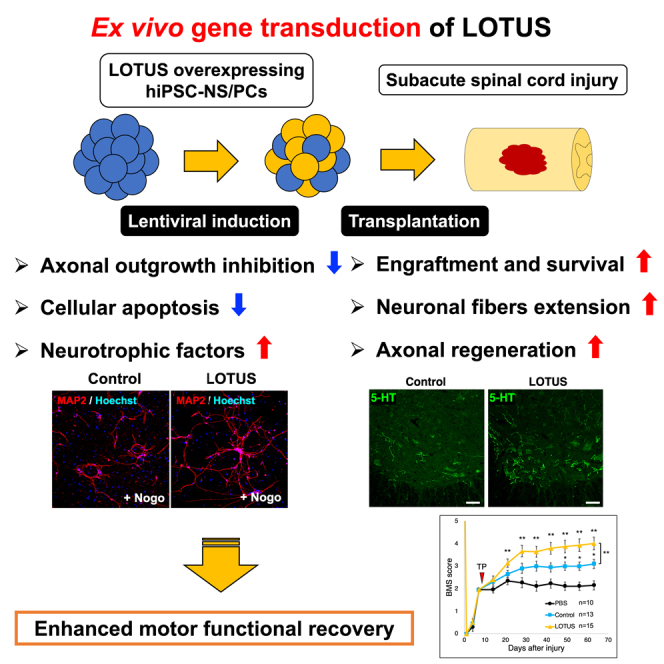
Summary
Functional recovery is still limited mainly due to several mechanisms, such as the activation of Nogo receptor-1 (NgR1) signaling, when human induced pluripotent stem cell-derived neural stem/progenitor cells (hiPSC-NS/PC) are transplanted for subacute spinal cord injury (SCI). We previously reported the neuroprotective and regenerative benefits of overexpression of lateral olfactory tract usher substance (LOTUS), an endogenous NgR1 antagonist, in the injured spinal cord using transgenic mice. Here, we evaluate the effects of lentiviral transduction of LOTUS gene into hiPSC-NS/PCs before transplantation in a mouse model of subacute SCI. The transduced LOTUS contributes to neurite extension, suppression of apoptosis, and secretion of neurotrophic factors in vitro. In vivo, the hiPSC-NS/PCs enhance the survival of grafted cells and enhance axonal extension of the transplanted cells, resulting in significant restoration of motor function following SCI. Therefore, the gene transduction of LOTUS in hiPSC-NS/PCs could be a promising adjunct for transplantation therapy for SCI.
Keywords: spinal cord injury, transplantation, iPSC, Nogo receptor, LOTUS, axonal regrowth, motor function, regenerative medicine, ex vivo gene therapy
Graphical abstract
Highlights
-
•
LOTUS-overexpressing hiPSC-NS/PCs induce axonal extension and apoptosis suppression
-
•
LOTUS-overexpressing hiPSC-NS/PCs increase secretion of neurotrophic factors
-
•
Transplanted LOTUS-overexpressing hiPSC-NS/PCs enhance motor functional recovery
-
•
Ex vivo gene therapy delivering LOTUS is beneficial for cell transplantation
In this article, Hideyuki Okano and his colleagues (Ito et al.) show that the transduced LOTUS contributes to neurite extension, apoptosis suppression, and secretion of neurotrophic factors in hiPSC-NS/PCs in vitro. They also show that transplanted LOTUS-overexpressing hiPSC-NS/PCs enhance survival of grafted cells and enhance axonal extension of the transplanted cells, resulting in significant restoration of motor function following SCI.
Introduction
Several studies have reported the efficacy of human induced pluripotent stem cell-derived neural stem/progenitor cell (hiPSC-NS/PC) transplantation at the subacute stage of spinal cord injury (SCI) (Fujimoto et al., 2012; Lu et al., 2014; Nori et al., 2011; Uezono et al., 2018). However, the functional recovery obtained by cell transplantation alone is still limited, and the establishment of a more effective therapeutic method is desirable (Fujimoto et al., 2012; Lu et al., 2014; Nori et al., 2011). Several factors are thought to limit recovery: poor survival of the transplanted cells and poor axonal outgrowth of the transplanted cells due to the unfavorable environment in the injured spinal cord. After SCI, myelin debris and glial scars produce ligands of Nogo receptor-1 (NgR1), such as Nogo (Chen et al., 2000; GrandPre et al., 2000; Prinjha et al., 2000), myelin-associated glycoprotein (MAG) (McKerracher et al., 1994; Mukhopadhyay et al., 1994; Savio and Schwab, 1990), oligodendrocyte myelin glycoprotein (OMgp) (Wang et al., 2002), B lymphocyte stimulator (BLyS) (Zhang et al., 2009), and chondroitin sulfate proteoglycans (CSPGs) (Dickendesher et al., 2012). These ligands bind to NgR1 and cause growth cone collapse by activating the Ras homolog gene family member A (Rho-A) and Rho-associated kinase (ROCK), thereby inhibiting neuronal regeneration (Dergham et al., 2002; Fournier et al., 2003; Niederost et al., 2002). Blocking the Rho-ROCK cascade that is upregulated by NgR1 activation, therefore, was postulated to be beneficial in the treatment of SCI (Forgione and Fehlings, 2014; Wu and Xu, 2016).
