Abstract
Haploidentical stem cell transplantation (haploSCT) has advanced to a common procedure for treating patients with hematological malignancies and immunodeficiency diseases. However, cure is seriously hampered by cytomegalovirus (CMV) infections and delayed immune reconstitution for the majority of haploidentical transplant recipients compared to HLA-matched stem cell transplantation. Three major approaches, including in vivo T-cell depletion (TCD) using antithymocyte globulin for haploSCT (in vivo TCD-haploSCT), ex vivo TCD using CD34 + positive selection for haploSCT (ex vivo TCD-haploSCT), and T-cell replete haploSCT using posttransplant cyclophosphamide (PTCy-haploSCT), are currently used worldwide. We provide an update on CMV infection and CMV-specific immune recovery in this fast-evolving field. The progress made in cellular immunotherapy of CMV infection after haploSCT is also addressed. Groundwork has been prepared for the creation of personalized avenues to enhance immune reconstitution and decrease the incidence of CMV infection after haploSCT.
Keywords: cytomegalovirus, infection, immune reconstitution, haploidentical, stem cell transplantation
Introduction
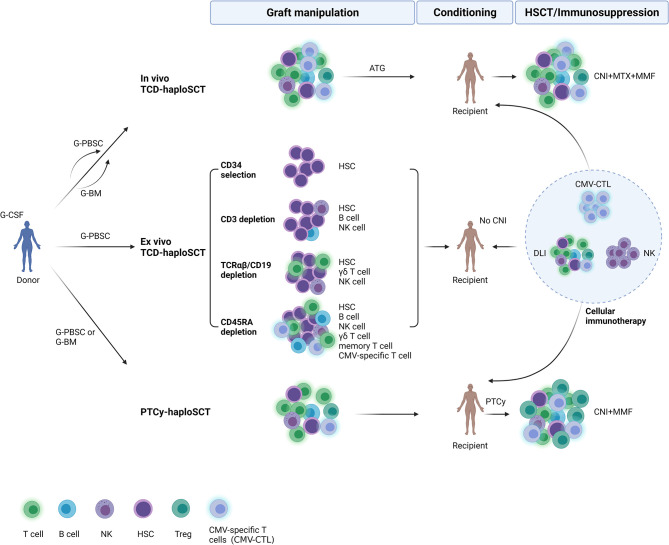
HLA-haploidentical stem cell transplantation (haploSCT) has spread rapidly worldwide in recent years. HLA-haploidentical donors sharing a single HLA haplotype with transplant recipients are almost always available, so haploSCT can be performed for patients who are lacking HLA-matched donors and/or are urgently needing transplantation. The major approaches for T-cell depletion are in vivo T-cell depletion using antithymocyte globulin (ATG) (in vivo TCD-haploSCT), ex vivo T-cell depletion (TCD) using CD34 + positive selection (ex vivo TCD-haploSCT), and T-cell replete haploSCT using posttransplant cyclophosphamide (PTCy-haploSCT). Compared with HLA-identical sibling transplantation, patients undergoing haploSCT usually receive more intensive immunosuppressors to guarantee engraftment and later prevent graft-versus-host disease (GVHD). Therefore, these patients always have impaired immune reconstitution after transplantation and a high incidence of CMV infection and CMV disease ( Figure 1 ). As the use of haploidentical transplantation has increased substantially, we summarize current data on CMV infection and its immune reconstitution after haploSCT during the last decade.
Figure 1.
Overview of immune reconstitution to cytomegalovirus and cellular immunotherapy after three major approaches of haploidentical stem cell transplantation (haploSCT). In vivo TCD-haploSCT, in vivo T-cell depletion (TCD) using antithymocyte globulin for haploSCT; Ex vivo TCD-haploSCT, ex vivo TCD using CD34 + positive selection for haploSCT; PTCy-haploSCT, T-cell replete haploSCT using posttransplant cyclophosphamide. G-CSF, granulocyte-colony stimulating factor; G-PBSC, G-CSF primed peripheral blood stem cells; G-BM, G-CSF primed bone marrow; HSC, hematopoietic stem cell; CMV, cytomegalovirus; CNI, calcineurin inhibitors; MTX, methotrexate; MMF, mycophenolate mofetil; DLI, donor lymphocyte infusion; NK cell, natural killer cell; Treg, regulatory T cell; HSCT, hematopoietic stem cell transplantation. Created with BioRender (https://biorender.com/).
Incidence of Cytomegalovirus Infection After haploSCT
In Vivo TCD-haploSCT (Anti-Thymocytic Globulin/ATG-Based)
Using the Beijing protocol at Peking University (1–7), there was a high incidence of CMV reactivation early after haploSCT (59.5-66%), whereas the rate of CMV disease was actually low (2.92-17%). CMV DNAemia was initially detected after a median of 35 days with a mean duration of positivity of 15 days (5, 6). Most (91.2%) cases of CMV gastroenteritis developed within 100 days, whereas most (90.3%) cases of CMV retinitis were late onset with the cumulative incidence of CMV retinitis at 2.3% one year (a median onset of 167 days) after haploSCT (6, 7). Einat Shmueli et al. from Israel designed a conditioning protocol for haploSCT including fludarabine, thiotepa, anti-thymocytic globulin, and total body irradiation (8). After receiving preemptive therapy, the incidence of CMV infection was 66.7% in haploSCT, and 11.6% of haploSCT transplant recipients with CMV reactivation developed CMV disease. Importantly, drug-resistance mutations and clinically suspected resistance were discovered only in haploSCT recipients (8), favoring prophylactic over preemptive treatment in high-risk patients and highlighting the need for better anti-CMV drugs.
It remains unclear whether primary disease affects CMV infection after haploSCT. Lan‐Ping Xu et al. from Peking University conducted studies to confirm the feasibility of haploidentical transplantation in patients with severe aplastic anemia (SAA) as salvage therapy (9–12). CMV viremia occurred in 51.7~84.00% of SAA patients. However, no difference in the rates of early CMV disease between haploidentical patients and matched related patients was found (9, 10). Consistently, several centers in China obtained similar results for SAA patients (13–15). The haploSCT cohorts with AML, MDS, or Ph+ ALL, including haplo-cord-HSCT, had higher CMV viremia than the HLA-matched HSCT cohorts (16–19), but the incidence of CMV disease was not significantly different between the two groups. Even in pediatric patients with MDS or patients with relapsed/refractory acute lymphoblastic leukemia after CAR-T therapy who underwent haploSCT, the incidence of CMV reactivation/infection was less than 60%, and very few patients developed CMV disease (20, 21).
Using a similar protocol, several transplant centers have reported promising results for unmanipulated haploidentical peripheral blood stem cell transplantation (PBSCT) (22, 23) or cotransplantation of unrelated cord blood (UCB) (24–26) or mesenchymal stem cells (MSCs) (27, 28). The 1-year cumulative incidence of CMV DNAemia in patients with hematologic malignancies was 23.5-41.7% in the matched sibling donor (MSD)-SCT group versus 62.1-81.0% in the haploSCT group with peripheral blood stem cells (PBSCs) (29, 30). The median time to the onset of CMV DNAemia in the haploSCT group was 33 days (range, 10–159 days) with the 1-year cumulative incidence of CMV disease at 7.9% (95% CI, 3.6–14.3%) (29). In addition, a total of 19.4%-92% of these patients experienced CMV reactivation after combination of haploSCT with UCB or MSCs (24–28). There was no statistical significance in the incidence of CMV viremia in terms of haplo-cord SCT vs HLA-matched donor SCT (MD-SCT) or haplo-cord SCT vs haploSCT (24–26).
As the use of ATG as a regimen for in vivo TCD and immunosuppressants is limited by impaired immune restoration and a high risk of severe infections, researchers are working on their impact after haploSCT. Peking University performed a study comparing 6 mg/kg ATG versus 10 mg/kg ATG in patients who underwent haploSCT (31). The 1-year cumulative incidence of CMV reactivation was similar between the ATG-6 and ATG-10 groups[(75.0% (66.8–83.2%) vs 78.6% (75.2–82.0%)]. Another multicenter study investigated the impact of 7.5 mg/kg and 10.0 mg/kg rabbit ATG on GVHD and virus reactivation after haploSCT (32). The 1-year incidence of CMV DNAemia was higher in the 10.0 mg/kg group [83.4% (77.5-87.9)] than in the 7.5 mg/kg group [73.4% (67.2-79.4)], whereas the 2-year incidence of CMV-associated diseases was also higher in the 10.0 mg/kg group [5.9% (3.2–9.7%)] than in the 7.5 mg/kg group [1.5% (0.4–4.0%)]. Yu Wang et al. recently extended follow-up from this original trial (33). They found that patients undergoing haploSCT benefit from 7.5 mg/kg ATG compared to 10.0 mg/kg ATG based on a balance between GVHD and infection control. The data supports ATG (7.5 mg/kg) is potentially the standard regimen in this platform. Researchers from Japan and the Republic of Korea later performed haploSCT using low-dose thymoglobulin at 5 mg/kg (34, 35). CMV reactivated in 41.67% and 72.7% of patients, but CMV disease developed in 0 and 19.4% of patients, respectively. A recent report from the Republic of Korea indicated that the cumulative incidence of CMV DNAemia at 3 years was 45.7% (30.7-59.4) for ATG (5-10 mg/kg)-based haploSCT (36). Moreover, a short-term tacrolimus regimen for the prophylaxis of GVHD in haploSCT did not increase the incidence of CMV infection compared with the Cyclosporine A regimen (39.5% vs. 37.5%, p = 0.783) (37).
Ex Vivo TCD-haploSCT
CD34+ selection was initially used as a method for TCD, but it resulted in delayed immune reconstitution and a high incidence of opportunistic infections and nonrelapse mortality. The ex vivo TCD techniques have developed from CD34+ selection, CD3+ cell depletion, and αβ+/CD19+ cell depletion to recent CD45RA+ depletion. Compared with CD34+ cell selection, after CD3+ cell depletion, the graft has more natural killer (NK) cells, monocytes, and other immunomodulating cells with better outcomes. Sameh Gaballa et al. retrospectively compared data on patients undergoing a two-step (a fixed T cell infusion followed 2 days later by cyclophosphamide, and then a CD34-selected stem cell product infused) haploidentical or matched related PBSCT for high-risk hematological malignancies and aplastic anemia (38). Compared with the matched related PBSCT group (matched related, 19%), the 100-day cumulative incidence of CMV viremia was higher in the haploidentical group (haploidentical, 67%). The median time to develop CMV reactivation was 26 days in the haploSCT group and 36 days in the matched related PBSCT group.
The cumulative incidence of CMV DNAemia in patients with acute leukemia was 73.5-81% after ex vivo αβ T cell-depleted haploSCT (39, 40). No patient developed CMV disease or died (39). A more recent study explored the role of interim-foscarnet prophylaxis in preventing CMV infection after ex vivo αβ T cell-depleted haploSCT in children between May 2012 and May 2018 (41). Forty (50.8%) of 81 patients developed CMV reactivation at a median of 41.3 days (range, 13–132) after haploSCT. The median duration of CMV reactivation was 28.5 days (range, 1–179), and the peak PCR level was 3.82 log copies/mL (range, 2.85–6.03) (41). In nonmalignant disease, ganciclovir/foscarnet significantly decreased CMV reactivation incidence (43.7% vs. 78.3%), whereas the prophylaxis strategy had no significant impact in patients with hematological malignancies. No significant difference was found in the rate of CMV disease according to prophylaxis method. It suggests that this intensified antiviral strategy may be necessary for αβ T cell-depleted haploSCT patients with nonmalignant disease who require higher doses of ATG.
Through TCR α+β+/CD19+ cell-depleted haploSCT, it is feasible to transfer to the transplant recipient both donor hematopoietic stem cells (HSCs) and hematopoietic progenitors as well as NK and γδ T cells, which could protect against leukemia and life-threatening infections, including posttransplant lymphoproliferative disease (PTLD). A total of 7.27-75% of patients undergoing TCR α+β+/CD19+ cell depleted HSCT experienced CMV reactivation (42–46). Most patients experienced CMV viremia during the first month after haploSCT (days +1 to +24) (45). In a report including three sickle cell disease and 11 thalassemia patients, Gaziev J et al. stated that viral reactivation occurred in the vast majority of patients after TCR α+β+/CD19+ cell–depleted haploSCT, with CMV reactivation in 64%, although no cases of CMV were noted (47).
After removal of potentially alloreactive CD45RA+ cell depletion, memory T cells, including virus-specific T cells left in grafts, could shorten viremia and reduce GVHD (48). B M Triplett et al. reported data from 17 patients with poor-prognosis hematologic malignancy who underwent haploSCT with CD45RA-depleted grafts after a reduced intensity conditioning regimen without TBI or serotherapy (49). Three patients of 17 received anti-CMV treatment after CMV reactivation. None of the patients experienced CMV disease, and all of them cleared CMV viremia without donor lymphocyte infusion (DLI). Early T-cell reconstitution was directly linked to the CD45RA-depleted graft content. This group then compared 41 patients receiving CD3‐depleted (CD3dep recipients) grafts with 26 receiving CD45RA‐depleted grafts (CD45dep recipients) after haploSCT (50). CD3dep recipients were more likely to develop CMV reactivation—23 (56%) vs 5 (19%). All CD3dep recipients with CMV received treatment, and eight (36%) were also infused with donor lymphocytes for CMV, whereas CMV treatment was needed for only three of the five CD45RAdep recipients. Although three CD3dep recipients died with active CMV viremia, CMV was not detected in CD45RAdep recipients at the time of death. It seems that CD45RA-depleted haploSCT confers enhanced T-cell recovery and reduced infection without increase in severe GVHD among these ex vivo TCD methods.
PTCy-haploSCT
PTCy is a method of in vivo T cell depletion that mainly acts on alloreactive T cells after haploSCT. CMV reactivation was noticed in 42%-69.2% of patients who underwent PTCy-haploSCT (51–58). A total of 2.8%-4.5% of patients experienced CMV-associated disease (51, 52). CMV reactivation occurred at a median time of 35-39 days (51, 52, 57). The median time to first episode of CMV DNAemia was 33 days (range, −7 to 123 days) after haploSCT (58). Moreover, the CMV DNA peak load was remarkably higher in haploSCT recipients, but the mortality by days 180 and 365 did not differ among comparison groups (55). García-Cadenas Irene et al. studied the impact of HLA donor matching on infection in patients receiving PTCy-based alloSCT (59). They found that haploSCT recipients had a higher incidence of CMV infection/reactivation at 18 months than other transplant modalities [(61% (95% CI: 41–74%) vs. 44% (95% CI: 31–54%)], whereas lethal infections were uncommon across all these groups. In their study, severe infections were common in transplant patients using PTCy. A more recent CIBMTR analysis reported (51) that PTCy increased the risk of CMV infection among CMV-seropositive recipients in both haploSCT and matched sibling donor HSCT compared with calcineurin inhibitor–based sibling donor transplantation, suggesting intensive CMV prevention strategies in all receiving PTCy. This is supported by the fact that an intensified method to prevent CMV reactivation correlated with a lower incidence of CMV reactivation (67% intensified group versus 81% traditional group) and less CMV disease (0% hybrid/intermediate dose versus 8% traditional dose) without increased toxicity after PTCy-haploSCT compared with a traditional antiviral prophylaxis regimen (60).
