Abstract
Introduction
Despite ongoing efforts to vaccinate communities against COVID-19, the necessity of face mask use in controlling the pandemic remains subject to debate. Several studies have investigated face masks and COVID-19, covering smaller and less diverse populations than this study's sample. This study examines a hypothesized association of face-covering mandates with COVID-19 mortality decline across 44 countries in 2 continents.
Methods
In a retrospective cohort study, changes in COVID-19‒related daily mortality rate per million population from February 15 to May 31, 2020 were compared between 27 countries with and 17 countries without face mask mandates in nearly 1 billion (911,446,220 total) people. Longitudinal mixed effect modeling was applied and adjusted for over 10 relevant demographic, social, clinical, and time-dependent confounders.
Results
Average COVID-19 mortality per million was 288.54 in countries without face mask policies and 48.40 in countries with face mask policies. In no mask countries, adjusted average daily increase was 0.1553 − 0.0017 X (days since the first case) log deaths per million, compared with 0.0900 − 0.0009 X (days since the first case) log deaths per million in the countries with a mandate. A total of 60 days into the pandemic, countries without face mask mandates had an average daily increase of 0.0533 deaths per million, compared with the average daily increase of 0.0360 deaths per million for countries with face mask mandates.
Conclusions
This study's significant results show that face mask mandates were associated with lower COVID-19 deaths rates than the rates in countries without mandates. These findings support the use of face masks to prevent excess COVID-19 deaths and should be advised during airborne disease epidemics.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) spread continues to impose a significant global public health burden.1 At the beginning of the COVID-19 pandemic, prominent health authorities were reluctant to support face masks as a preventive measure.2 , 3 Laboratory studies have revealed that COVID-19 is an airborne disease.4 , 5 A meta-analysis study of severe acute respiratory syndrome (SARS) coronavirus and Middle East respiratory syndrome coronavirus transmission showed that face masks could reduce infection risk.6, 7, 8, 9 Several studies showed that acute respiratory distress syndrome (ARDS) in influenza,10 , 11 SARS, and Middle East respiratory syndrome12 , 13 were induced by high initial viral load, which increased mortality risk. A study from Pujades et al.14 revealed the association between SARS coronavirus 2 (SARS-CoV-2) viral load and increased mortality.
There has been extensive debate around face mask effectiveness in controlling the COVID-19 pandemic.15, 16, 17, 18, 19, 20 Several studies have suggested near-universal face covering as an effective protective measure.7 , 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 From the U.S., numerous studies revealed that face masks averted COVID-19 disease incidence and mortality in several states.24 , 31, 32, 33, 34, 35, 36 Furthermore, in overpopulated and economically challenged communities where lockdowns and social distancing are not feasible, Tucho and colleagues37 recommended universal face covering to help virus containment.
In this study, an association of face-covering policy with COVID-19 mortality decline is hypothesized, and the impact of face mask policy on COVID-19 mortality rate is compared in 44 countries, comprising a population of almost 1 billion (911,446,220) persons in Europe and Asia. These findings are helpful for evidence-based policymaking in future rapid responses to airborne epidemics.
METHODS
Study Sample
This retrospective cohort study included 44 countries in Asia and Europe. COVID-19 mortality changes per million population (outcome) over time between countries with and those without a face mask policy (exposure) were compared.
Measures
The study period spanned from February 15, 2020, which was the date of the first confirmed COVID-19 death within the targeted countries, to May 31, 2020. This period was objectively selected because all countries, both with and without mask policies, had homogeneously implemented restrictions of movement and gathering. This created an ideal window with all nonpharmaceutical interventions in place so that they were automatically controlled for in this model, making it more parsimonious. Beyond this period, many countries lifted gathering and movement restrictions (Appendix 1, available online), or public compliance declined, hence relevant confounding impact potentially increased.
In all countries, COVID-19 mortality data were obtained from the first confirmed COVID-19 death to May 31, 2020, within each respective country. This provided a range of 54–107 daily observations per country.
To improve comparability of countries and reduce chances of selection bias, the top 50 countries of the UN Development Programme Human Development Index ranking in 2019 were targeted.38 These countries are also ranked as the first 50 countries on the WHO's Health System Performance index.39 Technical definitions are provided in Appendix 2 (available online).
The comparability of selected countries was improved by controlling health system performance confounders of quality of life, education level, and public compliance (social trust) with governments. Among the 50 selected countries, 4 countries in the Southern Hemisphere (New Zealand, Australia, Chile, and Argentina) were excluded to avoid potential seasonality characteristics. Furthermore, the U.S. and Canada were excluded owing to their state/province-based policies and lack of a unified national health policy. Table 1 lists the 44 countries included in this study.
