Abstract
Background
Pharmacological treatment of iron deficiency anaemia can reduce red blood cell (RBC) transfusions. Intravenous iron provides a more effective and quicker correction of iron deficiency anaemia than oral iron, and third-generation high-dose intravenous iron formulations allow the complete correction of iron deficiency with just one or two drug infusions, thus facilitating iron supplementation therapy and reducing transfusion requirement.
Material and methods
In an observational, retrospective study we compared RBC transfusion requirement during hospitalisation and within 3 months of hospital discharge in 88 patients with iron deficiency anaemia treated with high-dose ferric carboxymaltose and in 85 patients treated with ferric gluconate while hospitalised in the Internal Medicine unit of our Institution.
Results
Ferric carboxymaltose reduced the number of RBC units given to each transfused patient during hospitalisation (1.81±0.84 vs 2.39±1.49, p=0.011). At hospital discharge, fewer ferric carboxymaltose patients were prescribed home therapy with iron. No differences between treatment groups were observed in the proportion of patients or the number of RBC units transfused within 3 months of discharge. At one month from discharge, however, only 2 ferric carboxymaltose patients had been transfused compared with 7 ferric gluconate patients (p=0.078). Patients transfused post-discharge were more likely to have an underlying malignancy and/or higher serum creatinine concentrations.
Discussion
Treatment with ferric carboxymaltose reduced the number of RBC units per transfused patient. Larger studies are required to define risk factors associated with post-discharge transfusion requirement and to establish if home therapy with iron will reduce subsequent transfusions in patients treated with ferric carboxymaltose.
Keywords: iron deficiency anaemia, red blood cell transfusions, IV iron supplementation
INTRODUCTION
Iron deficiency is the most common cause of anaemia worldwide. It usually occurs as a result of bleeding from the gastrointestinal (GI) or the female genital tracts or due to iron malabsorption. More recently, the interest of clinicians has focused not only on patients with iron deficiency anaemia (IDA), but also on those with iron deficiency without anaemia, since iron deficiency may contribute to low quality of life and increased mortality1–4, the problem being most relevant in subjects >60 years of age5. Optimal treatment of IDA requires control of the underlying disease responsible for iron deficiency together with iron supplementation in order to accelerate the recovery from anaemia and the reconstitution of body iron stores. Iron supplementation is of particular importance for those who do not respond completely to treatment of the underlying disease, thus suffering from persistent blood loss or iron malabsorption which perpetuate iron deficiency and its harmful consequences. Owing to low cost and given its effectiveness, oral iron still represents the most widely used and accepted treatment for iron deficiency. However, oral iron is often associated with GI side-effects, such as abdominal pain, constipation, diarrhoea, nausea and vomiting, that limit efficacy and adherence to therapy. This is especially true in alimentary tract disorders, where oral iron often aggravates associated GI symptoms, is frequently ineffective due to concomitant malabsorption and/or inflammation, or can worsen the underlying disease through pro-inflammatory changes of the intestinal mucosa and alteration of the gut microbiota composition6,7.
IDA treatment has undergone recent changes following the introduction in clinical practice of new high-dose parenteral iron compounds (third-generation), such as ferric carboxymaltose (FCM), iron isomaltoside, ferumoxytol and low-molecular-weight iron dextran, that allow the safe administration of 500–1,000 mg of elemental iron as single intravenous (IV) injections8 and replacement of the amount of iron required to correct anaemia and reconstitute iron stores in one or two infusion sessions9,10. Safety and efficacy of high-dose IV iron formulations has been extensively reported and summarised in a number of reviews and meta-analyses11–18. Use of FCM in the Emergency Department has recently been shown to improve transfusion appropriateness and reduce the number of post-discharge transfusions19. Given the recent transition to FCM for parenteral iron supplementation in our institution, we retrospectively compared the effects of high-dose FCM and traditional ferric gluconate (FG) supplementation on the in-hospital and post-discharge need for red blood cell (RBC) transfusions among patients suffering from IDA and treated with parenteral iron during periods of hospitalisation in an Internal Medicine ward. The study endpoint was established based on the hypothesis that, through the use of FCM, supplementation with a larger amount of elemental iron during hospitalisation would reduce the need for RBC transfusions in the post-discharge period.