Lateral olfactory tract usher substance (LOTUS), also known as cartilage acidic protein-1B (Crtac1B) (Sato et al., 2011), has been reported to function as an endogenous antagonist of NgR1. LOTUS binds to NgR1 and inhibits all of the ligand proteins (Nogo-A, MAG, OMgp, BLys, and CSPGs). As a result, growth cone collapse is reduced, and neurite outgrowth is promoted (Kawakami et al., 2018; Kurihara et al., 2014). As shown in a recent study, pan neuronal LOTUS overexpression under control of the mouse synapsin-1 promoter enhances the regeneration of raphespinal tract fibers in dorsal hemisection model transgenic mice, thereby contributing to the recovery of motor function (Hirokawa et al., 2017). We further verified the effects of LOTUS on a more clinically relevant contusive SCI model using the same mice. Overexpression of LOTUS in the spinal cord exerted neuroprotective and regenerative effects, which contributed to a significant restoration of motor function and nerve conduction after SCI (Ito et al., 2018). Given the beneficial effects of LOTUS on both neural tissue protection and regeneration, we hypothesized that the induction of LOTUS expression in NS/PCs before transplantation would produce an even more beneficial outcome by increasing the survival of transplanted cells and promoting axonal extension in SCI. Furthermore, LOTUS was also reported to suppress the axonal growth-inhibiting receptor PIR-B, resulting in restraining growth cone collapse and neurite growth inhibition (Kurihara et al., 2020). PIR-B has been reported to function as a common receptor for Nogo, MAG, and OMgp, and inhibition of PIR-B activity partially restores neurite inhibition induced by these ligands (Atwal, 2008). Thus, LOTUS might exert stronger effects on axonal regeneration than NgR inhibitors by blocking both NgR1 and PIR-B.
In this study, we evaluated the efficacy of transplanting hiPSC-NS/PCs overexpressing LOTUS through lentiviral ex vivo gene transduction in subacute SCI. LOTUS suppressed the inhibition of axonal outgrowth and cellular apoptosis caused by NgR1 ligands and promoted the expression of neurotrophic factors in hiPSC-NS/PCs in vitro. Moreover, LOTUS enhanced the survival of graft cells and axonal extension in the injured spinal cord, contributing to further functional recovery. Thus, the combination of cell transplantation with ex vivo LOTUS gene transduction potentially represents a promising tool for the treatment of SCI.
Results
Establishment of 414C2 hiPSC-NS/PCs expressing LOTUS via lentiviral infection
In this study, we first established LOTUS-expressing hiPSC-NS/PCs using lentiviral induction to evaluate whether the NgR1 antagonist LOTUS enhances the efficacy of neural stem cell transplantation in SCI. We generated a lentiviral vector encoding mouse LOTUS (mLOTUS) with a fluorescent protein marker (Venus) driven by an EF-1α promoter (CSII-EF1α-mLOTUS-IRES-Venus) (Figure 1A). The hiPSC-NS/PCs generated from 414C2-hiPSCs were infected with the LOTUS-expressing lentivirus and identified as LOTUS-NS/PCs. The control-NS/PCs were prepared by a similar transduction of ffLuc lentivirus without the mLOTUS coding sequence (Hara-Miyauchi et al., 2012). Fourth-passage neurospheres were used in both the in vitro and in vivo experiments.
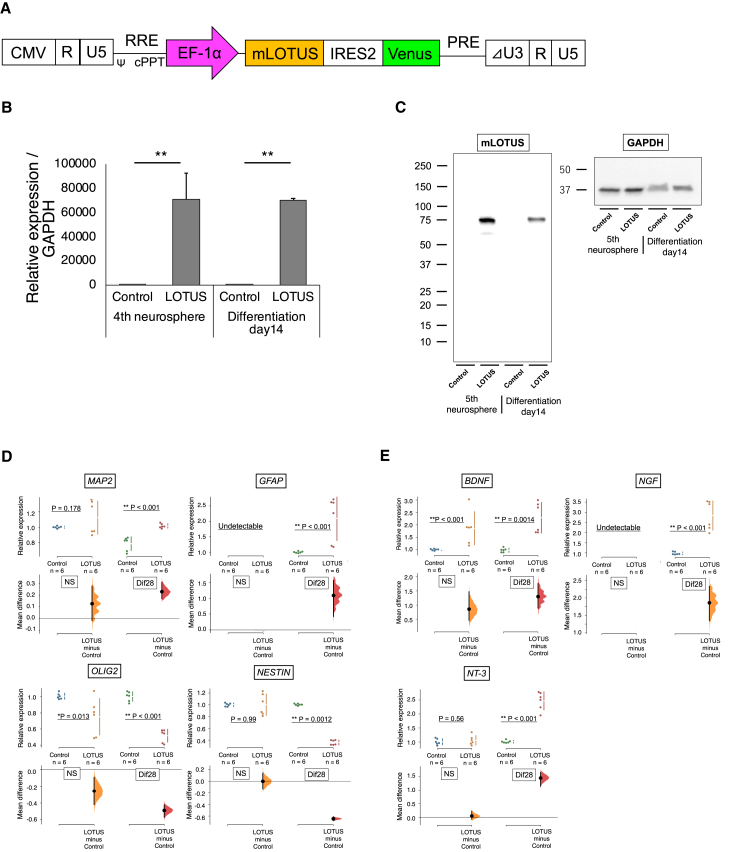
Figure 1.
Mouse LOTUS (mLOTUS) expression in neurospheres and differentiated cells derived from 414C2 hiPSC-NS/PCs and gene expression change of LOTUS-expressing NS/PCs in the differentiation marker and neurotrophic factors
(A) Schematic illustration of the lentiviral vector CSII-EF1α-mLOTUS-IRES-Venus, which expresses mLOTUS cDNA and Venus fluorescent protein gene connected by an internal ribosomal entry site (IRES) under the control of EF1α promoter.
(B) Representative images of the neurosphere derived from control-NS/PCs and LOTUS-NS/PCs visualized by fluorescence microscopy.
