Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been reported to trigger several autoimmune diseases. There are also recent reports of autoimmune diseases that develop after SARS-CoV-2 vaccines. Autoimmune hepatitis is a polygenic multifactorial disease, which is diagnosed using a scoring system.
A 61-year-old woman presented with malaise, fatigue, loss of appetite, nausea and yellow eyes. She had a Pfizer/BioNTech BNT162b2 mRNA vaccine a month ago. Her physical examination revealed jaundice all over the body, especially in the sclera. The laboratory tests showed elevated liver enzymes and bilirubin levels. Antinuclear antibody and anti-smooth muscle antibody were positive and immunoglobulin G was markedly elevated. The liver biopsy revealed histopathological findings consistent with autoimmune hepatitis (AIH). The patient was diagnosed with AIH and initiated on steroid therapy. She rapidly responded to steroid therapy.
A few cases of AIH have been reported after the COVID-19 vaccine so far. Although the exact cause of autoimmune reactions is unknown, an abnormal immune response and bystander activation induced by molecular mimicry is considered a potential mechanism, especially in susceptible individuals. As intensive vaccination against SARS-CoV-2 continues, we would like to emphasize that clinicians should be cautious and consider AIH in patients presenting with similar signs and symptoms.
Keywords: COVID-19, SARS-CoV-2 mRNA vaccine, Autoimmune hepatitis
1. Introduction and objective
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in late 2019 and has rapidly spread all over the world, has infected many people and caused millions of deaths worldwide. During this period, our knowledge of SARS-CoV-2 infection has increased day by day and the disease has been found to trigger many autoimmune diseases [1]. Although the exact mechanism by which autoimmune pathologies develop is unknown, one possible mechanism is believed to be the cross-reaction of the immune response developing after SARS-CoV-2 infection with human proteins resembling virus peptide sequences [2].
Vaccine studies were in full swing during this period and vaccines were produced in a short period of time that can be considered a record and granted an emergency use authorization [3]. The safety of vaccines is still a matter of debate, and vaccine-related complications are usually local, with rare reported cases of anaphylaxis [4].
Autoimmune hepatitis (AIH) is a multifactorial polygenic disorder that requires the interaction of hereditary, epigenetic, immunological, and environmental factors. Specific environmental factors, such as viral infections, may act as environmental triggers for loss of self-tolerance to autoantigens in individuals genetically susceptible to AIH [5,6].
We aimed to present our case of AIH developed following the Pfizer/BioNTech BNT162b2 mRNA vaccine in the light of the literature.
2. Case report
A 61-year-old woman with no previous complaints presented to our clinic with malaise, fatigue, anorexia, nausea, and yellow eyes persisting for 2 weeks. She had a history of Hashimoto's thyroiditis and hypertension and was on valsartan 160 mg/day and levothyroxine 100 mg/day for 10 years. Eight months ago, she had COVID-19 with mild symptoms. She had no known history of liver disease and did not use any hepatotoxic drug/agent, herbal remedies or alcohol. Her medical history revealed that she had a Pfizer/BioNTech BNT162b2 mRNA vaccine about a month ago. The patient's previous examinations in the national e-pulse system showed normal hepatic laboratory values.
On physical examination, she had jaundice throughout the body, especially in the sclera. The abdominal examination showed no defense or tenderness, with non-palpable liver and spleen. Other physical examination findings were normal.
