18±4×109/L; erythrocytes 3.7±0.4×1012/L; haemoglobin 12.9±1.6 g/dL and haematocrit 40.7±3.7%. Nine percent were discarded because of microbiological contamination.
Figure 2 shows the quality filters that would be applied to the total collected UCB samples in the blood bank: time to arrive at the blood bank, UCB volume (minimal 60 mL), tests for microbiological contamination and evaluation of cellularity for HPSC transplantation. It also shows the calculation of the potential volume of RBC available for transfusion based on previous information.
Figure 2. The calculation of the potentially available volume of red blood cells for transfusion after applying the quality filters.
UCB: umbilical cord blood; HPSC: haematopoietic stem cells; §CB-RBC: cord blood red blood cells.
Table II shows the similarity of the blood group distribution of the donated UCB samples and that of our study population.
Table II.
Blood group distribution of the study participants who were transfused and of the donated umbilical cord blood samples analysed for haematopoietic stem cell transplantation
| Blood group | Transfused neonates <32 weeks (N=454) | UCB samples (N=329) |
|---|---|---|
| 0+, % | 36.1 | 36.6 |
| A+, % | 36.2 | 33.5 |
| B+, % | 9.3 | 11.7 |
| AB+, % | 5.7 | 2.9 |
| 0−, % | 6.2 | 6.7 |
| A−, % | 5.7 | 7.1 |
| B−, % | 0.8 | 1.1 |
| AB−, % | None | 0.4 |
DISCUSSION
Treatment of anaemia in preterm infants continues to be a relevant health issue. Despite preventive strategies and strict guidelines for RBC transfusion, anaemia continues to be very frequent and RBC transfusions are much needed as our study shows (27.5% of preterm infants born earlier than <32 weeks of GA and 60% of infants born earlier than 28 weeks required at least one RBC transfusion).
The transfusion of RBC from adult donors has been repeatedly associated with BPD, ROP and, controversially, with NEC7–9,22–25. In this study significant associations were observed only between RBC transfusion and both NEC and BPD. However, this association does not establish causality since both NEC and BPD could be predisposing factors for RBC transfusion. The fact that ROP was not associated in this study with transfusion of adult RBC could be explained by the strict local guidelines on blood transfusion.
Different pathophysiological mechanisms have been proposed to explain the association between RBC transfusion and complications of prematurity. RBC products from UCB contain a higher concentration of HbF than those from adult donors and, in accordance with this, Teofili et al.26 have recently proven significantly higher HbF levels in preterm neonates transfused with UCB-RBC at 36 weeks of post-menstrual age than in those who received adult donor RBC transfusions. Compared to HbA, HbF has a greater affinity for oxygen, which affects its ability to release oxygen to tissues. Regarding the retina, this means that patients transfused with adult donor RBC experience a sudden increase in HbA levels and a high oxygen availability to the retinal tissues which leads to damage in the process of vascularisation in preterm infants12,27. An association between NEC and RBC transfusion in preterm infants is more controversial. Available evidence in published studies is inconclusive and the underlying mechanism of the putative association is unknown28–31. Oxygen release in the splanchnic vascular territory after RBC transfusion, following previous transient hypoxia due to anaemia, has been suggested as a possible component of the pathogenesis of NEC in these patients32–34. Finally, the association between RBC transfusion and BPD could be explained by mechanisms related to iron overload and inflammatory factors. The elevated levels of non-transferrin-bound iron in plasma after adult RBC transfusion could turn on oxidative stress mechanisms by releasing iron and other molecules which, in an excessive quantity, could be potentially harmful to the development of lungs35. RBC transfusion may also produce inflammatory mediators that make weaning from a ventilator difficult36.
Assessment of transfusion needs and morbidities of our study population led us to identify the ELGAN as the target group that would benefit most from UCB-RBC transfusions. This is explained by their much higher RBC transfusion rates (more than a half of them required at least one RBC transfusion) along with a higher incidence of complications of prematurity in this group of patients than in VPN. Furthermore, since the volume of blood that needs to be transfused depends on the recipient’s weight, smaller volumes are required in ELGAN, thereby minimising the number of blood donors to which each infant is exposed and decreasing the risk of sensitization and infection.