(C) Western blot analyses of mLOTUS protein expression in both NS/PCs (LOTUS-NS/PCs; n = 5 independent experiments, control-NS/PCs; n = 5 independent experiments).
(D) Quantitative real-time PCR analyses for the gene expression of MAP2, GFAP, OLIG2, and NESTIN in both NS/PC groups (LOTUS-NS/PCs; n = 6 independent experiments, control-NS/PCs; n = 6 independent experiments).
(E) Quantitative real-time PCR analyses for the gene expression of BDNF, NGF, and NT-3 in both NS/PC groups (LOTUS-NS/PCs; n = 6 independent experiments, control-NS/PCs; n = 6 independent experiments). Values are the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Values are the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Statistical analysis was performed using the Mann-Whitney U test in each real-time PCR analysis.
Next, we performed real-time PCR analyses to determine the expression of the Lotus gene in the LOTUS-NS/PCs. Compared with the control cells, the LOTUS-NS/PCs expressed higher levels of the Lotus gene during both the neurosphere stage and its subsequent derivatives (Figure 1B). Western blotting analysis also showed the expression of the mLOTUS protein in the LOTUS-NS/PCs before and after differentiation (Figure 1C).
Changes in gene expression of the LOTUS-expressing NS/PCs
We performed real-time PCR to examine the effects of LOTUS on the differentiation profiles and secretion of trophic factors by LOTUS-overexpressing NS/PCs. The qPCR analysis of the differentiated cells on day 28 showed that LOTUS expression did not affect the expression of NOGO and NGR1, an inhibitor of axonal regeneration and its receptor, respectively (Figure S1A). Among the markers of cell differentiation, MAP2 and GFAP were upregulated, while OLIG2 and NESTIN expression was downregulated in the LOTUS-NS/PCs (Figure 1D). Comparable expression levels of NESTIN, an indicator of undifferentiated neural cells, were detected. Notably, the expression of neurotrophic factors, such as BDNF, NT-3, and NGF, was upregulated in the LOTUS-NS/PCs (Figure 1E). An NgR1 ligand stimulation assay, followed by real-time PCR was performed to investigate the effects of the presence of NgR1 ligands, such as Nogo, MAG, and OMgp, on the expression of neurotrophic factors. At 14 days after seeding on the wells precoated with PBS and each NgR1 ligand, both control-NS/PCs and LOTUS-NS/PCs showed no significant change in the expression of each neurotrophic factor in the presence of NgR1 ligands. Therefore, the upregulated expression of neurotrophic factors may be an effect of LOTUS expression but not NgR1 signaling (Figure S2A).
The LOTUS-expressing NS/PCs suppressed NgR1 ligand-mediated axonal inhibition and apoptosis in vitro
LOTUS acts as an NgR1 antagonist and inhibits downstream RhoA/ROCK signaling, suggesting that LOTUS expression in NS/PCs may promote axonal outgrowth and suppress cellular apoptosis (Ito et al., 2018). To verify this, we performed immunocytochemistry following a 2-week differentiation of the control- and LOTUS-NS/PCs. We measured the neurite length in the MAP2-positive cells and found that significantly longer dendrites were observed in the LOTUS-NS/PCs than in the control-NS/PCs (Figures 2A and 2B).
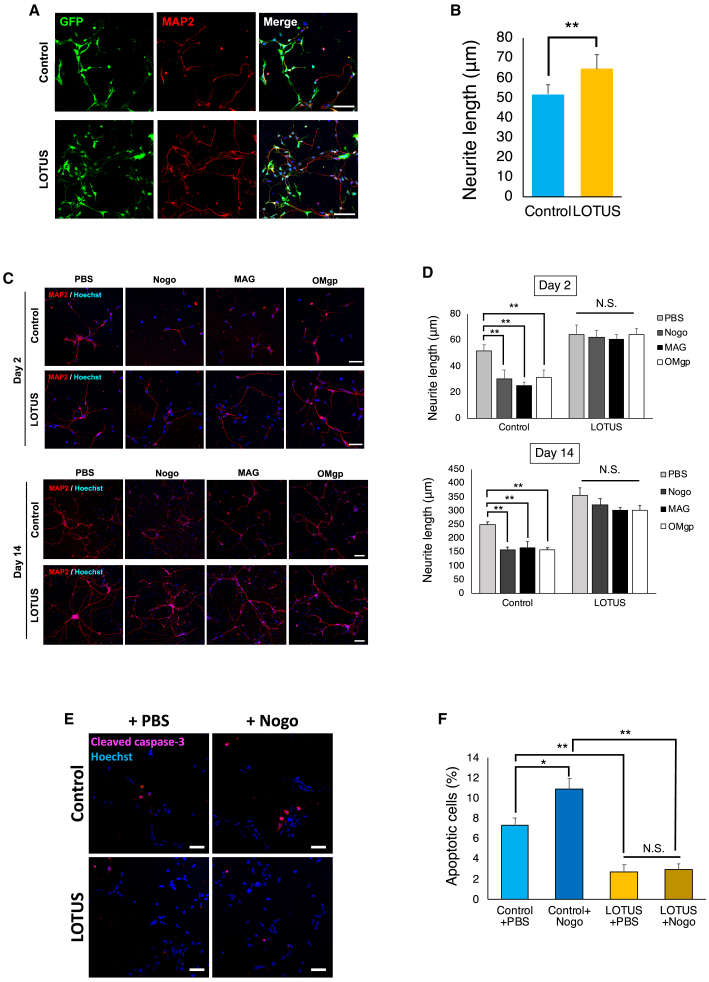
Figure 2.
