Dear Editor,
Since December 2019, when the first cases were reported in China until now the novel SARS-CoV-2 coronavirus has caused the death of more than 1,000,000 people around the world. In addition to the, already known, manifestations associated with the infection, other less common signs and symptoms are emerging that are important due to the potentially fatal nature.1, 2 We report three patients with chronic kidney disease (CKD) on renal replacement therapy (RRT) who suffered a stroke in relation to a COVID-19 infection. Between 10 March and 25 April 2020, 16 patients on RRT (one on peritoneal dialysis [PD]) positive for SARS-CoV-2 were admitted to our department. Of the 16 patients, 3 (18.7%) suffered a stroke and one of them died.
Patient 1: 85-year-old man with CKD secondary to nephroangiosclerosis, on RRT since 2014 (initially on PD and since 2017 on haemodialysis [HD]).The patient had hypertension, non-ischaemic dilated cardiomyopathy and severe chronic obstructive pulmonary disease and had suffered an atherothrombotic stroke two weeks earlier. He was admitted for moderate unilateral pneumonia due to SARS-CoV-2 and received hydroxychloroquine and lopinavir/ritonavir according to protocol. On the sixth day of admission, while following a suitable respiratory clinical course, he presented an episode of right hemiparesia and reduced consciousness from which he recovered hours later. Given his prior atherothrombotic stroke with the same signs and symptoms, the same location was assumed, and given that the patient was a candidate for conservative treatment with antiplatelet therapy only (which he was already receiving), imaging test was no repeated. On the eighth day, prior to the dialysis session, he presented an identical episode; respiratory impairment was observed and laboratory testing revealed significantly increased C-reactive protein (CRP) levels, worsening of lymphopenia and elevated D-dimer levels. The patient did not recover and died within 24 h. We believe that his first cerebrovascular accident may have been his initial presentation of COVID-19 two weeks before his pneumonia; this would be in line with other published series.3, 4, 5
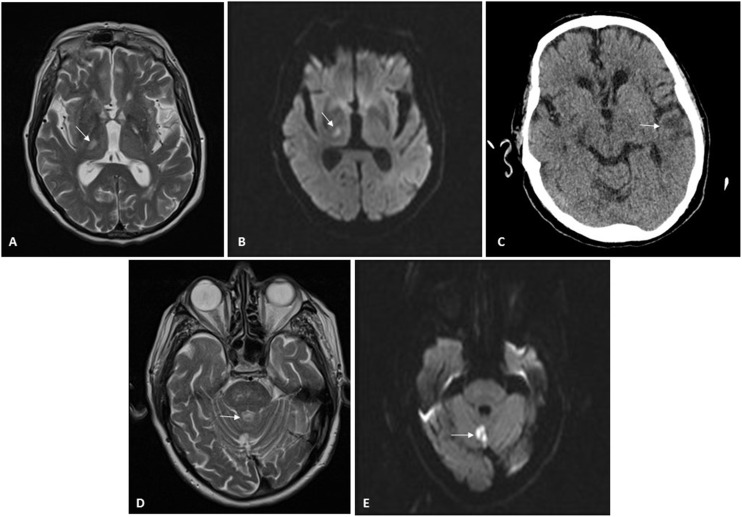
Patient 2: A 71-year-old man with CKD secondary to chronic interstitial nephropathy due to obstructive uropathy caused by urothelial bladder carcinoma. He had been on HD since 2015 and had experienced multiple problems with vascular access. At the time of his admission, he had a femoral Permcath. He had hypertension, squamous cell lung carcinoma and moderate to severe left ventricular dysfunction. He was admitted for diarrhoea, fever and mild bilateral pneumonia due to SARS-CoV-2. He received hydroxychloroquine for five days and lopinavir/ritonavir for seven days. He had a favourable clinical and laboratory course, except for a gradual increase in D-dimer up to six fold after the conclusion of the immunomodulatory therapy on the ninth day of admission. Hence, due to the high risk of thrombosis and according to protocol, we started heparin at 1 mg/kg/day. Few days later, the patient was discharged and was kept on heparin for two more weeks. Two weeks after heparin was suspended, he was admitted due to a sensory deficit of the left side of the body. As shown in Fig. 1 it was observed a right thalamic lacunar subacute stroke with no evidence of potentially embolic heart disease or carotid atheromatosis (Fig. 1). Antiplatelet therapy was added, and 11 days later, he was readmitted due to a second stroke, this time in the region of the left middle cerebral artery, with aphasia without a recovery. At the time of this writing, he remains in hospital.
