Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged at the end of 2019 and caused the pandemic of coronavirus disease 2019 (COVID-19). Basic and clinical investigations indicate that severe forms of COVID-19 are due in part to dysregulated immune responses to virus infection. The innate immune system is the first line of host defense against most virus infections, with pathogen recognition receptors detecting SARS-CoV-2 RNA and protein components and initiating pro-inflammatory and antiviral responses. Notwithstanding this response, SARS-CoV-2 proteins evade, inhibit, and skew innate immune signaling early in infection. In this review, we highlight the components of cell-based recognition of SARS-CoV-2 infection and the mechanisms employed by the virus to modulate these innate immune host defense pathways.
Current Opinion in Virology 2022, 52:30–38
This review comes from a themed issue on Viral pathogenesis
Edited by Michaela Gack and Susan Baker
For complete overview about the section, refer Viral Pathogenesis (2022)
Available online 11th November 2021
https://doi.org/10.1016/j.coviro.2021.11.002
1879-6257/© 2021 The Author(s). Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The SARS-CoV-2 pandemic has caused hundreds of millions of infections and resulted in over 5 million deaths as of November 2021 [1]. Multiple vaccines have been developed, approved, and deployed, yet vaccine hesitancy, inadequate global distribution, and emergence of resistant and more transmissible SARS-CoV-2 variants have sustained its spread [2]. SARS-CoV-2 causes a range of clinical syndromes in humans including asymptomatic infection, mild to moderate upper respiratory infection, pneumonia, acute respiratory distress syndrome, hyper-inflammatory disease, and long-term neurological/cognitive dysfunction [3]. The risk of developing severe and fatal COVID-19 depends on age, comorbidities, and genetic or acquired factors [4]. While most individuals infected with SARS-CoV-2 recover, many develop chronic debilitating health conditions [5].
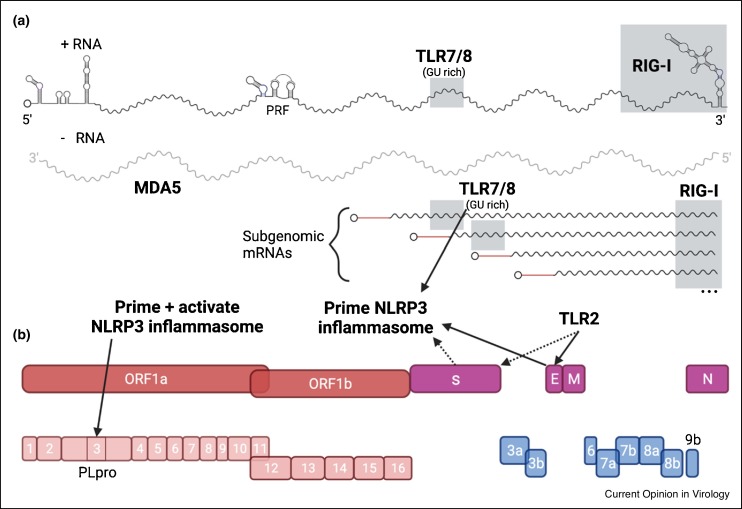
SARS-CoV-2 is a positive-sense single-stranded (ss)RNA Betacoronavirus in the Coronaviridae family. The ∼30 kb capped and methylated RNA genome encodes 4 major structural proteins and 16 nonstructural proteins (Nsps) that mediate virus replication, infection, and pathogenesis (Figure 1 ). Additional accessory proteins encoded at the 3ʹ end of the genome have structural or immune evasion roles and vary among individual coronaviruses (CoVs). SARS-CoV-2 uses its spike (S) protein to infect cells [6]. The primary receptor for SARS-CoV-2 on human cells is ACE2, although other receptors exist [7, 8, 9, 10, 11]. Proteolytic cleavage of S by the serine protease TMPRSS2 on the cell surface or cathepsins in the endosome triggers conformational changes in S and fusion with the host plasma or endosomal membrane [8,12, 13, 14]. Replication of the viral genome occurs in endoplasmic reticulum (ER)-associated replication complexes and generates full-length genomic RNA and nested subgenomic RNAs from which structural and accessory proteins are translated. Virion assembly and budding occurs along the ER-to-Golgi network, and new virions are released by exocytosis [6].
Figure 1.
SARS-CoV-2 replication components and PRR triggers.
(a) SARS-CoV-2 single-stranded RNA genome is capped and methylated. RNA structures of the 5′-UTR, 3′-UTR, and programmed ribosome frameshift (PRF) element are based off predicted structures in Ref. [83]. SARS-CoV-2 replication generates negative sense RNA intermediates and multiple subgenomic mRNAs. (b) SARS-CoV-2 nonstructural proteins (red) are translated as polyproteins and processed to indivual components. Structural proteins (purple) and accessory ORFs (blue) are translated from subgenomic mRNAs. Components that induce PRR pathways are labeled with PRR or indicated with solid arrows. Dashed arrows represent PRR induction after prior SARS-CoV-2 infection. Shaded regions are approximate. Created with BioRender.com.