Primary disease and conditioning regimen could also impact CMV infection after PTCy-haploSCT. CMV reactivation post engraftment was noted in 43.7% and 62% of transplant recipients with primary immune deficiency disorders (PIDs) (61) and relapsed/refractory SAA (62) undergoing PTCy-haploSCT, respectively. R V Raj et al. then investigated the effect of conditioning intensity on the incidence of viral infection after PTCy-haploSCT (63). Their study found that challenging viral infections after haploSCT cause significant morbidity in this patient population. It appears that the incidence of viral complications is higher following myeloablative doses of busulfan-containing conditioning regimens (63). Emmanuel Katsanis et al. recently performed a single center phase I study substituting day +4 PTCy with bendamustine (PT‐BEN) following myeloablative conditioning and T‐cell replete haploidentical bone marrow transplantation (64). CMV reactivation was notably less common in trial patients receiving PTCy/BEN, with one out of eight at-risk (seropositive recipient and/or seropositive donor) of experiencing CMV reactivation, whereas 71.4% of the at‐risk PTCy patients reactivated CMV.
Compared with bone marrow (BM) as a graft source, PBSCs could yield higher CD34+ cell counts but were possibly accompanied by increased GVHD; however, no difference in GVHD was observed in haploSCT (65). A total of 46-68% of patients with PTCy-haploSCT and PBSC grafts had posttransplant CMV viremia (65–69). The median time to viremia was 24 days (range: 3–68). CMV disease occurred in 17-28.8% of patients with CMV viremia (65, 68). Sirolimus with micophenolate mofetil (MMF) has recently been regarded as an alternative to calcineurin inhibitor-containing approaches, as this combination has a decreased risk of acute renal failure, decreased incidence of CMV reactivation, and better regulatory T cell reconstitution. Some groups have introduced PTCy plus sirolimus and MMF (PT-CY-Sir-MMF) as GVHD prophylaxis in allo-HSCT, regardless of donor type (70, 71). CMV DNAemia occurred in 52-63% of patients after haploSCT. The cumulative incidence of CMV DNAemia in patients who received pre-emptive antiviral therapy at one year was 39% (95% CI, 31–47%), and the 1-year cumulative incidence of CMV disease was 2.6% (95% CI, 0.09–5%) (70).
ATG+ PTCy-haploSCT
As ATG is usually used to reduce the risk of graft rejection and GVHD, it is assumed that ATG combined with PTCy in T-cell replete-haploSCT would minimize GVHD risk but not impact engraftment and risk of relapse. Princes Margaret Cancer Centre from Canada established unmanipulated haploidentical PBSC transplantation following RIC with ATG (total 4.5 mg/kg), PTCy (cyclophosphamide 50 mg/kg/day i.v. on days +3 and +4), and cyclosporine as a GVHD prevention strategy (72–74). CMV reactivation occurred in 74% of cases with CMV disease in 11.5% of cases (72). Cheng‐Hsien Lin et al. retrospectively compared the cumulative incidence of CMV DNAemia, two‐year OS, and leukemia‐free survival rates in acute leukemia patients with MSD, matched unrelated donor (MUD), and haploidentical donor allografts (ATG: 2 mg·kg-1 day-1, from day -3 to day -2; PTCy) (75). The cumulative incidences of CMV DNAemia at day 180 in the haploidentical groups were 85.7%, which were higher than those in the MSD and MUD allo‐HSCT groups. For the haploidentical groups, CMV DNAemia was detected at a median time of 29 days.
Yu Wang et al. from Peking University initiated a prospective study in patients with a standard-dose ATG/granulocyte colony-stimulating factor (G-CSF)-based regimen (ATG-PTCy) followed by low-dose PTCy (14.5 mg/kg on days 3 and 4) for haploSCT (76, 77). The 100-day cumulative incidence of CMV reactivation in the ATG-PTCy cohort was markedly higher than that in the ATG cohort (74% vs 30%), with a comparable incidence of CMV disease between the two cohorts (8% vs 8%) (77), indicating that dual T cell depletion with PTCy and ATG may bring about a higher incidence of CMV reactivation.
Comparison Among These Approaches
Published data have been inconsistent on the incidence of CMV reactivation and CMV disease after haploSCT ( Table 1 ). It indicates that haploSCT carries a substantially higher risk for CMV infection compared with HLA‐matched related or unrelated allo‐HSCTs, but this seemed not to impact overall and non‐relapse mortality. Hence, some data suggest the use of prophylactic anti-CMV antivirals when PTCy is used because a higher incidence of CMV reactivation was associated with the use of PTCy (51, 60). Surprisingly, a systematic review and meta-analysis of studies on haploSCT in idiopathic AA suggested that the addition of PTCy correlated with a lower risk of CMV viremia (10.4%) to a larger extent than MTX-containing (55.7%) and other (38.6%) regimens (79). The opposite results can be partly explained by the absence of an approved threshold of viral load to initiate anti-CMV treatment, considering the different transplant centers. The heterogeneous CMV serological status in the donor/recipient on account of geographical and ethnological characteristics is another possible explanation because the CMV seroprevalence is usually much higher (>=90%) in adult populations of China than in Europe and the USA (80–84). This issue could be better investigated in a future clinical trial.
Table 1.
Selected reports on CMV infection after haploidentical stem cell transplantation.
| Group | Year | Country | haploSCT Sample size | Primary Disease (n) | Stem cell source (n) | Graft manipulation | Dose of ATG | Conditioning (n) | GVHD prophylaxis | Assays measuring CMV DNAemia | Cutoff values for CMV reactivation or reactivation needing PET | CMV reactivation | CMV disease | Clinical outcome/Comments | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y Wang et al. | 2013 | China | 756 | AML (321); ALL (299); CML (136) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY | CsA+MMF+short-term MTX | Real-time PCR or with a CMV pp65 antigenemia test | NR | 100-day 64% | 4% | 2-year relapse (18%); 3-year OS (67%), LFS (63%), NRM (18%). More CMV-seropositive patients became antigenemia-positive than CMV-seronegative patients. | (4) |
| Y Chen et al. | 2016 | China | 248 | AL (201); CML (32); Others (15) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 or 1.5mg/kg×4d | Modified BUCY (241); TBI+CY+Me-CCNU (7) | CsA+MMF+short-term MTX | Real-time PCR (RT-PCR) | A viral load of >500 copies/ml for two consecutive readings 5 days apart | 59.50% | 6.85% | CMV DNAemia was found to be a poor prognostic factor in terms of NRM and OS. HBsAg seropositivity was associated with an increased risk of cytomegalovirus DNAemia. | (5) |
| CH Yan et al. | 2020 | China | 1466 | AML (801); ALL (490); MDS (175) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY (1416); TBI+CY+Me-CCNU (50) | CsA+MMF+short-term MTX | Automated, real-time, quantitative PCR assay | A detection threshold of >1000 copies/ml was defined as positive | 64.80% | 1-year CMVR 2.3% | CMVR was a rare complication after haploidentical HSCT but that the risk was greater in patients with multiple risk factors. | (6) |
| XY Meng el al. | 2020 | China | 3862 | AML (36); ALL (51); MDS (14); CML (4); SAA (2); Others (6) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY or TBI+CY+Me-CCNU BUCY (SAA) |
CsA+MMF+short-term MTX | Real-time PCR | A limit of detection of 509 IU/mL | NR | 2.92% | 1 year NRM 34.9% in patients with CMV diseases | (7) |
| LP Xu et al. | 2016 | China | 101 | SAA | BM+PBSC (100); BM (1) | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | BUCY | CsA+MMF+short-term MTX | NR | NR | 68.30% | 1% | 3-year OS (89.0%); FFS (86.8%) | (9) |
| LP Xu et al. | 2017 | China | 89 | SAA (69); VSAA (20) | BM+PBSC (78); BM (9); PBSC (2) | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | BUCY | CsA+MMF+short-term MTX | NR | NR | 51.70% | 1.12% | 3-year OS (86.1 ± 3.7%); FFS (85.0 ± 3.9%) | (10) |
| LP Xu et al. | 2018 | China | 51 | SAA | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | BUCY | CsA+MMF+short-term MTX | NR | NR | 84.00 ± 0.29% | 1.96% | 1- and 3-year OS 83.5 ± 5.4% (the probabilities of FFS were equal to the OS) | (11) |
| LP Xu et al. | 2017 | China | 52 pediatric patients | SAA (32); VSAA (20) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | BUCY | CsA+MMF+short-term MTX | NR | NR | 69.20% | NR | 3-year OS (84.5 ± 5.0%); FFS (82.7 ± 5.2%) | (12) |
| Y Lu et al. | 2018 | China | 41 | SAA | BM+PBSC | in vivo TCD-haploSCT | r-ATG 7.5 mg/kg (total dose) ATG-F 20mg/kg (total dose) | BUCY | Tacro+MMF+short-term MTX | PCR | Higher than 500 copies/mL | 65.90% | 4.88% | 3-year OS (80.3 ± 5.1%); FFS (76.4 ± 5.1%); GFFS (79.0 ± 8.6%) | (13) |
| L Liu et al. | 2020 | China | 146 | SAA (75); VSAA (71); SAA with PNH clone (15) | BM (15); PBSC (4); BM + PBSC (127) | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | BUCY | CsA+MMF+short-term MTX | Real-time PCR | NR | 42.47% | 2.05% | 4-year OS (81.4 ± 3.3%); GFFS (69.2 ± 3.9%) | (24) |
| Z Liu et al. | 2017 | China | 44 | SAA (31); VSAA (13) | BM+PBSC+MSCs | in vivo TCD-haploSCT | r-ATG 3.125 mg/kg×4d | BUCY | CsA+MMF+short-term MTX | NR | NR | 65.90% | 0 | 2-year OS 77.3% | (27) |
| Z Wang et al. | 2014 | China | 17 children and adolescents | SAA (11); VSAA (5); 2nd HSCT (1) | BM+PBSC+MSC | in vivo TCD-haploSCT | r-ATG 5mg/kg×4d (-4 to -1); ALG 20mg/kg/day d-4 to -1 | Flu+BUCY | CsA+MMF+short-term MTX+basiliximab | Real-time PCR | NR | 82.30% | 0 | 1-year OS 71.60 ± 17.00% | (14) |
| L Gao et al. | 2014 | China | 26 | SAA (16); VSAA (10) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Flu+CY | CsA+MMF+short-term MTX | PCR | NR | 23.08% | 3.85% | TRM 3.8% (100-day), 11.5% (1-year), 15.4% (2-year); OS 84.6% (follow-up of 1313.2 days) | (15) |
| Y Lu et al. | 2021 | China | 377 | AML | BM+PBSC | in vivo TCD-haploSCT | r-ATG 7.5-10mg/kg; ATG-F 20mg/kg | Modified BUCY, n=118; Intensified BU-based MAC, n=259 | CsA+MMF+short-term MTX | Real-time quantitative PCR | NR | 67.4 ± 5.1% | 1.06% | 3-year OS 74.9 ± 2.4%; LFS 73.8 ± 4.8%; relapse rates 14.3 ± 4.0%; NRM 12.3 ± 3.5% | (16) |
| Jiafu Huang et al. | 2020 | China | 75 patients aged over 50 years | AML (60); MDS (15) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 7.5-10mg/kg | BUCY or BF or TBI+CY | CsA+MMF+short-term MTX | PCR | NR | 64.00% | 4.00% | 2-year relapse 27.0% ± 5.6%; PFS 59.3% ± 5.8%; OS 63.0% ± 5.8%; GRFS 42.6% ± 5.9% | (17) |
| P Suo et al. | 2020 | China | 27 | MDS | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY | CsA+MMF+short-term MTX | Quantitative PCR | PET was given when a single CMV DNA > 1000 copies/mL or 600 copies/mL were observed twice. | 59.30% | 0 | 3-year DFS and 3-year OS 81.9% | (20) |
| P Ke et al. | 2018 | China | 48 | MDS | BM (9); PBSC (1); BM+PBSC (38); coinfusion of the cord blood | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY | CsA+MMF+short-term MTX | NR | NR | 42% | 0 | 2-year OS 64%; RFS 56%; relapse 12%; NRM 33% | (19) |
| L Gao et al. | 2015 | China | 47 | Ph+ ALL | BM+PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | TBI+Ara-C+CY | CsA+MMF+short-term MTX | NR | NR | 38.30% | 8.51% | 2-year OS 63.8%; LFS 59.5% | (18) |
| H Zhao et al. | 2020 | China | 55 | ALL | BM+PBSC or PBSC | in vivo TCD-haploSCT | NR | BUCY+TBI or nonmyeloablative regimens | NR | NR | NR | 56.10% | NR | 2-year LFS 65.6%; OS 77.0% | (21) |
| L Gao et al. | 2017 | China | 174 | AML (73); ALL (61); CML (22); MDS (18) | BM+PBSC | in vivo TCD-haploSCT | ATG-F 5mg/kg×4d | CCNU+BU+CY+Ara-C (AML,CML and MDS) CY+TBI+Ara-C (ALL) |
CsA/Tacro+MMF+short-term MTX | PCR | NR | 39.5% (Short-term Tacro); 37.5% (CsA) | NR | 2-year OS 59.3% (Short-term Tacro), 55.7% (CsA); 2-year DFS 65.1% (Short-term Tacro), 61.4% (CsA) | (37) |
| Y Wang et al. | 2014 | China | 224 | AML (106); ALL (91); CML (14); MDS (13) | BM+PBSC | in vivo TCD-haploSCT | r-ATG 1.5 mg/kg×4d, n=112; r-ATG 2.5 mg/kg×4d, n=112 | Modified BUCY, n=218; TBI based regimen, n=6 | CsA+MMF+short-term MTX | Real-time Taqman CMV DNA PCR | >600 copies/mL | 1-year 75.0% (ATG-6) and 78.6% (ATG-10) | 0.89% (ATG-6) and 5.36% (ATG-10) | 1-year relapse 7.6% (ATG-6), 4.6% (ATG-10); NRM 8.1% (ATG-6), 10.3% (ATG-10); OS 88.4% (ATG-6), 87.0% (ATG-10); DFS 84.3% (ATG-6); 86.0% (ATG-10) | (31) |
