Abstract
We examined the impact of pre-existing SARS-CoV-2-specific cellular immunity on BNT162b2 mRNA COVID-19 vaccine reactogenicity. Of 96 healthcare workers (HCWs), 76% reported any vaccine reaction (first dose: 70%, second dose: 67%), none of which was severe. Following first dose, systemic reactions were significantly more frequent among HCWs with past infection than in infection-naïve individuals, and among HCWs with pre-existing cellular immunity than in those without it. The rate of systemic reactions after second dose was 1.7 and 2.0-times higher than after first dose among infection-naïve HCWs and those without pre-existing cellular immunity, respectively. Levels of SARS-CoV-2-specific T-cells before vaccination were higher in HCWs with systemic reactions after the first dose than in those without them. BNT162b2 vaccine reactogenicity after first dose is attributable to pre-existing cellular immunity elicited by prior COVID-19 or cross-reactivity. Reactogenicity following second dose suggests an immunity-boosting effect. Overall, these data may reduce negative attitudes towards COVID-19 vaccines.
Study Registration.
The study was registered on clinicaltrials.gov, NCT04402827.
Keywords: SARS-CoV-2, COVID-19, BNT162b2 mRNA vaccine, Reactogenicity, T-cell responses, Booster effect
Abbreviations: BMI, body mass index; CI, Confidence Interval; HCWs, healthcare workers; PBMCs, peripheral blood mononuclear cells; RR, relative risk
1. Introduction
The BNT162b2 (Pfizer-BioNTech) mRNA coronavirus disease 2019 (COVID-19) vaccine showed to be highly effective in preventing COVID-19 in phase 3 clinical trials [1], and able to elicit robust humoral and cellular responses against SARS-CoV-2 [2]. From a safety standpoint, reactogenicity, defined as inflammatory events that occur soon after vaccination, was reported in up to 83% of previously uninfected individuals [1], [3]. Real-life studies observed variable rates of vaccine-induced reactions mostly after the second dose [4], [5], although the mechanisms underlying BNT162b2 mRNA COVID-19 vaccine reactogenicity are still poorly understood.
Cellular responses against SARS-CoV-2 epitopes are observed in almost all individuals after recovery from COVID-19, even in those with asymptomatic infection [6], or undetectable SARS-CoV-2-specific IgG [7]. Additionally, cellular responses were described in 40–60% of unexposed individuals, suggestive of cross-reactive immunity to endemic human coronaviruses [8], [9]. Hypothetically, cellular immunity against SARS-CoV-2 could be related to the emergence of vaccine reactogenicity. Therefore, we aimed to evaluate BNT162b2 mRNA COVID-19 vaccine reactogenicity in healthcare workers (HCWs) with past SARS-CoV-2 infection or pre-existing T-cell immunity to SARS-CoV-2 peptides.
2. Methods
The COVEX-2 study is an observational prospective cohort study that assessed the immune response and incidence of SARS-CoV-2 infection among HCWs at a tertiary university hospital. One hundred and twenty-six HCWs underwent blood test from May to October 2020 to evaluate humoral and T-cell responses to SARS-CoV-2 and completed a questionnaire about age, sex, body mass index (BMI), presence of comorbidities, smoking, alcohol intake, concomitant medications, time of exposure to COVID-19 patients, exposure to aerosol-generating procedures, COVID-19 symptoms and signs (fever, cough, anosmia, diarrhea, headache, or pneumonia), and disease severity. Mild disease was defined as the presence of symptoms attributable to COVID-19 in the absence of shortness of breath, dyspnea on exertion, or abnormal imaging. Moderate disease was defined as evidence of lower respiratory disease during clinical assessment or imaging, with oxygen saturation ≥ 94% on room air. Severe disease was considered in case of respiratory rate > 30 breaths per min, oxygen saturation of < 94% on room air, a ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air [PaO2/FiO2] ≤ 300, or lung infiltrates > 50% [10]. Additionally, the results of all SARS-CoV-2 RT-PCR and serologic tests performed before inclusion and during follow-up were collected. SARS-CoV-2 infection was defined as a positive RT-PCR test on nasopharyngeal swab, or/and seroconversion. Incident SARS-CoV-2 infections were self-reported prospectively.
During January-February 2021, 102 participants were vaccinated with two doses of BNT162b2 mRNA COVID-19 vaccine given 21 days apart whether or not had been previously infected. All participants received emails to initiate web-based health surveys after each dose of the BNT162b2 vaccine. A reminder was sent to HCWs that had not answered the survey after 8 weeks of the last dose. Participants were asked about the type and time of onset of reactions elicited by each vaccine dose. Additionally, the survey allowed participants to enter free-text information about their postvaccination experience.