Many steps of the CoV replication cycle co-opt host factors, reorganize subcellular structures, and generate pathogen associated molecular patterns (PAMPs). The cell-intrinsic host defense system uses multiple strategies to detect and restrict propagation of pathogens including SARS-CoV-2. Here, we review how host cellular sensors detect and induce an antiviral immune response to SARS-CoV-2 infection and describe some of the purported viral evasion strategies used to counteract these defenses. While substantial progress has been made eludcidating this host–pathogen interface, further research is needed to define immunomodulatory drug targets and their mechanisms of action, including ones currently being tested in humans [15, 16, 17, 18, 19].
Recognition by RIG-I-like-receptors
RIG-I like receptors (RLRs) are cytosolic pathogen recognition receptors (PRRs) that detect non-self RNA PAMPs. After RNA PAMP recognition, RIG-I and MDA5, the two most prominent RLR members, associate at the mitochondrial membrane with the shared adaptor protein MAVS and trigger a TBK1-IRF3 signaling cascade that induces a type I and III interferon (IFN) response [20,21]. Which RLR primarily responds depends on the particular RNA virus and cell type [20,22]. Whereas CoVs can be detected by both RIG-I and MDA5, certain CoVs have evolved strategies to prevent RLR detection or downstream signaling pathways [22,23]. SARS-CoV-2 can be recognized by both RIG-I and MDA5, although the primary RLR sensor is cell type-dependent and influenced by post-transcriptional RNA modifications [7,24,25•,26, 27, 28, 29, 30].
Although RIG-I recognizes the 5′ end of non-capped RNAs, other RNA PAMP motifs such as polyU sequences or short hairpin structures contribute to RIG-I binding [20]. Indeed, RIG-I detects the 3′ end of positive sense SARS-CoV-2 RNA (Figure 1), as was observed with other unrelated RNA viruses [25•,26,29,31]. Recognition of the SARS-CoV-2 3′ end may be inhibited by N-6-methyladenosine (m6A) post-transcriptional modification [24]. The m6A modification reduces binding of RIG-I to synthetic RNAs, and viral RNA mutated to be m6A deficient engage RIG-I more efficiently [32,33], induce a more robust IFN-β response, and are less pathogenic in vivo [33]. The SARS-CoV-2 genome has multiple m6A residues within the N gene, and viral RNAs lacking these modified nucleotides immunoprecipitate with RIG-I more efficiently and induce greater expression of inflammatory genes in Caco-2 cells [24]. Further investigation using replication competent genomic SARS-CoV-2 RNA with mutated m6A sites is needed to corroborate these findings.
An MDA5-mediated antiviral IFN response against SARS-CoV-2 requires viral replication [7,25•,26]. The SARS-CoV-2 negative strand RNA is likely the primary target of MDA5 recognition, as the negative strand RNA is more abundant than positive strand RNA in RNA immunoprecipitation experiments with MDA5 (Figure 1) [25•]. Furthermore, SARS-CoV-2 infection fails to induce IFNs and IFN-stimulated genes (ISG) in the presence of the RNA-dependent RNA polymerase inhibitor Remdesivir [26]. The negative stand RNA may form long hairpin structures providing the double stranded RNA motif typically recognized by MDA5. However, a specific motif or region of the SARS-CoV-2 viral RNA recognized by MDA5 has not yet been defined.
Recognition by Toll-like-receptors
Toll-like receptors (TLRs) are a family of integral membrane PRRs. The receptor domains of TLRs are located on the cell surface or in intracellular vesicles (e.g. endosomes) with the signaling domain on the cytosolic face of the membrane [34]. While there are twelve TLR family members, only some contribute to virus recognition: TLR3, TLR7, TLR8, and TLR9 recognize viral nucleic acids, and TLR2 and TLR4 detect viral glycoproteins [34]. Upon PAMP recognition, TLRs dimerize and recruit the adaptor proteins MyD88 or TRIF, which triggers a signaling cascade that activates MAP kinases and NF-κB to induce transcription of proinflammatory genes. TLR signaling can also promote nuclear translocation of the transcription factors IRF-3 and IRF-7, which directly stimulate type I IFN and ISG expression [34].
In patients diagnosed with severe COVID-19, transcripts levels for TLR1, TLR2, TLR4, TLR5, TLR8 and TLR9, along with the adaptor protein transcripts MYD88 and TRIF are elevated compared to healthy patients [35,36]. A separate study reported lower levels of TLR3 expression in the peripheral blood of COVID-19 patients [37], and mutations in the TLR3 gene were associated with severe disease [38]. Although TLR7 was elevated in patients with moderate but not severe COVID-19, other clinical reports suggest that individuals with TLR7 genetic mutations, particularly males since TLR7 is encoded on the X-chromosome, are at increased risk of severe COVID-19 [39, 40, 41, 42].
TLR2 and TLR7/8 have been evaluated for their roles in the host response to SARS-CoV-2 infection [36,43,44]. SARS-CoV-2 E protein induces TLR2 signaling and induction of pro-inflammatory cytokines (Figure 1) [36]. IL-6 levels, among other cytokines, are reduced in Tlr2−/− mice compared to wild-type (WT) mice after exogenous administration of the SARS-CoV-2 E protein. Furthermore, therapeutic administration of a TLR2 inhibitor reduced cytokine production and partially protected against SARS-CoV-2 induced disease in mice [36]. There is one report of SARS-CoV-2 S protein inducing TLR2 signaling [45]. Macrophages derived from patients previously infected with SARS-CoV-2 produce IL-1β when stimulated with SARS-CoV-2 S protein. TLR2 likely mediates this response, because IL-1β production is reduced when macrophages are pretreated with TLR2 inhibitors or blocking antibodies before S protein stimulation [45].
