1. Introduction
The Severe Acute Respiratory Coronavirus 2 (SARS-CoV2) pandemic emerged in 2019 and vaccine development began early and efficiently due to SARS-CoV2 genome availability and existing vaccine development platforms and technology. Pandemic preparedness allowed rapid vaccine development. Within 12 months of the first cases of human SARS-2-CoV pneumonia in Wuhan, China, three vaccines had reported vaccine efficacy (VE) from clinical trials, two using novel mRNA vaccine technology [1], [2], [3]. These vaccines underwent mass manufacture and deployment to meet global demand, however, demand exceeded manufacturing capacity in the short-term. Countries generally prioritised those at highest risk of severe illness and death for receipt of the first available vaccines.
The paucity of vaccine availability was problematic globally and so in January 2021, the UK Joint Committee on Vaccination and Immunisation (JCVI) and the Department of Health advised prioritisation of vulnerable groups, but also considered options for improving short-term impact of the national vaccine programme. This was in part due to the SARS-CoV2 alpha variant (B.1.1.7 first identified in the UK), rapidly spreading in December 2020/January 2021 to become the dominant strain with evidence of increased transmissibility and preliminary data suggesting an increased case fatality rate [4]. The JCVI advised administration of the first dose of the ChAdOx1 nCov-19 (AstraZeneca) and BNT162b2 COVID-19 (Pfizer) vaccines to as many people in the vulnerable groups as possible, thereby providing some protection to more people, while delaying the second dose of the vaccines from 3–4 weeks to 12 weeks [5]. The rationale for this decision was that high VE after one dose of both vaccines had been reported [2], [3], therefore more people could be protected against severe disease by widespread use of a single dose of vaccination. The reported VE for ChAdOx1 nCov-19 vaccine (AstraZeneca) against primary symptomatic COVID-19 more than 21 days after a single standard dose was 76·7% (95% CI 47·0–89·8) at 22–30 days (3–4 weeks) and 78·3% (95% CI 36·4–92) at 61–90 days (12 weeks) [3]. The reported VE for BNT162b2 COVID-19 vaccine (Pfizer) against confirmed COVID-19 21 days after the first dose was 52.4% (95% CI 29.5–68.4) [2]. Furthermore, it was thought that delaying second doses from a 3–4 week interval to 12 weeks might result in better protection, as was reported for the ChAdOx1 nCov-19 vaccine (AstraZeneca) [6]. The UK’s use of an altered dosing schedule was seen as controversial, and lacking an evidence base by some, but was justified by UK health agencies as a pragmatic and public health focussed approach in light of limitations to vaccine supply and the circulation of a new variant of concern (VOC). We review the progress being made in informing vaccine interval dosing, as it has major implications for increasing vaccine availability allowing for distribution to regions with poor vaccine uptake given the ongoing limited supply in 2021.
2. SARS-CoV2 vaccine intervals
At the time of writing there was a growing body of evidence for the use of post-vaccination antibody titers as the basis of a correlate of protection [7], [8], [9]. Neutralizing antibody titers were demonstrated to be predictive of immunological protection to SARS-CoV2 and there was strong correlation between neutralising antibody titers and VE for seven of the most widely used SARS-CoV2 vaccines: mRNA-1273 (Moderna); NVX-CoV2373 (Novavax); BNT162b2 (Pfizer); rAd26-S + rAd5-S (Gamaleya); ChAdOx1 nCoV-19 (AstraZeneca); Ad26.COV2.S (Johnson & Johnson); and CoronaVac (Sinovac) [8], [9]. Therefore, we sought available evidence to inform vaccine dosing intervals based on measurements of antibody responses post-vaccination for existing SARS-CoV2 vaccines in 2021, less than two years after the emergence of the pandemic coronavirus.