| S Kako et al. | 2017 | Japan | 12 | AML (5); ALL (1); CMML (1); Ph+ ALL (2); NHL (1); LCS (1); PMF (1) | PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×2d (-4 to -3) | BU+Mel, n=2; CY+TBI, n=6; Flu+Mel+TBI, n=3; Flu+BU+TBI, n=1 | CsA+short-term MTX | NR | NR | 41.67% | 0 | 1-year OS 33.3%, PFS 24.3%, RR 59.0%, and NRM 16.7% | (34) |
| GJ Min et al. | 2020 | Korea | 186 | AML | BM or PBSC | in vivo TCD-haploSCT | r-ATG 1.25 mg/kg×4d | Flu+BU+TBI | CsA+short-term MTX | Real-time quantitative-PCR | NR | 72.70% | 19.40% | OS 52.3% (mismatched) and 55.3% (matched); GRFS 40.6% (mismatched) and 42.2% (matched); Relapse 22.5% (mismatched) and 8.6% (matched); NRM 28.9% (mismatched) and 27.1% (matched) | (35) |
| L Zhu et al. | 2015 | China | 25 | AML (7); ALL (17); Bi-lineage AL (1) | BM+PBSC+MSC (21) or BM+MSC (4) | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d (-4 to -1) | BU+Ara-C+CY | CsA+MMF+short-term MTX | NR | NR | 92% | NR | 14-month OS 53.28% | (28) |
| J Xu et al. | 2020 | China | 72 | T-ALL | BM or PBSC or BM+PBSC combined with CB | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d (-4 to -1) | Modified BUCY | CsA+MMF+short-term MTX | PCR | NR | 19.40% | NR | 3-year OS (66.6 ± 6.2)%; RFS (62.0 ± 6.5)%; relapse (24.2 ± 6.4)%; NRM (16.9 ± 5.1)% | (25) |
| J Wang et al. | 2019 | China | 139 | AML (100); ALL (39) | BM+PBSC or BM+PBSC+UCB | in vivo TCD-haploSCT | ATG-F 5 mg/kg×4d | BUCY+Me-CCNU+FLAG/CLAG, n=96; TBI+CY+Me-CCNU+FLAG/CLAG, n=43 | CsA+MMF+short-term MTX | Real-time PCR | NR | 100-day 59.8% (Cord-HaploSCT) and 47.6% (HaploSCT) | 2.88% | 2-year relapse 25.9% (Cord-HaploSCT) and 53.2% (HaploSCT); NRM 38.8% (Cord-HaploSCT) and 24.6% (HaploSCT); OS 35.5% (Cord-HaploSCT) and 22.7% (HaploSCT); PFS 35.5% (Cord-HaploSCT) and 17.9% (HaploSCT) | (26) |
| XN Gao et al. | 2020 | China | 110 | AML (58); MDS (6); CML (4); MDS/MPN (1); ALL (38), NHL (3), PCL (1) | PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY, n=95; TBI+CY, n=3; Flu+BU, n=4; BU+FLAG, n=8 | CsA+MMF+short-term MTX | Real-time quantitative PCR | CMV DNA loads exceeded 1000 copies/mL | 1-year 55.0% | 1-year 7.9% | 3-year NRM 30.5% (CMV DNAemia+) and 13.7% (CMV DNAemia-); 3-year OS 55.0% (CMV DNAemia+) and 60.4% (CMV DNAemia-) | (29) |
| HH Li et al. | 2017 | China | 94 | AML (46); Therapy-related AML (6); MDS transformed AML (5); MDS-refractory anemia with excess blast (1); ALL (26); CML (5); Lymphoma (5) | PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg×4d | Modified BUCY, n=60; TBI+CY, n=28; BF, n=6 | CsA+MMF+short-term MTX | NR | NR | 1-year 62.1% | 1-year 8.1% | 3-year NRM 24.0% (HaploSCT) and 10.2% (MSD); relapse 39.0% (HaploSCT) and 22.6% (MSD); DFS 45.7% (HaploSCT) and 78.9% (MSD) | (30) |
| E Shmueli et al. | 2014 | Israel | 102 | Congenital disease; SAA; hematological malignancy; solid tumor | NR | in vivo TCD-haploSCT | ATG* | Flu+TT+TBI | NR | Real-time PCR | Higher than 50 copies/mL | 66.70% | 11.6% | The high rate of drug resistance as interlinked with severe disease in haplo-HSCT recipients. | (8) |
| SS Park et al. | 2021 | Korea | 46 | SAA | PBSC | in vivo TCD-haploSCT | r-ATG 5-10 mg/kg | TBI+Flu | Tacro+short-term course MTX | NR | NR | 45.70% | NR | 3-year OS 84.4%; 3-year TRM 11.2% | (36) |
| A Bertaina et al. | 2014 | Italy | 23 | SCID (8); SAA (4); FA (4); IPEX (1); CAMT (1); SDS (1); UNC13D-mutated HLH (1); DOCK-8-mutated HIEs (1); Osteopetrosis (1); Thalassemia (1) | PBSC | ex vivo TCD-haploSCT (αβ+ T and CD19+ B cells depletion) | r-ATG 4 mg/Kg×3d (-5 to -3) | BU+TT+Flu, n=3; Treo+TT+Flu, n=4; Treo+Flu, n=8; Flu+CY ± TBI, n=8 | No posttransplantation pharmacologic GVHD prophylaxis | NR | NR | 38% (CMV and adenovirus) | 4.35% | The 2-year probability of both OS and DFS was 91.1% | (42) |
| AE Hammerstrom et al. | 2018 | USA | 86 | Leukemia (75); Lymphoma (8); MM (1); AA (2) | BM (83); PBSC (3) | PTCy-haploSCT | No | Mel+TT+Flu | MMF+Tacro | pp65 CMV antigenemia assay or PCR. | CMV antigenemia with ≥1 cell/million or detectable CMV DNA | Traditional 81%; Hybrid 53%; Intermediatedose 71% | 8% (Traditional), 0% (Hybrid), and 0% (Intermediate dose) | 100-day NRM 0 (Traditional), 13% (Hybrid), and 13% (Intermediate dose); 100-day OS 100% (Traditional), 80% (Hybrid), and 87% (Intermediate dose) | (60) |
| R Mitchell et al. | 2019 | Australia | 19 | Primary immunodeficiency disease; HLH; FA; AML; ALL | PBSC; BM | ex vivo TCD-haploSCT (αβ+ T and CD19+ B cells depletion) | ATG* | Treo+Flu+TT; Bu+Flu+TT; Treo+Flu; Bu+CY+Flu; Flu+CY; Flu+Mel+TT; TBI+Flu+Mel+TT | MMF (n=11) or CsA (=3) or combination CsA/MMF (n=5), or no prophylaxis (n=1) | CMV PCR screening | NR | 50.00% | 5.26% | 100-day TRM 0% and 1-year TRM 15%; 5-years OS 80% | (43) |
| SH Kang et al. | 2021 | Korea | 81 | Malignant disease (45); Nonmalignant disease (36) | PBSC | ex vivo TCD-haploSCT (αβ T lymphocyte depletion) | Malignant disease r-ATG (2 mg/kg at -8d and 1 mg/kg at -7d); Nonmalignant disease r-ATG (2.5 mg/kg/day, -8d to -6d) | Flu+CY+TBI | NR | Quantitative real-time PCR | >2.49 log copies/mL | 50.8% (GCV/FCV 44.4% vs GCV 62.6%) | 15.4%; no significant difference in the incidence of CMV disease according to prophylaxis method | Interim-FCV prophylaxis effectively prevented CMV reactivation in those undergoing αβ T cell-depleted haploSCT. | (41) |
| I Airoldi et al. | 2015 | USA | 27 | ALL (9); AML (6); SCID (4); FA (3); Hyper-IgE syndrome (1); Refractory cytopenia of childhood (2); Kostmann syndrome (1); Osteopetrosis (1); SDS (1) | PBSC | ex vivo TCD-haploSCT (TCR-αβ+/CD19+ lymphocytes depletion) | No | TBI+TT+Mel; TBI+TT+CY; TBI+TT+Flu; Treo+TT+Mel; BU+TT+Flu; BU+CY+Mel; Treo+TT+Flu; Treo+Flu; TBI+CY+Flu; BU+Flu | No posttransplantation pharmacologic GVHD prophylaxis | NR | NR | 55.50% | NR | 81.5% survived at last follow-up | (44) |
| L Kaynar et al. | 2017 | Turkey | 34 | AML (24); ALL (10) | PBSC | ex vivo TCD-haploSCT (TcRαβ-depletion) | ATG-F 30 mg/kg (-12 to -9) | Flu+TT+Mel | MMF | PCR | NR | 73.5% (AML 66.7%; ALL 90.0%) | 0 | 1-year DFS 42%; OS 54% | (39) |
| HF Nazir et al. | 2020 | Oman | 12 | FHLH | PBSC | ex vivo TCD-haploSCT (CD3/CD19 depletion) | ATG-F 10 mg/kg (-6 to -3) | Treo+TT+Flu+Rituximab | CsA or Tacro or No pharmcologic prophylaxis | PCR | CMV viral load exceeded 500 copies/mL | 75.00% | 16.67% | 3-year DFS 58.3% | (45) |
| F Erbey et al. | 2018 | Turkey | 21 | ALL (14); AML (7) | PBSC | ex vivo TCD-haploSCT (TcRαβ-depletion) | r-ATG 20mg/kg (-13 to -9) | Flu+TT+Mel | MMF with or without CsA | PCR screening | NR | 81.00% | NR | 5-year OS 71.1%; RFS 86.9%; TRM 16.3% | (40) |
| S Gaballa et al. | 2016 | USA | 50 | AML (27); MDS or MPD (3); ALL (14); NHL (5); AA (1) | DLI + CD34-selected stem cell | PTCy-haploSCT | No | TBI (12 Gy over 4 day) | Tacro+MMF | PCR | NR | 100-day 67% | 0 | 3-year OS 70%; PFS 68%; NRM 10% | (38) |
| R Crocchiolo et al. | 2015 | Italy | 70 | HL (35); NHL (20); MM (2); AL (11); CLL (2) | BM (66); PBSC (4) | PTCy-haploSCT | No | NMA, n=48; RIC, n=16; MAC, n=6 | Tacro/CsA+MMF | PCR | Threshold of CMV viremia for PET was 3300 copies/mL | 54.00% | 4.29% | 2-year OS 48%, TRM 26% | (53) |
| J Gaziev et al. | 2018 | USA | 54 | Thalassemia (45); Sickle cell anemia (7); HbS-b thalassemia (2) | PBSC and/or BM | ex vivo TCD-haploSCT (CD34 selection of PBSCs and BM, n=32; CD34 selection of PBSCs and CD3/CD19 depletion of BM, n = 8; TCRαβ/CD19 depletion of PBSCs, n = 14) | r-ATG 12.5 mg/kg over 4 days, n=6; ATG-F 50 to 25 mg/kg over 5 days, n=48 | BUTT10CY200 preceded by HuAzFlu or BUTT10CY200 preceded by Flu with/without Rituximab prophylaxis | CsA +methylprednisolone or CsA+MMF | reverse-transcription PCR | NR | 64.00% | 0 | OS 78% (TCR group) and 84% (CD34 group); DFS 69% (TCR group) and 39% (CD34 group) | (47) |
| L Prezioso et al. | 2019 | Italy | 59 | AML (32); ALL (6); NHL (6); HL (8); MF (4); MDS (2); MM (1); PCL (1) | PBSC (24); CD34+ (35) | ex vivo TCD-haploSCT (αβTCR/CD19+ depletion or selection of the CD34+ cells) | r-ATG 1.5 mg/kg ×4d (-9 to -6) | Flu+TT | No posttransplantation pharmacologic GVHD prophylaxis | PCR | NR | 7.27% | 1.69% | 2-year OS 50.8% | (46) |
| D Huntley et al. | 2020 | Spain | 118 | AL (43); CL (9); Lymphoma (26) MDS/MM/Myelofibrosis (25); Other (15) |
PBSC (110); BM (8) | PTCy-haploSCT | Only one patient received ATG | MAC,n=35; RIC,n=83 | CsA or Tacro | RealTime CMV PCR | 31 IU/ml or 137 IU/ml at different centers | 63.90% | 4.50% | 1-year OS 70.3% | (55) |
| LJ Arcuri et al. | 2020 | USA | 87 | SAA | BM (81); PBSC (3); BM+PBSC (3) | PTCy-haploSCT | 12 patients received r-ATG | Flu+CY+TBI | CsA+MMF or Tacro+MMF | Positive antigenemia or PCR | NR | 100-day 61%, 1-year 62%, 2-year 62% | NR | 2-year OS 79%; 2-year EFS 70% | (62) |
| M Slade et al. | 2017 | USA | 104 | AML (70); ALL (11); MDS (11); Other (12) | PBSC | PTCy-haploSCT | NR | MAC, n=43; NMA, n=61 | CsA+MMF or Tacro+MMF | PCR | >40 000 IU/mL | 55.00% | 15% | 51% survived at last follow-up | (69) |
| E Katsanis et al. | 2020 | USA | 17 | AL,CML, NHL | BM | PTCy/BEN-haploSCT (9); PTCy-haploSCT (8) | No | TBI+Flu or BU+Flu+Mel | MMF+Tacro | PCR | NR | 12.5% in PTCy-BEN with 71.4% in PTCy | NR | 2-year OS 83.3% in PTCy-BEN with 58.3% in PTCy | (64) |
| GC Irene et al. | 2021 | Spain | 40 | AL/MDS (28); MPN (1); Lymphoid malignancies (9); Others (2) | PBSC or BM | PTCy-haploSCT | No | RIC,n=1;MAC,n=39; | Tacro | Quantitative PCR | PET: a level of DNAemia of >1000 IU/ml in one blood sample or two consecutive samples with a level of >500 IU/mL | 18-month 61% | 2.50% | 18-month OS 71.3%; PFS 67.4% with no differences by donor type | (59) |
| RV Raj et al. | 2016 | USA | 43 | AML/MDS (27); ALL (5); Myeloma (4); NHL/HL (4); Others (3) | BM (22); PBSC (21) | PTCy-haploSCT | No | Flu+CY+TBI, n=23; Flu+Bu+CY, n=15; Flu+Mel+TBI, n=5 | Tacro+MMF | Quantitative nucleic acid amplified tests (NAAT) | NR | RIC with 40% in MAC | 0 (RIC) and 7% (MAC) | NR | (63) |
| SR Goldsmith et al. | 2016 | USA | 138 | AML (93); MDS (15); Other (30) | PBSC | PTCy-haploSCT | No | MAC, n=58; RIC, n=80 | Tacro+MMF or other | Real-time qPCR | NR | 58.00% | 16.67% | Post-transplant CMV viremia was not associated with a statistical difference in overall survival | (65) |
| J Montoro et al. | 2020 | Spain | 42 | AL (15); MM (5); Lymphoproliferative disorders (13); MDS (5); MPD (4) | BM (5); PBSC (37) | PTCy-haploSCT | No | TBF-MAC, n=9; TBF-RIC, n=2; BU+Flu+CY, n=11 | MMF+Sirolimus | Quantitative real-time PCR assays | NR | 52.00% | 2.38% | 1-year NRM 14%; EFS 75%; OS 82%; GRFS 47%. A higher cumulative incidence of CMV DNAemia requiring pre-emptive antiviral therapy in the haploidentical cohort. | (70) |
| N Cieri et al. | 2015 | Italy | 40 | AML (22); ALL (5); MDS (1); CML (1); HL (6); NHL (5) | PBSC | PTCy-haploSCT | No | Flu+Treo+Mel | MMF+Sirolimus | Quantitative PCR | PET was started when CMV DNA copy number was more than 1000 copies/mL or increased more than.5 log in peripheral blood plasma. | 63.00% | 15% | 1-year OS 56%; DFS 48% | (71) |
| N Stocker et al. | 2020 | France | 19 | AML (10); MPN (1); MDS (1); ALL (4); NHL (3) | PBSC | PTCy-haploSCT | 2.5 mg/kg, n=3; 5 mg/kg, n=16 | RTC, n=13; TT+etoposide+CY+RIC, n=6 | CsA+MMF | Quantitative PCR | PET was initiated when CMV was above 1000 IU/mL | 46.00% | NR | 2-year Relapse 19% (Control group) and 19% (PTCy group); PFS 73% (Control group) and 70% (PTCy group); OS 78% (Control group) and 79% (PTCy group) | (67) |
| Crocchiolo R et al. | 2016 | Italy and France | 207 | AL (44); HL (54); NHL (61); MM (13); MDS/MPS (25); Drepanocytosis (1) | PBSC (111); BM (96) | PTCy-haploSCT | NR | NMA/RIC, n=181; MAC, n=26 | NR | NR | NR | 42.00% | 1.45% | Two-year OS 62% (Cohort 1); 65% (Cohort 2); 50% (Cohort 3); 42% (Cohort 4) | (56) |
| SR Goldsmith et al. | 2021 | USA | 757 | AML/ALL/MDS | BM or PBSC | PTCy-haploSCT | No | MAC or RIC/NMA | Tacro or CsA | PCR | NR | 180-day 42% | 100-day 2.8% | 2-year mortaligy 49.5% | (51) |