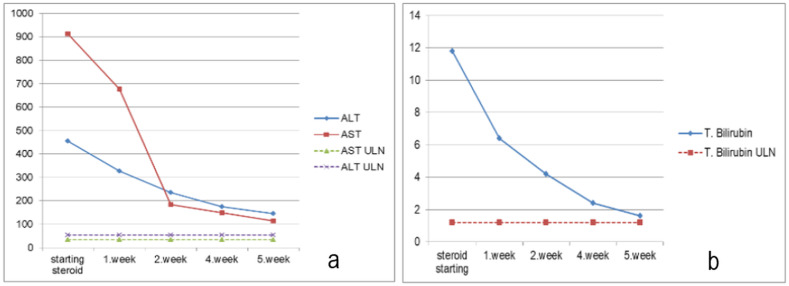
In laboratory tests, the complete blood count results were as follows: leukocyte, 8530/mm³; hemoglobin, 13.3 g/dl; platelet, 199,000/mm³. The biochemistry results were as follows: alanine aminotransferase (ALT), 455 IU/ml (upper limit of normal (ULN) 55 IU/ml); aspartate aminotransferase (AST), 913 IU/ml (ULN 34 IU/ml); gamma glutamyl transferase (GGT), 292 IU/ml (ULN 36 IU/ml); alkaline phosphatase (ALP), 436 IU/ml (normal range: 40–150 IU/ml); total bilirubin, 11.8 mg/dl (ULN 1,2 mg/dl); direct bilirubin, 9.18 mg/dl (ULN 0,5 mg/dl). The microbiology laboratory results were negative for hepatitis A, B, C, Ebstein-Barr virus and cytomegalovirus serology. Ceruloplasmin and serum copper levels were within the normal range. The result of the autoantibody study with immunofluorescence assay was positive for antinuclear antibody (ANA) 1/100 (<1/100 negative) and anti-smooth muscle antibody (ASMA) 1/100 (<1/100 negative). On microscopic examination, ANA was weakly granular and ASMA was positive. The immunoglobulin G was 4260 mg/dl (normal range: 552–1631 mg/dl).
The patient's ultrasound examination showed a normal liver size, with a homogeneous parenchymal echo pattern. The gallbladder was filled with many millimetric stones. The intrahepatic biliary tract and common bile duct were normal. The longitudinal axis of the spleen was 130 mm. No thrombus was present in the splenic vein and portal vein. The magnetic resonance cholangiopancreatography (MRCPI) showed that the bile ducts were of normal diameter, with no stone or sludge. The abdominal computed tomography (CT) showed no space-occupying formation.
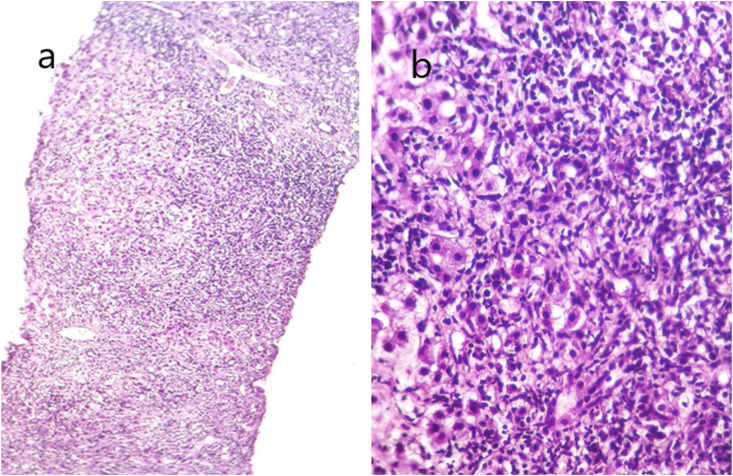
A percutaneous liver biopsy was performed, considering that the patient might have autoimmune hepatitis (AIH). The histopathological examination showed narrow sinusoids and lymphocyte infiltration, severe portal and periportal lymphocyte infiltration, periseptal interface hepatitis, 4–5 spotty necrosis in each high magnification field of view, and limiting plate disorder, but no confluent necrosis. There was mild fibrosis with Masson's trichrome staining. The patient's Ishak histological activity index was 10/18 and fibrosis score was stage 2/6 (Fig. 1 ).
Fig. 1.
Transaminases (a) and bilirubin (b) after steroid therapy.
The simplified autoimmune hepatitis score was found to be 7. The patient was diagnosed with AIH and initiated on oral prednisolone 40 mg. After 14 days, azathioprine (AZA) was added to the treatment and the steroid dose was incrementally reduced. The dose of prednisolone was reduced by 5 mg weekly, while the dose of AZA was increased to 100 mg. On the 35th day of the treatment, the patient did not have any complaints, with resolved icterus. Her laboratory results were insignificant other than mildly elevated transaminase and bilirubin levels (Fig. 2 ). She is currently receiving prednisolone 20 mg and AZA 100 mg.
Fig. 2.