The published data on SARS-CoV2 vaccines being used widely or in advanced clinical trials according to the WHO and LSHTM vaccine trackers (as of March 5th 2021) were reviewed. The ChAdOx1 nCov-19 vaccine (AstraZeneca) is composed of a chimpanzee adenovirus vector with the full length SARS-CoV2 spike DNA inserted. Early phase 1 animal studies identified enhanced immunogenicity with a prime-boost strategy as opposed to a single dose regimen, as measured by antibody and T cell responses [10]. This was also demonstrated in human phase 1/2 studies [11]. The ChAdOx1 nCov-19 vaccine was studied in four clinical trials, three single-blinded and a double-blinded phase RCTs. Initial results from assessment of 11,636 UK and Brazilian adult participants, of which 4,440 received two doses of ChAdOx1 nCov-19 vaccine four weeks apart and 4,455 controls, demonstrated VE of 62.1% (95% CI 41.0–75.7) [3]. Subsequent, combined, pooled data analysis evaluated 17,178 participants (8,597 received ChAdOx1 nCov-19 vaccine; 8,581 received control vaccine) to assess VE and differences in dosing schedules, though this was not part of the trial design, and was a post-hoc exploratory analyses due to the practical reality of delays in vaccine manufacture during a global pandemic [6]. Comparison of prime-boost intervals with two-dose schedules demonstrated higher VE with longer intervals, though these had overlapping confidence intervals; VE of 55.1% (95% CI 33.0–69.9) with <0, 6 week interval; 59·9% (95% CI 32·0–76·4) with 0, 6–8 week interval; 63·7% (95% CI 28·0–81·7) with a 0, 9–11 week interval; and 81.3% (95% CI 60.3–91.2) with a ≥0, 12 week interval. This incremental VE with increasing prime-boost intervals positively correlated with GMTs of anti-SARS-CoV2 spike IgG binding antibody [6].
The BNT162b2 COVID-19 vaccine (Pfizer), the first mRNA vaccine approved for use, was licensed with priming doses 3–4 weeks apart. However, subsequent analysis of 503 healthcare workers in the UK, who had the doses spaced at 0, 2–5 weeks (n = 75) or 0, 6–14 weeks (n = 428) showed a two to four fold increase in neutralizing antibody titers, assessed one month after the second dose, in those with the longer interval, no participants had previous COVID-19 [12]. A similar boost to antibody responses was seen with a longer duration in a study of >80 year olds (n = 172) measured two weeks after the standard 0, 3–4 week and 0, 12 week intervals [13]. The other SARS-CoV2 vaccines had not reported any formal data on variable dosing intervals at the time of writing .
3. Priming dose intervals – what can be learnt from other vaccines
Vaccine immunogenicity studies are not generally designed to address specific questions about vaccine priming intervals, however, information can be obtained by analysing the effect of different intervals on immunogenicity. We reviewed vaccines targeting viruses and bacteria to assess the evidence for the effect of different dosing schedules and prime-boost intervals. The immune responses measured as part of randomised controlled trials (RCTs) designed and powered to assess vaccine responses using different dosing schedules were assessed and are summarised in Fig. 1, Fig. 2, Fig. 3, Fig. 4 .
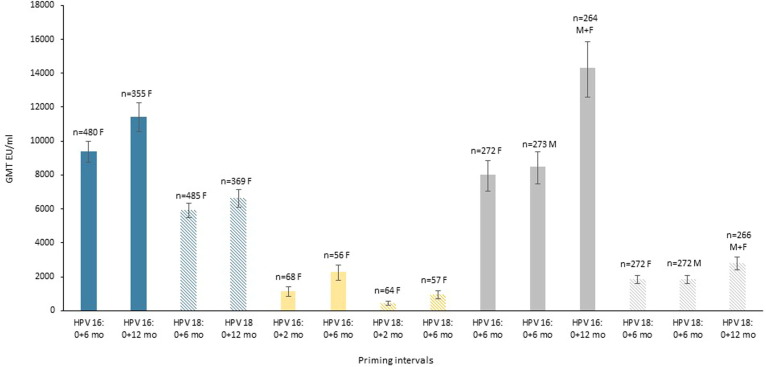
Fig. 1.
Immunogenicity as measured by geometric mean titres (GMT) post priming course, shown for HPV 16 (solid colour bar) and HPV18 (striped bar) at different priming intervals. Data from three trials are shown, teal represents the bivalent vaccine, HPV-16/18 AS04-adjuvanted vaccine (20 μg) Cervarix, GSK [16] GMT are one month post priming, yellow represents the bivalent vaccine, HPV-16/18 AS04-adjuvanted vaccine (40 μg), Cervarix, GSK [15] GMT are 24 months post priming, and grey represents nonavalent vaccine, Gardasil 9, Merck [17] GMT are one month post priming. All study participants were 9–14 years old. F = female, M = male. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
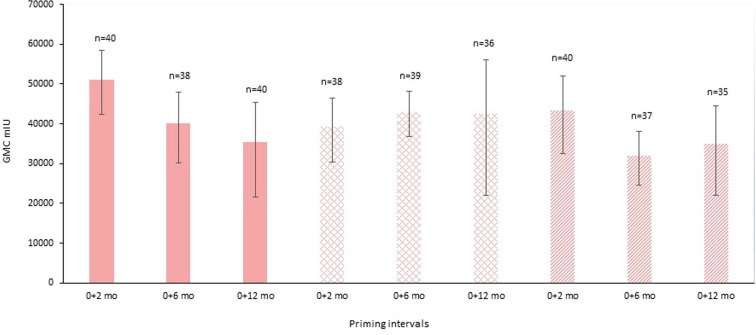
Fig. 2.