Vaccine-induced reactions were classified as local (pain, swelling, erythema at injection site) and systemic (fatigue, malaise, headache, insomnia, fever or chills, muscle or joint pain, enlargement of lymph nodes, nausea, or rash). For local reactions, the intensity was self-referred as mild (1–3), moderate (4–6), or severe (7–10) [11]. The use of analgesics, anti-inflammatory drugs, or medical attention was described.
HCWs with incident SARS-CoV-2 infection between blood collection and second vaccine dose were excluded to avoid misinterpretation of vaccine-induced reactions. We divided HCWs into two groups according to their history of SARS-CoV-2 infection at baseline. Secondarily, we compared individuals by the presence of pre-existing cellular immunity, as follows: (A) HCWs with past SARS-CoV-2 infection and pre-existing cellular immunity; (B) HCWs with past SARS-CoV-2 infection without pre-existing cellular immunity; (C) HCWs without past SARS-CoV-2 infection (negative RT-PCR and repeated IgG/A/M serologies) with pre-existing cellular immunity (infection-naïve cross-reactive group), and (D) HCWs without past SARS-CoV-2 infection (infection-naïve) nor pre-existing cellular immunity.
The study was approved by our ethics committee (EC162/20) and registered at the clinical trials repository (clinicaltrials.gov, NCT04402827). Written informed consent was obtained from all participants.
2.1. Laboratory procedures
The presence of cellular immune response was assessed at inclusion. SARS-CoV-2-specific CD4 + and CD8 + T-cells were measured using in vitro stimulation with SARS-CoV-2 peptide pools of viral proteins encompassing the spike, membrane, and nucleocapsid, followed by quantitation of CD4 + and CD8 + T-cell specific interferon (IFN)-γ in live-cell flow cytometry, using peripheral blood mononuclear cells (PBMCs) samples from all participants.
In detail, ethylenediaminetetraacetic acid (EDTA)-blood samples were collected from all individuals. After centrifugation at 200g for 10 min, plasma fraction was collected and again centrifuged at 1200g for 15 min, aliquoted, and stored at −80 °C. The cellular fraction was diluted with phosphate-buffered saline (PBS) and subjected to Ficoll density gradient centrifugation at 500g for 20 min. PBMCs were washed and frozen in fetal bovine serum (FBS) with 8% dimethyl sulfoxide (DMSO, Sigma, USA) in liquid nitrogen.
PBMCs were thawed and plated in 96-well flat-bottom plates at 106 cells/well in RPMI-1640 culture medium (Gibco, USA) supplemented with 10% human serum (AB serum, Sigma), 100 IU of penicillin/streptomycin/mL (Gibco, USA), 2 mM L-glutamine, and after 24 h cells were stimulated in five different conditions in the presence of 1 µg/ml purified anti-CD28 antibody (Miltenyi, Germany). Three wells were stimulated with each of the SARS-CoV-2 peptide pools S, M, and N at a concentration of 1 µg/ml. Each peptide pool was composed of 15-mers sequences with 11 amino acids overlap, covering the immunodominant sequence domains of the surface glycoprotein spike, the complete sequence of the membrane glycoprotein, and the complete nucleocapsid phosphoprotein of SARS-CoV-2 (PepTivator SARS-CoV-2 Prot S, M, and N, Miltenyi-Biotec, Cologne, Germany). In addition, one well was stimulated with culture medium alone as a negative control (unstimulated), and another well was stimulated adding 1.5 mg staphylococcal enterotoxin B (SEB, Sigma, Germany) as the positive control. An unresponsive sample to SEB would be excluded from the analysis. Stimulated PBMCs were incubated for two hours before adding brefeldin A (Rapid Cytokine Inspector CD4/CD8 T-cell kit, Miltenyi, Germany) into the medium to stop cytokine release and kept in culture for other 14 h. After stimulation, staining of the cells was carried out with the following fluorochrome-conjugated antibodies using Rapid Cytokine Inspector CD4/CD8 T-cell kit (Miltenyi, Germany): CD3-VioBlue, CD4-APC, CD8-FITC, CD14-PerCP, CD20-PerCP, IFN-γ-PE, and FcR blocking reagent. To exclude dead cells, viability 405/520 fixable dye staining (Milteny, Germany) was added for the last 10 min of incubation. Fixation and permeabilization were performed according to the manufactureŕs protocol. Samples were measured and analyzed by flow cytometry on a MACSQuant Analyzer 10 using MACSQuantify software. At least 105 cells were analyzed and gated with the following strategy: single (FSC-A/FSC-H dot plot) and live cells were first selected. Cell debris, monocytes, and B cells were excluded from the analysis with CD14- and CD20-PerCP antibodies. Then, lymphocytes were selected with an FSC-A/SSC-A dot plot, and CD3 + T-cells were gated. IFN-γ expression was finally analyzed separately for CD4 + and CD8 + T-cells and it was considered significantly reactive if the proportion of positive cells in stimulated wells was at least 2-fold higher in comparison with the negative control wells (unstimulated). Individuals with pre-existing cellular immunity were defined as those with significant T-cell reactivity to proteins S, M, and/or N.