MATERIALS AND METHODS
Study population
We performed an observational, retrospective study involving patients affected by IDA and treated with parenteral iron while hospitalised in the Internal Medicine unit of our institution during the period May 2013–April 2015, during which FG was used for IV iron supplementation, and July 2016–December 2018, when FCM was used. The temporal range May 2015–May 2016 was excluded, since during this period it was not always possible to establish which parenteral iron compound had been used for parenteral supplementation. The study was approved by the Institutional Research Ethics Committee (REC) of the Fondazione I.R.C.C.S. Policlinico San Matteo, Pavia, Italy, and was conducted in accordance with the Declaration of Helsinki. On admission to the hospital, all patients or their legal representatives signed a consensus form approved by the institutional REC (in compliance with the Good Clinical Practice Directive 2005/28/EC, local regulatory requirements, and legal requirements), authorising the use of the patient’s sensitive data for institutional activities concerning teaching and clinical research, including statistical analysis.
Patients treated with IV iron during hospitalisation were identified through examination of the electronic hospital records when one of the following International Classification of Diseases codes (ICD-9-CM) was present: 280.0 (chronic post-haemorrhagic anaemia), 280.1 (IDA due to inadequate alimentary iron intake), 280.9 (IDA) or 285.1 (acute post-haemorrhagic anaemia); confirmation that the patient had been treated with IV iron was subsequently obtained by analysis of patient hospital discharge letters. Patients receiving RBC transfusions in the post-discharge period were identified from the local blood bank transfusion records. Some patients might have been transfused in other hospitals once discharged, leading to an underestimation of post-discharge transfusions; most study patients, however, lived in the surrounding area and the blood bank supplies several local hospitals, allowing us to track most transfusions that may have been performed outside our centre.
Study endpoints
Demographic, clinical and laboratory data of the study patients during hospitalisation were analysed. The primary endpoint of the study was determination of the proportion of patients, among those treated with parenteral iron, who received RBC transfusions within 3 months of hospital discharge. Secondary endpoints included determination of the number of RBC units transfused during the study period, transfusion requirement during hospitalisation and within one month from hospital discharge, determination of factors influencing in-hospital and post-discharge RBC transfusions and mortality. A composite endpoint, represented by the proportion of patients who died and/or were transfused within 3 months of hospital discharge, was also considered. The burden of disease for individuals during hospitalisation was retrospectively evaluated through the cumulative illness rating scale (CIRS) and the number of different chronic conditions20,21. Estimated glomerular filtration rates (eGFR) were calculated using the 4-variables Modification of Diet in Renal Disease (MDRD-4) study equation and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation22,23. According to the World Health Organization (WHO), anaemia was defined by an haemoglobin (Hb) <130 g L−1 in males and <120 g L−1 in females24; moderate and severe anaemia were characterised by Hb <100 g L−1 and Hb <70 g L−1, respectively25.
Statistical analysis
Descriptive statistics were computed overall and by iron supplementation groups. Continuous variables were presented as mean and standard deviation (SD) or median and interquartile range; frequencies and proportions were used for categorical variables. Proportions of patients undergoing RBC transfusions after hospital discharge and mortality were compared with the χ2 test; for normally distributed variables, between group differences in laboratory parameters and clinical variables were tested by the Student’s t-test; otherwise the non-parametric Mann-Whitney U test was used. Variables associated with the need for RBC transfusion and the composite endpoint were evaluated in univariate and multivariate analysis; linear regression or logistic regression models were used depending on the outcome distributions. All tests were two-tailed. p<0.050 was considered statistically significant. The datasets analysed during the current study are available from the corresponding Author on reasonable request.