Immunogenicity as measured by geometric mean concentration (GMC) at one month post priming course, shown for different priming intervals of HZ/su, GSK, and at different age groups: 50–59 year old males and females (solid colour bars), 60–69 year old males and females (hatched bars), 70–79 year old males and females (striped bars), [22].
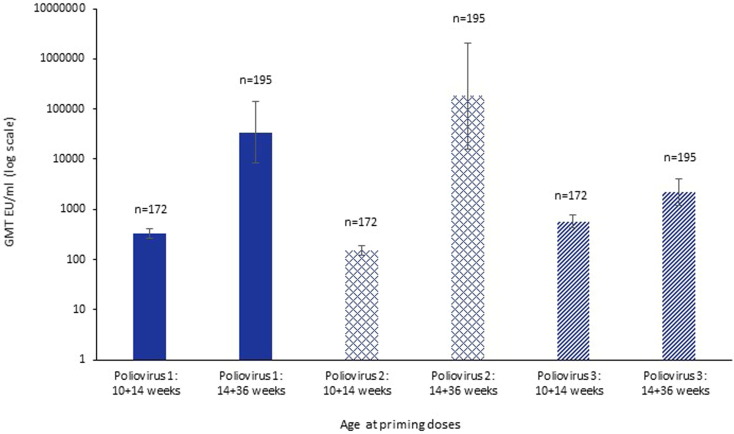
Fig. 3.
Immunogenicity as measured by geometric mean titres (GMT) one month post priming course of trivalent inactivated poliovirus vaccine given to six week old healthy infants using two schedules of 10 and 14 weeks of age or 14 and 36 weeks of age [23], for different poliovirus types: type 1 (solid colour bars), type 2 (hatched bars), type 3 (striped bars).
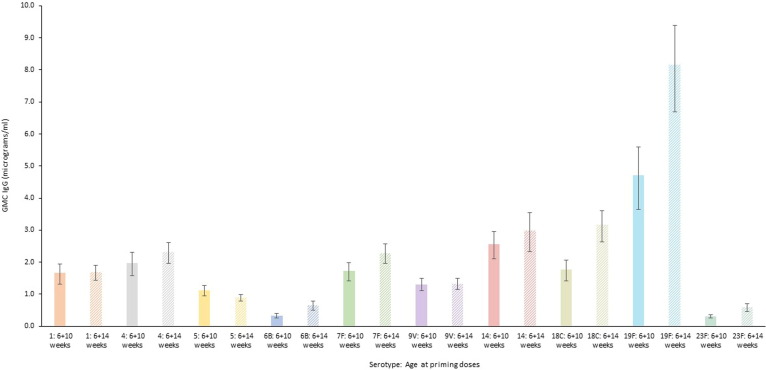
Fig. 4.