Autologous UCB transfusion in preterm infants has been evaluated in different studies with promising results in terms of collection and bank processing37–39. It is less immunogenic and carries a lower risk of infectious disease transmission than allogeneic transfusion40. In neonates born at term, the best results regarding the feasibility of autologous UCB transfusion have been obtained in those requiring peri-operative transfusion since this group of patients does not usually require repeated transfusions38. However, the availability of UCB for autologous transfusion in preterm infants is often insufficient, especially in preterm infants with a birth weight <1,000 g, since the collected volume to transfuse depends on birthweight and gestational age. Therefore, the feasibility of autologous UCB transfusion in preterm infants has not been shown to be greater than that of allogeneic transfusion13,39,41. Allogeneic transfusion of RBC obtained from term neonates involves a simpler procedure to collect the product and has proven to be feasible and safe in preterm infants14.
In this study, RBC transfusion requirements in preterm infants <32 weeks of GA were estimated to be just over 1.35 L/year. Currently, the blood bank has the capacity to produce 16 L of UCB-RBC per year, which guarantees the availability of enough allogeneic RBC to transfuse this group of preterm infants at the Hospital Clinic. Table II shows that the blood group distribution of the donated UCB samples was similar to that of the study population, which supports the capacity of our referral blood bank to supply enough matched samples. In terms of blood group-type bags, the most efficient approach could be to narrow the production of bags to type A or type O instead of preparing bags of each blood group in order to guarantee that as many bags as possible are used and to avoid bags of less frequent blood groups from expiring unused; this approach would maximise the cost-benefit ratio for the blood bank.
Collecting UCB carries a higher risk of bacterial contamination than collecting adult blood with rates of contamination of the former being up to 48%42, although this complication has decreased significantly in recent years. In our study 9% of samples were discarded because of microbiological contamination. Several sources of contamination have been described: vaginal, skin and gastrointestinal flora, poor aseptic techniques, use of contaminated material and others. The creation of an UCB bank for blood transfusion to ELGAN would require meticulously precise aseptic techniques, well-trained cord blood collectors and, ideally, the development of new collection techniques to establish in our unit. Because of the higher risk of contamination, it would be mandatory to perform microbiological tests on all UCB samples on top of the microbiological tests already carried out on the mothers’ samples. This would prolong the time necessary to evaluate the final product by 1 to 2 weeks compared to that necessary for standard RBC and would, therefore, reduce the period of time during which products are usable. More sensitive and faster microbiological tests are required to improve this aspect.
The required characteristics of UCB-RBC units must still be specified in order to introduce this product into daily practice of neonatal units. Teofili et al. have demonstrated the safety and efficacy of the product26. Based on their experience we will validate our UCB-RBC production to obtain bags with a minimum volume of 20 mL, a haematocrit of about 60% and an acceptable residual leukocyte content of <106. The expiry time of the product and time for pre-transfusion irradiation will also have to be established. All blood samples for neonatal transfusions in our unit are irradiated, in accordance with standard guidelines.
For research purposes adult kits can be used for the production of UCB-RBC bags, but the creation of an UCB-RBC ELGAN transfusion programme will require the development of specific blood fractionation kits with leukodepletion filters that can be used for small volumes. This will aid the safe production of blood components derived from placental blood. It will only be possible to calculate the average amount of blood lost during the leukodepletion process after validating these new fractionation kits.
The present study has some limitations. The retrospective nature of the study did not allow causality to be established between RBC transfusion and complications of prematurity, although it should be said that this was not the purpose of the study. Secondly, our study shows the feasibility of expanding an existing and well-established cord blood bank for haematopoietic cell transplantation in order to fulfil the transfusion needs of preterm infants in a specific area of health care, but for the project to be sustainable in practice, the process of cord blood collection and UCB-RBC production would have to meet the standard requirements of other reference hospitals with this programme already in place.
CONCLUSIONS
Considering the data obtained about RBC transfusion needs and morbidities, ELGAN have been identified as the target group of recipients who would benefit most from UCB-RBC transfusions. We have demonstrated that our reference blood bank is able to produce sufficient reserves of RBC from UCB.