Histological findings of the case who developed autoimmune hepatitis after SARS-CoV-2 vaccine. a) Medium magnification image(x40, hematoxylin-eosin) showing limiting plate disruption, severe periportal inflammation and fibrous tissue increase. b) High magnification(x100, hematoxylin-eosin) showing spotify necroses, intense inflammation, severe deteriotion of the limiting plate.
3. Discussion
In this report, we presented a case of AIH developed following the BioNTech-Pfizer mRNA vaccine and resolved by steroid treatment. Although virus-induced AIH has been well described in the literature, post-vaccination AIH is extremely rare [7]. Until now, few cases of AIH has been reported after COVID-19 vaccination in the literature [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17]], (Table 1 ).
Table 1.
Characteristics of patients with autoimmune hepatitis after SARS-CoV-2 vaccine.
| Study | Vaccine | Gender | Age | Autoimmune disease history | Antibodies | IgG | Biopsy | Steroid response |
|---|---|---|---|---|---|---|---|---|
| Bril et al | Pfizer-BioNTech | Female | 35 | None | ANA Anti-dsDNA |
Normal | Compatible | Yes |
| Londono et al | Moderna | Female | 41 | None | ANA SMA SLA LC-1 |
High | Compatible | Yes |
| Clayton-Chubb et al. | Oxford-AstraZeneca | Male | 36 | None | ANA | Normal | Compatible | Yes |
| Tan et al. | Moderna | Female | 56 | None | ANA SMA |
High | Compatible | Yes |
| McShane et al. | Moderna | Female | 71 | None | SMA | High | Compatible | Yes |
| Lodato et al. | Pfizer-BioNTech | Female | 43 | None | negative | Normal | Compatible | Yes |
| Rocco et al | Pfizer-BioNTech | Female | 80 | Hashimoto disease | ANA | High | Compatible | Yes |
| Rela et al (two cases) | Covishield | Female | 38 | None | ANA | High | Compatible | Yes |
| Male | 65 | None | – | – | Compatible | Yes | ||
|
Vuille-Lessard E et al Ghielmetti M et al |
Moderna Moderna |
Female Male |
76 63 |
Hashimoto disease None |
ANA ASMA anti-aktin ANCA ANA |
High High |
Compatible Compatible |
Yes Yes |
IgG, ımmunglobulin G; ANA, anti nuklear antikor; SMA, smooth muscle antibodies; dsDNA, double stranded DNA antibodies; LC1, liver sitozol antibody; anti-SLA, soluble liver antijen antibodies; ANCA, anti neutrophil cytoplasmic antibodies.
Although the exact mechanism of post-vaccination autoimmune diseases is unknown, possible mechanisms have been reported as follows: (1) An abnormal immune response induced by molecular mimicry, especially in susceptible individuals [18]; (2) Bystander activation through which microbial agents release sequestered self-antigens from host tissue that activate antigen-presenting cells and dormant autoreactive T-helper cells [19].
Autoimmune hepatitis is an immune-mediated chronic inflammatory disease with no specific diagnostic markers. The diagnosis is based on clinical and laboratory findings, histological abnormalities, increased serum IgG value, and the presence of one or more autoantibodies [20]. A scoring system is used for diagnosis, and the simplified scoring system provides the most accurate results for typical cases [21]. In our case, we used the simplified scoring system based on typical findings.
The first case of AIH after SARS-CoV-2 vaccination in the literature was presented by Bril F et al. [8]. The patient was a 35-year-old woman who had given birth 3 months ago. She presented with jaundice shortly after the mRNA vaccine. Unlike our case, she had no known autoimmune disease, and autoantibodies other than ANA were negative and IgG was normal. Afterward, the number of cases shared in the literature has increased (Table 1). Of note, most of the patients were female. The case presented by Lessard et al. was quite similar to our case. A 76-year-old patient developed AIH after the mRNA vaccine [15]. The patient had a history of Hashimoto's disease, as in our case. Autoantibodies were positive and IgG level was high. The patient had typical findings on biopsy and responded well to steroids.