TLR7 and 8 bind guanosine/uridine (GU) rich ssRNA [34]. Analysis of the SARS-CoV-2 genome highlights regions that could be recognized by TLR7 and 8, including motifs in the spike protein gene [44,46]. Synthetically generated SARS-CoV-2-specific GU rich RNAs act as TLR8 ligands in myeloid cells and were more pro-inflammatory than ligands produced from SARS-CoV-1 or HIV-1 RNA40, the latter a TLR8 ligand standard [44,46,47]. Although confirmatory evidence is required in cells and animal models of infection, the TLR8 response exhibited by SARS-CoV-2 synthetic RNAs and the likely abundance of ssRNA produced during SARS-CoV-2 infection could contribute to the pathological inflammation observed in some COVID-19 patients.
Inflammasome activation and recognition by NOD-like-receptors
Activation of the NLRP3 inflammasome requires two signals: (i) upregulation of NLRP3 expression after induction of a TLR/NF-κB pathway; and (ii) a trigger to assemble NLRP3 into the inflammasome complex, which can be a viral PAMP. After assembly of the NLRP3 inflammasome complex, pro-caspase-1 is cleaved into active caspase-1, which in turn cleaves pro-IL-1β and pro-IL-18 to their bioactive and secreted forms. Activation of the NLRP3 inflammasome also can lead to pyroptosis, a pro-inflammatory form of programmed cell death [48].
NLRP3 is upregulated and inflammasome formation is induced by multiple SARS-CoV-2 PAMPs, including GU-rich RNAs, E, and ORF3a proteins (Figure 1) [36,46,49]. In macrophages, synthetic GU-rich RNAs corresponding to SARS-CoV-2 sequences activate the NLRP3 inflammasome resulting in IL-1β production without induction of pyroptosis [46]. Activation of TLR2 by SARS-CoV-2 E protein in macrophages resulted in increased levels of NLRP3 mRNA and cleaved caspase-1 [36]. The viroporin ORF3a of SARS-CoV-2 both primes and activates the NLRP3 inflammasome through K+ efflux [49].
Although SARS-CoV-2 S protein fails to prime or activate the NLRP3 inflammasome in macrophages isolated from healthy mice and humans [37,46], one group reported that it can prime the NLRP3 inflammasome in cells derived from patients with prior SARS-CoV-2 or tuberculosis infection [36,45]. Macrophages isolated from previously infected individuals had distinct transcriptional signatures with epigenetic markers associated with innate immune memory. It is hypothesized that SARS-CoV-2 exposure leaves myeloid cells in a pre-primed state for assembling a functional NLRP3 inflammasome, and this can last several weeks after infection is cleared [45].
Like NLPR3, NOD1 is a member of the NOD-like-receptor (NLR) family but does not oligomerize to form an inflammasome. Instead, NOD1 activation leads to expression of pro-inflammatory cytokines and type I IFNs via NF-κB and IRF-3 or IRF-7 signaling. While usually described in the context of bacterial infection, NOD1 also serves as a pro-inflammatory mediator during viral infections [50]. Indeed, silencing of NOD1 expression in Calu-3 cells reduced the amount of IFN-β mRNA induced following SARS-CoV-2 infection and resulted in greater infection [26]. More studies are needed to define the role and mechanism of action for NLRs in the context of host defense against SARS-CoV-2 infection.
Activation of cGAS-STING signaling
An innate immune response also can be mounted by cytosolic sensors of damage associated molecular patterns (DAMPs). Cytosolic DNA, either from a pathogen, the nucleus, or the mitochondria, serves as a potent DAMPs during microbial infections. The cGAS-STING signaling pathway detects cytosolic DNA and produces type I IFNs [51]. This pathway occurs in two steps: (i) cGAS detects cytosolic DNA and produces the cyclic dinucleotide cGAMP; and (ii) cGAMP binds the mitochondrial associated receptor STING, which initiates proinflammatory and type I IFN gene expression. For RNA virus infections that cause mitochondrial damage, leakage of mitochondrial DNA into the cytoplasm can activate the cGAS-STING pathway [51, 52, 53, 54]. There are no reports that SARS-CoV-2 is directly detected by cGAS-STING, which is not surprising given that SARS-CoV-2 does not produce DNA intermediates during infection [55].
Activation of the STING pathway has been reported after SARS-CoV-2 infection of Calu-3 cells, although exogenously expressed SARS-CoV-2 proteins can inhibit cGAS-STING-induced IFN-β promoter activity [56,57]. Some studies assessing the importance of cGAS-STING signaling during SARS-CoV-2 infection focused on small molecule agonists of the pathway as a potential therapy [56,58,59•]. diABZI, an agonist of STING, had antiviral activity against SARS-CoV-2 both in cells and mice [56,58]. The antiviral activity of diABZI depended on JAK1/2 signaling, although another version of the drug, diABZI-4 promoted IFN-independent antiviral effects [56,59•]. Therapeutic administration of diABZI or diABZI-4 protected against SARS-CoV-2 induced disease in mice. Further investigation into the mechanism of cGAS-STING mediated protection by diABZI compounds appears warranted.