Immunogenicity as measured by geometric mean concentration (GMC) one month post priming course of the 10 valent pneumococcal conjugate vaccine (PCV10) when given to healthy infants aged 40–60 days old in two dosing schedules: 6 and 10 weeks of age (solid colour bars) and 6 and 14 weeks of age (striped bars) [28]. Four serotypes (6B, 18C, 19F, 23F) reached statistical significance p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For human papillomavirus (HPV) vaccines, which prevent genitourinary infection and subsequent risk of cervical cancer, antibody levels differed depending on the number of vaccine doses received, though seropositivity was high in all HPV vaccine recipients regardless of number of doses. Overall, longer dosing intervals were more immunogenic in the short (one month post priming) and longer term (24 months post priming), assessed by three separate studies [14], [15], [16], [17]. When bivalent and nonavalent HPV vaccine dosing intervals were assessed in young women and men, there was higher (non-inferior) immunogenicity with the longer interval (0, 12 months) compared to shorter intervals (0, 6 months) at short-term follow up (one month post priming) (Fig. 1). There was a 22% (HPV 16) and 12% (HPV 18) increase in GMT with the 0, 12 month intervals of Cervarix (GSK) in 9–14 year old girls, though the titres for HPV 18 had overlapping confidence intervals [8]. There was a 73% (HPV 16) and 50% (HPV 18) increase in GMT with the 0, 12 month schedule of Gardasil 9 (Merck) for 9–14 year old girls and boys [9]. At longer-term (24 months) follow up, a 0, 6 month interval of Cervarix (GSK) demonstrated higher titers in vaccinees than 0, 2 month schedules by 94% (HPV 16) and 117% (HPV 18), though this study had fewer participants and did not assess a 0, 12 month schedule [15]. These findings suggest that a longer priming intervals increase the antibody titres, by varying significant and non-significant extents in different studies, and this increase may impact the potential longevity of antibody mediated responses as waning starts from a higher antibody level. There is similar evidence for hepatitis A virus (HAV) vaccines, though not examined in RCTs, whereby a two-dose schedule is routinely given six months apart [18]. Observational studies identified that when the second HAV vaccine dose was delayed in adolescents and adults by between 20 to 77 months, antibody responses to the booster dose were not reduced, providing evidence for persistent immune memory [19], [20], [21].
Varicella zoster virus (VZV) vaccines can contribute to reducing the burden of herpes zoster and associated post-herpetic neuralgia in the elderly population, whose natural cellular immunity has waned. Although antibody responses are only a reflection of the boost the vaccines give cellular immunity, they do serve as an indicator of efficacy. The evidence from VZV subunit vaccines suggests that longer intervals between doses may not enhance antibody levels but can be non-inferior to shorter intervals, though intervals are limited to a maximum of six months. A 0, 6 month schedule using the subunit VZV vaccine (HZ/su, GSK) was identified to be non-inferior to 0, 2 month dosing, but the longer interval of 0, 12 month was inferior at four weeks post-vaccination across all three age groups examined (Fig. 2) [22]. As the age of participants varied in these studies this may be important to consider in the context of immunosenescence as well as the potential benefits of short-term versus long-term protection in this particular population.
Inactivated poliovirus (IPV) vaccines have largely been used in high-income countries, but there is an increasing need for their use in conjunction with live oral poliovirus vaccines with potential global polio elimination in sight. Hence, optimising scheduling of these vaccines is important in low- and middle-income settings. Multiple dosing schedules were assessed in six-week old infants in Panama and in the Dominican Republic. Giving two doses later and further apart was superior for two of the three poliovirus types. A study showed that GMTs measured four weeks after second doses for a two-dose strategy at 14 and 36 weeks of age (0, 22 weeks) were substantially higher than two doses given at 10 and 14 weeks of age (0, 4 weeks) for poliovirus type 1 (329.3 (264.7–409.5) vs 34397.9 (8508.1–139070.1)), poliovirus type 2 (150.7 (119.0–190.9) vs 183211.9 (16152.3–2078134.4)), and poliovirus type 3 (569.2 (424.1–764.0) vs 2194.5 (1212.3–3972.5)) (Fig. 3) [23]. The only setting where a three-dose schedule (10, 14, 36 weeks) gave better immunogenicity than the two dose 14, 36 weeks schedule, was for type 3 poliovirus for which overall GMTs at 40 weeks was higher, but the two-dose 14, 36 weeks regimen was optimal for type 1 and 2 polioviruses. In this study, it was in not possible to fully determine whether the improved responses were due to the infants increased age at 14 and 36 weeks compared to those at 10 and 14 weeks, or the effect of reduced maternal antibody levels at the later 36 week timepoint.
Vaccines against bacterial infections can also have varying efficacy with alternative dosing schedules that may be required in different global settings. Pneumococcal conjugate vaccines (PCV) are part of routine infant immunisation programs recommended by the World Health Organization as either 2 + 1 or 3 + 0 options, with implementation decisions based on the epidemiology of invasive pneumococcal disease and programmatic design [24]. Two-month spacing of the priming interval in the prime-boost regimen is preferred in infants [25], [26], [27]. However, for programmatic reasons, different PCV10 priming dosing in children in Nepal was investigated, suggesting that priming doses given at six and ten weeks of age (0, 1 month schedule) were non-inferior to a schedule of six and 14 weeks of age (0, 2 month schedule) following a booster dose at nine months, but until the boosting dose a two month priming interval showed better immunogenicity against six of ten serotypes examined (1, 4, 6B, 7F, 18C, and 23F). Geometric mean concentrations favoured a 0, 2 month schedule for nine serotypes (Fig. 4), of which four serotypes (6B, 18C, 19F, 23F) reached statistical significance [28]. The heterogeneity of the pneumococcal serotype-specific responses is a well-recognised phenomenon, for reasons that are incompletely understood but could include the role of breastfeeding, maternal antibody and infant demographics [29].