| Y Lu et al. | 2018 | China | 41 | SAA (28)/VSAA (13) | BM+PBSC | in vivo TCD-haploSCT | ATG-r 7.5 mg/kg, n=42; ATG-F 20 mg/kg, n=47 | BU+Flu+CY | Tacro+MMF+short-term MTX | PCR | Higher than 500 copies/mL in plasma | 65.90% | 4.88% | 3-year OS 80.3% ± 5.1%; 3-year FFS 76.4% ± 5.1% | (13) |
| W-R Huang et al. | 2016 | China | 130 | AML; ALL; CML; Lymphoma | PBSC | in vivo TCD-haploSCT | r-ATG 2.5 mg/kg/day -5d to -2d | Modified BUCY, n=90; Modified BF, n=32; TBI+CY, n=8 | CsA+MMF+short-term MTX | PCR | NR | 1-year 61.0 ± 5.3% | 1-year 8.0% ± 2.9% | 3-year OS 45.6% ± 5.6%; LFS 44.2% ± 5.9% | (23) |
| BM Triplett et al. | 2015 | USA | 17 | ALL (6); AML (9); MLL (1); MDS (1) | PBSC | ex vivo T-cell depletion (CD45RA-depletion) | No | TLI+Flu+CY+TT+Mel | Sirolimus or MMF | PCR | NR | 17.65% | 0 | 76.5% survived at a median of 223 days | (49) |
| BM Triplett et al. | 2018 | USA | 67 | ALL (28); AML (22); MLL (4); MDS (8); Lymphoma (3); CML (2) | PBSC | ex vivo T-cell depletion (CD3-depletion,n=41; CD45RA-depletion,n=26) | No | CD3-depleted: Flu+TT+Mel+OKT3 (n = 21) or alemtuzumab (n=20)+Rituximab CD45RA-depleted: Flu+TT+Mel+lymphoid irradiation+CY |
a short (<60 days) course of MMF | Quantitative PCR | NR | CD3-depleted 56%, CD45RA-depleted 19% | NR | 180-day mortality CD3dep recipients 22% vs CD45RAdep recipients 15.4% | (50) |
| A Fayard et al. | 2019 | France | 381 | AL/MDS (208); HL/NHL (115); MPN (31); MM/solitary plasmacytoma (15); chronic leukemia (10); bone marrow failure syndrome (2) | BM (103); PBSC (278) | PTCy-haploSCT | No | RIC, n=307; MAC, n=73 | an anticalcineurin +MMF | A single pp65 antigen-positive leukocyte or a positive viremia in peripheral blood | NR | 48.80% | 4.50% | Median of PFS 19.9 months; Median of OS 33.5 months | (52) |
| A Esquirol et al. | 2021 | Spain | 236 | AML (76); MDS (39); ALL (22); NHL (39); HL (31); CLL (8); CML/MPN (12); MM (5); biphenotypic acute leukemia (2); aplasia (1); prolymphocytic leukemia (1) | BM (45); PBSC (191) | PTCy-haploSCT | NR | Flu+BU; Flu+Bu+CY; TBF; Other | CsA+MMF or Tacro alone | PCR | >1000 IU/mL | 69.00% | 2.12% | 12-month OS 64%; 12-month PFS 57% | (54) |
| Monzr M. Al Malki et al. | 2017 | USA | 119 | Acute leukemia (80); bone marrow failure (15); lymphoma (11); chronic leukemia (6); hemoglobinopathies (5); MM (2) | PBSC (81); BM (38) | PTCy-haploSCT | NR | MAC, n=46; RIC/NMA, n=73 | Tacro/MMF | PCR | NR | 100-day 69.2% | 0 | CMV reactivation was not associated with OS, RFS, relapse incidence, or NRM. | (57) |
| D Huntley et al. | 2020 | Spain | 71 | Acute leukemia (24); Chronic leukemia (6); Lymphoma (15); Myelofibrosis/MDS (18); Other (5) | PBSC (65); BM (6) | PTCy-haploSCT | No | MAC, n=17; RIC, n=54 | Tacro-based, n=41; MMF-based, n=15 | Real-time PCR | Higher than 600 IU/ml or higher than IU/ml at different centers | 59.70% | 4.23% | PTCy-haploSCT recipients may reconstitute CMV-specific T-cell immunity to the same extent as patients undergoing HLA-matched allo-HSCT | (58) |
| R Uppuluri et al. | 2019 | India | 16 | Primary immune deficiency disorder | BM (6); PBSC (10) | PTCy-haploSCT | NR | Flu+Mel, n=5; Flu+Treo, n=3; Treo+Flu+TBI, n=3; Treo+Flu, n=1; Flu+Treo+TBI, n=4 | NR | NR | NR | 43.70% | 6.25% | Overall mortality 37.5%; OS 62.5%; Cytokine release syndrome (CRS) 75% | (61) |
| SR Solomon et al. | 2015 | USA | 30 | AML (16); ALL (6); CML (5); MDS (1); NHL (2) | PBSC | PTCy-haploSCT | No | Flu+TBI | Tacro+MMF | Quantitative CMV PCR | PET was initiated if viral reactivation was detected (higher than 400 copies/mL) | 58.00% | 0 | 2-year OS 78%; 2-year DFS 73% | (66) |
| C Oltolini et al. | 2020 | Italy | 145 | Myeloid disorders (106); Lymphoid disorders (39) | PBSC | PTCy-haploSCT | No | MAC, n=110; RIC, n=35 | sirolimus+MMF, n=141; CsA+MMF, n=3 | PCR | PET was started when plasmatic CMVDNA higher than 1000 copies/mL or increased >0.5 log. | 61% (68%, haploSCT) | 13.79% | Relapse 44% | (68) |
| AD Law et al. | 2018 | Canada | 50 | AML (28); MDS (8); MPN (6); ALL (2); Lymphoma (5); BPDCN (1) | PBSC | PTCy-haploSCT | r-ATG 4.5 mg/kg | Flu+BU+TBI | CsA | NR | NR | 74% | 8% | 1-year OS 48.1%; NRM 38.2% | (72) |
| MQ Salas et al. | 2020 | Canada | 52 | AML (29); MDS (8); MPN (5); ALL (3); Lymphoproliferative disease (6); BPDCN (1) | PBSC | PTCy-haploSCT | r-ATG 4.5 mg/kg | Flu+BU+TBI | CsA | Quantitative PCR | >200 copies/ml | 58% | 4% | 1-year OS 58.8 (44–70.9)%; 1-year RFS 53.3 (38.8–65.8)% | (73) |
| J Tischer et al. | 2015 | Germany | 55 | AML (33); CML (2); ALL (7); SAA (1); NHL (14); CLL (2) | BM+PBSC | ex vivo T-cell depletion (cTCR/TCD: CD6-depleted G-CSF-mobilized peripheral blood stem cells); PTCy-haploSCT | cTCR/TCD: r-ATG 20 mg/kg for 5 days; TCR/PTCY: No ATG | RIC or MAC | CsA+MTX or Tacro+MMF or MMF | Quantitative real-time PCR | NR | cTCR/TCD 42.9%; TCR/PTCy 14.8% | 7.14% (cTCR/TCD) and 0 (TCR/PTCy) | cTCR/TCD: 1-year OS 39%, RFS 38%; TCR/PTCY: 1-year OS 59%; RFS 55% | (78) |
HaploSCT, haploidentical stem cell transplantation; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; AL, acute leukemia; MDS, myelodysplastic syndromes; AA, aplastic anemia; SAA, severe aplastic anemia; VSAA, very severe aplastic anemia; Ph+, Philadelphia chromosome-positive; PNH, paroxysmal nocturnal hemoglobinuria; CMML, chronic myelomonocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; PCR, polymerase chain reaction; PET, preemptive therapy; LCS, Langerhans cell sarcoma; PMF, primary myelofibrosis; BPDCN, blastic plasmacytoid dendritic cell neoplasm; PCL, plasma cell leukemia; SCID, severe combined immunodeficiency; FA, Fanconi anemia; IPEX, immunodeficiency with polyendocrinopathy and enteropathy X-linked; CAMT, congenital amegakaryocytic thrombocytopenia; SDS, Shwachmann-Diamond syndrome; HLH, hemophagocytic lymphohistiocytosis; UNC13D-mutated HLH, UNC13D-mutated hemophagocytic lymphohistiocytosis; DOCK-8-mutated HIEs, DOCK-8–mutated hyper-IgE syndrome; FHLH, familial hemophagocytic lymphohistiocytosis; MPD, myeloproliferative disease; HL, Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; CL, chronic leukemia; MPN, myeloproliferative neoplasm; MPS, myeloproliferative syndrome; MLL, mixed lineage leukemia; BM, bone marrow; PBSC, peripheral blood stem cells; HSCT, hematopoietic stem cell transplant; MSC, mesenchymal stem cell; CB, cord blood; UCB, umbilical cord blood; DLI, donor lymphocyte infusion; TCD, T-cell depletion; PTCy, posttransplant cyclophosphamide; ATG, anti-thymocyte globulin; ATG-F, ATG-Fresenius; r-ATG, ATG-Genzyme; BU, busulfan; CY, cyclophosphamide; BUCY, busulfan cyclophosphamide regimen; CCNU, lomustine; Me-CCNU, simustine; Ara-c, cytosine arabinoside; BF, busulfan fludarabine regimen; FLAG, fludarabine+ cytarabine + granulocyte colony-stimulating factor; CLAG, cladribine + cytarabine + granulocyte colony-stimulating factor; Flu, fludarabine; TT, thiotepa; Treo, treosulfan; Mel, melphalan; Az, azathioprine; Hu, hydroxyurea; TBI, total body irradiation; TBF, thiotepa busulfan fludarabine; MAC, myeloablative conditioning, NMA, non-myeloablative; RIC, reduced-intensity conditioning; RTC, reduced toxicity conditioning; TLI, total lymphoid irradiation; CMV, cytomegalovirus; CsA, cyclosporine A; Tacro, tacrolimus; MMF, mycophenolate mofetil; MTX, methotrexate; GCV, ganciclovir; FCV, foscarnet; TCR, T-cell-replete; HLA, human leukocyte antigen; CMVR, cytomegalovirus retinitis; RRM, relapse-related mortality; OS, overall survival; LFS, leukemia-free survival; NRM, non-relapse mortality; TRM, transplant-related mortality; GVHD, graft-versus-host disease; aGVHD, acute graft-versus-host disease; FFS, failure-free survival; GFFS, GVHD-free and relapse-free survival; GRFS, GVHD-free relapse-free survival; PFS, progression-free survival; EFS, event-free survival; DFS, disease-free survival; RFS, relapse-free survival; RR, relapse rate; MSD, matched sibling donor; NR, not reported.
*The dose of ATG is not mentioned in the paper.
Johanna Tischer et al. retrospectively compared the incidence of virus infections and outcome in two different haploSCT settings (78). The first approach was the combination of T cell repletion and T cell depletion (CD6 deletion) using ATG prior to haploSCT (cTCR/TCD group). The second was T cell repletion (TCR) using high-dose posttransplantation cyclophosphamide (TCR/PTCy group). CMV reactivation occurred more frequently in the cTCR/TCD group (57%) than in the TCR/PTCy group (31%). Furthermore, pre-emptive treatment of CMV reactivation was successful in the TCR/PTCy group, whereas CMV DNA became undetectable in only 50% of the cTCR/TCD group.
CMV-Specific Immune Reconstitution and Its Association With CMV Reactivation After haploSCT
CMV-Specific T Cell (CTL)
We previously investigated CMV-specific T cell (CMV-CTL) reconstitution post in vivo TCD-haploSCT (85–87). The CD8+ T cell number in transplant recipients was comparable to that of controls at day 60 after transplantation. The median number of CMV-CTLs and their subsets was equal to those of the controls from day 30 to day 360. In addition, haploSCT recipients had a high frequency of CMV-CTLs with strong proliferation capacities and interferon-γ production at one year after transplantation (86). CMV-specific T cells with the central memory CD45RO+CD62L+ cell phenotype were significantly expanded when CMV was reactivated early after transplantation (87). Ruri Kato et al. demonstrated that there were considerably lower maximum numbers of CMV-CTLs in the CMV antigenemia resolved group than in the persistent group (median, 22.15 vs. 50 cells/μl) (88). Nevertheless, CMV-CTLs reached a peak more quickly in the resolved group than in the persistent group (median, 21 vs. 78 days) (88).
M Noviello et al. retrospectively explored either CD34 selection or posttransplant sirolimus as GVHD prophylaxis for haploSCT recipients (89). At 30 days, 21.7% of patients had CMV-specific T cells higher than 1 sfc/μL measured by enzyme-linked immunosorbent spot (ELISPOT), whereas CMV viremia occurred in only one patient who received anti-CMV treatment. At 90 days, 29.0% of patients reached this threshold, and no patients experienced clinically relevant viremia. At 180 days, 52.9% of patients finally reached the threshold, and none of them experienced CMV viremia. They found the protective value of 1 CMV sfc/μL against CMV reactivation posttransplant (89).
Dixie Huntley et al. performed a multicenter observational study to monitor CMV-specific T cell kinetics in PTCy-haploSCT patients and compared it with HLA-matched transplantation (58). In their analysis, CMV DNAemia developed at a similar frequency with equal numbers of CMV-specific T-cell at most time points examined between PTCy-haploSCT and MRD/MUD recipients. CMV DNAemia did not affect CMV-specific CD8+ and CD4+ T-cell reconstitution by the end of the follow-up period (day +180) in either allo-HSCT modality. They claimed that PTCy-haploSCT recipients may restore CMV-specific T-cell immunity to the same extent as HLA-matched allo-HSCT patients (58). The same group also reported that CMV infection was related to high levels of CD27−CD28− T cells, which behave like Tregs (90). They found a suboptimal correlation between CMV-specific CD4+ or CD8+ T cells and Tregs in peripheral blood (PB), which was weaker in patients with CMV reactivation prior to immunological monitoring. This suggests that recovery of PB Tregs and that of CMV-specific T-cell subsets show distinct kinetics, particularly after CMV reactivation.
More recently, Jasper J. P. van Beek et al. conducted longitudinal analysis of high-dimensional T-cell immunophenotypes in 21 recipients of PTCy-haploSCT (91). CMV-specific T-cells were primed early after PTCy-haploSCT and initially showed a proliferating/activated phenotype, that was quickly replaced by a terminal effector phenotype, while uncontrolled viral replication associated with lower abundance of distinct CMV-specific CD4+ T-cell immunophenotypes, hinting at a possible role of these cells in CMV control. CMV-specific T-cell features were similar to those of the CMV-seropositive donor one year posttransplantation, implying reestablishment of physiological homeostasis.