ISGs
A major response to IFN signaling is the production of ISGs [21]. ISGs can modulate the infection of many viruses, although the mechanism of action for many of these proteins remains undefined [53,60]. There are a few specific antiviral ISGs that have been investigated in the context of SARS-CoV-2 replication [61,62,63••].
ISG15 has multiple ascribed antiviral functions including ISGylation, the covalent attachment of ISG15 to a target protein [64]. ISG15 is upregulated rapidly in airway epithelial cells after SARS-CoV-2 infection [63••,65•] and appears required for optimal MDA5 signaling [63••]. The SARS-CoV-2 papain like protease (PLpro) of Nsp3 antagonizes MDA5 signaling by removing ISG15 from the MDA5 caspase activation and recruitment domains (CARD), and inhibiting CARD oligomerization and downstream signaling [63••,65•]. PLpro also cleaves ISG15 from other host proteins resulting in elevated levels of free ISG15 in macrophages [65•], which results in increased expression of pro-inflammatory cytokines including IL-1β and IL-6 and reduced antigen presentation [65•].
LY6E is an ISG that restricts infection of SARS-CoV-2 and other CoVs by blocking entry into cells in contrast to phenotypes with unrelated viruses, where its expression enhanced infection [61,62,66, 67, 68]. Although data on the role LY6E in SARS-CoV-2 pathogenesis is lacking, studies the distantly related murine hepatitis virus showed that mice lacking LY6E expression in immune cells sustained greater infection in the spleen, weight loss, and fatal disease than in WT, congenic animals [61].
Innate immune evasion strategies of SARS-CoV-2
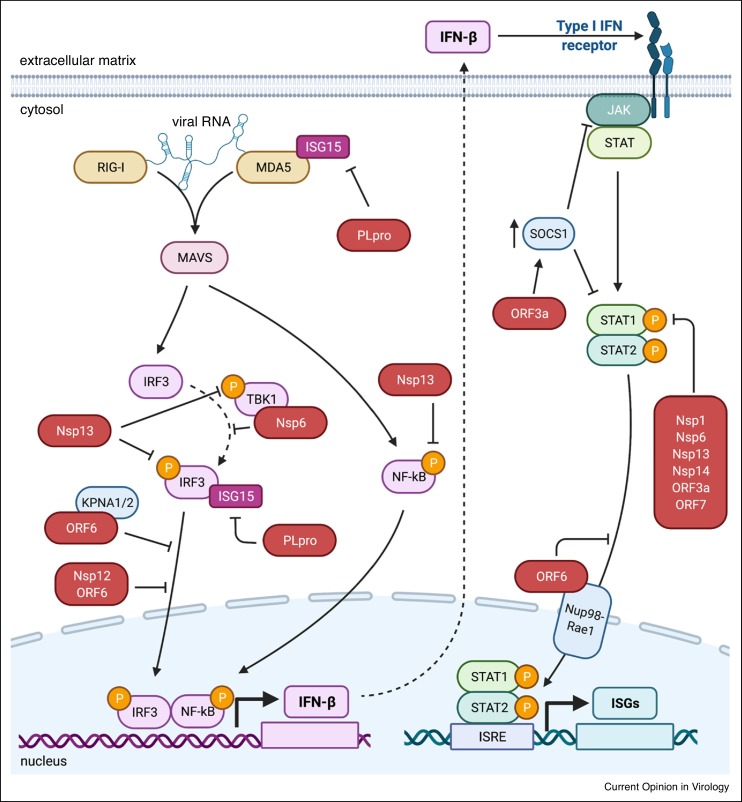
SARS-CoV-2 has multiple strategies to evade the host innate immune system similar to other pathogenic CoVs (Figure 2 ) [23,69]. Limiting the immune response by reducing IFN levels is advantageous for SARS-CoV-2, as it appears more susceptible to type I IFN than other pathogenic CoVs [70]. Multiple SARS-CoV-2 proteins reportedly inhibit type I IFN induction, signaling, or both [71•,72,73].
Figure 2.
SARS-CoV-2 inhibition of IFN induction and signaling.
Key components of IFN induction and signaling are shown with solid arrows denoting signaling pathway. SARS-CoV-2 proteins (red) that are repotyed to inhibit different steps in the pathway. Direct interacting partners of SARS-CoV-2 proteins are shown as overlapping. SARS-CoV-2 proteins that inhibit phosphorylation steps are shown with flat head arrows toward phosphorylation symbols (yellow circles). Proteins that inhibit translocation events are shown with flat arrows towards translocation path. Created with BioRender.com.
Host translation shut-off
The Nsp1 protein is a potent IFN antagonist that blocks IFN induction and signaling [71•,72,73]. This is achieved by interacting with ribosomes to prevent translation of host mRNAs [71•,72,73, 74, 75]. SARS-CoV-2 specific mRNAs evade translation inhibition because of a stem loop structure in the 5ʹ untranslated region (UTR) of all viral transcripts [75]. Nsp14 also may inhibit cellular translation in a manner that is enhanced by Nsp10 [74].