Future steps will be to validate the processing and storage conditions to enable the initiation of clinical studies to demonstrate the safety and efficacy of these products on a routine basis in our NICU. Clinical trials are necessary to test the hypothesis that the use of UCB as a source of RBC for transfusion in ELGAN is more beneficial than RBC from adult donors.
Footnotes
AUTHORSHIP CONTRIBUTIONS
EG: study concept, data management, methodology, formal analysis, writing of the original draft; MA: study concept, data management, methodology, formal analysis, writing of the original draft; DS: blood bank methodology and results; VA-B: methodology; MT-P: writing and review of the article; EF: blood bank methodology; JF-A: methodology; MDS-R: study concept, methodology, writing and review of the article; SQ: study concept, methodology, writing and review of the article.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Rabe H, Diaz-Rossello JL, Duley L, et al. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012;8:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Jeon GW, Sin JB. Risk factors of transfusion in anemia of very low birth weight infants. Yonsei Med J. 2013;54:366–73. doi: 10.3349/ymj.2013.54.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss RG. Managing the anemia of prematurity: red blood cell transfusions versus recombinant erythropoietin. Transfus Med Rev. 2001;15:213–23. doi: 10.1053/tmrv.2001.24592. [DOI] [PubMed] [Google Scholar]
- 4.Widness JA, Madan A, Grindeanu LA, et al. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299–306. doi: 10.1542/peds.2004-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews. 2008;9:e520. doi: 10.1542/neo.9-11-e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YC, Chan OW, Chiang MC, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr Neonatol. 2017;58:216–22. doi: 10.1016/j.pedneo.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Dani C, Reali MF, Bertini G, et al. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Hum Dev. 2001;62:57–63. doi: 10.1016/s0378-3782(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Huang X, Lu H. Association between red blood cell transfusion and bronchopulmonary dysplasia in preterm infants. Sci Rep. 2014;4:1–5. doi: 10.1038/srep04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir A, Aziz K, McMillan D, et al. Red blood cell transfusions at 21 days of age or older in previously transfusion-naive very preterm infants: association with neonatal outcomes. Am J Perinatol. 2015;32:1139–44. doi: 10.1055/s-0035-1549295. [DOI] [PubMed] [Google Scholar]
- 10.Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Haueux V, Truttmann A, Gagnon C, Bard H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm Infants during the first week of life. Semin Perinatol. 2002;26:411–5. doi: 10.1053/sper.2002.37313. [DOI] [PubMed] [Google Scholar]
- 12.Stutchfield CJ, Jain A, Odd D, et al. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: a pilot prospective cohort study. Eye. 2017;31:1451–5. doi: 10.1038/eye.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khodabux CM, Von Lindern JS, Van Hilten JA, et al. A clinical study on the feasibility of autologous cord blood transfusion for anemia of prematurity. Transfusion. 2008;48:1634–43. doi: 10.1111/j.1537-2995.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi M, Giannantonio C, Spartano S, et al. Allogeneic umbilical cord blood red cell concentrates: an innovative blood product for transfusion therapy of preterm infants. Neonatology. 2015;107:81–6. doi: 10.1159/000368296. [DOI] [PubMed] [Google Scholar]
- 15.Falck AJ, Medina AE, Cummins-Oman J, et al. Mercury, lead, and cadmium exposure via red blood cell transfusions in preterm infants. Pediatr Res. 2020;87:677–82. doi: 10.1038/s41390-019-0635-x. [DOI] [PubMed] [Google Scholar]
- 16.Klebermass-Schrehof K. Vermont Oxford Network. Manuals and forms. [Accessed on 18/05/2020]. Available at https://public.vtoxford.org/manuals-forms/
- 17.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 18.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn GE. The international classification of retinopathy of prematurity revisited: An international committee for the classification of retinopathy of prematurity. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 20.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 21.Programa Concordia. [Accessed on 18/05/2020]. Available at: https://www.bancsang.net/professionals/es_concordia/