4. Discussion
Here we have summarised available evidence on the influence of vaccine priming intervals on immune responses and efficacy of vaccines. For many vaccines, dosing intervals have not been specifically tested and not in the optimal RCT setting. The changing nature of disease epidemiology and improvements in understanding infectious disease pathogenesis meant that altering and optimising vaccine interval dosing after licensure may be warranted. For regulatory reasons, demonstration of non-inferiority of new regimens compared to existing ones is important in this regard. Despite the lack of RCTs to specifically assess dosing intervals, analysis of the available data from both vaccines against SARS-CoV2 and against other infections are consistent in that longer intervals between first and second doses result in better priming and higher antibody titers post-dose two, at least if the second dose is given within six months. Of course, considerations of public health and adherence to booster doses weigh heavily in the decision regarding optimal schedules. Further, the timing of booster doses should also follow this argument and be given six months after initial priming.
An important caveat to many vaccine efficacy studies is that correlates of protection against clinical infection are not always well-defined, for example antibody level versus immune memory as a marker for preventing clinical infection. In this review,the use of antibody levels as a marker of protection against SARS-CoV2 infection was used due to the growing evidence that this may be a reliable correlate of protection [9]. Further, antibody levels are relatively easy to quantify and have served as a strong surrogate for protection through alternative immune mechanisms. The host T-cell mediated response has also been important for many of the vaccines discussed here, SARS-CoV2 included, but this has not been measured or assessed in this paper with respect to priming intervals.
Another consideration of the data presented here, is that the viral and bacterial vaccines assessed vary in terms of their constituents and vaccine platform design, i.e. HPV and VZV vaccines are subunit vaccines, poliovirus vaccines are inactivated (killed) vaccines, and pneumococcal vaccines are conjugate polysaccharides which all lead to different immune responses which may alter with variably timed dosing intervals. The SARS-CoV2 vaccines represent an array of novel and established vaccine platforms and we continue to learn about the immune responses mediated to SARS-CoV2 especially by novel mRNA vaccines. Nevertheless, data published to date suggest that in the short-term prevention may be better served by giving one dose to as many as possible where vaccine supply is inadequate, but the general conclusion that longer intervals between doses increase immunogenicity as measured by antibody levels, has implications on global supply of available vaccines and distribution to those most in need.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Stanley Plotkin reports a relationship with Major vaccine manufacturers and biotechnology companies that includes: consulting or advisory.
Acknowledgements
No formal acknowledgements.
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horby P, Huntley C, Davies N, Edmunds J, Ferguson N, Medley G, et al. NERVTAG note on B.1.1.7 severity. In: Scientific Advisory Group for Emergencies, editor; 2021.
- 5.Department of Health & Social Care. Optimising the COVID-19 vaccination programme for maximum short-term impact. UK: UK Government; 2021.