The LOTUS-expressing NS/PCs suppressed the axonal outgrowth inhibition extension and apoptosis in vitro
(A) Representative images of immunostaining for GFP and MAP2 at 2 days after differentiation.
(B) Quantitative analysis of the neurite length in both NS/PCs (LOTUS-NS/PCs; n = 5 independent experiments, control-NS/PCs; n = 5 independent experiments).
(C) Representative images of neurite outgrowth inhibition due to Nogo, MAG, and OMgp, compared with PBS as control at 2 and 14 days post-differentiation.
(D) Quantitative analysis of the neurite length on the NgR ligands compared with PBS (LOTUS-NS/PCs; n = 5 independent experiments, control-NS/PCs; n = 5 independent experiments, for each ligand).
(E) Representative images of apoptotic cells immunostained with anti-cleaved caspase-3 antibody at 2 days post-differentiation.
(F) Quantitative analysis of the percentage of cleaved caspase-3-positive apoptotic cells (LOTUS-NS/PCs; n = 5 independent experiments, control-NS/PCs; n = 5 independent experiments). Values are the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Statistical analysis was performed using Mann-Whitney U test in (B) and one-way ANOVA followed by the Tukey-Kramer test in (D and F). Scale bars, 50 μm in (A and E) and day 2 in (C), 100 μm day 14 in (C).
To determine whether LOTUS expression suppresses the inhibitory action of NgR1 ligands on neurite outgrowth, we performed a neurite outgrowth assay using the control-NS/PCs and the LOTUS-NS/PCs. The neurospheres of both NS/PC groups were dissociated into single cells and seeded on dishes precoated with PBS, Nogo, MAG, or OMgp. The lengths of the extended neurons were measured at 2 and 14 days after differentiation. In the presence of Nogo, MAG, or OMgp, the length of the neurite outgrowth was significantly shorter than that in PBS in the control-NS/PCs. However, there was no significant inhibition of axonal outgrowth on these ligand coatings in the LOTUS-NS/PCs at 2 and 14 days after seeding (Figures 2C and 2D). These results suggest that LOTUS prevented the inhibition of neurite outgrowth as an antagonist of NgR1.
We further examined the induction of apoptotic cell death by Nogo in the NS/PCs with or without LOTUS overexpression. The dissociated single cells of both NS/PCs were plated on dishes precoated with PBS or Nogo, and the apoptotic cells were counted 2 days post-plating. Immunostaining of cleaved caspase-3, a marker for apoptosis, revealed significantly fewer apoptotic cells in the LOTUS-NS/PCs than in the control-NS/PCs. Furthermore, the addition of Nogo significantly increased the apoptotic cells in the control-NS/PCs, whereas the induction of apoptosis was suppressed even in the presence of Nogo in the LOTUS-NS/PCs (Figures 2E and 2F).
The LOTUS-NS/PCs showed enhanced survival after transplantation and good differentiation into the three neural lineages
Previous in vitro data indicated that LOTUS suppresses cell apoptosis, promotes the secretion of trophic factors, and enhances axonal outgrowth in NS/PCs. We first examined the efficacy of LOTUS on promoting the survival of NS/PCs transplanted into the injured spinal cord by conducting bioluminescence imaging (BLI), as described in previous reports (Itakura et al., 2015; Kojima et al., 2019; Okada et al., 2005; Uezono et al., 2018). The BLI analysis revealed that the photon counts of the transplanted control-NS/PCs and LOTUS-NS/PCs and that the cells survived for up to 49 days after transplantation (Figures 3A and 3B). The engraftment rate of the transplanted LOTUS-NS/PCs reached 14.7% from the starting point to 49 days after transplantation, which was higher than 10.6% for the control-NS/PCs (Figure 3A). The histological analysis performed on day 54 revealed the presence of transplanted cells around the scar area that had migrated rostrally and caudally (Figure 3C).
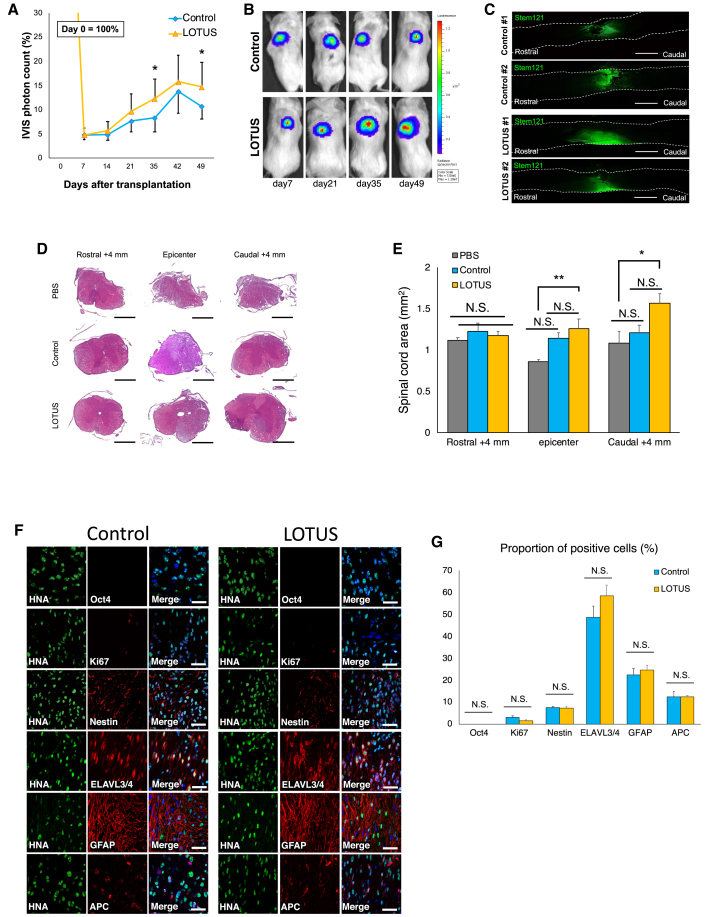
Figure 3.