Host protein cleavage
SARS-CoV-2 PLpro is a protease domain of Nsp3 that cleaves both viral and host proteins. PLpro antagonizes MDA5 signaling through de-ISGylation of MDA5 and IRF3 [65•,76]. While PLpro has some de-ubiquitination activity, it appears more specific for ISG15ylated than ubiquitinated substrates [63••,76,77].
Inhibition of phosphorylation
Multiple SARS-CoV-2 proteins reportedly reduce phosphorylation of PRR and IFN signaling pathway intermediates. Nsp1, Nsp6, Nsp13, Nsp14, ORF3a, ORF7a, and ORF7b all have been reported to prevent or reduce phosphorylation of STAT1 and 2 [71•,78]. Nsp6 also directly binds to TBK1 to block phosphorylation of IRF3 [71•]. Nsp13 also inhibits the phosphorylation of IRF3, in addition to TBK1 and NF-κB, although the mechanism remains uncharacterized [73].
Preventing transcription factor translocation
Because many innate immune signaling pathways converge on transcription factors, they are commonly targeted by viral proteins. SARS-CoV-2 proteins Nsp12 and ORF6 prevent the nuclear translocation of IRF3, and Nsp13 prevents translocation of NF-κB [72,73,79,80]. ORF6 also prevents translocation of STAT1 into the nucleus [71•,72,81]. The C-terminal domain of ORF6 is required to prevent translocation of both proteins [71•,72,73], however the direct binding partners to prevent the translocation of STAT1 and IRF3 differ. ORF6 binds the nuclear importin KPNA2 and possibly KPNA1 to block translocation of IRF3, whereas ORF6 interacts with the Nup98-Rae1 nuclear pore complex to disrupt nuclear import of STAT1 [71•,81].
Regulating host protein expression
ORF3a can antagonize IFN signaling by promoting JAK2 ubiquitination and degradation, and upregulating the negative regulator SOCS1 at both the transcript and protein level [82]. Silencing of SOCS1 alleviated the ORF3a inhibition of STAT1 phosphorylation and JAK2 degradation [82].
Despite intensive study on the mechanisms of antagonism and evasion of IFN induction and signaling by SARS-CoV-2 proteins, many questions remain. To date, all of the studies have used ectopic expression systems in cells, and usually a single SARS-CoV-2 protein is expressed at a time. Several of these findings must be corroborated with infectious virus in primary cells. Moreover, exploring synergistic relationships between evasion proteins (e.g. Nsp10 and Nsp14 [74]) may explain how SARS-CoV-2 infection evades host defense responses in specific cell types.
Conclusions
Much has been learned about the interface between cell-intrinsic immunity and and SARS-CoV-2 infection in the past 22 months. The rapidity of discovery has been aided by decades of research on other CoVs before the pandemic. Nonetheless, many questions remain, the answers of which could impact the development of drugs against SARS-CoV-2 including those targeting innate immunity. While detection of SARS-CoV-2 by RLRs, NLRs, TLRs, the inflammasome, and responses by the cGAS-STING pathway all contribute to the initial response to infection, multiple SARS-CoV-2 proteins antagonize IFN induction and subsequent signaling, which likely delays the control of virus replication and spread. Beyond the early host defense response, an overexuberant innate immune reaction in the later stages of COVID-19 likely contributes to more severe disease. Indeed, limiting the innate immune response with the JAK inhibitor, baricitinib in combination with antiviral treatment improved the clinical status of hospitalized COVID-19 patients [18,19]. A better understanding of the mechanisms of innate immune restriction of SARS-CoV-2 and the viral pathways of evasion could facilitate deployment of targeted therapies that restrict SARS-CoV-2 replication more rapidly without causing pathological inflammation.
Conflict of interest statement
M.S.D. is a consultant for Inbios, Vir Biotechnology, and Carnival Corporation, and on the Scientific Advisory Boards of Moderna and Immunome. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Vir Biotechnology, Moderna, and Emergent BioSolutions.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This study was supported by grants and contracts from N.I.H. (R01 AI157155, HHSN75N93019C00074, and NIAID Centers of Excellence for Influenza Research and Response (CEIRR) contract 75N93021C00014. E.A.M. was supported by a W.M. Keck Postdoctoral Fellowship from Washington University.