Table 2.
| Non pharmaceutical interventions | Variations |
|---|---|
| Face coverage | Facemask, Face cloth |
| Restriction of movement | Quarantine (voluntary, mandatory) Home stay (voluntary, mandatory) Travel restriction (voluntary, mandatory) |
| Social distancing | Physical distancing Cancellation of public event Schools’ closure Business closure Restriction of gathering above 2 persons Restriction of gathering above 5 persons Restriction of gathering above 50 persons Restriction of gathering above 500 persons Restriction of gathering above 1000 persons |
| Testing and tracking | Early detection Contact tracing |
| Hospital triage | Traffic Control Bundling (eTCB) |
| Case isolation | After disease manifestation Upon arrival from abroad After departing the contaminated location |
| Hand washing | Hand sanitation Personal hygiene Hand hygiene Surface hygiene |
| Public education | Public information Changing public behavior Public awareness raising |
This study did not involve any individually identifiable data, and did not impose any risk of violating the Declaration of Helsinki; therefore, ethics committee approval was not required. Country-level COVID-19 mortality, number of performed COVID-19 tests and cases, and each country's date of first COVID-19 case and death report were retrieved from Our World in Data database.40 Each country's data and timeline on face mask policies and quarantine were obtained from a given country's official online resources (Appendix 1, available online). Demographic and socioeconomic data, including population size, median population age, percentage urban population, percentage population aged >65 years, sex ratio, population density (per km2), health expenditure per capita, net migrants index, intensive care unit beds per 100,000 population, and hospital beds per 1,000 population were collected from the World Bank.41 The Infectious Disease Vulnerability Index42 was used as a proxy for social trust between governments and citizens.
Potential confounders between face mask policy and mortality reduction, such as nonpharmaceutical interventions, were identified. Other studies suggest that face covering is effective in combination with social distancing–related measures.7 These interventions are highlighted in Table 2 ,7 , 21 , 22 , 40 , 43 and relevant dates are detailed in Appendix 1 (available online). Reliable data on hospital triage, contact tracing, and personal hygiene were unavailable for all countries during the study period.
Table 1.
Sociodemographic Parameters Included in the Study
| Parameter | Countries with national mask policya | Countries with no national mask policyb | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | p-value | |
| Total population, n | 557,916,212 | 353,530,008 | |
| Average population per country (2020) | 5,850,343 (8,327,314) | 9,449,321 (29,392,625) | 0.46 |
| Median age, years | 41.9 (6.9) | 42.0 (2.8) | 0.80 |
| Sex (males per 100 female population) | 97.75 (8.34) | 98.46 (3.73) | 0.72 |
| Population aged >65 years, % | 16.25 (5.37) | 19.48 (2.92) | 0.14 |
| Urban population, % | 77.31 (24.06) | 82.25 (9.8) | 0.72 |
| Migrants (net) | 10,000 (38807.5) | 28,000 (39,270) | 0.30 |
| Density (population count/Km2) | 113.13 (215.72) | 122.58 (167.39) | 0.52 |
| Health expenditure, USD | 2,619 (1,080.5) | 4,228 (2,998) | 0.07 |
| ICU beds per 100,000 | 9.1 (3.3) | 9.2 (3.0) | 0.87 |
| Hospital bed per 1,000 | 4.69 (3.59) | 3.32 (1.99) | 0.22 |
| Overall social trust score | 0.80 (0.11) | 0.87 (0.18) | 0.30 |
Austria, Bahrain, Cyprus, Czech Republic, Estonia, Germany, Greece, Hong Kong, Iceland, Ireland, Hungary, Israel, Japan, South Korea, Latvia, Lithuania, Luxembourg, Malta, Oman, Poland, Portugal, Qatar, Russia, Singapore, Slovakia, Slovenia, United Arab Emirates.
Andorra, Belarus, Belgium, Brunei, Croatia, Denmark, Finland, France, Italy, Liechtenstein, Netherlands, Norway, Saudi Arabia, Spain, Sweden, Switzerland, United Kingdom.
USD, U.S. dollar; ICU, intensive care unit.
Quarantine (including all alternatives reflected in Table 2 7 , 21 , 22 , 40 , 43) was operationalized as a binary variable in this model. In longitudinal analysis, at the time of each mortality data point, if quarantine was active, the variable took the value of 1 and 0 otherwise.
The time intervals, by days, between the first COVID-19 confirmed case in each country and all mortality data points were calculated. The time interval variable was used in longitudinal analysis to adjust for the impact of face mask policy duration on COVID-19 spread and mortality and other time-dependent confounders such as increased public awareness.
Statistical Analysis
Data imputation, due to missing observations, was performed using multivariate imputation by chained equation44 for health expenditure per capita, intensive care unit beds per 100,000 population, hospital beds per 1,000 population, and social trust score. Then, bivariate analysis was performed on the variables mentioned earlier using the Wilcoxon rank sums test to evaluate any differences between countries with and without a face mask policy (Table 1). For modeling of mortality rate, a longitudinal mixed effect model was constructed with random intercept and random slope for time interval variable (Days) to assess change of mortality per million population. A random intercept was adjusted for variability within each country, and a random slope was adjusted for variation of mortality rates across different countries. An autoregressive model of order 1 was imposed as a correlation structure to account for data dependency within each country. For variables with collinearity, those with higher scientific plausibility were selected. Natural log transformation on the output variable helped to ensure that observations were on the same scale. The final model carried the following form:
Ln(Mortality Per Million)ij = B0 + (Group)iB1 + (National Face Mask Policy)ij B2 + (Days)ij B3 + (Days2)ij B4 + (Monday)ijB5 + (Population Density)i B6 + (Population Aged >65 Years)i B7 + (Urban Ratio)i B8 + (Sex)i B9 + (Quarantine Policy)ij B10 + (Migrant Index)i B11 + (Health Expenditure)i B12 + (Intensive Care Unit Beds)i B13 + (Hospital Beds)i B14 + (Social Trust)i B15 + [(Group)i × (Days)ij]B16 + [(Group)ij × (Days2)ij]B17 + [(National Face Mask Policy)ij × (Days)ij]B18 + [(National Face Mask Policy)ij × (Days2)ij]B19 + µi + (Days)ij vi + €ij.