RESULTS
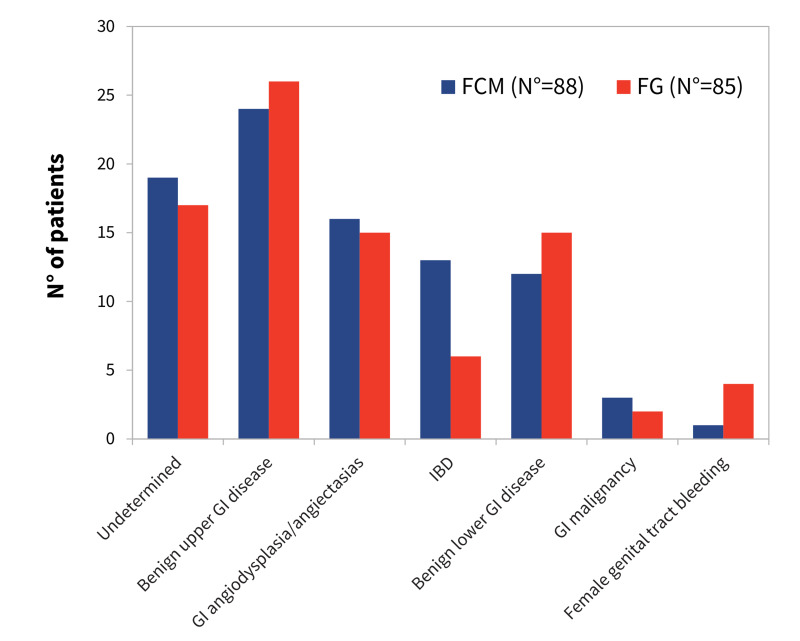
One-hundred and seventy-three patients were treated with IV iron while hospitalised in the Internal Medicine unit of our institution during the study periods: 85 patients were treated with FG, 88 with FCM. Table I shows the baseline characteristics of the study patients. The FCM and the FG cohorts were similar in terms of burden of disease, hospital length of stay, and main laboratory parameters. The most common cause of IDA, computed overall and by iron supplementation group, was bleeding from benign upper GI tract disorders (Figure 1), although for a significant number of cases the main mechanism of anaemia or the bleeding site remained undetermined. In some cases, this occurred because, given the advanced age or severely compromised status, it was not considered worthwhile to pursue a definitive diagnosis through invasive and/or expensive procedures. Patients undergoing in-hospital RBC transfusions were older than untransfused patients (77±15 years vs 66±22, p=0.015) and had lower nadir Hb concentrations during hospitalisation (67±12 g L−1 vs 88±13 g L−1, p<0.001) (Table I). At hospital discharge, however, Hb levels were similar in the two groups (96±10 and 99±12 g L−1, respectively, p=0.142). Globally, more of those treated with FCM required RBC transfusions, 76 vs 69% in the FG group, but with a lower total number of RBC units (116 vs 139). The net result was that each transfused patient treated with FCM received a lower number of RBC units compared with transfused patients treated with ferric gluconate (1.81±0.84 vs 2.39±1.49 RBC units, respectively, p =0.011), although there was no significant difference between their nadir Hb concentrations during hospitalisation and Hb levels at hospital discharge. In addition, transfused patients treated with FCM were older than those treated with ferric gluconate (Table I). Since the number of RBC units per transfused patient was also related to age, nadir Hb concentration and mean corpuscular volume (MCV), a multiple regression analysis including these parameters and iron supplementation modality as independent variables was performed, and confirmed the correlation of iron supplementation modality, age, nadir Hb concentration and MCV with the number of RBC units per transfused patient (Table II). At hospital discharge, fewer FCM patients were prescribed home therapy with oral iron, whereas no differences between treatment groups were observed in the proportions of patients discharged with moderate/severe anaemia or prescribed home therapy with antiplatelet and/or anticoagulant drugs (Table I).
Table I.