NK
NK cells similarly play an essential role in defense against infections and leukemia relapse after hapoSCT. Fengyan Jin et al. explored NK cell dynamics in 29 patients after haploSCT between August 2011 and November 2014 (92). IFNγ-producing NK cells expanded in 19 patients after CMV reactivation, and the percentages of IFNγ-producing NK cells in these patients greatly increased from day 60 to 180 after transplantation compared to those of their donors. The percentage of KIR-expressing NK cells and IFNγ-producing NKG2C+ NK cells was significantly higher in haploSCT recipients with CMV reactivation than in those without CMV reactivation. Moreover, CMV reactivation was associated with expansion of the CD56brightCD16dim/−DNAM1+ NK cell subset between days 30 and 90 after haploSCT (93). Patients with increased CD56brightCD16dim/−DNAM1+ NK cells also had a remarkably higher CMV viral load (93).
Letizia Muccio et al. reported that CMV reactivation boosted the arrival of mature NK cells in pediatric patients with hematological malignancies receiving HLA-haploSCT after removal of both αβT cells and CD19 B cells (94). A memory-like NK cell subset expressing NKG2C and CD57 progressively expanded in most children. NKG2C+CD57+ NK cells were detected by month 3 after allo-SCT and expanded until at least month 12. These cells characteristically expressed high levels of killer Ig-like receptors (KIRs) and leukocyte inhibitory receptor 1 (LIR-1) and low levels of Siglec-7, NKG2A and interleukin-18Rα. Additionally, they poorly secreted interferon-γ in response to interleukin-12 and interleukin-18. The compromised response to these cytokines as well as their highly differentiated profile may reflect their skewing toward immune control of human cytomegalovirus.
Xiang‐Yu Zhao et al. from Peking University previously found that donor-recipient KIR ligand matching decreased CMV reactivation and refractory CMV infection by day 100 post-transplantation (95). This indicates that donor-recipient KIR ligand matching might improve the NK cell licensing process and promote NK cell-mediated control of CMV reactivation. The same group then prospectively assessed NK cell reconstitution in patients undergoing matched sibling transplantation and haploSCT (96). CD107a was increasingly expressed in NK cells after versus before CMV reactivation at days 60, 100, and 180 after transplantation, but CMV reactivation did not impact the maturation process of NK cells after transplantation. In addition, KIR expression and NKp30 expression were lower on NK cells in patients with CMV reactivation than in those without CMV reactivation at day 30. The NK-to-T-cell (NK/T) ratio was persistently higher in patients with CMV reactivation than in those without CMV reactivation from 30 days to one year after haploSCT.
An emerging report from Elisa Zaghi et al. demonstrated impaired adaptive NK cells expanded after CMV reactivation in PTCy-haploSCT (97). By a longitudinal single-cell computational profiling of multiparametric flow cytometry, they found that CMV accelerates NK cell immune recovery with the expansion of CD158b1b2jpos/NKG2Aneg/NKG2Cpos/NKp30lo NK cells. The number of this subset is associated with CMV reactivation, further increases in recipients with multiple viral reactivations and persists for months after the infection. The transcriptional characteristics of FACS-sorted CD158b1b2jpos NK cells confirmed the capacity of CMV to deregulate NKG2C, NKG2A, and NKp30 gene expression, thus mediating the expansion of NK cells with adaptive traits. These results imply that the dysfunction/exhaustion of “adaptive” KIRpos NK cells in patients with CMV reactivated is induced, at least partially, by the CMV-induced expression of checkpoint inhibitors.
γδ T
Fifty pediatric patients undergoing αβ T cell-depleted haploSCT between August 2012 and December 2015 were analyzed (98). CMV reactivation developed in 19 transplantations at a median of 30 days (range, 13-318 days) after haploSCT. Higher γδ T cells were observed in patients without CMV reactivation than in patients with CMV reactivation at day 30 (197.8 ± 153.9 vs 53.9 ± 58.7). There was a significantly higher incidence of CMV reactivation in patients with a low percentage of γδ T cells at day 30 than in patients with a high percentage of γδ T cells (78.0 ± 15.3% vs 22.2 ± 13.9%). No difference in day 30 γδ T cells was found between patients with and without CMV disease.
Irma Airoldi et al. prospectively monitored the functional and phenotypic characteristics of γδ T cells up to 7 months after αβ+ T cells and CD19+ B cells depleted haploSCT in 27 children (44). They reported that γδ T cells are the foremost T-cell population in patients during the first weeks and are mainly derived from the graft content and expanded in vivo after transplantation. Central memory cells predominated very early after haploSCT for both the Vδ1 and the Vδ2 subsets. Vδ1 cells are specifically expanded in patients with CMV reactivation and are more cytotoxic than those of children without reactivation.
CMV-specific T-cell, NK cell, and γδ T-cell are vital to immune control of CMV infection post haploSCT, but it seems that γδ T-cell is more likely responsible for viral reactivation in the context of ex vivo TCD-haploSCT. Although NK cells and γδ T cells are the first lymphocytes that recover after transplantation, CMV-specific T cells are dominant in number in case of viral infection. The majority of studies state that impaired T-cell and NK-cell reconstitution and increased risk of CMV infection after haploSCT, so seeking factors influencing CMV-specific immune reconstitution and interventions to improve immune reconstitution is urgent at the moment. Although data from Dixie Huntley et al. supported similar incidence of CMV infection and restored CMV-specific T cells after PTCy-haploSCT compared to MRD/MUD transplantation (58), the scarce number of MUD and MRD recipients and more sirolimus used in PTCy-haploSCT group preclude any definitive conclusion and further studies are warranted to validate their findings.
Cellular Immunotherapy of CMV Infection
Delayed CMV-specific immune reconstitution has been consistently associated with the development of CMV infection and CMV disease after allo-SCT. Accordingly, adoptive transfer of CMV-specific T cells has been employed to treat CMV infection. Several clinical trials and case reports have confirmed the safety and efficacy of this strategy for the prophylaxis and treatment of CMV infection after haploSCT. Table 2 lists cellular approaches currently in clinical trials and serves as evidence that CMV-targeting immune-based interventions could provide a safe, novel treatment option while offering clinical benefit to CMV reactivated recipients after haploSCT.
Table 2.
Ongoing clinical trials using cytomegalovirus-specific cellular immunotherapy for allo-SCT patients including haploidentical SCT (accessed on 5 Oct 2021, ClinicalTrials.gov).
| Intervention | Patients | Enrollment | Phase | Duration | NCT number | Status |
|---|---|---|---|---|---|---|
| Donor-derived viral specific T-cells (VSTs) | Stem cell transplant recipients who have evidence of viral infection or reactivation | 450 | Phase 1/Phase 2 | 2014-2024 | NCT02048332 | Recruiting |
| HLA-matched VSTs | EBV, CMV, adenovirus, and BK infections post allogeneic SCT | 47 | Phase 1 | 2021-2024 | NCT04013802 | Recruiting |
| Multivirus (CMV, EBV, AdV)-specific T cells | Chemo-refractory viral infections after allo-HSCT | 149 | Phase 3 | 2019-2022 | NCT04832607 | Recruiting |
| Third party donor derived CMVpp65 specific T-cells | CMV Infection or persistent CMV viremia after allogeneic hematopoietic stem cell transplantation | 41 | Phase 2 | 2014-2022 | NCT02136797 | Recruiting |
| Adaptive NK cells infusion post transplantation | CMV infection in patients post haploidentical transplantation | 30 | Not Applicable | 2020-2021 | NCT04320303 | Recruiting |
| CMV-specific T cells | Relapsing or therapy refractory CMV infection after allogeneic stem cell transplantation | 20 | Phase 2 | 2016-2022 | NCT03067155 | Recruiting |
| CMV cytotoxic T cells (CTLs) manufactured with the Miltenyi CliniMACS Prodigy Cytokine Capture System | Refractory cytomegalovirus (CMV) infection post allogeneic hematopoietic stem cell transplantation (AlloHSCT), with primary immunodeficiencies (PID) or post solid organ transplant | 20 | Phase 2 | 2018-2023 | NCT03266640 | Recruiting |
| Direct infusions of donor-derived virus-specific T-cells using the Cytokine Capture System | Recipients of hematopoietic stem cell transplantation with post-transplant viral infections | 12 | Phase 2 | 2014-2022 | NCT02007356 | Recruiting |
| Emergency access to CMV pp65/IE-1 specific cytotoxic T lymphocytes | Recipients of allogeneic stem cell transplants with persistent or therapy refractory Infections | 20 | Phase 1 | 2008-2014 | NCT00769613 | Active, not recruiting |
| Viral specific T-Lymphocytes by Cytokine Capture System (CCS) | Infection with adenovirus, cytomegalovirus or Epstein-Barr Virus after hematopoietic cell transplantation or solid organ transplantation and in patients with compromised immunity | 25 | Phase 1/Phase 2 | 2021-2028 | NCT04364178 | Recruiting |
| CMV specific adoptive t-cells | Opportunistic cytomegalovirus infection occurring after stem cell transplant | 20 | Early Phase 1 | 2016-2022 | NCT02982902 | Recruiting |
| Virus specific T-cell (VST) infusion | Enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation | 60 | Phase 1/Phase 2 | 2018-2023 | NCT03475212 | Active, not recruiting |
| CMV-specific T-cells | CMV in pediatric and adult immunocompromised patients or recipients of allogeneic stem cell transplantation | 20 | Phase 1 | 2020-2026 | NCT03798301 | Recruiting |
| Allogeneic cytomegalovirus-specific cytotoxic T lymphocytes | CMV reactivation or infection in participants who have undergone stem cell transplant or solid organ transplant | 10 | Early Phase 1 | 2020-2021 | NCT03665675 | Recruiting |
| Adoptive cell immunotherapy | Prophylaxis of cytomegalovirus infection in haploidentical transplantation of hematopoietic progenitors | 15 | Phase 2 | 2021-2022 | NCT04056533 | Not yet recruiting |
| Adoptive transfer of selected cytomegalovirus-specific cytotoxic T lymphocytes (CMV-CTL) | Patients at risk of CMV Disease after allogeneic stem cell transplantation (SCT) | 78 | Phase 2 | 2009-2013 | NCT00986557 | Recruiting |
| Donor derived cytomegalovirus specific T lymphocytes | Treatment of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation | 30 | Phase 4 | 2016-2021 | NCT03004261 | Recruiting |
Therapeutical CMV-Specific T-Cell Approaches
Feuchtinger T et al. treated 18 patients after allo-SCT from HLA–mismatched/haploidentical or HLA–matched unrelated donors with polyclonal CMV-specific T cells (99). These T cells were generated by isolation of interferon-γ–producing cells after stimulation with pp65 antigen. Patients with refractory CMV disease or viremia received a mean of 21 × 103/kg pp65-specific T cells. CMV infection was cleared, or viral burden was significantly decreased in 83% of these patients, even in patients with CMV encephalitis. Viral control was related to improved antiviral T-cell reconstitution and in vivo expansion of CMV-specific T cells in 12 of 16 evaluable cases without inducing GVHD or acute side effects.
In another CMV infection refractory cohort (100), 27 of 32 treated patients after haploSCT cleared CMV within four weeks after adoptive T-cell therapy without recurrence. After cellular transfer, CMV-specific T cells expanded in vivo with improved cytokine production and proliferation ability. In addition, the expression of programmed death-1 (PD-1) on CMV-specific T cells was reduced. In the early effective group, patients who cleared viremia within four weeks after T-cell infusion, CMV-specific CD8+ IFN-γ+ and CD4+ IFN-γ+ T cells were rapidly and massively expanded in vivo, whereas in the late effective group, there was no significant expansion of CMV-specific T cells. Xiang-Yu Zhao et al. further evaluated the safety and efficacy of donor-derived CMV-specific cytotoxic T cells (CTLs) as a first-line therapy for CMV infection after haploSCT (101). They observed that first-line therapy with CTLs significantly reduced the incidence of CMV infection with lower 1-year treatment-related mortality and better 1-year overall survival. Moreover, first-line therapy with CTLs promoted the recovery of CTLs in patients, which correlated with CMV clearance.
A case report described two patients with drug-resistant CMV encephalitis after haploSCT successfully received donor CMV-specific CTLs (102). In the first case, a 27-year-old male developed CMV encephalitis during ganciclovir maintenance treatment after haploSCT. After administering foscarnet and donor CMV-specific CTLs, CMV-DNA of his cerebrospinal fluid (CSF) was negative by RT-PCR, and the lesions on brain magnetic resonance imaging (MRI) were reduced. Another case, a 57-year-old female, also experienced CMV encephalitis during maintenance treatment with ganciclovir after haploSCT. After intrathecal treatment with donor CMV-specific CTLs, the CMV load of the CSF was reduced.
Prophylactic DLI
Prophylactic and therapeutic DLI are administered to improve posttransplant immune restoration to reduce both infectious complications and disease relapse. Michael Maschan et al. investigated low-dose memory (CD45RA-depleted) donor lymphocyte infusion (mDLI) after αβ T-cell depleted HSCT (103–105). The incidence of CMV reactivation was 45-50% in the experimental mDLI arm and 54-55% in the control arm. The median duration of CMV viremia was 3 weeks (range, 1-9) in the prospective cohort and 4 weeks (range, 1-26) in the historical cohort (105). Memory DLI was associated with improved CMV-specific T-cell reconstitution in a subcohort of CMV IgG seropositive recipients. Analysis of a subcohort of CMV seropositive recipients indicated remarkably better CMV-specific T-cell reconstitution on day 30 in the experimental arm (104). Compared to that of the historical cohort, restoration of CMV-specific immunity at day 30 was significantly enhanced in the prospective cohort (40% versus 25%) (105). Luca Castagna et al. prospectively evaluated a CD45RA+ depleted DLI in terms of reducing viral infection early after PTCy-haploSCT (106). CMV reactivation occurred in 28% of patients. Although the majority of the patients received the planned three infusions, only one patient developed grade 2 acute GVHD, and two patients had moderate chronic GVHD.
Therapeutic DLI
Park HJ et al. reported the successful treatment of refractory CMV colitis after PTCy-haploSCT using CD45RA+ depleted DLI (107). After failure of ganciclovir and foscarnet, granulocyte colony-stimulating factor-primed, CD45RA+ depleted DLI was administered to treat refractory CMV colitis. CMV pp65-specific CTLs were found in recipients four weeks after DLI. Meanwhile, diffuse wall thickening involving the entire colon was also normalized in the abdominal CT scan.
As manipulated DLI approaches are still not widely used due to high cost and intensive labor, unmanipulated donor lymphocytes (U-DLIs), if feasible by harvesting CTLs directly from the peripheral blood of seropositive donors, are used for refractory or relapsed patients with CMV infection. Researchers from Turkey enrolled five pediatric patients receiving U-DLI for CMV infection after transplantation (108). Among them, three patients underwent haploSCT. One patient who was transplanted from an unrelated donor received U-DLI from his haploidentical mother. CMV titers were dramatically reduced after U-DLI in these patients.