2.2. Statistical analysis
Characteristics of HCWs were presented globally, by past SARS-CoV-2 infection and by pre-existing cellular immunity to SARS-CoV-2. Continuous variables were expressed as the median, 25th, and 75th percentiles or mean and ranges, as specified. Categorical variables were displayed as frequencies and percentages. Comparisons between groups were performed using two-tailed statistical tests, χ2 or Fisher’s exact tests for categorical variables where appropriate, and Mann-Whitney U test for continuous variables. Relative risks (RR) with 95% confidence intervals (95 %CI) of presenting systemic reactions according to subgroups of participants were calculated. Statistical significance was defined as two-sided P-values < 0.05. Statistical analyses were performed by Stata 16.0 software (StataCorp, College Station, TX, USA).
3. Results
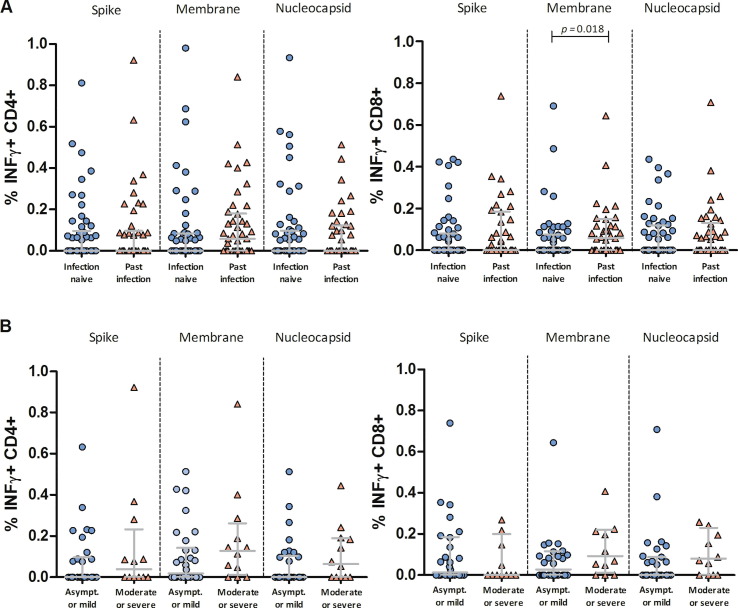
A total of 96 HCWs completed the survey and were included in this analysis. The mean age was 44 years (range 26–65) and 66% were females. At baseline, 54 (56%) participants were infection-naïve and 42 (44%) had past SARS-CoV-2 infection, of whom 3 (7%), 27 (64%), 5 (12%), and 7 (17%) had asymptomatic infection, mild, moderate, and severe COVID-19, respectively (Table 1 ). Using in vitro stimulation of PBMCs with SARS-CoV-2 spike, nucleocapsid, and membrane peptide pools, we observed that 73 (76%) participants had pre-existing T-cell immunity to any SARS-CoV-2 peptides, of which 33 had been previously infected and 40 were considered to have cross-reactive responses. Interestingly, the percentage of spike, membrane, and nucleocapsid-specific INFγ + CD4 + and INFγ + CD8 + T-cells was not significantly different between participants with cross-reactivity and those with past infection, except for membrane-specific INFγ + CD8 + T-cells, which were higher in HCWs with past infection (Fig. 1 A). Similarly, HCWs with a history of asymptomatic infection or mild COVID-19 and those with moderate or severe COVID-19 had comparable percentages of virus-specific INFγ + CD4 + and INFγ + CD8 + T-cells (Fig. 1 B).
Table 1.
Baseline clinical characteristics and laboratory findings of the study population.