Figure 1.
Neuroradiology imaging. A) MRI of patient 2. Hyperintense lesion on T2-weighted sequences located in lateral nuclei of the thalamus/posterior limb of the right internal capsule, consistent with lacunar infarction. B) MRI of patient 2. Restricted diffusion sequence imaging confirming stroke from prior image with an acute/subacute course. C) CT scan of patient 2. Cortical/subcortical hypodensity in the left temporal region consistent with ischaemic lesion. D) MRI of patient 3. Hyperintense lesion on T2-weighted sequences located in the cerebellar vermis consistent with infarction. E) MRI of patient 3. Diffusion-weighted imaging identifying a focus of signal abnormality in the cerebellar vermis, which restricts on an apparent diffusion coefficient (ADC) map, consistent with acute/subacute infarction in the location reported on T2-weighted imaging in the prior image.
Patient 3: An 80-year-old man with a single functional kidney due to ischaemia that had been on PD since 2015. He suffered from grade 2A chronic ischaemia of both legs and had undergone a carotid endarterectomy in 2011. He was admitted for moderate bilateral pneumonia due to SARS-CoV-2 and treated with hydroxychloroquine for five days and lopinavir/ritonavir for seven days. He progressed favourably and was discharged two weeks after admission; prophylactic heparin was continued for another 10 days. Twenty-four hours after discharge, he presented a rash with papular erythematous lesions on his trunk that mostly spared his legs and spared his arms entirely. He was assessed by dermatology, which, after biopsy, diagnosed him with exanthema probably secondary to COVID-19. After two weeks of antithrombotic prophylaxis, heparin was suspended. Five days later, the patient developed monoparesis of the right leg of sudden onset. He was admitted to Neurology, and magnetic resonance imaging revealed an acute infarction in the cerebellar vermix (Fig. 1). An ultrasound showed no evidence of embolic heart disease, and a neurosonology study found no evidence of intracranial or extracranial stenosis. The patient recovered mobility, remained asymptomatic, and he was discharged (Table 1 ).
Table 1.
Demographic, laboratory and clinical data.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age (years) | 85 | 71 | 80 |
| Gender | M | M | M |
| HTN | Yes | Yes | Yes |
| DM | No | No | No |
| DL | No | No | No |
| Ischaemic cardiopathy | No | No | No |
| Peripheral arteriopathy | Yes | No | Yes |
| Prior CVA | Yes | No | No |
| Atrial fibrillation | No | No | No |
| Artificial valve | No | No | No |
| Prior anticoagulation | No | No | No |
| Prior kidney transplant | No | No | No |
| Time on renal replacement therapy (years) | 5.3 | 4.4 | 4.4 |
| Renal replacement therapy type | HD | HD | PD |
| Venous access | AVF | Permanent catheter | N/A |
| EPO (IU/week) | 2000 | 15,000 | 6000 |
| ASA | Yes | Yes | Yes |
| Pneumonia | Moderate unilateral | Mild bilateral | Moderate bilateral |
| Days of admission | 9 | 12 | 15 |
| ICU admission | No | No | No |
| Immunomodulatory therapy | Hydroxychloroquine, lopinavir/ritonavir | Hydroxychloroquine, lopinavir/ritonavir | Hydroxychloroquine, lopinavir/ritonavir |
| Duration of immunomodulatory therapy (days) | 9 | 5 + 7 | 5 + 7 |
| Treatment with steroids | No | No | No |
| Prophylactic heparin on admission (mg/24 h) | 20 | 20 | 20 |
| High risk of thrombosis (D-dimer >6 times monitoring level) | No | Yes (day 9) | No |
| Dose of heparin due to high risk of thrombosis (mg/day) | N/A | 60 | N/A |
| Duration of intermediate dose heparin treatment (days) | 0 | 18 | 0 |
| Total heparin treatment duration (days) | 9 | 27 | 25 |
| CRP (mg/l) on admission/maximum (ref. 0.0−0.5) | 87.1/216 | 85.7/116.1 | 15.9/103.4 |
| Ferritin (μg/l) on admission/maximum (ref. 30.0–400.0) | NA | 454/502 | 236/328 |
| LDH (U/l) on admission/maximum (ref. 135−225) | 228/249 | 229/313 | 160/194 |
| Hb (g/dl) on admission (ref. 13.0−17.5) | 12 | 10.9 | 10.8 |
| Platelets (103/μl) on admission (ref. 150−450) | 222 | 130 | 134 |
| Lymphopenia (103/μl) on admission/maximum (ref. 1.00−4.50) | 520/180 | 1007/780 | 1222/760 |