In this model, μi and vi are random intercepts and random slopes, respectively. Days (time-interval variable) was defined as the number of days since the first COVID-19 incidence in each country i until observation j. The Days2 variable was included to account for nonlinearity in mortality rate. The Monday variable captures whether the observation is collected on a Monday or otherwise to adjust for any delayed reporting of mortality data over the weekend. The Group variable indicates whether the country of interest belongs to the masked country group or otherwise, and the Mask variable indicates whether at time j country has mask implementation or not. For face mask countries, observations before mask implementation were considered as pre-exposure data points. By contrast, the remaining data points were considered as exposure outcome (in face mask countries) and nonexposure outcome (in no face mask countries). To fit this model, within the statistical software R environment, version 3.6.3, the lme function in the nlme package was employed.45
RESULTS
Table 1 shows the sociodemographic characteristics of the 2 cohorts of countries with and without face mask policies. No significant difference between the 2 cohorts was found concerning their population and healthcare system variables.
From the first confirmed COVID-19 case until May 31, there were 2,167,664 confirmed COVID-19 cases in the 44 study countries. Of these, 1,253,757 cases were in no face mask countries, and 913,907 cases were in face mask countries; this difference was statistically significant (p<0.01).
The average COVID-19 mortality per million population was 288.54 in countries without face mask policies and 48.40 in countries with face mask policies. The earliest first confirmed case was on January 20, 2020 in South Korea, and the latest was on March 9, 2020 in Cyprus.
Among the 44 countries in this study, Russia (February 7, 2020) and South Korea (February 15, 2020) were the first 2 countries to set a face mask policy, whereas Greece (May 4, 2020) and Ireland (May 5, 2020) were the last. In all countries, there was movement restriction, except in Bahrain, Japan, and South Korea in the face mask group and Andorra, Belarus, and Brunei in the no face mask group.
Table 3 shows the longitudinal mixed effect model. Interaction terms National Facemask Policy Days, Group Days, and Group Days2 were all statistically significant, revealing a significant difference in mortality growth rate in countries with a face mask policy compared with that in those without. Consolidating results, a parsimonious longitudinal model (as shown below) was generated to focus on only grouping face mask policy and days while holding all other covariates constant:
Table 3.
The Longitudinal Multivariate Analysis of Mortality Change per Million
| Full model |
mAIC |
|||||
|---|---|---|---|---|---|---|
| Parameter | Coefficient | SE | p-value | Coefficient | SE | p-value |
| Intercept | ‒0.94 | 1.72 | 0.58 | ‒4.28 | 1.42 | <0.01 |
| Group (mask vs no mask) | 1.33 | 0.48 | <0.01a | 0.65 | 0.49 | 0.19 |
| National mask policy (yes vs no) | 0.34 | 0.10 | <0.01 | 0.34 | 0.10 | <0.01 |
| Number of days since the first COVID-19 case | 0.16 | <0.01 | <0.01 | 0.16 | <0.01 | <0.01 |
| Number of days since the first COVID-19 case (squared) | >‒0.01 | <0.01 | <0.01 | >‒0.01 | <0.01 | <0.01 |
| Monday (yes vs no) | >‒0.01 | <0.01 | <0.01 | >‒0.01 | <0.01 | <0.01 |
| Density (population count/Km2) | >‒0.01 | <0.01 | 0.22 | |||
| Population aged >65 years, % | 0.10 | 0.04 | 0.02 | |||
| Urban population, % | >‒0.01 | <0.01 | 0.72 | |||
| Sex (female vs male) | <0.01 | <0.01 | 0.59 | |||
| National movement restriction (yes vs no) | 0.03 | 0.01 | <0.01 | |||
| Net migrants index | >‒0.01 | <0.01 | 0.34 | |||
| Health expenditure per capita | <0.01 | <0.01 | <0.01 | |||
| Number of ICU beds per 100,000 population | 0.02 | 0.03 | 0.57 | |||
| Number of hospital beds per 1,000 population | ‒0.16 | 0.06 | <0.01 | |||
| Social trust score | ‒5.52 | 2.01 | <0.01 | 2.43 | 1.63 | 0.14 |
| Group x days since the first COVID-19 case | ‒0.05 | <0.01 | <0.01 | ‒0.05 | <0.01 | <0.01 |
| Group x days since the first COVID-19 case (squared) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| National mask policy number of days since the first COVID-19 case | ‒0.01 | <0.01 | 0.01 | ‒0.01 | <0.01 | 0.02 |
| National mask policy number of days since the first COVID-19 case (squared) | <0.01 | <0.01 | 0.10 | <0.01 | <0.01 | 0.10 |
| R2 marginal | 0.68 | 0.47 | ||||
| R2 conditional | 0.97 | 0.97 | ||||
Note: Boldface indicates statistical significance (p<0.05). Numbers are displayed up to 2 decimal points. Numbers within the range of 0.01 and ‒0.01 have been displayed accordingly.
mAIC, marginal Akaike Information Criterion; ICU, intensive care unit.