In vivo imaging analyses of changes in the transplanted cells and histological analyses of spinal cord volume and differentiation profile
(A) Quantitative analyses of the IVIS photon counts of the transplanted cells as percentage changes compared with the day of transplantation (LOTUS group; n = 8, control group; n = 6).
(B) Representative images of the IVIS photon counts up to 49 days.
(C) Representative images of immunostaining for STEM121 in the sagittal sections 54 days after transplantation.
(D) Representative images of H&E staining of the axial sections at 4 mm rostral, epicenter and 4 mm rostral to the epicenter.
(E) Quantitative analyses of the spinal cord area (LOTUS group; n = 5, control group; n = 5, PBS group n = 5).
(F) Representative images of immunostaining for each group at the injury site. HNA-positive transplanted cells were stained with Ki67, Nestin, HuC/HuD, GFAP, and APC. Nuclei were stained with Hoechst.
(G) The percentages of each marker-positive cells among the HNA-positive transplanted cells at 54 days after transplantation (LOTUS group; n = 5, control group; n = 5). Scale bars, 1,000 μm in (C), 500 μm in (D), and 20 μm in (F). Values are the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Statistical analysis was performed using one-way ANOVA followed by the Tukey-Kramer test in (E) and Mann-Whitney U test in (G).
We performed a histological analysis using H&E staining at 54 days after transplantation to evaluate the protective and regenerative effects of transplantation of LOTUS-expressing cells on the injured spinal cord (Figure 3D). Quantitative analyses revealed a significantly larger cross-sectional area of the spinal cord at the injury epicenter and 4 mm caudal to the epicenter in the LOTUS group than in the PBS group (Figure 3E), while no significant difference was observed between the areas in the control group and the PBS group.
Next, we evaluated the differentiation characteristics and tumorigenic risk of the transplanted cells using immunohistochemistry. Both the control-NS/PCs and the LOTUS-NS/PCs were differentiated into three neural lineages: ELAVL3/4-positive mature neurons, GFAP-positive astrocytes, and APC-positive oligodendrocytes (Figure 3F). Quantitative analyses revealed the differentiation rate of the transplanted cells. The proportions of ELAVL3/4-positive cells (control group: 48.6% ± 5.1%; LOTUS group: 58.6% ± 4.9%), GFAP-positive cells (control group: 22.4% ± 2.9%; LOTUS group: 24.6% ± 2.0%), and APC-positive cells (control group: 12.6% ± 2.2%; LOTUS group: 12.4% ± 0.6%) were not significantly different between the control group and the LOTUS group (Figure 3G). We also detected Ki-67+ and Nestin+ cells (Figure 4F). Immunostaining for various cell markers was examined 89 days after transplantation and subjected to quantitative analyses to evaluate the differentiation phenotype of the transplanted cells in vivo. Furthermore, Oct4-positive undifferentiated hiPSCs were not detectable and the percentages of Ki67+ proliferating cells (control group, 3.1% ± 0.7%; LOTUS group, 1.6% ± 0.3%) and Nestin+ cells (control group, 7.5% ± 2.2%; LOTUS group, 7.4% ± 0.6%) were low in each group without a significant difference, indicating that LOTUS did not increase the risk of tumorigenesis (Figure 3G).
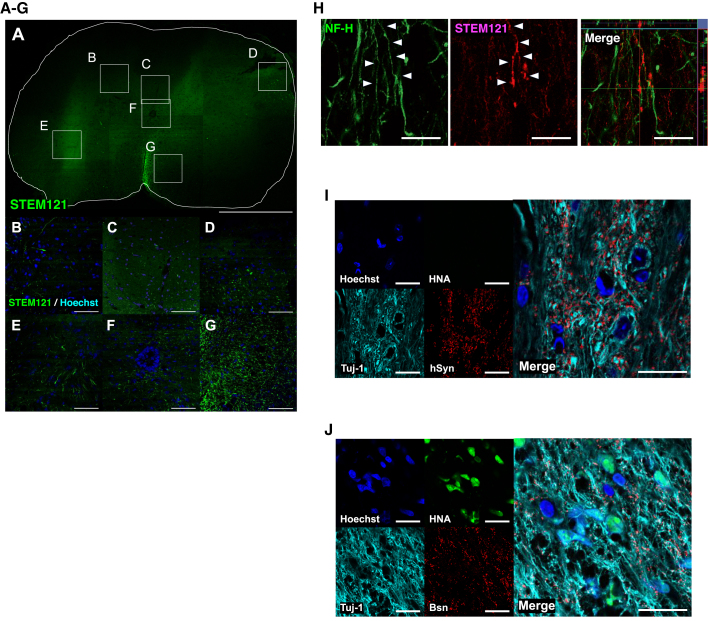
Figure 4.
The transplanted LOTUS-NS/PCs were migrated and differentiated into the serotonergic neuronal fibers, and formed synaptic connection
(A) Representative images of immunostaining for STEM121 at the axial sections at 4 mm rostral to the epicenter in the LOTUS group.
(B–G) Enlarged images of the area in the white box in (A); posterior horn in (B), dorsal funiculus in (C), dorsal funiculus in (D), anterior horn in (E), central canal in (F), and ventral funiculus in (G).
(H) Representative images of immunostaining for STEM121 and NF-H at the sagittal sections in the LOTUS group.