Baseline characteristics of patients treated with intravenous iron during hospitalisation
| Characteristics | Patient groups | |||
|---|---|---|---|---|
|
| ||||
| Total (n=173) | FG (n=85) | FCM (n=88) | p | |
|
| ||||
| Age, mean (SD), years | 71 (18) | 69 (18) | 74 (17) | 0.071 |
|
| ||||
| Male sex, n (%) | 61 (35) | 34 (40) | 27 (31) | 0.202 |
|
| ||||
| Patient burden of disease | ||||
| CIRS, mean (SD) | 24 (4) | 24 (4) | 24 (4) | 0.607 |
| Number of different chronic conditions, n (SD) | 4 (2) | 4 (2) | 4 (2) | 0.524 |
|
| ||||
| Hospital length of stay, days (SD) | 13.4 (7.2) | 13.2 (6.7) | 13.6 (7.7) | 0.706 |
|
| ||||
| Patients with malignant neoplasms, n (%) | 28 (16) | 16 (19) | 12 (14) | 0.3395 |
|
| ||||
| Post-discharge iron supplementation, n (%) | 82 (48) | 66 (78) | 16 (19) | <0.001 |
|
| ||||
| Antithrombotic therapy at hospital discharge, n (%) | ||||
| antiplatelet | 47 (27) | 21 (25) | 26 (30) | 0.475 |
| anticoagulant | 34 (20) | 16 (19) | 18 (20) | 0.787 |
| antiplatelet plus anticoagulant | 5 (3) | 1 (1) | 4 (5) | 0.368 |
| antiplatelet and/or anticoagulant | 81 (47) | 38 (45) | 48 (55) | 0.196 |
|
| ||||
| Laboratory values, means (SD) | ||||
| Hb nadir, g L−1 | 73 (15) | 73 (15) | 73 (16) | 0.826 |
| Hb at hospital discharge, g L−1 | 97 (11) | 99 (11) | 96 (11) | 0.095 |
| Moderate/severe anaemia at hospital discharge, n (%) | 101 (58) | 47 (55) | 54 (61) | 0.418 |
| Serum ferritin, μg L−1 | 17 (8–48)* | 16 (8–41)* | 20 (8–49)* | 0.175** |
| Serum creatinine, mg L−1 | 9.4 (7.3–1.27)* | 7.0 (9.3–11.7)* | 9.5 (7.3–12.7)* | 0.540 |
|
| ||||
| In-hospital transfusions | ||||
| transfused patients, n (%) | 126 (72) | 59 (69) | 67 (76) | 0.320 |
| Age, mean (SD), years | 73 (16) | 69 (17) | 77 (15) | 0.008 |
| Male sex, n (%) | 46 (37) | 26 (44) | 20 (30) | 0.242 |
| Transfused patients Hb nadir, g L−1 | 67 (11) | 66 (13) | 68 (11) | 0.350 |
| Total n of RBC units | 255 | 139 | 116 | 0.412** |
| n of units/transfused patient, mean (SD) | 2 (1–3)* | 2 (1–3)* | 2 (1–2)* | 0.011 ** |
n: number; CIRS: Cumulative Illness Rating Scale; Hb: haemoglobin; FG: ferric gluconate; FCM: ferric carboxymaltose; SD: standard deviation; RBC: red blood cells. p-values refer to differences between the FG and the FCM groups.
Medians and interquartile ranges.
Mann-Whitney U test; statistical significance in bold.
Figure 1. Causes of anaemia in patients treated with intravenous (IV) iron during hospitalisation.
The prevalence of different conditions leading to iron deficiency anaemia (IDA) was similar in the ferric carboxymaltose (FCM) and the ferric gluconate (FG) groups. Benign upper gastrointestincal (GI) tract disease include esophagitis, stomach and duodenal ulcers-erosions; benign lower GI tract disease include haemorrhoids and large bowel diverticula and polyps. IBD: inflammatory bowel disease.
Table II.