- 6.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Cold Spring Harbor Laboratory; 2021. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 9.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham S.P., McLean R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines. 2020;5(1) doi: 10.1038/s41541-020-00221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 12.Payne R, Longet S, Austin J, Skelly D, Dejnirattisai W, Adele S, et al. S ustained T cell immunity, protection and boosting using extended dosing intervals of BNT162b2 mRNA vaccine; 2021. [DOI] [PMC free article] [PubMed]
- 13.Parry H., Bruton R., Stephens C., Brown K., Amirthalingam G., Hallis B., et al. Cold Spring Harbor Laboratory; 2021. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman H., Buckley B.S., Villanueva G., Petkovic J., Garritty C., Lutje V., et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romanowski B., Schwarz T.F., Ferguson L.M., Peters K., Dionne M., Schulze K., et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. 2011;7:1374–1386. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puthanakit T., Huang L.-M., Chiu C.-H., Tang R.-B., Schwarz T.F., Esposito S., et al. Randomized Open Trial Comparing 2-Dose Regimens of the Human Papillomavirus 16/18 AS04-Adjuvanted Vaccine in Girls Aged 9–14 Years Versus a 3-Dose Regimen in Women Aged 15–25 Years. J Infect Dis. 2016;214(4):525–536. doi: 10.1093/infdis/jiw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iversen O.-E., Miranda M.J., Ulied A., Soerdal T., Lazarus E., Chokephaibulkit K., et al. Immunogenicity of the 9-Valent HPV Vaccine Using 2-Dose Regimens in Girls and Boys vs a 3-Dose Regimen in Women. JAMA. 2016;316(22):2411. doi: 10.1001/jama.2016.17615. [DOI] [PubMed] [Google Scholar]
- 18.Whitworth H.S., Schiller J., Markowitz L.E., Jit M., Brisson M., Simpson E., et al. Continued HPV vaccination in the face of unexpected challenges: A commentary on the rationale for an extended interval two-dose schedule. Vaccine. 2021;39(6):871–875. doi: 10.1016/j.vaccine.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Iwarson S., Lindh M., Widerström L. Excellent booster response 4–6 y after a single primary dose of an inactivated hepatitis A vaccine. Scand J Infect Dis. 2002;34(2):110–111. doi: 10.1080/00365540110077362. [DOI] [PubMed] [Google Scholar]
- 20.Landry P., Tremblay S., Darioli R., Genton B. Inactivated hepatitis A vaccine booster given >/=24 months after the primary dose. Vaccine. 2000;19:399–402. doi: 10.1016/s0264-410x(00)00188-2. [DOI] [PubMed] [Google Scholar]
- 21.Williams J., Fox-Leyva L., Christensen C., Fisher D., Schlicting E., Snowball M., et al. Hepatitis A vaccine administration: comparison between jet-injector and needle injection. Vaccine. 2000;18(18):1939–1943. doi: 10.1016/s0264-410x(99)00446-6. [DOI] [PubMed] [Google Scholar]
- 22.Lal H., Poder A., Campora L., Geeraerts B., Oostvogels L., Vanden Abeele C., et al. Immunogenicity, reactogenicity and safety of 2 doses of an adjuvanted herpes zoster subunit vaccine administered 2, 6 or 12 months apart in older adults: Results of a phase III, randomized, open-label, multicenter study. Vaccine. 2018;36(1):148–154. doi: 10.1016/j.vaccine.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Bandyopadhyay A.S., Gast C., Rivera L., Sáez-Llorens X., Oberste M.S., Weldon W.C., et al. Safety and immunogenicity of inactivated poliovirus vaccine schedules for the post-eradication era: a randomised open-label, multicentre, phase 3, non-inferiority trial. Lancet Infect Dis. 2021;21(4):559–568. doi: 10.1016/S1473-3099(20)30555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney C.G., Goldblatt D., O'Brien K.L. Dosing schedules for pneumococcal conjugate vaccine: considerations for policy makers. Pediatr Infect Dis J. 2014;33(Suppl 2):S172–S181. doi: 10.1097/INF.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spijkerman J., Veenhoven R.H., Wijmenga-Monsuur A.J., Elberse K.E.M., van Gageldonk P.G.M., Knol M.J., et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine administered according to 4 different primary immunization schedules in infants: a randomized clinical trial. JAMA. 2013;310(9):930. doi: 10.1001/jama.2013.228052. [DOI] [PubMed] [Google Scholar]
- 26.Fleming-Dutra K.E., Conklin L., Loo J.D., Knoll M.D., Park D.E., Kirk J., et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J. 2014;33(Supplement 2):S152–S160. doi: 10.1097/INF.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldblatt D., Southern J.o., Ashton L., Richmond P., Burbidge P., Tasevska J., et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25(4):312–319. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 28.Kandasamy R., Gurung M., Thorson S., Yu L.-M., Galal U., Voysey M., et al. Comparison of two schedules of two-dose priming with the ten-valent pneumococcal conjugate vaccine in Nepalese children: an open-label, randomised non-inferiority controlled trial. Lancet Infect Dis. 2019;19(2):156–164. doi: 10.1016/S1473-3099(18)30568-1. [DOI] [PubMed] [Google Scholar]
- 29.Lipsitch M., Li L.M., Patterson S., Trammel J., Juergens C., Gruber W.C., et al. Serotype-specific immune responses to pneumococcal conjugate vaccine among children are significantly correlated by individual: Analysis of randomized controlled trial data. Vaccine. 2018;36(4):473–478. doi: 10.1016/j.vaccine.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]