Summary and Outlook
Despite the use of prophylactic or preemptive treatments, CMV infection remains an obstacle for successful haploSCT and the improvement of immunologic reconstitution is the primary strategy for infection prevention. A higher rate of CMV reactivation occurred early after haploSCT compared to HLA-matched HSCT, but CMV disease rates were low after haploSCT, particularly in in vivo TCD-haploSCT and PTCy-haploSCT. It results from expansion of CMV-specific central memory T-cells in the setting of CMV antigenemia or acceptable CMV-specific T-cell reconstitution. Traditional ex vivo TCD-haploSCT successfully prevents lethal GVHD without any posttransplantation immunosuppression, but the small number of T cells in the graft results in impaired immune recovery, which could be overcome by novel ex vivo TCD-haploSCT and adoptive cellular therapy. In vivo TCD-haploSCT and PTCy-haploSCT indicated low treatment‐related mortality (TRM) and an acceptable safety profile, which appears to compare favorably with ex vivo TCD-haploSCT in terms of infections. However, synergistic immunosuppression by PTCy and ATG has led to a higher incidence of CMV infection. We now have a better understanding of CMV reactivation and immune reconstitution post haploSCT. Our data demonstrate that novel ex vivo TCD techniques followed by prophylactic and therapeutic DLI, a low dose of ATG, an intensified antiviral prophylaxis regimen, sirolimus-containing immunosuppressors and CMV-specific cellular immunotherapy can boost immune recovery and decrease the incidence of CMV reactivation. Furthermore, the majority of patients receiving the RIC regimen might be less susceptible to infections (63). In this context, it would be essential to perform a prospective study comparing the risk of infectious complications after in vivo TCD-haploSCT vs. ex vivo TCD-haploSCT or PTCy-haploSCT in patients who received a similar conditioning regimen.
CMV reactivation is associated with delayed immune reconstitution, although this reactivation could also leave a profound imprint on the recovering T cell compartment long-term following allo-SCT (91, 109, 110). Several studies have reported that CMV serostatus and CMV reactivation may be more predictive of T-cell restoration after allo-SCT than GVHD, highlighting the deep impact of this virus on reconstituting T-cells, considering the high incidence of CMV reactivation after haploSCT. More importantly, CMV infection is increasingly recognized as an immunomodulator in cancer patients (111), even in the context of allo-SCT, which is associated with a decreased risk of leukemia relapse, although it is still conflicting (112–115). There is evidence of a bidirectional relationship between CMV reactivation and acute GVHD (116, 117). We should take these into account and balance the merit and disadvantage of taking steps to enhance CMV-specific immune reconstitution and decrease CMV infection.
Author Contributions
X-HL wrote the first draft of the manuscript, conducted the literature search, reviewed the abstracts, performed analysis and contributed to the final draft. YZ contributed to revising the manuscript and provided scientific input. Y-TC and L-PS conducted the literature search. LL revised and wrote the final draft, and contributed to the analysis. All authors contributed to the article and approved the submitted version.
Funding
X-HL was supported by the National Natural Science Foundation of China [grant 81100388, grant 81470344].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors apologize to all authors whose work could not be cited because of length restrictions of this update on CMV infection and CMV-specific immune reconstitution after haploidentical stem cell transplantation.
References
- 1. Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning Including Antithymocyte Globulin Followed by Unmanipulated HLA-Mismatched/Haploidentical Blood and Marrow Transplantation can Achieve Comparable Outcomes With HLA-Identical Sibling Transplantation. Blood (2006) 107:3065–73. doi: 10.1182/blood-2005-05-2146 [DOI] [PubMed] [Google Scholar]
- 2. Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical Hematopoietic Stem Cell Transplantation Without In Vitro T-Cell Depletion for the Treatment of Hematological Malignancies. Bone Marrow Transplant (2006) 38:291–7. doi: 10.1038/sj.bmt.1705445 [DOI] [PubMed] [Google Scholar]
- 3. Xiao-Jun H, Lan-Ping X, Kai-Yan L, Dai-Hong L, Yu W, Huan C, et al. Partially Matched Related Donor Transplantation can Achieve Outcomes Comparable With Unrelated Donor Transplantation for Patients With Hematologic Malignancies. Clin Cancer Res (2009) 15:4777–83. doi: 10.1158/1078-0432.CCR-09-0691 [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Liu D-H, Liu K-Y, Xu L-P, Zhang X-H, Han W, et al. Long-Term Follow-Up of Haploidentical Hematopoietic Stem Cell Transplantation Without In Vitro T Cell Depletion for the Treatment of Leukemia. Cancer (2013) 119:978–85. doi: 10.1002/cncr.27761 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Xu LP, Liu KY, Chen H, Chen YH, Zhang XH, et al. Risk Factors for Cytomegalovirus DNAemia Following Haploidentical Stem Cell Transplantation and Its Association With Host Hepatitis B Virus Serostatus. J Clin Virol (2016) 75:10–5. doi: 10.1016/j.jcv.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 6. Yan CH, Wang Y, Mo XD, Sun YQ, Wang FR, Fu HX, et al. Incidence, Risk Factors, and Outcomes of Cytomegalovirus Retinitis After Haploidentical Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2020) 55:1147–60. doi: 10.1038/s41409-020-0790-z [DOI] [PubMed] [Google Scholar]
- 7. Meng X-Y, Fu H-X, Zhu X-L, Wang J-Z, Liu X, Yan C-H, et al. Comparison of Different Cytomegalovirus Diseases Following Haploidentical Hematopoietic Stem Cell Transplantation. Ann Hematol (2020) 99:2659–70. doi: 10.1007/s00277-020-04201-4 [DOI] [PubMed] [Google Scholar]
- 8. Shmueli E, Or R, Shapira MY, Resnick IB, Caplan O, Bdolah-Abram T, et al. High Rate of Cytomegalovirus Drug Resistance Among Patients Receiving Preemptive Antiviral Treatment After Haploidentical Stem Cell Transplantation. J Infect Dis (2014) 209:557–61. doi: 10.1093/infdis/jit475 [DOI] [PubMed] [Google Scholar]
- 9. Xu L-P, Wang S-Q, Wu D-P, Wang J-M, Gao S-J, Jiang M, et al. Haplo-Identical Transplantation for Acquired Severe Aplastic Anaemia in a Multicentre Prospective Study. Br J Haematol (2016) 175:265–74. doi: 10.1111/bjh.14225 [DOI] [PubMed] [Google Scholar]
- 10. Xu L-P, Jin S, Wang S-Q, Xia L-H, Bai H, Gao S-J, et al. Upfront Haploidentical Transplant for Acquired Severe Aplastic Anemia: Registry-Based Comparison With Matched Related Transplant. J Hematol Oncol (2017) 10:25. doi: 10.1186/s13045-017-0398-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu LP, Xu ZL, Wang FR, Mo XD, Han TT, Han W, et al. Unmanipulated Haploidentical Transplantation Conditioning With Busulfan, Cyclophosphamide and Anti-Thymoglobulin for Adult Severe Aplastic Anaemia. Bone Marrow Transplant (2018) 53:188–92. doi: 10.1038/bmt.2017.237 [DOI] [PubMed] [Google Scholar]
- 12. Xu LP, Zhang XH, Wang FR, Mo XD, Han TT, Han W, et al. Haploidentical Transplantation for Pediatric Patients With Acquired Severe Aplastic Anemia. Bone Marrow Transplant (2017) 52:381–7. doi: 10.1038/bmt.2016.281 [DOI] [PubMed] [Google Scholar]
- 13. Lu Y, Sun R-J, Zhao Y-L, Xiong M, Cao X-Y, Zhang J-P, et al. Unmanipulated Haploidentical Hematopoietic Stem Cell Transplantation Achieved Outcomes Comparable With Matched Unrelated Donor Transplantation in Young Acquired Severe Aplastic Anemia. Biol Blood Marrow Transplant (2018) 24:1881–7. doi: 10.1016/j.bbmt.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Zheng X, Yan H, li D, Wang H. Good Outcome of Haploidentical Hematopoietic SCT as a Salvage Therapy in Children and Adolescents With Acquired Severe Aplastic Anemia. Bone Marrow Transplant (2014) 49:1481–5. doi: 10.1038/bmt.2014.187 [DOI] [PubMed] [Google Scholar]
- 15. Gao L, Li Y, Zhang Y, Chen X, Gao L, Zhang C, et al. Long-Term Outcome of HLA-Haploidentical Hematopoietic SCT Without In Vitro T-Cell Depletion for Adult Severe Aplastic Anemia After Modified Conditioning and Supportive Therapy. Bone Marrow Transplant (2014) 49:519–24. doi: 10.1038/bmt.2013.224 [DOI] [PubMed] [Google Scholar]
- 16. Lu Y, Zhao YL, Zhang JP, Xiong M, Cao XY, Liu DY, et al. Comparable Outcomes Among Unmanipulated Haploidentical, Matched Unrelated, and Matched Sibling Donors in BU-Based Myeloablative Hematopoietic Stem Cell Transplantation for Intermediate and Adverse Risk Acute Myeloid Leukemia in Complete Remission: A Single-Center Study. Ann Hematol (2021) 100(6):1579–91. doi: 10.1007/s00277-020-04355-1 [DOI] [PubMed] [Google Scholar]
- 17. Huang J, Huang F, Fan Z, Xu N, Xuan L, Liu H, et al. Haploidentical Related Donor vs Matched Sibling Donor Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome Aged Over 50 Years: A Single-Center Retrospective Study. Cancer Med (2020) 9:6244–55. doi: 10.1002/cam4.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao L, Zhang C, Gao L, Liu Y, Su Y, Wang S, et al. Favorable Outcome of Haploidentical Hematopoietic Stem Cell Transplantation in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Multicenter Study in Southwest China. J Hematol Oncol (2015) 8:90. doi: 10.1186/s13045-015-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ke P, Bao XB, Hu XH, Zhuang J, Wu XJ, Liu YJ, et al. Myeloablative Conditioning Regimens With Combined of Haploidentical and Cord Blood Transplantation for Myelodysplastic Syndrome Patients. Bone Marrow Transplant (2018) 53:162–8. doi: 10.1038/bmt.2017.229 [DOI] [PubMed] [Google Scholar]
- 20. Suo P, Wang S, Xue Y, Cheng Y, Kong J, Yan C, et al. Unmanipulated Haploidentical Hematopoietic Stem Cell Transplantation for Children With Myelodysplastic Syndrome. Pediatr Transplant (2020) 24:e13864. doi: 10.1111/petr.13864 [DOI] [PubMed] [Google Scholar]
- 21. Zhao H, Wei J, Wei G, Luo Y, Shi J, Cui Q, et al. Pre-Transplant MRD Negativity Predicts Favorable Outcomes of CAR-T Therapy Followed by Haploidentical HSCT for Relapsed/Refractory Acute Lymphoblastic Leukemia: A Multi-Center Retrospective Study. J Hematol Oncol (2020) 13:42. doi: 10.1186/s13045-020-00873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-Cell-Replete Haploidentical HSCT With Low-Dose Anti-T-Lymphocyte Globulin Compared With Matched Sibling HSCT and Unrelated HSCT. Blood (2014) 124:2735–43. doi: 10.1182/blood-2014-04-571570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang WR, Li HH, Gao CJ, Bo J, Li F, Dou LP, et al. Haploidentical, Unmanipulated G-CSF-Primed Peripheral Blood Stem Cell Transplantation for High-Risk Hematologic Malignancies: An Update. Bone Marrow Transplant (2016) 51:1464–9. doi: 10.1038/bmt.2016.166 [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Qiu H, et al. Combination of Haploidentical Haematopoietic Stem Cell Transplantation With an Unrelated Cord-Blood Unit in Patients With Severe Aplastic Anemia: A Report of 146 Cases. Bone Marrow Transplant (2020) 55:2017–25. doi: 10.1038/s41409-020-0874-9 [DOI] [PubMed] [Google Scholar]
- 25. Xu J, Zhao R, Yang L, Gong H, Ma S, Chen J, et al. Haploidentical Stem Cells Combined With a Small Dose of Umbilical Cord Blood Transplantation Exert Similar Survival Outcome of HLA-Matched Stem Cells Transplantation in T-Cell Acute Lymphoblastic Leukemia. Bone Marrow Transplant (2020) 55:1197–9. doi: 10.1038/s41409-019-0666-2 [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Wang Z, Wei W, Zhang W, Zhang T, Cheng H, et al. Cord Haploidentical Non-In Vitro T Cell Depletion Allogeneic Hematopoietic Stem Cell Transplantation Reduces Relapse of Refractory Acute Leukemia. Biol Blood Marrow Transplant (2019) 25:121–8. doi: 10.1016/j.bbmt.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Zhang Y, Xiao H, Yao Z, Zhang H, Liu Q, et al. Cotransplantation of Bone Marrow-Derived Mesenchymal Stem Cells in Haploidentical Hematopoietic Stem Cell Transplantation in Patients With Severe Aplastic Anemia: An Interim Summary for a Multicenter Phase II Trial Results. Bone Marrow Transplant (2017) 52:704–10. doi: 10.1038/bmt.2016.347 [DOI] [PubMed] [Google Scholar]
- 28. Zhu L, Wang Z, Zheng X, Ding L, Han D, Yan H, et al. Haploidentical Hematopoietic Stem Cell Transplant With Umbilical Cord-Derived Multipotent Mesenchymal Cell Infusion for the Treatment of High-Risk Acute Leukemia in Children. Leuk Lymphoma (2015) 56:1346–52. doi: 10.3109/10428194.2014.939970 [DOI] [PubMed] [Google Scholar]
- 29. Gao XN, Lin J, Wang LJ, Li F, Li HH, Wang SH, et al. Risk Factors and Associations With Clinical Outcomes of Cytomegalovirus Reactivation After Haploidentical Versus Matched-Sibling Unmanipulated PBSCT in Patients With Hematologic Malignancies. Ann Hematol (2020) 99:1883–93. doi: 10.1007/s00277-020-04156-6 [DOI] [PubMed] [Google Scholar]
- 30. Li H-H, Li F, Gao C-J, Huang W-R, Bo J, Dou L-P, et al. Similar Incidence of Severe Acute GVHD and Less Severe Chronic GVHD in PBSCT From Unmanipulated, Haploidentical Donors Compared With That From Matched Sibling Donors for Patients With Haematological Malignancies. Br J Haematol (2017) 176:92–100. doi: 10.1111/bjh.14331 [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of Two Different Doses of Antithymocyte Globulin in Patients With Standard-Risk Disease Following Haploidentical Transplantation: A Randomized Trial. Bone Marrow Transplant (2014) 49:426–33. doi: 10.1038/bmt.2013.191 [DOI] [PubMed] [Google Scholar]