Factors influencing the number of red blood cell (RBC) units/transfused patient used during hospitalisation
| Continuous variables | Transfused patients n=126 |
Correlation coefficients | Univariate analysis p-value |
Multivariate analysis p-value |
|---|---|---|---|---|
|
| ||||
| Age, mean (SD), y | 73 (16) | r=−0.2388 | 0.007 | 0.004 |
|
| ||||
| Age, mean (SD), y | 73 (16) | r=−0.2388 | 0.007 | 0.004 |
| Patient burden of disease | ||||
| CIRS, mean (SD) | 24 (4) | r=−0.1216 | 0.175 | - |
| n. of different chronic conditions, n (SD) | 4 (2) | r=−0.1315 | 0.142 | - |
| Patients with malignant neoplasms, n (%) | 21 (17) | r=0.0439 | 0.625 | - |
| Laboratory values | ||||
| Hb nadir, g L−1 | 67 (11) | r=−0.4562 | <0.001 | <0.001 |
| Hb at hospital discharge, g L−1 | 97 (13) | r=−0.0482 | 0.592 | - |
| MCV, fL | 80 (9) | r=0.1926 | 0.031 | 0.002 |
|
| ||||
| Categorical variables | n of patients (%) | RBC units/transfused patient, mean (SD) | ||
|
| ||||
| Gender | ||||
| Males | 46 (36) | 2.24 (1.53) | 0.267 | - |
| Women | 80 (64) | 1.90 (0.97) | ||
|
| ||||
| In-hospital iron treatment | ||||
| FG | 59 (47) | 1.81 (0.84) | 0.004 | 0.038 |
| FCM | 67 (53) | 2.39 (1.49) | ||
n: number; CIRS: Cumulative Illness Rating Scale; Hb: haemoglobin; MCV: mean corpuscular volume; FG: ferric gluconate; FCM: ferric carboxymaltose; CI: confidence intervals; SD: standard deviation. Statistical significance in bold.
As far as the primary endpoint of the study is concerned, 22 patients were transfused within 3 months from hospital discharge; no difference in either the proportion of patients transfused or the number of transfused RBC units was observed between iron treatment groups (Table III). Although the number of reported events was low, more patients in the FG group were transfused at one month from discharge (7 vs 2 in the FCM group, p=0.078). More patients in the post-discharge transfusion group were males, had a compromised renal function (as expressed by higher serum creatinine concentrations), or an underlying malignancy (Table IV). Considering the composite endpoint, death and/or RBC transfusions within 3 months from discharge, the relations of serum creatinine and prevalence of malignancy with this endpoint were even more significant (p=0.001 for creatinine, p<0.001 for malignancy); the composite endpoint occurred more commonly among patients with heart disease, i.e. ischemic heart disease, heart failure and/or atrial fibrillation (20 of 79 patients with heart disease either died or were transfused vs 10 of 94 without heart disease, p=0.011). These associations were further investigated by multivariate analysis using a logistic regression model in which serum creatinine, cancer, age, sex, iron supplementation modality and heart disease were examined as covariates predictive of 3-month RBC transfusion requirement or of the composite endpoint. However, under multivariate analysis, only serum creatinine and malignancy maintained significant associations with the endpoints (p=0.008 for serum creatinine and p=0.003 for malignancy when the 3-month transfusion requirement was considered; p=0.007 for serum creatinine and p<0.001 for malignancy when the composite endpoint of death and/or RBC transfusions was considered). Compared to serum creatinine, eGFR as an index of renal function, computed either by the MDRD-4 or the CKD-EPI equations, did not improve prediction of RBC transfusion requirement or of the composite endpoint in either univariate or multivariate analysis (data not shown). No significant associations were found between cause of anaemia and post-discharge transfusion requirement, although a higher proportion of patients with angiodysplasia/angiectasias required transfusions (6 of 31 patients, 19%) compared with those affected by benign upper or lower GI disease (7 of 77 patients, 9%), but the difference was not statistically significant (p=0.138).
Table III.
Red blood cell (RBC) transfusions at 1 and 3 months following hospital discharge
| Total patient number (n=173) | FG (n=85) | FCM (n=88) | p | |
|---|---|---|---|---|
|
| ||||
| 1-month transfused patients, n (%) | 9 (5) | 7 (8) | 2 (2) | 0.078 |
|
| ||||
| 3-month transfused patients, n (%) | 22 (13) | 11 (13) | 11 (12) | 0.931 |
|
| ||||
| 3-month RBC transfused units, n | 51 | 23 | 28 | |
| n of units/patient (95% CI) | 0.29 (0.23–0.37) | 0.27 (0.18–0.38) | 0.32 (0.22–0.43) | 0.944* |
| n of units/transfused patient (SD) | 2 (1–2) | 2 (1.5–3.5) | 2 (1–2) | 0.741* |
|
| ||||
| Composite endpoint,** n (%) | 29 (17) | 13 (15) | 16 (18) | 0.611* |
n: number; FG: ferric gluconate; FCM: ferric carboxymaltose; CI: confidence intervals; SD: standard deviation.