Logarithmic Mortality per Million Population = 1.3328(Group) + 0.3402(Mask) + 0.1561(Days) – 0.0009(Days2) – 0.0543(GroupDays) + 0.0003(GroupDays2) – 0.0113(Mask Days) + 0.0001(Mask Days2).
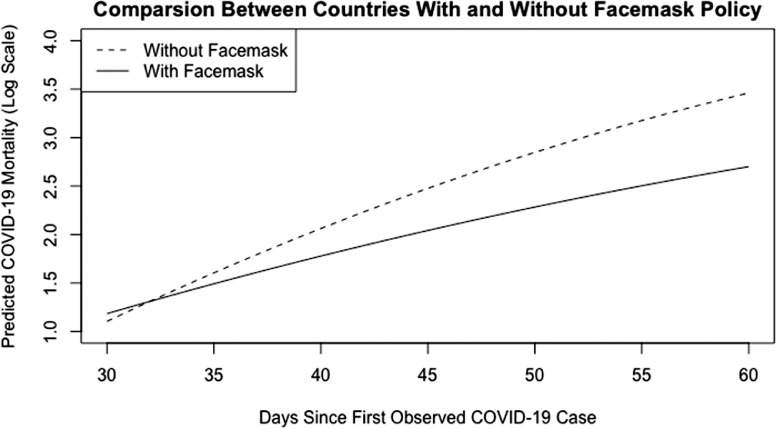
This equation translates into a daily average increase of 0.1553–0.0017 (days since the first case) log deaths per million in no face mask countries compared with a daily average increase of 0.0900–0.0009 (days since the first case) log deaths per million in facemask countries. Figure 1 shows these results, showing a significantly lower increase of daily log mortality in face mask countries. For instance, 60 days into the pandemic, no mask countries had an average daily increase of 0.0533 deaths per million. This increase is significantly larger than the average daily increase of mask countries, which was 0.0360 deaths per million.
Figure 1.
Predicted daily COVID-19 mortality over time in countries with and without facemask policy.
Note: This figure illustrates the daily mortality trend. The rate of increase of daily mortality per million population is significantly lower in countries with a facemask policy compared to the rate of increase in countries without a facemask policy. The illustration shows the gap between countries with and without facemask policy widens across time.
Over time, mortality rate gaps became smaller between countries with and without face mask policies. This may be due to gradually implementing stricter restriction guidelines. It is also expected to see faster declines when the initial mortality rate is much higher.
The full model explained the complete relationship between the predictors and response. Model selection was performed to check the necessity of including all predictors. The Akaike information criterion was used to screen out unnecessary predictors. For the mixed effect model, the marginal Akaike information criterion was used to select fixed-effect predictors.46 During model selection procedures, the variables Monday, Days, Days2, Group, Mask Policy, and the 4 interaction terms appearing in all potential models were fixed because they were the main study variables. On running selection algorithms, no significant difference was found between the reduced and full model; hence, the full model was omitted for brevity. Table 3 shows the reduced model. For both the full and reduced model, marginal and conditional R 2-values are presented to assess goodness of fit.47
To further assess the validity of the model, the authors also investigated fitting the same data using a fixed-effect model. Although such a model may not fully capture the associations among the predictors and response, it is useful to confirm the results. The details are reflected in Appendix 3 (available online).
DISCUSSION
This study supports evidence in favor of face mask mandates as a strategy to decrease COVID-19 mortality. The results reveal a significant association between public face-covering policy and COVID-19 mortality rate reduction in 27 countries with face mask policies. Several studies support these results,7 , 21 , 24 , 32 , 34 , 35 but most examined smaller and less diverse populations than this study, and none performed a longitudinal mortality study. Few measured direct mask policy impact on COVID-19 mortality. In a recent comparison of daily county-level COVID-19 growth rates among U.S. states with and without state face-covering policy, multivariate difference-in-difference analysis revealed a significant daily decline in COVID-19 growth rate of 0.9–2.0 percentage points.34 However, the investigators did not include mortality rates before and after states’ mandates. In a meta-analysis of 44 observational studies across 16 countries with a total sample size of 25,697 patients, mask adoption decreased incidence rate with AOR=0.15 (95% CI=0.09, 0.38)7; however, the focus of this meta-analysis was not on the impact of face masks on mortality. Coclite et al.48 and Leffler and colleagues49 noted a mortality decrease after mask adoption but did not find statistical significance with their modeling.
In selecting this study sample, the authors focused on countries with the highest health systems efficacy ranking to control for potential confounding due to unassured data.39 Although comparability among included countries was improved, these findings may not be generalizable to countries with lower Human Development Index and health systems scores.38 , 39 However, Abdullahi et al.50 and others37 , 51 suggest that universal face covering is beneficial for virus containment in developing countries.
In terms of modeling assumptions, it is necessary to show that data satisfy the parallel trend assumption, which in turn reveals the significance of the effect of the intervention. A dynamic modeling approach52 was used to verify this assumption for this data set. This model is similar to the main model presented in this paper while replacing the Group and Mask Policy variables with time-dependent indicator variables (). The goal is to show these indicator variables are statistically insignificant before the implementation of a mask policy, indicating that no pretrend exists in the data. This investigation verified that the data do indeed confirm the nonexistence of a pretrend. Further explanations are provided in Appendix 4 (available online).