- 32. Lin R, Wang Y, Huang F, Fan Z, Zhang S, Yang T, et al. Two Dose Levels of Rabbit Antithymocyte Globulin as Graft-Versus-Host Disease Prophylaxis in Haploidentical Stem Cell Transplantation: A Multicenter Randomized Study. BMC Med (2019) 17:156. doi: 10.1186/s12916-019-1393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Liu Q-F, Lin R, Yang T, Xu Y-J, Mo X-D, et al. Optimizing Antithymocyte Globulin Dosing in Haploidentical Hematopoietic Cell Transplantation: Long-Term Follow-Up of a Multicenter, Randomized Controlled Trial. Sci Bull (2021). doi: 10.1016/j.scib.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 34. Kako S, Akahoshi Y, Harada N, Nakano H, Kameda K, Ugai T, et al. HLA-Mismatched Haploidentical Transplantation Using Low-Dose Anti-Thymocyte Globulin (ATG: Thymoglobulin). Hematology (2017) 22:129–35. doi: 10.1080/10245332.2016.1231968 [DOI] [PubMed] [Google Scholar]
- 35. Min GJ, Kim HJ, Kim TG, Hyun YS, Hyun SJ, Baek IC, et al. Specific Donor HLA Allotypes as Predictors of Cytomegalovirus Disease Risk in Acute Myeloid Leukemia. Hla (2020) 96:445–55. doi: 10.1111/tan.13966 [DOI] [PubMed] [Google Scholar]
- 36. Park SS, Min GJ, Park S, Lee SE, Yoon JH, Shin SH, et al. Comparable Outcomes Between Unrelated and Haploidentical Stem Cell Transplantation in Adult Patients With Severe Aplastic Anemia. Transplantation (2021) 105(5):1097–105. doi: 10.1097/tp.0000000000003342 [DOI] [PubMed] [Google Scholar]
- 37. Gao L, Liu J, Zhang Y, Chen X, Gao L, Zhang C, et al. Low Incidence of Acute Graft-Versus-Host Disease With Short-Term Tacrolimus in Haploidentical Hematopoietic Stem Cell Transplantation. Leuk Res (2017) 57:27–36. doi: 10.1016/j.leukres.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 38. Gaballa S, Palmisiano N, Alpdogan O, Carabasi M, Filicko-O’Hara J, Kasner M, et al. A Two-Step Haploidentical Versus a Two-Step Matched Related Allogeneic Myeloablative Peripheral Blood Stem Cell Transplantation. Biol Blood Marrow Transplant (2016) 22:141–8. doi: 10.1016/j.bbmt.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 39. Kaynar L, Demir K, Turak EE, Öztürk ÇP, Zararsız G, Gönen ZB, et al. Tcrαβ-Depleted Haploidentical Transplantation Results in Adult Acute Leukemia Patients. Hematology (2017) 22:136–44. doi: 10.1080/10245332.2016.1238182 [DOI] [PubMed] [Google Scholar]
- 40. Erbey F, Akçay A, Atay D, Ovalı E, Öztürk G. Comparison of Outcomes After HLA-Matched Unrelated and αβ T-Cell-Depleted Haploidentical Hematopoietic Stem Cell Transplantation for Children With High-Risk Acute Leukemia. Pediatr Transplant (2018) 22:e13192. doi: 10.1111/petr.13192 [DOI] [PubMed] [Google Scholar]
- 41. Kang SH, Yoo JW, Seo JK, Kim H, Koh KN, Choi ES, et al. The Role of Interim-Foscarnet Prophylaxis in Preventing Cytomegalovirus Infection After Ex Vivo αβ T Cell-Depleted Haploidentical Hematopoietic Cell Transplant in Children. Bone Marrow Transplant (2021) 56(2):505–7. doi: 10.1038/s41409-020-01020-z [DOI] [PubMed] [Google Scholar]
- 42. Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-Haploidentical Stem Cell Transplantation After Removal of αβ+ T and B Cells in Children With Nonmalignant Disorders. Blood (2014) 124:822–6. doi: 10.1182/blood-2014-03-563817 [DOI] [PubMed] [Google Scholar]
- 43. Mitchell R, Cole T, Shaw PJ, Mechinaud F, O’Brien T, Fraser C. TCR α+β+/CD19+ Cell–Depleted Hematopoietic Stem Cell Transplantation for Pediatric Patients. Pediatr Transplant (2019) 23:e13517. doi: 10.1111/petr.13517 [DOI] [PubMed] [Google Scholar]
- 44. Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, et al. γδ T-Cell Reconstitution After HLA-Haploidentical Hematopoietic Transplantation Depleted of TCR-αβ+/CD19+ Lymphocytes. Blood (2015) 125:2349–58. doi: 10.1182/blood-2014-09-599423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nazir HF, Ba Alawi FS, Al Hosni S, Al Rawas A, Dennison D. T Cell Depleted Haploidentical Hematopoietic Stem Cell Transplantation for Patients With Familial Hemophagocytic Lymphohistiocytosis Who Do Not Have Matched Family Donors: Experience in Oman. Biol Blood Marrow Transplant (2020) 26:1119–23. doi: 10.1016/j.bbmt.2020.02.014 [DOI] [PubMed] [Google Scholar]
- 46. Prezioso L, Manfra I, Bonomini S, Schifano C, Segreto R, Monti A, et al. Haploidentical Hematopoietic Stem Cell Transplantation in Adults Using the αβtcr/CD19-Based Depletion of G-CSF-Mobilized Peripheral Blood Progenitor Cells. Bone Marrow Transplant (2019) 54:698–702. doi: 10.1038/s41409-019-0608-z [DOI] [PubMed] [Google Scholar]
- 47. Gaziev J, Isgrò A, Sodani P, Paciaroni K, De Angelis G, Marziali M, et al. Haploidentical HSCT for Hemoglobinopathies: Improved Outcomes With Tcrαβ(+)/CD19(+)-Depleted Grafts. Blood Adv (2018) 2:263–70. doi: 10.1182/bloodadvances.2017012005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of Acute Leukemia Patients Transplanted With Naive T Cell–Depleted Stem Cell Grafts. J Clin Invest (2015) 125:2677–89. doi: 10.1172/JCI81229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Triplett BM, Shook DR, Eldridge P, Li Y, Kang G, Dallas M, et al. Rapid Memory T-Cell Reconstitution Recapitulating CD45RA-Depleted Haploidentical Transplant Graft Content in Patients With Hematologic Malignancies. Bone Marrow Transplant (2015) 50:968–77. doi: 10.1038/bmt.2014.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Triplett BM, Muller B, Kang G, Li Y, Cross SJ, Moen J, et al. Selective T-Cell Depletion Targeting CD45RA Reduces Viremia and Enhances Early T-Cell Recovery Compared With CD3-Targeted T-Cell Depletion. Transplant Infect Dis (2018) 20:e12823. doi: 10.1111/tid.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goldsmith SR, Abid MB, Auletta JJ, Bashey A, Beitinjaneh A, Castillo P, et al. Posttransplant Cyclophosphamide Is Associated With Increased Cytomegalovirus Infection: A CIBMTR Analysis. Blood (2021) 137:3291–305. doi: 10.1182/blood.2020009362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fayard A, Daguenet E, Blaise D, Chevallier P, Labussière H, Berceanu A, et al. Evaluation of Infectious Complications After Haploidentical Hematopoietic Stem Cell Transplantation With Post-Transplant Cyclophosphamide Following Reduced-Intensity and Myeloablative Conditioning: A Study on Behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-Tc). Bone Marrow Transplant (2019) 54:1586–94. doi: 10.1038/s41409-019-0475-7 [DOI] [PubMed] [Google Scholar]
- 53. Crocchiolo R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E, et al. Infections After T-Replete Haploidentical Transplantation and High-Dose Cyclophosphamide as Graft-Versus-Host Disease Prophylaxis. Transplant Infect Dis (2015) 17:242–9. doi: 10.1111/tid.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Esquirol A, Pascual MJ, Kwon M, Pérez A, Parody R, Ferra C, et al. Severe Infections and Infection-Related Mortality in a Large Series of Haploidentical Hematopoietic Stem Cell Transplantation With Post-Transplant Cyclophosphamide. Bone Marrow Transplant (2021) 56:2432–44. doi: 10.1038/s41409-021-01328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huntley D, Giménez E, Pascual MJ, Hernández-Boluda JC, Gago B, Vázquez L, et al. Incidence, Features, and Outcomes of Cytomegalovirus DNAemia in Unmanipulated Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation With Post-Transplantation Cyclophosphamide. Transpl Infect Dis (2020) 22:e13206. doi: 10.1111/tid.13206 [DOI] [PubMed] [Google Scholar]
- 56. Crocchiolo R, Castagna L, Furst S, Devillier R, Sarina B, Bramanti S, et al. The Patient’s CMV Serological Status Affects Clinical Outcome After T-Cell Replete Haplo-HSCT and Post-Transplant Cyclophosphamide. Bone Marrow Transplant (2016) 51:1134–6. doi: 10.1038/bmt.2016.69 [DOI] [PubMed] [Google Scholar]
- 57. Al Malki MM, Dadwal S, Yang D, Mokhtari S, Cao T, Gendzekhadze K, et al. High Incidence of CMV Reactivation After Haploidentical Donor Hematopoietic Cell Transplantation Using High-Dose Post-Transplant Cyclophosphamide, and Its Impact on Transplant Outcomes. Blood (2017) 130:4494–4. doi: 10.1182/blood.V130.Suppl_1.4494.4494 [DOI] [Google Scholar]
- 58. Huntley D, Giménez E, Pascual MJ, Remigia MJ, Amat P, Vázquez L, et al. Reconstitution of Cytomegalovirus-Specific T-Cell Immunity Following Unmanipulated Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation With Posttransplant Cyclophosphamide. Bone Marrow Transplant (2020) 55:1347–56. doi: 10.1038/s41409-020-0865-x [DOI] [PubMed] [Google Scholar]
- 59. Irene G-C, Albert E, Anna B-V, Rahinatu A, Silvana N, Silvana S, et al. Patterns of Infection and Infectious-Related Mortality in Patients Receiving Post-Transplant High Dose Cyclophosphamide as Graft-Versus-Host-Disease Prophylaxis: Impact of HLA Donor Matching. Bone Marrow Transplant (2021) 56(4):818–27. doi: 10.1038/s41409-020-01092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hammerstrom AE, Lombardi LR, Pingali SR, Rondon G, Chen J, Milton DR, et al. Prevention of Cytomegalovirus Reactivation in Haploidentical Stem Cell Transplantation. Biol Blood Marrow Transplant (2018) 24:353–8. doi: 10.1016/j.bbmt.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 61. Uppuluri R, Sivasankaran M, Patel S, Swaminathan VV, Ramanan KM, Ravichandran N, et al. Haploidentical Stem Cell Transplantation With Post-Transplant Cyclophosphamide for Primary Immune Deficiency Disorders in Children: Challenges and Outcome From a Tertiary Care Center in South India. J Clin Immunol (2019) 39:182–7. doi: 10.1007/s10875-019-00600-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arcuri LJ, Nabhan SK, Cunha R, Nichele S, Ribeiro AAF, Fernandes JF, et al. Impact of CD34 Cell Dose and Conditioning Regimen on Outcomes After Haploidentical Donor Hematopoietic Stem Cell Transplantation With Post-Transplantation Cyclophosphamide for Relapsed/Refractory Severe Aplastic Anemia. Biol Blood Marrow Transplant (2020) 26:2311–7. doi: 10.1016/j.bbmt.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 63. Raj RV, Hari P, Pasquini M, Epperla N, D’Souza A, Fenske T, et al. Impact of Haploidentical Hematopoietic Cell Transplantation Conditioning Intensity on the Incidence and Severity of Post-Transplantation Viral Infections. Bone Marrow Transplant (2016) 51:1602–4. doi: 10.1038/bmt.2016.216 [DOI] [PubMed] [Google Scholar]
- 64. Katsanis E, Maher K, Roe DJ, Simpson RJ. Progressive Substitution of Posttransplant Cyclophosphamide With Bendamustine: A Phase I Study in Haploidentical Bone Marrow Transplantation. eJHaem (2020) 1:286–92. doi: 10.1002/jha2.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Goldsmith SR, Slade M, DiPersio JF, Westervelt P, Lawrence SJ, Uy GL, et al. Cytomegalovirus Viremia, Disease, and Impact on Relapse in T-Cell Replete Peripheral Blood Haploidentical Hematopoietic Cell Transplantation With Post-Transplant Cyclophosphamide. Haematologica (2016) 101:e465–8. doi: 10.3324/haematol.2016.149880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Total Body Irradiation–Based Myeloablative Haploidentical Stem Cell Transplantation Is a Safe and Effective Alternative to Unrelated Donor Transplantation in Patients Without Matched Sibling Donors. Biol Blood Marrow Transplant (2015) 21:1299–307. doi: 10.1016/j.bbmt.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 67. Stocker N, Gaugler B, Labopin M, Farge A, Ye Y, Ricard L, et al. High-Dose Post-Transplant Cyclophosphamide Impairs γδ T-Cell Reconstitution After Haploidentical Haematopoietic Stem Cell Transplantation Using Low-Dose Antithymocyte Globulin and Peripheral Blood Stem Cell Graft. Clin Trans Immunol (2020) 9:e1171. doi: 10.1002/cti2.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oltolini C, Greco R, Galli L, Clerici D, Lorentino F, Xue E, et al. Infections After Allogenic Transplant With Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biol Blood Marrow Transplant (2020) 26:1179–88. doi: 10.1016/j.bbmt.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 69. Slade M, Goldsmith S, Romee R, DiPersio JF, Dubberke ER, Westervelt P, et al. Epidemiology of Infections Following Haploidentical Peripheral Blood Hematopoietic Cell Transplantation. Transpl Infect Dis (2017) 19:e12629. doi: 10.1111/tid.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montoro J, Piñana JL, Hernández-Boluda JC, Hernani R, Lorenzo I, Pérez A, et al. Uniform Graft-Versus-Host Disease Prophylaxis With Posttransplant Cyclophosphamide, Sirolimus, and Mycophenolate Mofetil Following Hematopoietic Stem Cell Transplantation From Haploidentical, Matched Sibling and Unrelated Donors. Bone Marrow Transplant (2020) 55:2147–59. doi: 10.1038/s41409-020-0921-6 [DOI] [PubMed] [Google Scholar]
- 71. Cieri N, Greco R, Crucitti L, Morelli M, Giglio F, Levati G, et al. Post-Transplantation Cyclophosphamide and Sirolimus After Haploidentical Hematopoietic Stem Cell Transplantation Using a Treosulfan-Based Myeloablative Conditioning and Peripheral Blood Stem Cells. Biol Blood Marrow Transplant (2015) 21:1506–14. doi: 10.1016/j.bbmt.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 72. Law AD, Salas MQ, Lam W, Michelis FV, Thyagu S, Kim DDH, et al. Reduced-Intensity Conditioning and Dual T Lymphocyte Suppression With Antithymocyte Globulin and Post-Transplant Cyclophosphamide as Graft-Versus-Host Disease Prophylaxis in Haploidentical Hematopoietic Stem Cell Transplants for Hematological Malignancies. Biol Blood Marrow Transplant (2018) 24:2259–64. doi: 10.1016/j.bbmt.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Salas MQ, Prem S, Atenafu EG, Datt Law A, Lam W, Al-Shaibani Z, et al. Dual T-Cell Depletion With ATG and PTCy for Peripheral Blood Reduced Intensity Conditioning Allo-HSCT Results in Very Low Rates of GVHD. Bone Marrow Transplant (2020) 55:1773–83. doi: 10.1038/s41409-020-0813-9 [DOI] [PubMed] [Google Scholar]
- 74. Salas MQ, Prem S, Atenafu EG, Law AD, Lam W, Al-Shaibani Z, et al. Reduced Intensity Allogeneic Stem Cell Transplant With Anti-Thymocyte Globulin and Post-Transplant Cyclophosphamide in Acute Myeloid Leukemia. Eur J Haematol (2019) 103:510–8. doi: 10.1111/ejh.13321 [DOI] [PubMed] [Google Scholar]