(I) Representative images of immunostaining for HNA, β-tubulin-3, and Bassoon (Bsn) (mouse presynaptic marker). HNA/β-tubulin-3 double-positive neurons, which were derived from transplanted cells, contacted Bsn-positive host cells.
(J) Representative images of immunostaining for HNA, β-tubulin-3 and hSyn (human-specific presynaptic marker). hSyn-positive boutons contacted HNA-negative and β-tubulin-3-positive host mouse neurons.
Scale bars, 500 μm in (A), 50 μm in (B–G, I, and J), and 20 μm in (H).
The transplanted LOTUS-NS/PCs extensively spread into the injured spinal cord, differentiated into nerve fibers, and formed synaptic connections
We performed immunohistological analyses to identify the localization of the migrated LOTUS-NS/PCs at the caudal site of the injury, which is considered important for the assessment of the descending motor pathway. The immunostaining of STEM121 antibody, which is specific to human cytoplasm, in the axial section 4 mm caudal to the injury showed that the grafted LOTUS-NS/PCs spread to the dorsal and ventral columns, anterior and posterior horn, and central canal (Figures 4A–4G). The STEM121-positive fibers derived from the transplanted cells were co-stained with neurofilament 200 kDa (NF-H), suggesting that these fibers differentiated into neurofilament-positive neuronal axons (Figure 4H). Finally, triple immunostaining of HNA (a human-specific nuclear antigen), β-tubulin-3 and mouse-specific Bassoon (Bsn), a presynaptic marker, was performed to determine whether the differentiated transplanted LOTUS-NS/PCs were incorporated into the host neural circuit. The β-tubulin-3 and HNA double-positive cells that were differentiated from the LOTUS-NS/PCs were in contact with the Bsn-positive synaptic boutons of the host neurons (Figure 4I). Furthermore, triple immunostaining of HNA, β-tubulin-3, and human-specific synaptophysin (hSyn) demonstrated that HNA-negative and β-tubulin-3-positive host neural cells were in contact with the hSyn-positive boutons (Figure 4J). These results suggest synaptic interactions between the graft-derived neurons and host neuronal cells.
LOTUS enhanced axonal elongation of graft cells into the injured spinal cord and promoted regrowth of raphespinal serotonergic fibers
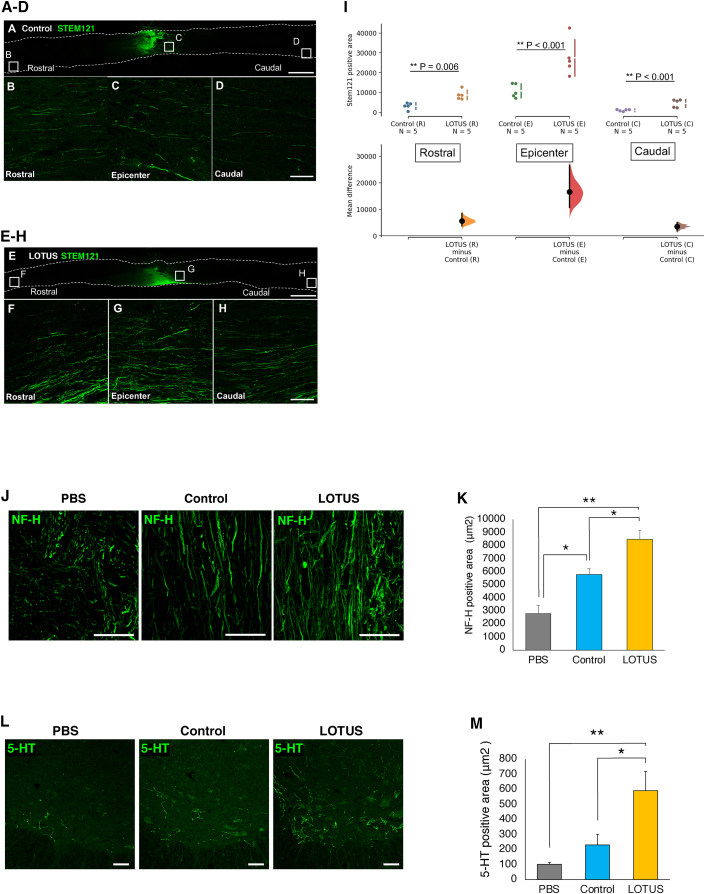
To examine whether LOTUS promotes axonal outgrowth of transplanted cells in the injured spinal cord, we performed a detailed histological evaluation using STEM121 immunostaining. An enlarged image of STEM121 immunostaining showed axonal elongation of the transplanted cells of NS/PCs that had migrated from the lesion epicenter to the rostral and caudal site on the ventral aspect of the spinal cord (Figures 5A–5H). The LOTUS-NS/PCs showed more neuronal fibers derived from the transplanted cells than the control-NS/PCs in all images (rostral, epicenter, and caudal), and quantitative analysis revealed that a significantly larger STEM121-positive area was observed on the epicenter, rostral, and caudal sides (Figure 5I). These results suggested that LOTUS promoted neuronal elongation of grafted cells in the injured spinal cord.
Figure 5.
LOTUS enhanced axonal extension of the transplanted cells and serotonergic neuronal regeneration in the injured spinal cord
(A) Representative images of immunostaining for STEM121 at the sagittal sections in the control group.
(B–D) Enlarged images of the area in the white box in (A); rostral in (B), epicenter in (C), and caudal in (D).
(E) Representative images of immunostaining for STEM121 at the sagittal sections in the LOTUS group.
(F–H) Enlarged images of the area in the white box in (E); rostral in (F), epicenter in (G), and caudal in (H).