Moreover, to better assess the association of face mask policies and mortality with the presence of various nonpharmaceutical interventions, separate models that include these policies were fitted. Because the results remain consistent, the details are deferred to Appendix 5 (available online).
Focusing on the public compliance with mask mandates, there are variations across different countries. For instance, in April 2020, Eikenberry and colleagues32 estimated that near-universal (80%) adoption of moderately (50%) effective masks could reduce COVID-19 mortality rates in New York and Washington by 17%–45% and 24%–65%, respectively. Rosenstrom et al.35 also suggest a significant decrease in COVID-19 prevalence and mortality if masks are adopted by 70% of the public. A literature search on public face mask compliance, as a COVID-19 preventive measure, yielded data for some target countries but not homogeneous data for all countries. Consequently, these could not be applied in this model, but it can be assumed that even 50% public compliance results in a significant difference as suggested by Rosenstrom and colleagues.35 As a controlling proxy measure, countries with a higher level of public social trust in governmental measures (ranging from 0.6 to 1, median=0.8151) were chosen, and data on social trust were applied in this modeling to reflect the impact of public compliance.42
The literature on public knowledge, attitude, and practice measures as well as compliance with face covering as a COVID-19 preventive measure were sought for targeted countries, and studies on 13 countries were found. Results of a positive response from participants is summarized as Hong Kong (96.6%),53 , 54 Saudi Arabia (90%),55 , 56 Japan (80.9%),57 , 58 Singapore (67.2%),59 Greece (60%),60 South Korea (48.8%),61 Croatia (52.8%),62 and Italy (89%).63 Margraf et al.64 reported that 91.7% of participants in their knowledge, attitude, and practice study from 8 countries (France, Germany, Poland, Russia, Spain, Sweden, United Kingdom, and the U.S.) adhered to governmental measures, and Ganczak and colleagues65 reported 65.7%–73.6% adherence from Poland.
In an English-language search, no comprehensive data source on availability and type of face masks in studied countries was found. Asadi et al.15 reported that both surgical masks and unvented KN95 respirators reduced particle emission by 90% and 74%, respectively, on average compared with wearing no mask, whereas Ngonghala and colleagues24 argued that even adoption of masks with <30% efficacy by 80% of the population can avert COVID-19 burden.
From the bioimmunological standpoint, clinical and laboratory evidence indicates that COVID-19 infection may lead to a cytokine storm, an overwhelming immune response to a pathogen, causing collateral damage (e.g., ARDS).66, 67, 68, 69, 70 In patients with COVID-19, the association between cytokine storm and ARDS has been documented by several studies.66 , 67 , 69 Yang et al.71 showed a relationship among SARS-CoV-2 exposure load, proinflammatory cytokine production, and COVID-19 disease severity. Other studies have revealed a relationship between SARS-CoV-2 viral load and mortality.14 , 72, 73, 74, 75, 76 Not using a face mask results in exposure to higher initial SARS-CoV-2 viral loads, thus increasing the probability of a proinflammatory cytokine reaction by the immune system.71 , 77 This is associated with mortality in cases of severe COVID-19 infection.72 , 73 Furthermore, it is in line with Milton and colleagues’78 findings associating face masks with decreased influenza viral load.
Two studies have argued that initial SARS-CoV-2 viral load does not predict COVID-19 severity and mortality.79 , 80 However, these studies assessed viral load at hospital admission time, several days after initial virus contact, which may be dependent on not only the initial viral load but also on patient–virus interactions and disease etiology.
The primary objective of this article was to assess the lessons learned from this pandemic to better prepare for future potential epidemics of airborne diseases before pharmaceutical interventions are available. Given that at this stage of the pandemic, countries are moving toward vaccinating their populations, there are 2 points to consider with regard to the necessity of face masks in this ongoing SARS-CoV-2 pandemic. Primarily, delays in vaccination continue to challenge health systems across the globe, and this work adds to evidence that before81 and even after82 full vaccination of the population, face masks continue to be a preventive measure against COVID-19. Secondly, across variants, vaccines may reduce mortality but not necessarily morbidity, and face masks continue to protect against both.
Strengths of this study include its longitudinal mixed effect model that was employed to explore COVID-19 mortality rates before and after face mask mandates, compared with that in countries without mandates. Applying a mixed-effect model with random intercept and slope adjusted for random variation across countries concerning these measures. The model impact was exercised on nearly 1 billion people. Detection of the unique ideal window for analysis mentioned earlier may also be seen as a strength of this paper. Finally, the main outcome variable was mortality, which represents a highly robust outcome.
Limitations
Regarding limitations, first, this model is not causal. Second, social and health system–related unmeasured confounders were limited by including only countries with the highest Human Development Index scores because data reliability from other countries was unassured. Third, reliable data on public compliance with face covering for all targeted countries in the study was unavailable. This was managed by applying a conservative 50% compliance suggested by Rosenstrom et al.35 Finally, the literature review was conducted only in the English language.
CONCLUSIONS
The results of this study show a significant association between face mask mandates and reductions in COVID-19 mortality. This evidence supports the positive impact of face mask policies on saving peoples’ lives.