|
All groups (n = 96) |
HCWs with past SARS-CoV-2 infection (n = 42) |
Infection- naïve HCWs (n = 54) |
p-value | |
|---|---|---|---|---|
| Age [years], mean (range) | 44 (26–65) | 43 (26–63) | 46 (30–65) | 0.240 |
| Gender | ||||
| Female | 63 (66) | 24 (57) | 39 (72) | 0.120 |
| Male | 33 (34) | 18 (43) | 15 (28) | |
| Body mass index [kg/m2] | 23.5 (21.5–26.7) | 23.4 (21.6–24.6) | 23.7 (21.4–27.1) | 0.880 |
| Smoking, ever | 42 (44) | 13 (31) | 29 (54) | 0.026 |
| Comorbidities | 37 (39) | 12 (29) | 25 (46) | 0.077 |
| Hypertension | 11 (12) | 6 (14) | 5 (9) | 0.440 |
| Diabetes mellitus | 3 (3) | 0 (0) | 3 (6) | 0.120 |
| Chronic lung disease | 6 (6) | 1 (2) | 5 (9) | 0.170 |
| T-cell immunity at baseline | 73 (76) | 33 (79) | 40 (74) | 0.610 |
| CD4 + reactive | 67 (70) | 28 (67) | 39 (72) | 0.560 |
| CD8 + reactive | 62 (65) | 31 (74) | 31 (74) | 0.710 |
| Past COVID-19 diagnosis at baseline | – | – | ||
| Asymptomatic infection | 3 (3) | 3 (7) | – | – |
| Mild disease | 27 (28) | 27 (64) | – | – |
| Moderate disease | 5 (5) | 5 (12) | – | |
| Severe disease | 7 (7) | 7 (17) | – | – |
| History of positive RT-PCR at baseline | 35 (36) | 35 (83) | – | – |
| SARS-CoV-2 IgG positive at baseline | 22 (23) | 22 (52) | – | – |
| Time from COVID-19 to first vaccine dose [days] | 306 (290–312) | 306 (290–312) | – | – |
| Time from inclusion to vaccination [days] | ||||
| First dose | 101 (87–114) | 102 (87–118) | 101 (84–112) | 0.910 |
| Second dose | 122 (108–135) | 123 (108–139) | 122 (105–133) | 0.930 |
COVID-19, coronavirus disease 2019; HCWs, healthcare workers; RT-PCR, reverse transcription-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. All categorical variables are expressed as number (%) and quantitative variables as median (p25-p75) unless specified.
Fig. 1.
Quantitative analysis of SARS-CoV-2-specific T-cell immunity in healthcare workers before vaccination according to past infection. Percentage of interferon gamma-producing (INFγ + ) CD8 + and CD4 + T-cells induced by peptides spanning the immunogenic domains of the SARS-CoV-2 spike, membrane, and nucleocapsid proteins in peripheral blood mononuclear cells of healthcare workers before vaccination. Data are stratified by history of SARS-CoV-2 infection among all participants (A), and by disease severity among participants with past infection (B). Asympt. = asymptomatic SARS-CoV-2 infection. The horizontal lines represent medians with interquartile ranges. Comparisons between groups were performed using the Mann-Whitney U test.
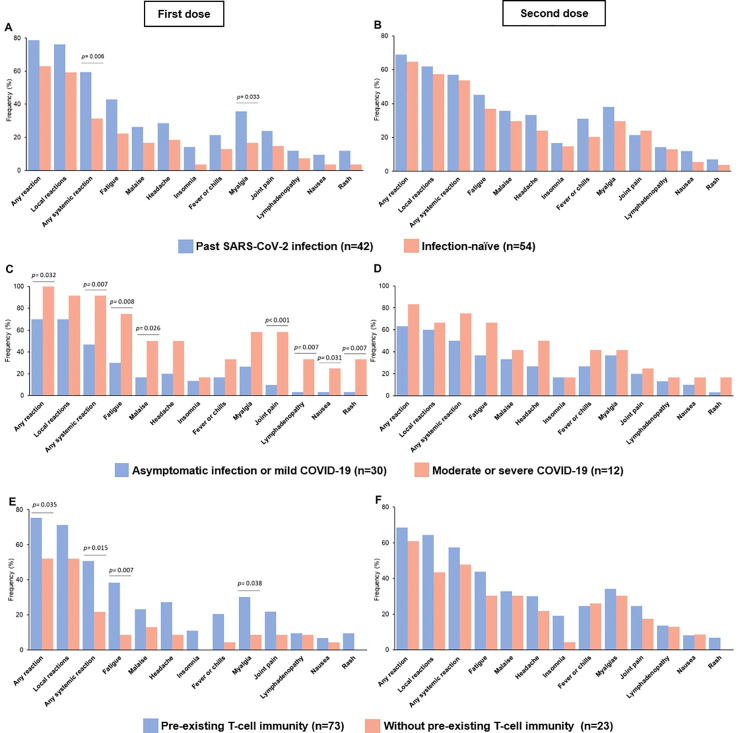
Globally, 73 (76%) HCWs presented any reaction after vaccination (first dose: 70%, second dose: 67%). Most participants reported local reactions (first dose: 67%, second dose: 59%) or systemic reactions (first dose: 44%, second dose: 55%). The most frequently reported symptoms were injection site reactions, fatigue, and myalgias after both doses. No event resulted in hospitalization or death. The rate of local and systemic symptoms after any vaccine dose was similar for participants with past SARS-CoV-2 infection compared with infection-naïve participants (79% versus 74%, p = 0.61). However, HCWs with past SARS-CoV-2 infection had a significantly higher frequency of systemic reactions after the first vaccine dose compared with infection-naïve participants (60% versus 31%, p = 0.006, Fig. 2 A). Indeed, among HCWs with past SARS-CoV-2 infection, reactogenicity after the first dose was significantly higher for participants with previous moderate or severe COVID-19 compared with those with asymptomatic or mild infection (any reaction: 100% versus 70%, p = 0.032; systemic reactions: 92% versus 47%, p = 0.007, respectively; Fig. 2 C).