References
- 1.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman R., Shah S., Jeurissen P., Jit M., Mossialos E. COVID-19 vaccine challenges: what have we learned so far and what remains to be done? Health Policy. 2021;125:553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., Labrique A., Mohan D. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199:113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puray-Chavez M., LaPak K.M., Schrank T.P., Elliott J.L., Bhatt D.P., Agajanian M.J., Jasuja R., Lawson D.Q., Davis K., Rothlauf P.W., et al. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11 doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey A.L., Diamond M.S. A crisp(r) new perspective on SARS-CoV-2 biology. Cell. 2020;184:15–17. doi: 10.1016/j.cell.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang J., Wan Y., Liu C., Yount B., Gully K., Yang Y., Auerbach A., Peng G., Baric R., Li F. Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F. Structure, function, and evolution of coronavirus spike proteins. Ann Rev Virol. 2015;3:1–25. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021;296 doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neubauer A., Johow J., Mack E., Burchert A., Meyn D., Kadlubiec A., Torje I., Wulf H., Vogelmeier C.F., Hoyer J., et al. The janus-kinase inhibitor ruxolitinib in SARS-CoV-2 induced acute respiratory distress syndrome (ARDS) Leukemia. 2021;10:2917–2923. doi: 10.1038/s41375-021-01374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guimarães P.O., Quirk D., Furtado R.H., Maia L.N., Saraiva J.F., Antunes M.O., Filho R.K., Junior V.M., Soeiro A.M., Tognon A.P., et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385:406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titanji B.K., Farley M.M., Mehta A., Connor-Schuler R., Moanna A., Cribbs S.K., O’Shea J., DeSilva K., Chan B., Edwards A., et al. Use of baricitinib in patients with moderate and severe COVID-19. Clin Infect Dis. 2020;72 doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2020;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang T.N., Pino M., Boddapati A.K., Viox E.G., Starke C.E., Upadhyay A.A., Gumber S., Nekorchuk M., Busman-Sahay K., Strongin Z., et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184:460–475.e21. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loo Y.-M., Gale M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazear H.M., Schoggins J.W., Diamond M.S. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo Y.-M., Fornek J., Crochet N., Bajwa G., Perwitasari O., Martinez-Sobrido L., Akira S., Gill M.A., García-Sastre A., Katze M.G., et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindler E., Thiel V., Weber F. Chapter seven interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N., Hui H., Bray B., Gonzalez G.M., Zeller M., Anderson K.G., Knight R., Smith D., Wang Y., Carlin A.F., et al. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Yamada T., Sato S., Sotoyama Y., Orba Y., Sawa H., Yamauchi H., Sasaki M., Takaoka A. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat Immunol. 2021;22:820–828. doi: 10.1038/s41590-021-00942-0. [DOI] [PubMed] [Google Scholar]; Description of RIG-I induced abortive infection and mapping RIG-I binding sites of SARS-CoV-2.
- 26.Yin X., Riva L., Pu Y., Martin-Sancho L., Kanamune J., Yamamoto Y., Sakai K., Gotoh S., Miorin L., Jesus P.D.D., et al. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampaio N.G., Chauveau L., Hertzog J., Bridgeman A., Fowler G., Moonen J.P., Dupont M., Russell R.A., Noerenberg M., Rehwinkel J. The RNA sensor MDA5 detects SARS-CoV-2 infection. Sci Rep. 2021;11:13638. doi: 10.1038/s41598-021-92940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorne L.G., Reuschl A., Zuliani-Alvarez L., Whelan M.V.X., Turner J., Noursadeghi M., Jolly C., Towers G.J. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40 doi: 10.15252/embj.2021107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouwaki T., Nishimura T., Wang G., Oshiumi H. RIG-I-like receptor-mediated recognition of viral genomic RNA of severe acute respiratory syndrome coronavirus-2 and viral escape from the host innate immune responses. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.700926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebendenne A., Valadão A.L.C., Tauziet M., Maarifi G., Bonaventure B., McKellar J., Planès R., Nisole S., Arnaud-Arnould M., Moncorgé O., et al. SARS-CoV-2 triggers an MDA-5-dependent interferon response which is unable to control replication in lung epithelial cells. J Virol. 2021;95 doi: 10.1128/JVI.02415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David R.Y.S., Combredet C., Sismeiro O., Dillies M.-A., Jagla B., Coppée J.-Y., Mura M., Galla M.G., Despres P., Tangy F., et al. Comparative analysis of viral RNA signatures on different RIG-I-like receptors. eLife. 2016;5:1065. doi: 10.7554/eLife.11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durbin A.F., Wang C., Marcotrigiano J., Gehrke L. RNAs containing modified nucleotides fail to trigger RIG-I conformational changes for innate immune signaling. mBio. 2016;7 doi: 10.1128/mBio.00833-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M., Zhang Z., Xue M., Zhao B.S., Harder O., Li A., Liang X., Gao T.Z., Xu Y., Zhou J., et al. N6-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat Microbiol. 