Acknowledgments
ACKNOWLEDGMENTS
This study is dedicated to healthcare workers worldwide who, despite global shortages of personal protective equipment, made personal sacrifices in fighting and reducing the burden of the COVID-19 pandemic.
The study protocol, statistical code, and data sets used and analyzed in this study are available from the corresponding author (bmohit1@alumni.jh.edu) on reasonable request.
No financial disclosures were reported by the authors of this paper.
CRediT AUTHOR STATEMENT
Sahar Motallebi: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Writing - original draft; Writing - review & editing. Rex C.Y. Cheung: Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing - original draft; Writing - review & editing. Babak Mohit: Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing - original draft; Writing - review & editing. Shahram Shahabi: Investigation; Writing - original draft; Writing - review & editing. Amir Alishahi Tabriz: Data curation; Formal analysis; Investigation; Methodology; Writing - original draft. Syamak Moattari: Data curation; Supervision; Writing - review & editing.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2021.09.019.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time [published correction appears in Lancet Infect Dis. 2020;20(9):e215] Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369:m1435. doi: 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- 3.WHO . WHO; Geneva, Switzerland: 2020. Mask use in the context of COVID-19.https://www.who.int/publications-detail/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus- Accessed October 7, 2021. [Google Scholar]
- 4.Pyankov OV, Bodnev SA, Pyankova OG, Agranovski IE. Survival of aerosolized coronavirus in the ambient air. J Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad MDF, Wahab S, Ali Ahmad F, et al. A novel perspective approach to explore pros and cons of face mask in prevention the spread of SARS-CoV-2 and other pathogens. Saudi Pharm J. 2021;29(2):121–133. doi: 10.1016/j.jsps.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8(5):434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone TE, Kunaviktikul W, Omura M, Petrini M. Facemasks and the Covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22(2):339–342. doi: 10.1111/nhs.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memoli MJ, Czajkowski L, Reed S, et al. Validation of the wild-type influenza A human challenge model H1N1pdMIST: an A(H1N1)pdm09 dose-finding investigational new drug study. Clin Infect Dis. 2015;60(5):693–702. doi: 10.1093/cid/ciu924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Wang D, Gao R, et al. Biological features of novel avian influenza A (H7N9) virus. Nature. 2013;499(7459):500–503. doi: 10.1038/nature12379. [DOI] [PubMed] [Google Scholar]
- 12.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mubarak A, Alturaiki W, Hemida MG. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019 doi: 10.1155/2019/6491738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8(9):e70. doi: 10.1016/S2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asadi S, Cappa CD, Barreda S, Wexler AS, Bouvier NM, Ristenpart WD. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities Sci Rep.2020:10(1):15665. https://doi.org/10.1038/s41598-020-72798-7. [DOI] [PMC free article] [PubMed]
- 16.Chou R, Dana T, Jungbauer R, Weeks C, McDonagh MS. Masks for prevention of respiratory virus infections, including SARS-CoV-2, in health care and community settings: a living rapid review. Ann Intern Med. 2020;173(7):542–555. doi: 10.7326/M20-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai AN, Aronoff DM. Masks and coronavirus disease 2019 (COVID-19) JAMA. 2020;323(20):2103. doi: 10.1001/jama.2020.6437. [DOI] [PubMed] [Google Scholar]
- 18.Javid B, Weekes MP, Matheson NJ. COVID-19: should the public wear face masks? BMJ. 2020;369:m1442. doi: 10.1136/bmj.m1442. [DOI] [PubMed] [Google Scholar]
- 19.Mahase E. COVID-19: what is the evidence for cloth masks? BMJ. 2020;369:m1422. doi: 10.1136/bmj.m1422. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y, Bayham J, Richter A, Fenichel EP. Risk compensation and face mask mandates during the COVID-19 pandemic Sci Rep. 2021:11(1):3174. doi:10.1038/s41598-021-82574-w. [DOI] [PMC free article] [PubMed]