Fig. 2.
Reactogenicity of BNT162b2 mRNA COVID-19 vaccine among healthcare workers. Comparison of vaccine-induced local and systemic reactions after the first dose (A, C, and E) and second dose (B, D, and F) of BNT162b2 mRNA vaccine. Data are presented as percentages and stratified by history of SARS-CoV-2 infection (A and B), COVID-19 severity (C and D), and pre-existing cellular immunity (E and F). Comparisons between groups were performed using the Mann-Whitney U test.
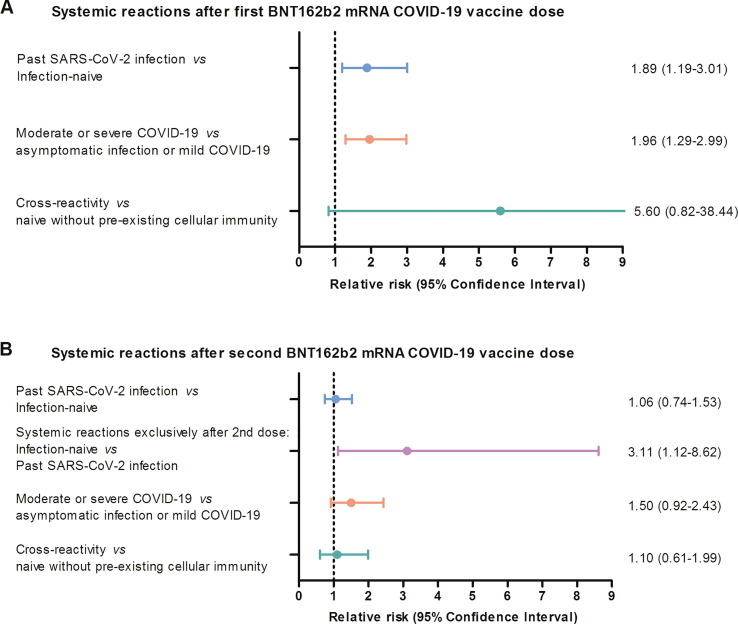
Likewise, HCWs with pre-existing cellular immunity to SARS-CoV-2 peptides had significantly greater reactogenicity after the first dose compared with participants without pre-existing cellular immunity (75% versus 52%, p = 0.035; Fig. 2 E). Particularly, systemic reactions such as fatigue, myalgias, and headache were more often observed among HCWs with pre-existing cellular immunity. Fever, rash, and lymphadenopathy were also more commonly reported after the first dose by participants with pre-existing cellular immunity, although it was not statistically significant given the small sample size (Fig. 2 E). Furthermore, in a subgroup analysis among infection-naïve HCWs, participants with cross-reactivity had a higher risk of systemic reactions after the first dose compared with those without cellular immunity (RR 5.6, 95 %CI 0.82–38.44, Fig. 4 and Table 2 ).
Fig. 4.
Risk of presenting systemic reactions after the BNT162b2 mRNA COVID-19 vaccine among healthcare workers by subgroups. Relative risk and 95% Confidence Interval for comparisons based on history of SARS-CoV-2 infection, COVID-19 severity, and pre-existing cellular immunity for the first (A) and second (B) vaccine dose.
Table 2.
Reactogenicity of BNT162b2 mRNA COVID-19 vaccine according to past SARS-CoV-2 infection and pre-existing cellular immunity.