2020;5:584–598. doi: 10.1038/s41564-019-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng M., Karki R., Williams E.P., Yang D., Fitzpatrick E., Vogel P., Jonsson C.B., Kanneganti T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menezes M.C.S., Veiga A.D.M., de Lima T.M., Ariga S.K.K., Barbeiro H.V., de Lucena Moreira C., Pinto A.A.S., Brandao R.A., Marchini J.F., Alencar J.C., et al. Lower peripheral blood Toll-like receptor 3 expression is associated with an unfavorable outcome in severe COVID-19 patients. Sci Rep-uk. 2021;11:15223. doi: 10.1038/s41598-021-94624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q., Bastard P., Liu Z., Pen J.L., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solanich X., Vargas-Parra G., van der Made C.I., Simons A., Schuurs-Hoeijmakers J., Antolí A., del Valle J., Rocamora-Blanch G., Setién F., Esteller M., et al. Genetic screening for TLR7 variants in young and previously healthy men with severe COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallerini C., Daga S., Mantovani S., Benetti E., Picchiotti N., Francisci D., Paciosi F., Schiaroli E., Baldassarri M., Fava F., et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. eLife. 2021;10 doi: 10.7554/eLife.67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asano T., Boisson B., Onodi F., Matuozzo D., Moncada-Velez M., Renkilaraj M.R.L.M., Zhang P., Meertens L., Bolze A., Materna M., et al. X-linked recessive TLR7 deficiency in ∼1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., van den Heuvel G., Mantere T., Kersten S., van Deuren R.C., Steehouwer M., van Reijmersdal S.V., Jaeger M., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno-Eutimio M.A., López-Macías C., Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020;22:226–229. doi: 10.1016/j.micinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salvi V., Nguyen H.O., Sozio F., Schioppa T., Gaudenzi C., Laffranchi M., Scapini P., Passari M., Barbazza I., Tiberio L., et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theobald S.J., Simonis A., Georgomanolis T., Kreer C., Zehner M., Eisfeld H.S., Albert M., Chhen J., Motameny S., Erger F., et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med. 2021;13 doi: 10.15252/emmm.202114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell G.R., To R.K., Hanna J., Spector S.A. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience. 2021;24 doi: 10.1016/j.isci.2021.102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 48.Jo E.-K., Kim J.K., Shin D.-M., Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13:148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H., Chitre S.A., Akinyemi I.A., Loeb J.C., Lednicky J.A., McIntosh M.T., Bhaduri-McIntosh S. SARS-CoV-2 viroporin triggers the NLRP3 inflammatory pathway. bioRxiv. 2020 doi: 10.1101/2020.10.27.357731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coutermarsh-Ott S., Eden K., Allen I.C. Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure. J Gen Virol. 2016;97:825–838. doi: 10.1099/jgv.0.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopfner K.-P., Hornung V. Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat Rev Mol Cell Bio. 2020;21:501–521. doi: 10.1038/s41580-020-0244-x. [DOI] [PubMed] [Google Scholar]
- 52.Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell. 2020;319:C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoggins J.W., MacDuff D.A., Imanaka N., Gainey M.D., Shrestha B., Eitson J.L., Mar K.B., Richardson R.B., Ratushny A.V., Litvak V., et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun B., Sundström K.B., Chew J.J., Bist P., Gan E.S., Tan H.C., Goh K.C., Chawla T., Tang C.K., Ooi E.E. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci Rep. 2017;7:3594. doi: 10.1038/s41598-017-03932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan B., Chakravorty S., Mirabelli C., Wang L., Trujillo-Ochoa J.L., Chauss D., Kumar D., Lionakis M.S., Olson M.R., Wobus C.E., et al. Host-virus chimeric events in SARS-CoV-2-infected cells are infrequent and artifactual. J Virol. 2021;95 doi: 10.1128/JVI.00294-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M., Ferretti M., Ying B., Descamps H., Lee E., Dittmar M., Lee J.S., Whig K., Kamalia B., Dohnalová L., et al. Pharmacological activation of STING blocks SARS-CoV-2 infection. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rui Y., Su J., Shen S., Hu Y., Huang D., Zheng W., Lou M., Shi Y., Wang M., Chen S., et al. Unique and complementary suppression of cGAS-STING and RNA sensing- triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduct Target Ther. 2021;6:123. doi: 10.1038/s41392-021-00515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W., Reyes H.M., Yang J.F., Li Y., Stewart K.M., Basil M.C., Lin S.M., Katzen J., Morrisey E.E., Weiss S.R., et al. Activation of STING signaling pathway effectively blocks human coronavirus infection. J Virol. 2021;95 doi: 10.1128/JVI.00490-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Humphries F., Shmuel-Galia L., Jiang Z., Wilson R., Landis P., Ng S.-L., Parsi K.M., Maehr R., Cruz J., Morales A., et al. A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abi9002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Activation of STING provides IFN-dependent and -independent therapeutic protection against SARS-CoV-2 challenge in mice.
- 60.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., RICE C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfaender S., Mar K.B., Michailidis E., Kratzel A., Boys I.N., V’kovski P., Fan W., Kelly J.N., Hirt D., Ebert N., et al. LY6E impairs coronavirus fusion and confers immune control of viral disease. Nat Microbiol. 2020;5:1330–1339. doi: 10.1038/s41564-020-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao X., Zheng S., Chen D., Zheng M., Li X., Li G., Lin H., Chang J., Zeng H., Guo J.-T. LY6E restricts entry of human coronaviruses, including currently pandemic SARS-CoV-2. J Virol. 2020;94 doi: 10.1128/JVI.00562-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Liu G., Lee J.-H., Parker Z.M., Acharya D., Chiang J.J., Gent M., van, Riedl W., Davis-Gardner M.E., Wies E., Chiang C., et al. ISG15-dependent activation of the sensor MDA5 is antagonized by the SARS-CoV-2 papain-like protease to evade host innate immunity. Nat Microbiol. 2021;6:467–478. doi: 10.1038/s41564-021-00884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mechanistic description of the direct role that ISG15 has in MDA5 signal activation and antagonism by SARS-CoV-2 papain-like protease.