Although it is not certain whether AIH was triggered by SARS-CoV-2 vaccine in our case, the absence of a history of hepatotoxic drug/agent use for liver disease, negative viral serology, a history of Hashimoto's disease, biochemical/histopathological findings consistent with AIH, simplified AIH score, and good response to immunosuppressive treatment suggest that this relationship is not a coincidence. However, in our case, a fibrosis score of 2 raises the question of underlying silent autoimmune hepatitis. The patient's past records from the national registry system showed no pathological findings. In the literature, 25–34% of autoimmune hepatitis patients are asymptomatic, with spontaneous laboratory improvement in approximately 12% of symptomatic patients [22,23]. Therefore, we could not rule out the presence of an underlying silent autoimmune hepatitis in our case.
In conclusion, while intensive vaccination against SARS-CoV-2 continues, the diagnosis of AIH secondary to mRNA vaccines should be included in the differential diagnosis in cases of acute hepatitis of unexplained etiology.
Data availability
Data will be made available on request.
References
- 1.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oldstone M.B. Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon. Antibodies Immunodiagn. Immunother. 2014;33(3):158–165. doi: 10.1089/mab.2013.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.E Oliver S., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The advisory committeeon immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine: United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allergic reactions including anaphylaxis After receipt of the first dose of pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christen U., Hintermann E. Pathogen infection as a possible cause for autoimmune hepatitis. Int. Rev. Immunol. 2014;33:296–313. doi: 10.3109/08830185.2014.921162. [DOI] [PubMed] [Google Scholar]
- 6.Floreani A., Leung P.S., Gershwin M.E. Environmental basis of autoimmunity. Clin. Rev. Allergy Immunol. 2016;50:287–300. doi: 10.1007/s12016-015-8493-8. [DOI] [PubMed] [Google Scholar]
- 7.Perumalswami P., Peng L., Odin J.A. Vaccination as a triggering event for autoimmune hepatitis. Semin. Liver Dis. 2009;29:331–334. doi: 10.1055/s-0029-1233537. [DOI] [PubMed] [Google Scholar]
- 8.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan C.K., Wong Y.J., Wan L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcshane C., Kiat C., Rigby J., Crosbie O. The mRNA Covid-19 vaccine-A rare trigger of autoimmune hepatitis? J. Hepatol. 2021 doi: 10.1016/j.hep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Londono M.C., Gratacos-Gines J., Saez-Penataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination.Still casualty? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton-Chubb D., Schneider D., Freeman E., Kemp W., Roberts S.K. Comment to the letter of Bril F et al. “Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty?”. J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.06.014. [DOI] [Google Scholar]
- 13.Lodato F., Larocca A., D'Errico A., Cennamo V. An anusual case of acute cholestatic hepatitis after m-RNABNT162b2 (comirnaty) SARS-COV-2 vaccine: coincidence,autoimmunity or drug related liver injury? J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J. Hepatol. 2021:728–729. doi: 10.1016/j.jhep.2021.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lessard E.V., Montani M., Bosch J., Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rela M., Jothimani D., Vij M., Rajakumar A., Rammohan A. Auto-immune hepatitis following COVID vaccination. J. Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 17.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., Beretta-Piccoli T.B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination:A novel clinical entitiy? J. Autoimmun. 2021 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perumalswami P., Peng L., Odin J.A. Vaccination as a triggering event for autoimmune hepatitis. Semin. Liver Dis. 2009;29:331–334. doi: 10.1055/s-0029-1233537. [DOI] [PubMed] [Google Scholar]
- 19.Vadala M., Poddighe D., Laurino C., Palmieri B. Vaccination and autoimmune diseases:is prevention of adverse health effects on the horizon? EPMA J. 2017;8:295–311. doi: 10.1007/s13167-017-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manns M.P., Czaja A.J., Gorham J.D., Krawitt E.L., Mieli-Vergani G., Vergani D., Vierling J.M. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 21.Czaja A.J. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatoloy. 2008;48:1540–1548. doi: 10.1002/hep.22513. [DOI] [PubMed] [Google Scholar]
- 22.Kogan J J., Safadi R., Ashur Y., Shouval D., Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J. Clin. Gastroenterol. 2002;35:75–81. doi: 10.1097/00004836-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Czaja A.J. Features and consequences of untreated type 1 autoimmune hepatitis. Liver Int. 2009;29:816–823. doi: 10.1111/j.1478-3231.2008.01904.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.