Mann-Whitney U test.
Composite endpoint included death and/or transfusion requirement within 3 months of hospital discharge
Table IV.
Factors predictive of the need for red blood cell transfusions within 3 months of hospital discharge
| Variables | Non-transfused patients n=151 |
Transfused patients n=22 |
Univariate analysis p-value |
Multivariate analysis p-value |
|---|---|---|---|---|
|
| ||||
| Age, mean (SD), years | 71 (18) | 73(18) | 0.682 | 0.411 |
|
| ||||
| Male sex, n (%) | 49 (32) | 12 (55) | 0.043 | 0.121 |
|
| ||||
| In-hospital iron treatment | ||||
| Ferric gluconate, n (%) | 74 (49) | 11 (50) | 0.931 | 0.635 |
| FCM, n (%) | 77 (51) | 11 (50) | ||
|
| ||||
| Patient burden of disease | ||||
| CIRS, mean (SD) | 24 (4) | 25 (4) | 0.240 | - |
| Number of different chronic conditions, n (SD) | 4 (2) | 4 (2) | 0.717 | - |
| Hospital length of stay, days (SD) | 13.2 (7) | 14.6 (10.0) | 0.380 | - |
|
| ||||
| Patients with malignant neoplasms, n (%) | 19 (13) | 9 (41) | 0.001 | 0.003 |
|
| ||||
| Laboratory values | ||||
| Hb nadir, g L−1 | 73 (15) | 71 (14) | 0.566 | - |
| Hb at hospital discharge, g L−1 | 97 (11) | 97 (13) | 0.940 | - |
| Moderate/severe anaemia at hospital discharge, n (%) | 88 (58) | 13 (59) | 0.943 | - |
| Serum creatinine, mg L−1 | 10.2 (4.5) | 13.7 (7.9) | 0.003 | 0.008 |
n: number; CIRS: Cumulative Illness Rating Scale; Hb: haemoglobin; MCV: mean corpuscular volume; FG: ferric gluconate; FCM: ferric carboxymaltose; CI: confidence intervals; SD: standard deviation. Statistical significance in bold.
DISCUSSION
RBC transfusions are frequently used as life-saving therapies in conditions characterised by severe anaemia or acute bleeding associated with haemodynamic instability. However, their use must be restricted owing to high costs, limited supply, and risk of infectious and non-infectious adverse events, the problem being most relevant in low- and middle-income countries26,27. Ways to reduce RBC use in clinical practice include implementation of patient blood management programmes28, prevention of severe anaemia by optimal therapy/prevention of pharmacologically treatable forms, such as IDA, and adherence to published guidelines on the use of blood products, the latter suggesting that, in most clinical situations, restrictive pre-transfusion Hb thresholds (down to 70 g L−1 in haemodynamically stable patients) are advisable29,30. In the present study, we investigated whether in-hospital IV iron supplementation of IDA patients with FCM (which allows the administration of up to 1 g of elemental iron with a single injection) compared to FG (maximal suggested IV daily dose 125 mg of elemental iron) reduces RBC transfusion requirement after hospital discharge. The study involved patients hospitalised in the Internal Medicine unit of our institution where advanced age (mean age 71±18 years in our series), a significant burden of disease (a mean of 4±2 different chronic conditions and a CIRS score 24±4) or an underlying heart disease/acute coronary syndrome, for which higher pre-transfusion Hb thresholds are suggested, are commonly observed. No differences between treatment groups were found in the prevalence of transfusions (primary endpoint) and number of transfused RBC units per patient within 3 months from hospital discharge. Among the laboratory and clinical parameters investigated, only serum creatinine concentration and the presence of cancer correlated with post-hospitalisation RBC transfusion requirement when tested in multivariate analysis. Even factors associated with in-hospital RBC transfusions, such as older age and lower nadir Hb concentrations, were not predictive of post-discharge transfusions.