- 21.Howard J, Huang A, Li Z, et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci U S A. 2021;118(4) doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo H, Miller GF, Sunshine G, et al. Decline in COVID-19 hospitalization growth rates associated with statewide mask mandates −10 states, March‒October 2020 [published correction appears in MMWR Morb Mortal Wkly Rep. 2021;70(8):293] MMWR Morb Mortal Wkly Rep. 2021;70(6):212–216. doi: 10.15585/mmwr.mm7006e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang M, Gao L, Cheng C, et al. Efficacy of face mask in preventing respiratory virus transmission: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngonghala CN, Iboi E, Eikenberry S, et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel coronavirus. Math Biosci. 2020;325 doi: 10.1016/j.mbs.2020.108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abboah-Offei M, Salifu Y, Adewale B, Bayuo J, Ofosu-Poku R, Opare-Lokko EBA. A rapid review of the use of face mask in preventing the spread of COVID-19. Int J Nurs Stud Adv. 2021;3 doi: 10.1016/j.ijnsa.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoertel N, Blachier M, Blanco C, et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France [published correction appears in Nat Med. 2020;26(11):1801] Nat Med. 2020;26(9):1417–1421. doi: 10.1038/s41591-020-1001-6. [DOI] [PubMed] [Google Scholar]
- 27.Liao M, Liu H, Wang X, et al. A technical review of face mask wearing in preventing respiratory COVID-19 transmission. Curr Opin Colloid Interface Sci. 2021;52 doi: 10.1016/j.cocis.2021.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raina MacIntyre C, Jay Hasanain S. Community universal face mask use during the COVID 19 pandemic-from households to travellers and public spaces. J Travel Med. 2020;27(3) doi: 10.1093/jtm/taaa056. taaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SH, Teoh JYC, Leung CH, et al. COVID-19 and public interest in face mask use. Am J Respir Crit Care Med. 2020;202(3):453–455. doi: 10.1164/rccm.202004-1188LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worby CJ, Chang HH. Face mask use in the general population and optimal resource allocation during the COVID-19 pandemic. Nat Commun. 2020;11(1):4049. doi: 10.1038/s41467-020-17922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chernozhukov V, Kasahara H, Schrimpf P. Causal impact of masks, policies, behavior on early covid-19 pandemic in the U.S. J Econom. 2021;220(1):23–62. doi: 10.1016/j.jeconom.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eikenberry SE, Mancuso M, Iboi E, et al. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Liu B, Liu SH, Ji J, Li Y. Evaluating the impact of New York's executive order on face mask use on COVID-19 cases and mortality: a comparative interrupted times series study. J Gen Intern Med. 2021;36(4):985–989. doi: 10.1007/s11606-020-06476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyu W, Wehby GL. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the U.S. Health Aff (Millwood). 2020;39(8):1419‒1425. https://doi.org/10.1377/hlthaff.2020.00818. [DOI] [PubMed]
- 35.Rosenstrom E, Oruc BE, Hupert N, et al. High-quality masks reduce COVID-19 infections and deaths in the U.S. medRxiv. January 28, 2021 doi: 10.1101/2020.09.27.20199737. Preprint. [DOI] [Google Scholar]
- 36.Shen M, Zu J, Fairley CK, et al. Effects of New York's executive order on face mask use on COVID-19 infections and mortality: a modeling study. J Urban Health. 2021;98(2):197–204. doi: 10.1007/s11524-021-00517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucho GT, Kumsa DM. Universal use of face masks and related challenges during COVID-19 in developing countries. Risk Manag Healthc Policy. 2021;14:511–517. doi: 10.2147/RMHP.S298687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Human development index (HDI). United Nations Development Programme (UNDP). http://hdr.undp.org/en/content/human-development-index-hdi. Updated May 3, 2021. Accessed October 7, 2021.
- 39.Tandon A, Murray CJL, Lauer JA, Evans DB. Measuring overall health system performance for 191 countries. Geneva, Switzerland: WHO. https://www.who.int/healthinfo/paper30.pdf. Accessed July 2, 2020.
- 40.Coronavirus pandemic (COVID-19). Our World in Data. https://ourworldindata.org/coronavirus. Updated September 1, 2021. Accessed September 3, 2021.
- 41.World Bank open data. The World Bank. https://data.worldbank.org/. Updated November 8, 2020. Accessed October 7, 2021.
- 42.Moore M, Gelfeld B, Okunogbe A, Paul C. Identifying future disease hot spots: infectious disease vulnerability index. Santa Monica, CA: RAND Corporation. https://www.rand.org/pubs/research_reports/RR1605.html. Published 2016. Accessed April 15, 2020. [PMC free article] [PubMed]
- 43.Zheng Q, Jones FK, Leavitt SV, et al. HIT-COVID, a global database tracking public health interventions to COVID-19. Sci Data. 2020;7(1):286. doi: 10.1038/s41597-020-00610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: linear and Nonlinear Mixed Effects Models. R package version 3.1-148. Vienna, Austria: The Comprehensive R Archive Network. https://CRAN.R-project.org/package=nlme. Published 2020. Accessed October 7, 2021.