| All groups (n = 96) |
Past SARS-CoV-2 infection |
Infection-naïve |
|||||
|---|---|---|---|---|---|---|---|
|
Pre-existing cellular immunity (n = 33) |
Without pre-existing cellular immunity (n = 9) |
p-value |
Pre-existing cellular immunity (n = 40) |
Without pre-existing cellular immunity (n = 14) |
p-value | ||
| Reactions after any dose | |||||||
| Any reaction | 73 (76) | 27 (82) | 6 (67) | 0.326 | 30 (75) | 10 (71) | 0.793 |
| Local reactions (pain, swelling or erythema) | 70 (73) | 27 (82) | 6 (67) | 0.326 | 29 (73) | 8 (57) | 0.287 |
| Systemic reactions | 62 (65) | 24 (73) | 5 (56) | 0.323 | 26 (65) | 7 (50) | 0.322 |
| Reactions after the first dose | |||||||
| Any reaction | 67 (70) | 27 (82) | 6 (67) | 0.326 | 28 (70) | 6 (43) | 0.070 |
| Local reactions (pain, swelling or erythema) | 64 (67) | 26 (81) | 6 (67) | 0.449 | 26 (65) | 6 (43) | 0.147 |
| Systemic reactions | 42 (44) | 21 (64) | 4 (44) | 0.298 | 16 (40) | 1 (7) | 0.023 |
| Use of analgesic or anti-inflammatory drugs | 33 (34) | 16 (49) | 4 (44) | 0.737 | 12 (30) | 1 (7) | 0.231 |
| Reactions after the second dose | |||||||
| Any reaction | 64 (67) | 25 (76) | 4 (44) | 0.072 | 25 (63) | 10 (71) | 0.547 |
| Local reactions (pain, swelling or erythema) | 57 (59) | 23 (70) | 3 (33) | 0.046 | 24 (60) | 7 (50) | 0.515 |
| Systemic reactions | 53 (55) | 20 (61) | 4 (44) | 0.385 | 22 (55) | 7 (50) | 0.747 |
| Use of analgesic or anti-inflammatory drugs | 48 (50) | 21 (64) | 3 (33) | 0.658 | 18 (45) | 6 (43) | 0.490 |
COVID-19, coronavirus disease 2019; HCWs, healthcare workers. All categorical variables are expressed as numbers (%). Comparisons between groups were performed using two-tailed statistical tests, χ2 or Fisher’s exact tests for categorical variables where appropriate.
The percentage of systemic reactions after the second dose was numerically greater than after the first dose (Table 2). Specifically, infection-naïve HCWs and those without pre-existing cellular immunity had a 1.7 and 2.0-times higher rate of systemic reactions after the second dose, respectively (Fig. 2 B and F). Moreover, infection-naïve participants had a higher risk of systemic reactions exclusively after the second dose compared to those with past infection (RR 3.11, 95 %CI 1.12–8.62, Fig. 4). Other comparisons between subgroups of HCWs yielded similar rates of systemic reactogenicity after the second vaccine dose (Fig. 4 and Table 2).
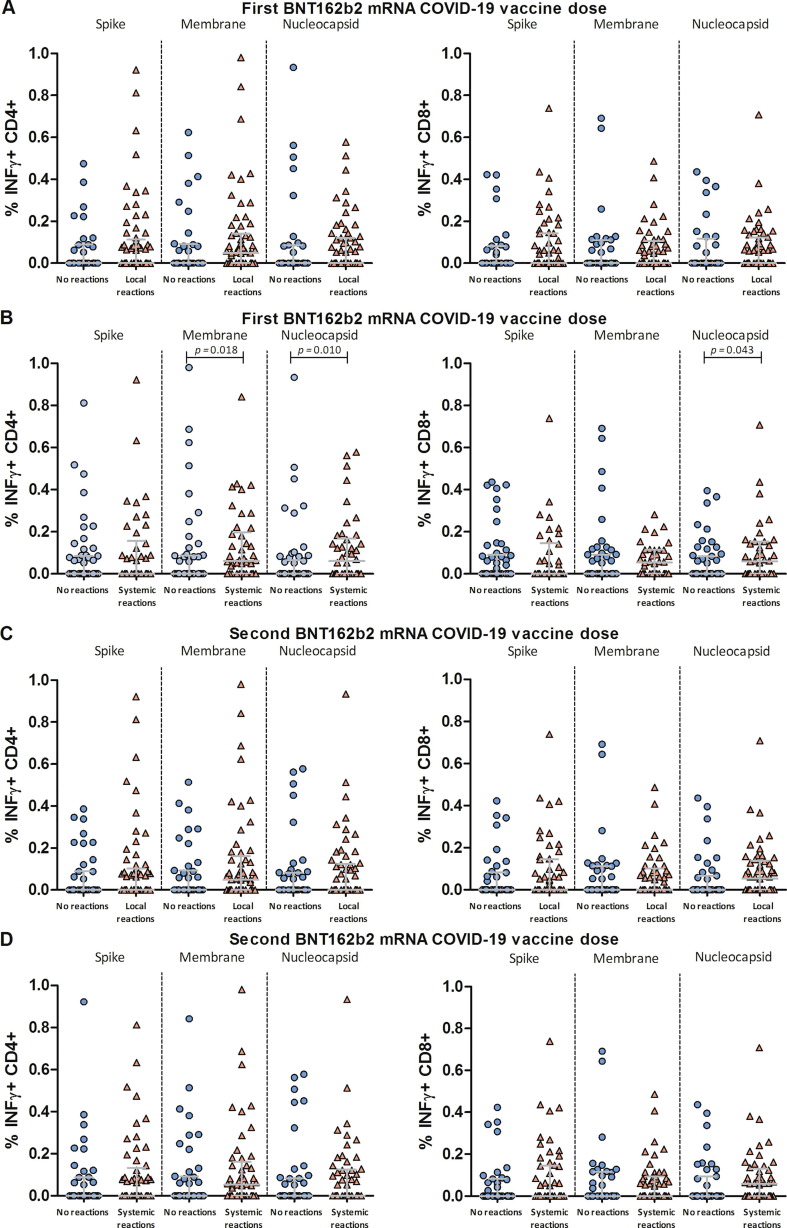
In the quantitative analysis of pre-existing T-cell immunity, percentages of virus-specific INFγ + CD4 + and INFγ + CD8 + T-cells were higher among participants who developed systemic reactions after the first dose (Fig. 3 B), while they were comparable among HCWs with and without systemic symptoms after the second dose (Fig. 3 D). Percentages of virus-specific T-cells among participants with and without local reactions after each vaccine dose were similar (Fig. 3 A and C).