(I), Quantitative analysis of the STEM121-positive area (LOTUS group; n = 5, control group; n = 5).
(J) Representative images of immunostaining for NF-H at mid-sagittal sections at 4 mm caudal to the epicenter.
(K) Quantitative analysis of NF-H-positive area (LOTUS group; n = 5, control group; n = 5, PBS group; n = 5).
(L) Representative images of immunostaining for 5-HT at the axial sections at 4 mm caudal to the epicenter.
(M) Quantitative analysis of 5-Ht-positive area (LOTUS group; n = 5, control group; n = 5, PBS group; n = 5).
Scale bars, 1,000 μm in (A and E), 100 μm in (B–D and F–H), and 50 μm in (J and L). Values are the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Statistical analysis was performed using Mann-Whitney U test in (I) and one-way ANOVA followed by the Tukey-Kramer test in (K and M).
To determine the regrowth of neurofilament and raphespinal serotonergic fibers (which are thought to contribute to the recovery of motor function after SCI in rodents) (Kaneko et al., 2006; Kim et al., 2004), we performed immunoreactions with the NF-H antibody in the mid-sagittal sections and the 5-hydroxytrytamine (5-HT) antibody in the axial sections at 4 mm caudal to the epicenter in the PBS, control, and LOTUS groups. Histological analyses demonstrated that cell transplantation caused an increase in the NF-H fibers or 5-HT-positive raphespinal serotonergic fibers in the raphe nucleus at the lumbar enlargement in all groups (Figures 5J and 5L). Quantitative analysis revealed that the LOTUS group had significantly greater NF-H or 5-HT-positive areas than the PBS and control groups (Figures 5K and 5M).
LOTUS enhanced the recovery of motor function following cell transplantation
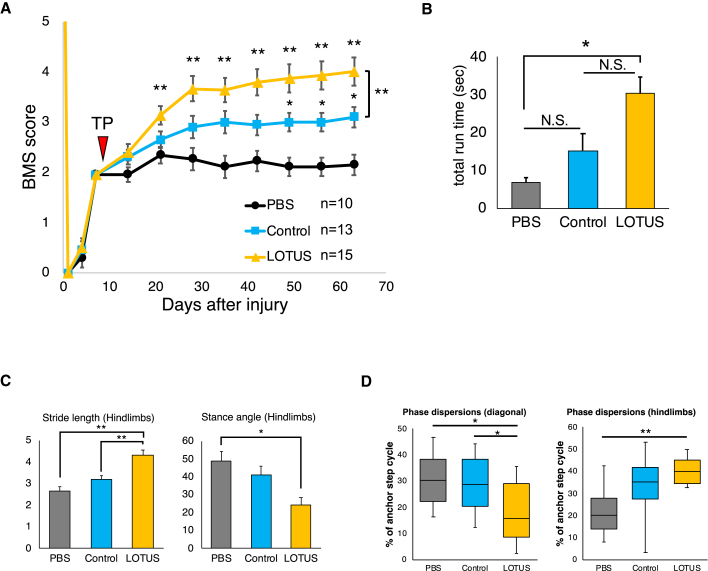
Recovery of motor function following cell transplantation or PBS injection was assessed using the Basso mouse scale (BMS) scoring system, the rotarod test, and DigiGait footprint analysis. The BMS scores in the LOTUS group were significantly improved by 12 and 40 days after transplantation in the PBS group and control group, respectively (Figure 6A). Furthermore, the LOTUS group had a significantly higher BMS score than the control group at the final follow-up (Figure 6A). The LOTUS group also exhibited a significantly longer total run time in the rotarod test 54 days after transplantation than the PBS group (Figure 6B). Although the times were generally better in the LOTUS group than the control group, there was no significant difference (Figure 6B). In the treadmill gait analyses, using the DigiGait system, we observed a significantly longer hindlimb stride length and narrower hindlimb stance angle in the LOTUS group than the PBS group 54 days following transplantation. Regarding stride length, the LOTUS group showed a significantly greater improvement than the control group (Figure 6C). Phase dispersion, an indicator of coordination measured using DigiGait analysis, demonstrated that the LOTUS group exhibited significantly better improvements than the PBS group in diagonal and hindlimb coordination. A significantly improved diagonal coordination was also observed in the LOTUS group than the control group (Figure 6D). These transplanted cell-derived neurons were tentatively suggested to form synaptic connections with host neurons, leading to significant improvements in motor function. Since the ex vivo gene transduction of LOTUS in hiPSC-NS/PCs further enhances the effects following transplantation, this could be a promising adjunct in the treatment of subacute SCI with cell transplantation.
Figure 6.
Evaluation of motor functional recovery
(A) Hindlimb motor function was evaluated weekly for 63 days after SCI (54 days after transplantation) by BMS scores in the LOTUS group, the control group and the PBS group (LOTUS group; n = 15, control group; n = 13, PBS group; n = 10).
(B) The rotarod test was performed 54 days after transplantation, and quantitatively analysis of total run time was evaluated (LOTUS group; n = 15, control group; n = 13, PBS group; n = 10).
(C) Treadmill gait analyses using a DigiGait system were examined 54 days after transplantation, and quantitative analyses of the stride length and stance angle were performed (LOTUS group; n = 15, control group; n = 13, PBS group; n = 10).
(D) Hindlimb coordination was analyzed by phase dispersions as the diagonal/hindlimbs coordination in DigiGait analyses (LOTUS group; n = 15, control group; n = 13, PBS group; n = 10). Values are the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Statistical analysis was performed using two-way repeated-measures ANOVA with Tukey's test in the analysis of the BMS score and one-way ANOVA followed by the Tukey-Kramer test in the rotarod test and DigiGait analyses.