- 75. Lint CH, Su YJ, Hsu CY, Wang PN, Teng CL. Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation Increases the Risk of Cytomegalovirus Infection in adult Patients With Acute Leukemia. Transpl Infect Dis (2019) 21(4):e13096. [DOI] [PubMed] [Google Scholar]
- 76. Wang Y, Chang Y-J, Chen L, Xu L-P, Bian Z-L, Zhang X-H, et al. Low-Dose Post-Transplant Cyclophosphamide can Mitigate GVHD and Enhance the G-CSF/ATG Induced GVHD Protective Activity and Improve Haploidentical Transplant Outcomes. OncoImmunology (2017) 6:e1356152. doi: 10.1080/2162402X.2017.1356152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang Y, Wu D-P, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, et al. Low-Dose Post-Transplant Cyclophosphamide and Anti-Thymocyte Globulin as an Effective Strategy for GVHD Prevention in Haploidentical Patients. J Hematol Oncol (2019) 12:88. doi: 10.1186/s13045-019-0781-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tischer J, Engel N, Fritsch S, Prevalsek D, Hubmann M, Schulz C, et al. Virus Infection in HLA-Haploidentical Hematopoietic Stem Cell Transplantation: Incidence in the Context of Immune Recovery in Two Different Transplantation Settings. Ann Hematol (2015) 94:1677–88. doi: 10.1007/s00277-015-2423-y [DOI] [PubMed] [Google Scholar]
- 79. ElGohary G, El Fakih R, de Latour R, Risitano A, Marsh J, Schrezenmeier H, et al. Haploidentical Hematopoietic Stem Cell Transplantation in Aplastic Anemia: A Systematic Review and Meta-Analysis of Clinical Outcome on Behalf of the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (SAAWP of EBMT). Bone Marrow Transplant (2020) 55:1906–17. doi: 10.1038/s41409-020-0897-2 [DOI] [PubMed] [Google Scholar]
- 80. Li J-J, Huang X, Wang H, Guo X-Y, Ge S-X, Zhang J. Baseline Antibody Level may Help Predict the Risk of Active Human Cytomegalovirus Infection in a HCMV Seropositive Population. Eur J Clin Microbiol Infect Dis (2017) 36:863–8. doi: 10.1007/s10096-016-2873-8 [DOI] [PubMed] [Google Scholar]
- 81. Lu S-C, Chin L-T, Wu F-M, Hsieh G-J, Haung S-P, Chen J-C, et al. Seroprevalence of CMV Antibodies in a Blood Donor Population and Premature Neonates in the South-Central Taiwan. Kaohsiung J Med Sci (1999) 15:603–10. [PubMed] [Google Scholar]
- 82. Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the Worldwide Seroprevalence of Cytomegalovirus: A Systematic Review and Meta-Analysis. Rev Med Virol (2019) 29:e2034. doi: 10.1002/rmv.2034 [DOI] [PubMed] [Google Scholar]
- 83. Lachmann R, Loenenbach A, Waterboer T, Brenner N, Pawlita M, Michel A, et al. Cytomegalovirus (CMV) Seroprevalence in the Adult Population of Germany. PloS One (2018) 13:e0200267. doi: 10.1371/journal.pone.0200267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Antona D, Lepoutre A, Fonteneau L, Baudon C, Halftermeyer-Zhou F, Le Strat Y, et al. Seroprevalence of Cytomegalovirus Infection in France in 2010. Epidemiol Infect (2017) 145:1471–8. doi: 10.1017/S0950268817000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Luo XH, Chang YJ, Huang XJ. Improving Cytomegalovirus-Specific T Cell Reconstitution After Haploidentical Stem Cell Transplantation. J Immunol Res (2014) 2014. doi: 10.1155/2014/631951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Luo XH, Huang XJ, Li D, Liu KY, Xu LP, Liu DH. Immune Reconstitution to Cytomegalovirus Following Partially Matched-Related Donor Transplantation: Impact of In Vivo T-Cell Depletion and Granulocyte Colony-Stimulating Factor-Primed Peripheral Blood/Bone Marrow Mixed Grafts. Transplant Infect Dis (2013) 15:22–33. doi: 10.1111/j.1399-3062.2012.00722.x [DOI] [PubMed] [Google Scholar]
- 87. Luo X-H, Huang X-J, Liu K-Y, Xu L-P, Liu D-H. Protective Immunity Transferred by Infusion of Cytomegalovirus-Specific CD8+ T Cells Within Donor Grafts: Its Associations With Cytomegalovirus Reactivation Following Unmanipulated Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2010) 16:994–1004. doi: 10.1016/j.bbmt.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 88. Kato R, Tamaki H, Ikegame K, Yoshihara S, Kaida K, Taniguchi K, et al. Early Detection of Cytomegalovirus-Specific Cytotoxic T Lymphocytes Against Cytomegalovirus Antigenemia in Human Leukocyte Antigen Haploidentical Hematopoietic Stem Cell Transplantation. Ann Hematol (2015) 94:1707–15. doi: 10.1007/s00277-015-2446-4 [DOI] [PubMed] [Google Scholar]
- 89. Noviello M, Forcina A, Veronica V, Crocchiolo R, Stanghellini MT, Carrabba M, et al. Early Recovery of CMV Immunity After HLA-Haploidentical Hematopoietic Stem Cell Transplantation as a Surrogate Biomarker for a Reduced Risk of Severe Infections Overall. Bone Marrow Transplant (2015) 50:1262–4. doi: 10.1038/bmt.2015.132 [DOI] [PubMed] [Google Scholar]
- 90. Huntley D, Giménez E, Pascual MJ, Vázquez L, Amat P, Remigia MJ, et al. Peripheral Blood Regulatory T Cells and Occurrence of Cytomegalovirus DNAemia After Unmanipulated Haploidentical Allogeneic Hematopoietic Stem Cell Transplantation With Posttransplant Cyclophosphamide. Bone Marrow Transplant (2020) 55:1493–6. doi: 10.1038/s41409-020-0950-1 [DOI] [PubMed] [Google Scholar]
- 91. van Beek JJP, Puccio S, Roberto A, De Paoli F, Graziano G, Salviato E, et al. Single-Cell Profiling Reveals the Dynamics of Cytomegalovirusspecific T-Cells in Haploidentical Hematopoietic Stem Cell Transplantation. Haematologica (2021) 106(10):2768–73. doi: 10.3324/haematol.2020.276352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jin F, Lin H, Gao S, Wang H, Yan H, Guo J, et al. Characterization of Ifnγ-Producing Natural Killer Cells Induced by Cytomegalovirus Reactivation After Haploidentical Hematopoietic Stem Cell Transplantation. Oncotarget (2017) 8:51–63. doi: 10.18632/oncotarget.13916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jang JE, Hwang DY, Chung H, Kim SJ, Eom JI, Jeung HK, et al. Early Cytomegalovirus Reactivation and Expansion of CD56(bright)CD16(dim/-)DNAM1(+) Natural Killer Cells Are Associated With Antileukemia Effect After Haploidentical Stem Cell Transplantation in Acute Leukemia. Biol Blood Marrow Transplant (2019) 25:2070–8. doi: 10.1016/j.bbmt.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 94. Muccio L, Bertaina A, Falco M, Pende D, Meazza R, Lopez-Botet M, et al. Analysis of Memory-Like Natural Killer Cells in Human Cytomegalovirus-Infected Children Undergoing αβ+T and B Cell-Depleted Hematopoietic Stem Cell Transplantation for Hematological Malignancies. Haematologica (2016) 101:371–81. doi: 10.3324/haematol.2015.134155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhao XY, Luo XY, Yu XX, Zhao XS, Han TT, Chang YJ, et al. Recipient-Donor KIR Ligand Matching Prevents CMV Reactivation Post-Haploidentical T Cell-Replete Transplantation. Br J Haematol (2017) 177:766–81. doi: 10.1111/bjh.14622 [DOI] [PubMed] [Google Scholar]
- 96. Hu L-J, Cao X-H, Yu X-X, Liu X-F, Zhao X-S, Chang Y-J, et al. NK Cell Reconstitution Following Unmanipulated HLA-Mismatched/Haploidentical Transplantation Compared With Matched Sibling Transplantation. Sci China Life Sci (2020) 63:781–4. doi: 10.1007/s11427-018-9565-5 [DOI] [PubMed] [Google Scholar]
- 97. Zaghi E, Calvi M, Puccio S, Spata G, Terzoli S, Peano C, et al. Single-Cell Profiling Identifies Impaired Adaptive NK Cells Expanded After HCMV Reactivation in Haploidentical-HSCT. JCI Insight (2021) 6(12):e146973. doi: 10.1172/jci.insight.146973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Park M, Im HJ, Lee Y-J, Park N, Jang S, Kwon SW, et al. Reconstitution of T and NK Cells After Haploidentical Hematopoietic Cell Transplantation Using αβ T Cell-Depleted Grafts and the Clinical Implication of γδ T Cells. Clin Transplant (2018) 32:e13147. doi: 10.1111/ctr.13147 [DOI] [PubMed] [Google Scholar]
- 99. Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, et al. Adoptive Transfer of Pp65-Specific T Cells for the Treatment of Chemorefractory Cytomegalovirus Disease or Reactivation After Haploidentical and Matched Unrelated Stem Cell Transplantation. Blood (2010) 116:4360–7. doi: 10.1182/blood-2010-01-262089 [DOI] [PubMed] [Google Scholar]
- 100. Pei XY, Zhao XY, Chang YJ, Liu J, Xu LP, Wang Y, et al. Cytomegalovirus-Specific T-Cell Transfer for Refractory Cytomegalovirus Infection After Haploidentical Stem Cell Transplantation: The Quantitative and Qualitative Immune Recovery for Cytomegalovirus. J Infect Dis (2017) 216:945–56. doi: 10.1093/infdis/jix357 [DOI] [PubMed] [Google Scholar]
- 101. Zhao X-Y, Pei X-Y, Chang Y-J, Yu X-X, Xu L-P, Wang Y, et al. First-Line Therapy With Donor-Derived Human Cytomegalovirus (HCMV)–specific T Cells Reduces Persistent HCMV Infection by Promoting Antiviral Immunity After Allogenic Stem Cell Transplantation. Clin Infect Dis (2019) 70:1429–37. doi: 10.1093/cid/ciz368 [DOI] [PubMed] [Google Scholar]
- 102. Ke P, Bao X, Zhou J, Li X, Zhuang J, He X, et al. Donor CMV-Specific Cytotoxic T Lymphocytes Successfully Treated Drug-Resistant Cytomegalovirus Encephalitis After Allogeneic Hematopoietic Stem Cell Transplantation. Hematology (2020) 25:43–7. doi: 10.1080/16078454.2019.1710945 [DOI] [PubMed] [Google Scholar]
- 103. Maschan M, Blagov S, Shelikhova L, Shekhovtsova Z, Balashov D, Starichkova J, et al. Low-Dose Donor Memory T-Cell Infusion After TCR Alpha/Beta Depleted Unrelated and Haploidentical Transplantation: Results of a Pilot Trial. Bone Marrow Transplant (2018) 53:264–73. doi: 10.1038/s41409-017-0035-y [DOI] [PubMed] [Google Scholar]
- 104. Dunaikina M, Zhekhovtsova Z, Shelikhova L, Glushkova S, Nikolaev R, Blagov S, et al. Safety and Efficacy of the Low-Dose Memory (CD45RA-Depleted) Donor Lymphocyte Infusion in Recipients of αβ T Cell-Depleted Haploidentical Grafts: Results of a Prospective Randomized Trial in High-Risk Childhood Leukemia. Bone Marrow Transplant (2021) 56:1614–24. doi: 10.1038/s41409-021-01232-x [DOI] [PubMed] [Google Scholar]
- 105. Shelikhova L, Glushkova S, Nikolaev R, Dunaikina M, Zhekhovtsova Z, Blagov S, et al. Serotherapy-Free Regimen Improves Non-Relapse Mortality and Immune Recovery Among the Recipients of αβ TCell–Depleted Haploidentical Grafts: Retrospective Study in Childhood Leukemia. Transplant Cell Ther (2021) 27:330.e1–.e9. doi: 10.1016/j.jtct.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 106. Castagna L, Valli V, Timofeeva I, Capizzuto R, Bramanti S, Mariotti J, et al. Feasibility and Efficacy of CD45RA+ Depleted Donor Lymphocytes Infusion After Haploidentical Transplantation With Post-Transplantation Cyclophosphamide in Patients With Hematological Malignancies. Transplant Cell Ther (2021) 27:478.e1–.e5. doi: 10.1016/j.jtct.2021.03.010 [DOI] [PubMed] [Google Scholar]
- 107. Park HJ, Hong KT, Yun SO, Ahn HY, Choi JY, Shin HY, et al. Successful Treatment of Refractory CMV Colitis After Haploidentical HSCT With Post-Transplant Cyclophosphamide Using CD45RA+ Depleted Donor Lymphocyte Infusion. Bone Marrow Transplant (2020) 55:1674–6. doi: 10.1038/s41409-019-0685-z [DOI] [PubMed] [Google Scholar]
- 108. Uygun V, Karasu G, Daloğlu H, Öztürkmen S, Yalçın K, Çelen SS, et al. Use of Low Cell Dose for Unmanipulated Donor Lymphocyte for Management of Cytomegalovirus Infection: A Single-Center Experience. Pediatr Transplant (2020) 24:e13882. doi: 10.1111/petr.13882 [DOI] [PubMed] [Google Scholar]
- 109. Lugthart G, van Ostaijen-ten Dam MM, Jol - van der Zijde CM, van Holten TC, Kester MGD, Heemskerk MHM, et al. Early Cytomegalovirus Reactivation Leaves a Specific and Dynamic Imprint on the Reconstituting T Cell Compartment Long-Term After Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2014) 20:655–61. doi: 10.1016/j.bbmt.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 110. Raphael I, Marie R, Helene M-T, Marc D, Marc B, Aliénor X, et al. Cytomegalovirus Shapes Long-Term Immune Reconstitution After Allogeneic Stem Cell Transplantation. Haematologica (2015) 100:114–23. doi: 10.3324/haematol.2014.113415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Luo XH, Meng Q, Rao M, Liu Z, Paraschoudi G, Dodoo E, et al. The Impact of Inflationary Cytomegalovirus-Specific Memory T Cells on Anti-Tumour Immune Responses in Patients With Cancer. Immunology (2018) 155:294–308. doi: 10.1111/imm.12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang Y-L, Zhu Y, Xiao Q, Wang L, Liu L, Luo X-H. Cytomegalovirus Infection is Associated With AML Relapse After Allo-HSCT: A Meta-Analysis of Observational Studies. Ann Hematol (2019) 98:1009–20. doi: 10.1007/s00277-018-3585-1 [DOI] [PubMed] [Google Scholar]
- 113. Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early Human Cytomegalovirus Replication After Transplantation is Associated With a Decreased Relapse Risk: Evidence for a Putative Virus-Versus-Leukemia Effect in Acute Myeloid Leukemia Patients. Blood (2011) 118:1402–12. doi: 10.1182/blood-2010-08-304121 [DOI] [PubMed] [Google Scholar]
- 114. Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, et al. CMV Reactivation After Allogeneic HCT and Relapse Risk: Evidence for Early Protection in Acute Myeloid Leukemia. Blood J Am Soc Hematol (2013) 122:1316–24. doi: 10.1182/blood-2013-02-487074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, et al. Early Cytomegalovirus Reactivation Remains Associated With Increased Transplant-Related Mortality in the Current Era: A CIBMTR Analysis. Blood (2016) 127:2427–38. doi: 10.1182/blood-2015-11-679639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cantoni N, Hirsch HH, Khanna N, Gerull S, Buser A, Bucher C, et al. Evidence for a Bidirectional Relationship Between Cytomegalovirus Replication and Acute Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2010) 16:1309–14. doi: 10.1016/j.bbmt.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 117. Lönnqvist B, Ringdén O, Wahren B, Gahrton G, Lundgren G. Cytomegalovirus Infection Associated With and Preceding Chronic Graft-Versus-Host Disease. Transplantation (1984) 38:465–8. doi: 10.1097/00007890-198411000-00004 [DOI] [PubMed] [Google Scholar]