- 22.Wang YC, Chan OW, Chiang MC, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr Neonatol. 2017;58:216–22. doi: 10.1016/j.pedneo.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham KE, Okolo FC, Baker R, et al. Red blood cell transfusion in premature infants leads to worse necrotizing enterocolitis outcomes. J Surg Res. 2017;213:158–65. doi: 10.1016/j.jss.2017.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alverson DC. The physiologic impact of anemia in the neonate. Clin Perinatol. 1995;22:609–25. [PubMed] [Google Scholar]
- 25.Ghirardello S, Dusi E, Cortinovis I, et al. Effects of red blood cell transfusions on the risk of developing complications or death: an observational study of a cohort of very low birth weight infants. Am J Perinatol. 2017;34:88–95. doi: 10.1055/s-0036-1584300. [DOI] [PubMed] [Google Scholar]
- 26.Teofili L, Papacci P, Orlando N, et al. Allogeneic cord blood transfusions prevent fetal haemoglobin depletion in preterm neonates. Results of the CB-TrIP study. Br J Haematol. 2020;91:263–8. doi: 10.1111/bjh.16851. [DOI] [PubMed] [Google Scholar]
- 27.Lust C, Vesoulis Z, Jackups R, Jr, et al. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J Perinatol. 2019;39:393–400. doi: 10.1038/s41372-018-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–40. doi: 10.1542/peds.2011-2872. [DOI] [PubMed] [Google Scholar]
- 29.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155:331–7. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faraday C, Hamad S, Jones KD, et al. Characteristics and incidence of transfusion-associated necrotizing enterocolitis in the UK. J Matern Neonatal Med. 2020;33:398–403. doi: 10.1080/14767058.2018.1494147. [DOI] [PubMed] [Google Scholar]
- 31.Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. 2006;23:451–8. doi: 10.1055/s-2006-951300. [DOI] [PubMed] [Google Scholar]
- 32.Luban NLC. Neonatal red blood cell transfusions. Vox Sang. 2004;87(Suppl 2):184–8. doi: 10.1111/j.1741-6892.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen HL, Tseng HI, Lu CC, et al. Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatr Neonatol. 2009;50:110–6. doi: 10.1016/S1875-9572(09)60045-0. [DOI] [PubMed] [Google Scholar]
- 34.Wardle SP, Weindling AM. Peripheral fractional oxygen extraction and other measures of tissue oxygenation to guide blood transfusions in preterm infants. Semin Perinatol. 2001;25:60–4. doi: 10.1053/sper.2001.23200. [DOI] [PubMed] [Google Scholar]
- 35.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. 2006;66:355–64. doi: 10.1016/j.mehy.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Keir AK, McPhee AJ, Andersen CC, et al. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr Res. 2013;73:75–9. doi: 10.1038/pr.2012.144. [DOI] [PubMed] [Google Scholar]
- 37.Jansen M, Brand A, Von Lindern JS, et al. Potential use of autologous umbilical cord blood red blood cells for early transfusion needs of premature infants. Transfusion. 2006;46:1049–56. doi: 10.1111/j.1537-2995.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 38.Brune T, Garritsen H, Witteler R, et al. Autologous placental blood transfusion for the therapy of anaemic neonates. Biol Neonate. 2002;81:236–43. doi: 10.1159/000056754. [DOI] [PubMed] [Google Scholar]
- 39.Kotowski M, Litwinska Z, Klos P, et al. Autologous cord blood transfusion in preterm infants - could its humoral effect be the key to control prematurity-related complications? A preliminary study. J Physiol Pharmacol. 2017;68:921–7. [PubMed] [Google Scholar]
- 40.Garritsen HSP, Brune T, Louwen F, et al. Autologous red cells derived from cord blood: Collection, preparation, storage and quality controls with optimal additive storage medium (Sag-mannitol) Transfus Med. 2003;13:303–10. doi: 10.1046/j.1365-3148.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 41.Strauss RG, Widness JA. Is there a role for autologous/placental red blood cell transfusions in the anemia of prematurity? Transfus Med Rev. 2010;24:125–9. doi: 10.1016/j.tmrv.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bello-López JM, Noguerón-Silva J, Castañeda-Sánchez JI, Rojo-Medina J. Molecular characterization of microbial contaminants isolated from umbilical cord blood units for transplant. Braz J Infect Dis. 2015;19:571–7. doi: 10.1016/j.bjid.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]