We found an important, though not statistically significant, difference between treatment groups in the number of patients transfused within one month of hospital discharge (7 of 85 FG patients vs 2 of 88 FCM patients); the difference disappeared at the 3-month post-discharge time point. Of note, contrary to the FG group, most patients treated with FCM did not receive post-discharge iron supplementation (72 of 88 vs 19 of 85 in the ferric gluconate group) (Table I). It has been hypothesised that the in-hospital use of FCM reduces the need for RBC transfusion in the short term (within one month of discharge), whereas during subsequent months a significant proportion of these patients will face worsening of anaemia and transfusion requirement, possibly due to ongoing bleeding and relapse of iron deficiency. This is particularly true in the case of patients with angiodysplasia/angiectasias in whom endoscopic treatment of the culprit lesions is often effective in the prevention of massive bleeding episodes, but may not be able to prevent small, chronic bleeding that could eventually lead to severe anaemia and transfusion requirement. Post-discharge iron supplementation in the FG group may have compensated bleeding-related iron loss and reduced transfusion requirement between 1 and 3 months post-discharge. Although in this study only 22 patients required RBC transfusions within 3 months of discharge, this represents a substantial proportion of the study population (13% of the total, 95% confidence intervals 9–19%) and may justify prescribing iron supplementation in the post-discharge period, either in oral form or as IV iron. Larger studies will be needed for a deeper investigation of risk factors associated with development of post-discharge severe anaemia and transfusion requirement; hopefully, this will allow an individualised approach to iron treatment, thus reducing costs and side-effects, and help optimise blood component use following hospital discharge. A strict follow-up is the key to reduce transfusion requirement in the medium-long term after hospitalisation, especially in patients with GI bleeding. Given the retrospective nature of this study, we did not address the problem of hypophosphataemia which occurs in up to 75% of those treated with FCM; although its clinical importance has yet to be determined, moderate-to-severe FCM-induced hypophosphataemia must be prospectively considered as a potential and harmful complication of the increasing use of FCM in clinical practice31.
Patients transfused during hospitalisation had lower nadir Hb concentrations than non-transfused patients. These were similar to pre-transfusion concentrations recently reported in a retrospective cohort of patients discharged from Northern California hospitals (72 g L−1 in 2014)25, and in a retrospective blood management study from Western Australia (73 g L−1 in 2013–2014)28. Although there are substantial differences in the designs of these studies, and we investigated only anaemic patients treated with in-hospital parenteral iron supplementation, these data suggest that RBC transfusion practices in the management of anaemia during hospitalisation are similar in western countries, and pre-transfusion Hb thresholds now adopted in clinical practice approach those suggested by the American Association of Blood Banks and the 2018 Frankfurt Consensus Conference29,30.
CONCLUSIONS
The study was characterised by a low rate of events defining the primary and secondary endpoints after hospital discharge (proportion of transfused patients and number of RBC units transfused at 1 and 3 months post-discharge) and was, therefore, underpowered to detect factors influencing post-discharge transfusion requirement, with the exception of serum creatinine concentration and of an underlying malignancy. The lack of data on the dose of IV iron infused during hospitalisation represents a further limitation of this study. We provide, however, valuable insights into the in-hospital use of third-generation high-dose IV iron formulations that, in our experience, reduced the number of RBC units per transfused patient. If associated with the subsequent prescription of iron home therapy, such an approach may reduce the requirement for post-discharge RBC transfusions.
ACKNOWLEDGEMENT
We thank the patients who participated in the study. The work was supported by Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Footnotes
AUTORSHIP CONTRIBUTIONS
All Authors contributed to the design of the study, acquisition, analysis, and interpretation of data. GB, AL, CP and ADS wrote the manuscript. NA, CB, EB, VDR, MG, LG and CM revised the manuscript.
The Authors declare no conflict of interest.