Fig. 3.
Quantitative analysis of SARS-CoV-2-specific pre-existing T-cell immunity in healthcare workers with and without BNT162b2 mRNA COVID-19 vaccine reactogenicity. Percentage of interferon gamma-producing (INFγ + ) CD8 + and CD4 + T-cells induced by peptides spanning the immunogenic domains of the SARS-CoV-2 spike, membrane, and nucleocapsid proteins in peripheral blood mononuclear cells of healthcare workers before vaccination. Data are presented as self-reported local (A and C) and systemic reactions (B and D) after the first (A and B) and second (C and D) vaccine doses. Comparisons between groups were performed using the Mann-Whitney U test.
4. Discussion
This study performed in a real-life setting indicates for the first time to our knowledge a close association between pre-existing cellular immunity to SARS-CoV-2 and BNT162B2 mRNA COVID-19 vaccine reactogenicity. Indeed, 78% of all reactogenicity was attributable to detectable T-cell responses before vaccination. This observation was illustrated by the predominance of systemic reactions after the first dose in HCWs with pre-existing cellular immunity secondary to past infection or cross-reactivity. Moreover, infection-naïve participants were more likely to have systemic reactions only following the second dose, when the adaptative immune response has already been primed.
We found an overall frequency of self-reported reactions to the BNT162b2 mRNA COVID-19 vaccine of 76%, mainly local reactions, fatigue, and myalgia. These observations are consistent with the results of phase 3 clinical trials and nationwide surveillance reports [1], [4]. Recently, a large-scale study in the UK reported that females, people aged 55 years or younger, and individuals with prior COVID-19 were more likely to have systemic reactions than males, people older than 55 years, or SARS-CoV-2 naïve individuals, respectively [5]. Although the sample size precluded us to perform age-stratified analysis, our data confirm that individuals with past infection are expected to have reactogenicity. This is in line with previous observations of greater antibody titers and stronger T-cell responses after a single BNT162b2 vaccine dose in individuals with past COVID-19 compared with infection-naïve individuals, indicating secondary memory responses to SARS-CoV-2 antigens [12], [13]. Since only six individuals of our cohort had SARS-CoV-2 infection within the six months before vaccination, we were unable to assess the influence of time since COVID-19 infection on reactogenicity.
Notably, reactogenicity after the first vaccine dose was particularly higher among individuals who had presented a moderate or severe disease. Broader and more durable T-cell responses have been described in individuals with a severe disease which may be the result of higher viral loads or dysfunctional T-cell responses and may contribute to disease severity [14], [15]. Though, the percentage of SARS-CoV-2 specific T-cells before vaccination was not different based on COVID-19 severity in our small cohort.
Furthermore, we observed that systemic reactions after the first dose were more frequent among individuals with pre-existing cellular immunity to SARS-CoV-2, even among infection-naïve participants. Indeed, individuals with systemic reactions after the first dose had stronger virus-specific CD4 + and CD8 + T-cell responses before vaccination than those without systemic symptoms. Kramer et al. described that seropositive vaccine recipients had a higher rate of systemic events than seronegative individuals suggesting a correlation between humoral immunity and reactogenicity [16]. However, reports of cellular correlates of reactogenicity for SARS-CoV-2 vaccines as well as other vaccines are scarce [17]. Burny et al. observed associations between the CD4 + T-cell responses elicited by hepatitis B virus surface antigen combined with different adjuvants and systemic reactions [17]. Globally, our data demonstrate that BNT162b2 mRNA vaccine-induced systemic reactogenicity is explained by the presence of cellular immunity before vaccination either in individuals with past SARS-CoV-2 infection and in those with cross-reactive T-cell responses. In these individuals, the first vaccine dose acts as a booster dose that enhances immunity and therefore leads to reactogenicity [12].