| D-dimer (mg/l) on admission/maximum (ref. <0.5) | 3.2/3.2 | 2.1/5.3 | 1.11/1.11 |
| Fibrinogen (mg/dl) on admission/maximum (ref. 200.0−400.0) | NA | 536/565 | 456/956 |
| Time from onset of symptoms to CVA (days) | 16 | 40 | 31 |
| Time from SARS-CoV-2 diagnosis to CVA (days) | 9 | 38/52 | 30 |
| Time from virus CRP becoming negative to CVA (days) | N/A | 17/31 | 16 |
| Time from end of heparin to CVA (days) | N/A | 15/29 | 5 |
| CVA type | Ischaemic | Ischaemic/ischaemic | Ischaemic |
| Location | M2 branch of left MCA | Right thalamic lacunar/left MCA | Cerebellar vermis |
| Other late signs of COVID-19 | No | No | Exanthema |
| Death | Yes | No | No |
5 + 7: five days of hydroxychloroquine and seven days of lopinavir/ritonavir; ASA: acetylsalicylic acid; AVF: arteriovenous fistula; CRP: C-reactive protein; CVA: cerebrovascular accident; DL: dyslipidaemia; DM: diabetes mellitus; EPO: erythropoietin; HD: haemodialysis; HTN: arterial hypertension; ICU: intensive care unit; LDH: lactate dehydrogenase; MCA: middle cerebral artery; N/A: not applicable; NA: not available; PD: peritoneal dialysis; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Although the specific incidence of stroke in patients with COVID-19 is unknown, the literature on this subject is growing exponentially.1 According to Mao et al.,6 in a cohort of 214 patients admitted for SARS-CoV-2, 5.7% presented stroke (in the final stage of the infection). Li et al.7 recently published an incidence of 5% (in early stages of the infection) in patients with risk factors such as hypertension, diabetes, coronary disease and stroke. A series by Beyrouti et al.4 reported six cases of stroke with moderate to severe COVID-19 that presented between day 0 and day 24 after the onset of symptoms. Lodigiani et al.8 compiled a cohort of 388 patients hospitalised due to SARS-CoV-2 and found an incidence of ischaemic stroke of 2.5%. Although the pathophysiological mechanisms are not clear,4 the factors that appear to be involved are: hypoxia, an hypercoagulable state,3 disseminated intravascular coagulation, thrombotic microangiopathy and occasional positivity for anticardiolipin antibodies and lupus anticoagulant appear.9, 10 There are no references in the literature in the dialysis population, which means that this is the first publication to address this matter; the findings point to a probable higher incidence than in the general population.
Footnotes
Please cite this article as: de Lorenzo A, Espinel L, Revilla A, Corbalán T, Martins J, Naya MT, et al. Ictus isquémico asociado a COVID-19 en pacientes en diálisis. Nefrologia. 2021;41:590–593.
References
- 1.Hess D.C., Eldahshan W., Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11:322–325. doi: 10.1007/s12975-020-00818-9. [Epub ahead of print] PubMed PMID: 32378030; PubMed Central PMCID: PMC7202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal G., Lippi G., Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;20 doi: 10.1177/1747493020921664. 1747493020921664. [DOI] [PubMed] [Google Scholar]
- 3.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., Glaser A., et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.077. pii: S0889-1591(20)30685-1. [Epub ahead of print] PubMed PMID: 32360439; PubMed Central PMCID: PMC7187846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. pii: jnnp-2020-323586. [Epub ahead of print] PubMed PMID: 32354768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 doi: 10.1056/NEJMc2009787. [Epub ahead of print] PubMed PMID: 32343504; PubMed Central PMCID: PMC7207073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;10:e201127. doi: 10.1001/jamaneurol.2020.1127. [Epub ahead of print]. PubMed PMID: 32275288. PubMed Central PMCID: PMC7149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Wang M, Zhou Y, Chang J. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. [Accessed 2 May 2020]. Available from: https://ssrn.com/abstract=3550025. [DOI] [PMC free article] [PubMed]
- 8.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [Epub ahead of print] PubMed PMID: 32353746; PubMed Central PMCID: PMC7177070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]