- 46.Donohue MC, Overholser R, Xu R, Vaida F. Conditional Akaike information under generalized linear and proportional hazards mixed models. Biometrika. 2011;98(3):685–700. doi: 10.1093/biomet/asr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson PC. Extension of Nakagawa & Schielzeth's R2GLMM to random slopes models. Methods Ecol Evol. 2014;5(9):944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coclite D, Napoletano A, Gianola S, et al. Face mask use in the community for reducing the spread of COVID-19: a systematic review. Front Med (Lausanne) 2021;7 doi: 10.3389/fmed.2020.594269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leffler CT, Ing E, Lykins JD, Hogan MC, McKeown CA, Grzybowski A. Association of country-wide coronavirus mortality with demographics, testing, lockdowns, and public wearing of masks. Am J Trop Med Hyg. 2020;103(6):2400–2411. doi: 10.1101/2020.05.22.20109231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdullahi L, Onyango JJ, Mukiira C, et al. Community interventions in low-and middle-income countries to inform COVID-19 control implementation decisions in Kenya: a rapid systematic review. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0242403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siewe Fodjo JN, Pengpid S, Villela EFM, et al. Mass masking as a way to contain COVID-19 and exit lockdown in low- and middle-income countries. J Infect. 2020;81(3):e1–e5. doi: 10.1016/j.jinf.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borusyak K, Jaravel X. Revisiting event study designs. SSRNJ. May 8, 2017 doi: 10.2139/ssrn.2826228. Preprint. Online. [DOI] [Google Scholar]
- 53.Cheng VC, Wong SC, Chuang VW, et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020;81(1):107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong CL, Chen J, Chow KM, et al. Knowledge, attitudes and practices towards COVID-19 amongst ethnic minorities in Hong Kong. Int J Environ Res Public Health. 2020;17(21):7878. doi: 10.3390/ijerph17217878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Hanawi MK, Angawi K, Alshareef N, et al. Knowledge, attitude and practice toward COVID-19 among the public in the Kingdom of Saudi Arabia: a cross-sectional study. Front Public Health. 2020;8:217. https://doi.org/10.3389/fpubh.2020.00217. [DOI] [PMC free article] [PubMed]
- 56.Bazaid AS, Aldarhami A, Binsaleh NK, Sherwani S, Althomali OW. Knowledge and practice of personal protective measures during the COVID-19 pandemic: a cross-sectional study in Saudi Arabia. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatabu A, Mao X, Zhou Y, et al. Knowledge, attitudes, and practices toward COVID-19 among university students in Japan and associated factors: an online cross-sectional survey. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machida M, Nakamura I, Saito R, et al. Incorrect use of face masks during the current COVID-19 pandemic among the general public in Japan. Int J Environ Res Public Health. 2020;17(18):6484. doi: 10.3390/ijerph17186484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheok GJW, Gatot C, Sim CHS, et al. Appropriate attitude promotes mask wearing in spite of a significant experience of varying discomfort. Infect Dis Health. 2021;26(2):145–151. doi: 10.1016/j.idh.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mouchtouri VA, Agathagelidou E, Kofonikolas K, et al. Nationwide survey in Greece about knowledge, risk perceptions, and preventive behaviors for COVID-19 during the general lockdown in April 2020. Int J Environ Res Public Health. 2020;17(23):8854. doi: 10.3390/ijerph17238854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee M, Kang BA, You M. Knowledge, attitudes, and practices (KAP) toward COVID-19: a cross-sectional study in South Korea. BMC Public Health. 2021;21(1):295. doi: 10.1186/s12889-021-10285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marendić M, Bokan I, Buljan I, Dominiković P, Suton R, Kolčić I. Adherence to epidemiological measures and related knowledge and attitudes during the coronavirus disease 2019 epidemic in Croatia: a cross-sectional study. Croat Med J. 2020;61(6):508–517. doi: 10.3325/cmj.2020.61.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallè F, Sabella EA, Da Molin G, et al. Understanding knowledge and behaviors related to CoViD-19 epidemic in Italian undergraduate students: the EPICO Study. Int J Environ Res Public Health. 2020;17(10):3481. doi: 10.3390/ijerph17103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Margraf J, Brailovskaia J, Schneider S. Behavioral measures to fight COVID-19: an 8-country study of perceived usefulness, adherence and their predictors. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganczak M, Pasek O, Duda-Duma Ł, Świstara D, Korzeń M. Use of masks in public places in Poland during SARS-Cov-2 epidemic: a covert observational study. BMC Public Health. 2021;21(1):393. doi: 10.1186/s12889-021-10418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 68.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 69.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. 2020;20(5):277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y, Wong G, Yang L, et al. Comparison between human infections caused by highly and low pathogenic H7N9 avian influenza viruses in wave five: clinical and virological findings. J Infect. 2019;78(3):241–248. doi: 10.1016/j.jinf.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, Shen C, Li J, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller EH, Zucker J, Castor D, et al. Pretest symptom duration and cycle threshold values for severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction predict coronavirus disease 2019 mortality. Open Forum Infect Dis. 2021;8(2) doi: 10.1093/ofid/ofab003. ofab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hue S, Beldi-Ferchiou A, Bendib I, et al. Uncontrolled innate and impaired adaptive immune responses in patients with COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(11):1509–1519. doi: 10.1164/rccm.202005-1885OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han A, Czajkowski LM, Donaldson A, et al. A dose-finding study of a wild-type influenza A(H3N2) virus in a healthy volunteer human challenge model. Clin Infect Dis. 2019;69(12):2082–2090. doi: 10.1093/cid/ciz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El Saleeby CM, Devincenzo JP. Respiratory syncytial virus load and disease severity in the community. J Med Virol. 2011;83(5):904–905. doi: 10.1002/jmv.22039. [DOI] [PubMed] [Google Scholar]
- 76.El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis. 2011;204(7):996–1002. doi: 10.1093/infdis/jir494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9(3) doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carrasquer A, Peiró ÓM, Sanchez-Gimenez R, et al. Lack of association of initial viral load in SARS-CoV-2 patients with in-hospital mortality. Am J Trop Med Hyg. 2020;104(2):540–545. doi: 10.4269/ajtmh.20-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Borgne P, Solis M, Severac F, et al. SARS-CoV-2 viral load in nasopharyngeal swabs in the emergency department does not predict COVID-19 severity and mortality. Acad Emerg Med. 2021;28(3):306–313. doi: 10.1111/acem.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Your guide to masks. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/about-face-coverings.html. Updated March 31, 2021. Accessed October 7, 2021.
- 82.When you've been fully vaccinated. How to protect yourself and others. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html. Updated October 15, 2021. Accessed October 15, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.