- 64.Perng Y.-C., Lenschow D.J. ISG15 in antiviral immunity and beyond. Nat Rev Microbiol. 2018;16:423–439. doi: 10.1038/s41579-018-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Munnur D., Teo Q., Eggermont D., Lee H.H.Y., Thery F., Ho J., Leur S.W., van, Ng W.W.S., Siu L.Y.L., Beling A., et al. Altered ISGylation drives aberrant macrophage-dependent immune responses during SARS-CoV-2 infection. Nat Immunol. 2021;22:1416–1427. doi: 10.1038/s41590-021-01035-8. [DOI] [PubMed] [Google Scholar]; Description of elevated levels of free ISG15 during SARS-CoV-2 infection and consequent pro-inflammatory skewing of macrophages.
- 66.Mar K.B., Rinkenberger N.R., Boys I.N., Eitson J.L., McDougal M.B., Richardson R.B., Schoggins J.W. LY6E mediates an evolutionarily conserved enhancement of virus infection by targeting a late entry step. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J., Liang C., Liu S.-L. Interferon-inducible LY6E protein promotes HIV-1 infection. J Biol Chem. 2017;292:4674–4685. doi: 10.1074/jbc.M116.755819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majdoul S., Compton A.A. Lessons in self-defence: inhibition of virus entry by intrinsic immunity. Nat Rev Immunol. 2021:1–14. doi: 10.1038/s41577-021-00626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frieman M., Heise M., Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133:101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lokugamage K.G., Hage A., Vries M., de, Valero-Jimenez A.M., Schindewolf C., Dittmann M., Rajsbaum R., Menachery V.D. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol. 2020;94 doi: 10.1128/JVI.01410-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Xia H., Cao Z., Xie X., Zhang X., Chen J.Y.-C., Wang H., Menachery V.D., Rajsbaum R., Shi P.-Y. Evasion of type I interferon by SARS-CoV-2. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Screen of multiple SARS-CoV-2 proteins for IFN antagonism at induction and signaling steps.
- 72.Lei X., Dong X., Ma R., Wang W., Xiao X., Tian Z., Wang C., Wang Y., Li L., Ren L., et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vazquez C., Swanson S.E., Negatu S.G., Dittmar M., Miller J., Ramage H.R., Cherry S., Jurado K.A. SARS-CoV-2 viral proteins NSP1 and NSP13 inhibit interferon activation through distinct mechanisms. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu J.C.-C., Laurent-Rolle M., Pawlak J.B., Wilen C.B., Cresswell P. Translational shutdown and evasion of the innate immune response by SARS-CoV-2 NSP14 protein. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2101161118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tidu A., Janvier A., Schaeffer L., Sosnowski P., Kuhn L., Hammann P., Westhof E., Eriani G., Martin F. The viral protein NSP1 acts as a ribosome gatekeeper for shutting down host translation and fostering SARS-CoV-2 translation. RNA. 2020;27 doi: 10.1261/rna.078121.120. rna.078121.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osipiuk J., Wydorski P.M., Lanham B.T., Tesar C., Endres M., Engle E., Jedrzejczak R., Mullapudi V., Michalska K., Fidelis K., et al. Dual domain recognition determines SARS-CoV-2 PLpro selectivity for human ISG15 and K48-linked di-ubiquitin. bioRxiv. 2021 doi: 10.1101/2021.09.15.460543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayn M., Hirschenberger M., Koepke L., Nchioua R., Straub J.H., Klute S., Hunszinger V., Zech F., Bozzo C.P., Aftab W., et al. Systematic functional analysis of SARS-CoV-2 proteins uncovers viral innate immune antagonists and remaining vulnerabilities. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang W., Zhou Z., Xiao X., Tian Z., Dong X., Wang C., Li L., Ren L., Lei X., Xiang Z., et al. SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation. Cell Mol Immunol. 2021;18:945–953. doi: 10.1038/s41423-020-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura I., Konno Y., Uriu K., Hopfensperger K., Sauter D., Nakagawa S., Sato K. Sarbecovirus ORF6 proteins hamper the induction of interferon signaling. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miorin L., Kehrer T., Sanchez-Aparicio M.T., Zhang K., Cohen P., Patel R.S., Cupic A., Makio T., Mei M., Moreno E., et al. SARS-CoV-2 Orf6 hijacks Nup98 to block STAT nuclear import and antagonize interferon signaling. Proc Natl Acad Sci U S A. 2020;117:28344–28354. doi: 10.1073/pnas.2016650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang R., Yang X., Chang M., Xue Z., Wang W., Bai L., Zhao S., Liu E. ORF3a protein of severe acute respiratory syndrome coronavirus 2 inhibits interferon-activated janus kinase/signal transducer and activator of transcription signaling via elevating suppressor of cytokine signaling 1. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.752597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huston N.C., Wan H., Strine M.S., de Cesaris Araujo Tavares R., Wilen C.B., Pyle A.M. Comprehensive in vivo secondary structure of the SARS-CoV-2 genome reveals novel regulatory motifs and mechanisms. Mol Cell. 2021;81:584–598.e5. doi: 10.1016/j.molcel.2020.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]