Please pay attention: the correct doi number is 10.2450/2020.0167-20 instead of 10.2450/2020.00167-20 (10.2450/2020.00167-20)
REFERENCES
- 1.Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–82. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012;14:423–29. doi: 10.1093/eurjhf/hfs017. [DOI] [PubMed] [Google Scholar]
- 3.Çekiç C, Ipek S, Aslan F, et al. The effect of intravenous iron treatment on quality of life in inflammatory bowel disease patients with nonanemic iron deficiency. Gastroenterol Res Pract. 2015;2015:582163. doi: 10.1155/2015/582163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philip KEJ, Sadaka AS, Polkey MI, et al. The prevalence and associated mortality of non-anaemic iron deficiency in older adults: a 14 years observational cohort study. Br J Haematol. 2020;189:566–72. doi: 10.1111/bjh.16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wouters HJCM, van der Klauw MM, de Witte T, et al. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica. 2019;104:468–76. doi: 10.3324/haematol.2018.195552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeggi T, Kortman GA, Moretti D, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64:731–42. doi: 10.1136/gutjnl-2014-307720. [DOI] [PubMed] [Google Scholar]
- 7.Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66:863–71. doi: 10.1136/gutjnl-2015-309940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avni T, Bieber A, Grossman A, et al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clinic Proceedings. 2015;90:12–23. doi: 10.1016/j.mayocp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Camaschella C. Iron-deficiency anemia. New Engl J Med. 2015;372:1832–43. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 10.Bergamaschi G, Di Sabatino A, Corazza GR. Pathogenesis, diagnosis and treatment of anaemia in immune-mediated gastrointestinal disorders. Br J Haematol. 2018;182:319–29. doi: 10.1111/bjh.15254. [DOI] [PubMed] [Google Scholar]
- 11.Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20:125–33. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
- 13.Batchelor EK, Kapitsinou P, Pergola PE, et al. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31:456–68. doi: 10.1681/ASN.2019020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematol. 2019;142:30–6. doi: 10.1159/000496728. [DOI] [PubMed] [Google Scholar]
- 15.Powers JM, Buchanan GR. Disorders of iron metabolism: new diagnostic and treatment approaches to iron deficiency. Hematol Oncol Clin North Am. 2019;33:393–408. doi: 10.1016/j.hoc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31–8. doi: 10.1002/ajh.24201. [DOI] [PubMed] [Google Scholar]
- 17.Auerbach M, Gafter-Gvili A, Macdougall IC. Intravenous iron: a framework for changing the management of iron deficiency. Lancet Haematol. 2020;7:e342–50. doi: 10.1016/S2352-3026(19)30264-9. [DOI] [PubMed] [Google Scholar]
- 18.Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287:153–70. doi: 10.1111/joim.13004. [DOI] [PubMed] [Google Scholar]
- 19.Beverina I, Razionale G, Ranzini M, et al. Early intravenous iron administration in the Emergency Department reduces red blood cell unit transfusion, hospitalisation, re-transfusion, length of stay and costs. Blood Transfus. 2020;18:106–16. doi: 10.2450/2019.0248-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 21.Friedman B, 1, Jiang HJ, Elixhauser A, Segal A. Hospital inpatient costs for adults with multiple chronic conditions. Med Care Res Rev. 2006;63:327–46. doi: 10.1177/1077558706287042. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanc B, Finch CA, Hallberg L, et al. Nutritional anaemias. Report of a WHO Scientific Group. WHO Tech Rep Ser. 1968;405:1–40. [PubMed] [Google Scholar]
- 25.Roubinian NH, Murphy EL, Mark DG, et al. Long-term outcomes among patients discharged from the hospital with moderate anemia: a retrospective cohort study. Ann Intern Med. 2019;170:81–9. doi: 10.7326/M17-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts N, James S, Delaney M, Fitzmaurice C. The global need and availability of blood products: a modelling study. Lancet Haematol. 2019;6:e606–e15. doi: 10.1016/S2352-3026(19)30200-5. [DOI] [PubMed] [Google Scholar]
- 27.Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. 2019;133:1831–9. doi: 10.1182/blood-2018-10-833988. [DOI] [PubMed] [Google Scholar]
- 28.Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57:1347–58. doi: 10.1111/trf.14006. [DOI] [PubMed] [Google Scholar]
- 29.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–35. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]