Consistent with previous results, systemic reactions were more frequent after the second dose of the BNT162b2 mRNA vaccine [1], [4]. Our findings further indicate that the increment in systemic reactogenicity after the second dose occurs mainly among infection-naïve individuals and those without pre-existing cellular immunity. This is supported by the notion that the second vaccine dose predominantly boosts the adaptative immunity among infection-naïve individuals [18], [19]. Alternatively, it may reflect a contraction of cellular immunity after the second dose in previously infected individuals due to activation-induced cell death or functional exhaustion, as hypothesized by Camara et al [19]. Although the amount of virus-specific T-cells before vaccination was similar in individuals with and without reactogenicity after the second dose, we did not analyze T-cell responses in the period between the first and second doses which might have detected differences between subgroups.
Our study has several limitations, including the small sample size and the relatively young mean age of our population, precluding the generalization of results to older populations. Second, HCWs with past infection might have been misclassified as being infection-naive considering that some individuals do not build humoral or cellular responses after SARS-CoV-2 infection. However, this issue was minimized by repeating highly sensitive serological tests.
In conclusion, most of BNT162b2 mRNA COVID-19 vaccine reactogenicity is attributable to pre-existing cellular immunity to SARS-CoV-2 either elicited by prior COVID-19 or cross-reactivity. Further studies should demonstrate whether reactogenicity is also related to vaccine immunogenicity. Anyway, our findings provide insights into the mechanisms underlying vaccine-induced reactions and may reduce negative attitudes towards vaccines that challenge their impact on public health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Ana Abad for database management.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contribution
Pilar Vizcarra: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Johannes Haemmerle: Data curation, Investigation, Methodology, Validation, Visualization, Writing – review & editing. Hector Velasco: Investigation, Methodology, Validation, Visualization, Writing – review & editing. Tamara Velasco: Investigation, Methodology, Validation, Visualization, Writing – review & editing. Marina Fernández-Escribano: Conceptualization, Investigation, Validation, Visualization, Writing – review & editing. Alejandro Vallejo: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. José L. Casado: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 3.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019;4(1) doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapin-Bardales J., Gee J., Myers T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA. 2021;383:2603–2615. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 5.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekine T., Perez-potti A., Rivera-ballesteros O., Strålin K. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. MedRxiv. 2020 doi: 10.1101/2020.06.29.174888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarzkopf S., Krawczyk A., Knop D., Klump H., Heinold A., Heinemann F.M., et al. Cellular immunity in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2–specific IgG. Emerg Infect Dis. 2021;27(1):122–129. doi: 10.3201/eid2701.203772. [DOI] [PubMed] [Google Scholar]
- 8.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 10.National Institutes of Health. Clinical Spectrum of SARS-CoV-2 Infection n.d. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/#:∼:text=Patients with COVID-19 are,may experience rapid clinical deterioration. (accessed April 22, 2021).
- 11.Nalamachu S., Robinson R.L., Viktrup L., Cappelleri J.C., Bushmakin A.G., Tive L., et al. Pain severity and healthcare resource utilization in patients with osteoarthritis in the United States. Postgrad Med. 2021;133(1):10–19. doi: 10.1080/00325481.2020.1841988. [DOI] [PubMed] [Google Scholar]
- 12.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauzin A, Nayrac M, Benlarbi M, Gong SY, Gasser R, Beaudoin-Bussières G, Brassard N, Laumaea A, Vézina D, Prévost J, Anand SP, Bourassa C, Gendron-Lepage G, Medjahed H, Goyette G, Niessl J, Tastet O, Gokool L, Morrisseau C, Arlotto P, Stamatatos L, McGuir FA. A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses 2021. https://doi.org/10.1101/2021.03.18.435972.
- 14.Casado J.L., Vizcarra P., Velasco H., Hammerle J., McGee A., Fernandez-Escribano M., et al. Progressive and parallel decline of humoral and T cell immunity in convalescent health care workers with asymptomatic or mild-moderate SARS-CoV-2 infection. J Infect Dis. 2021:1–31. doi: 10.1093/infdis/jiab242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burny W., Callegaro A., Bechtold V., Clement F., Delhaye S., Fissette L., et al. Different Adjuvants Induce Common Innate Pathways That Are Associated with Enhanced Adaptive Responses against a Model Antigen in Humans. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samanovic A.M.I., Cornelius A.R., Gray-gaillard S.L., Allen J.R., Karmacharya T., Wilson J.P., et al. Robust immune responses after one dose of BNT162b2 mRNA vaccine dose in SARS-CoV-2 experienced individuals. BioRxiv. 2021:1–37. doi: 10.1101/2021.02.07.21251311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camara C, Lozano-Ojalvo D, Lopez-Granados E, Paz-Artal E, Pion M, Correa-Rocha R, et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naïve and COVID-19 recovered individuals. BioRxiv 2021:2021.03.22.436441. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.