Discussion
In this study, we evaluated the efficacy of transplanting hiPSC-NS/PCs transduced with the mLOTUS gene via lentivirus transduction in the treatment of subacute SCI. In vitro, the LOTUS-NS/PCs showed an increased neurite length compared with the control-NS/PCs after differentiation. LOTUS-NC/PCs also suppressed the inhibition of axonal outgrowth caused by NgR1 ligands and Nogo-induced cellular apoptosis. RT-PCR analyses revealed the upregulated gene expression of neurotrophic factors, such as BDNF, NGF, and NT-3, in the LOTUS-NS/PCs. In vivo, the LOTUS-NS/PCs showed increased engraftment and survival following transplantation and protected the injured spinal cord tissues while successfully differentiating into all three lineages. Importantly, in the LOTUS group, the transplanted cells significantly extended their neuronal fibers to the rostral and caudal sites in the host spinal cord with significant regrowth of raphespinal serotonergic fibers, which are known to be responsible for functional recovery after the SCI in the rodents (Kaneko et al., 2006). These transplanted cell-derived neurons are tentatively suggested to form synaptic connections with host neurons, leading to significant improvements in motor function. Since the ex vivo gene transduction of LOTUS in hiPSC-NS/PCs further enhances the effects following transplantation, this could be a promising adjunct in the treatment of subacute SCI with cell transplantation.
In this study, LOTUS functioned as an NgR1 antagonist, resulting in the suppression of axonal outgrowth inhibition, even in the presence of NgR1 ligands in vitro. Consequently, these effects contributed to significant axonal outgrowth in the injured spinal cord after cell transplantation. NgR1 is suggested to play a critical role in axonal regeneration after SCI. Numerous studies have demonstrated and suggested that the blocking of NgR1 and its downstream signals should be considered when treating SCI. For instance, depressing NgR1 activation by administering NEP1-40, a Nogo-66 antagonist, led to axonal outgrowth with functional recovery after hemisection SCI (GrandPre et al., 2002; Li and Strittmatter, 2003). Similar results were observed using an SCI model of NgR1-deficient mice (Kim et al., 2004). Furthermore, the Rho inhibitor C3 transferase, which inhibits the downstream cascade of NgR1, resulted in axonal regrowth with recovery of locomotor function (Wu and Xu, 2016). Recently, Kurihara et al. (2020) reported that NgR1 antagonist LOTUS also suppressed axonal growth-inhibiting receptor PIR-B, resulting in restraining growth cone collapse and axonal growth inhibition. PIR-B has been reported as a common receptor for Nogo, MAG, and OMgp, and interfering with PIR-B activity partially rescued neurite inhibition by these ligands (Atwal, 2008). Thus, LOTUS could exert a stronger role for axonal regeneration than NgR1 inhibitors through blocking both NgR1 and PIR-B. In addition to the beneficial impact of LOTUS on host neural tissue, this study showed its effects on neurite extension in the transplanted cells themselves. Therefore, induction of the LOTUS gene for NS/PCs can be a beneficial option for improving neuronal regeneration.
NgR1 is known to initiate neuronal apoptosis by activating the Rho-A and ROCK pathways after SCI (Zhang et al., 2007). According to previous reports, NEP1-40 and Rho inhibitors suppress neuronal apoptosis in injury models of the central nervous system (CNS) (Dubreuil et al., 2003; Wang et al., 2008; Zhang et al., 2007). Our previous study also reported a neuroprotective effect of LOTUS on a transgenic mouse model of SCI, including a reduction in the number of apoptotic cells and preservation of the spinal tract and myelination (Ito et al., 2018). We therefore recognize that inhibition of apoptosis itself is one of the effects of LOTUS on SCI. In this study, we demonstrate the prevention of neuronal apoptosis in NS/PCs expressing LOTUS in vitro, and these results led to a significant increase in cell survival and host tissue protection after transplantation. Thus, inhibition of apoptosis by expressing LOTUS in NS/PCs was probably attributed to further functional restoration after SCI.
RT-PCR analysis showed that LOTUS upregulated the expression of the neurotrophic factors BDNF, NGF, and NT-3 in the NS/PCs. These factors are already known for their positive attributes following transplantation therapy for CNS injuries. Lu et al. evaluated the efficacy of BDNF gene transfection in bone marrow stromal cells (MSCs), which were transplanted into the injured spinal cord. The transplanted BDNF-overexpressing MSCs promoted host axonal extension into the grafted cells (Lu et al., 2005). Another study demonstrated that BDNF-hypersecreting human MSCs enhanced the regrowth of host corticospinal and raphespinal tract fibers after SCI with significantly improved locomotor function (Sasaki et al., 2009). Kamei et al. showed the effectiveness of not only BDNF but also NGF and NT-3 secreted from NS/PCs and cocultured with organoids using the neonatal brain cortex and spinal cord. Transplantation of these cells achieved axonal sprouting of the corticospinal tract in organoids, but administration of antibodies against each trophic factor canceled this sprouting (Kamei et al., 2007). Together, these results from previous studies allowed us to hypothesize the favorable outcomes in this study: the three trophic factors potentially contribute to axonal outgrowth and functional recovery after SCI.
In this study, LOTUS antagonized NgR1 and upregulated neurotrophic factors, which facilitated the axonal elongation of grafted NS/PCs. These NS/PCs differentiated into nerve fibers such as neurofilament, contributing to the increase in raphespinal fibers in the lumbar enlargement of the spinal cord. Although the raphespinal tract is recognized for its modulatory effects on sensory activity (Mason, 2001), it can function as an alternative circuit, connecting to the motor circuit after SCI (Liang et al., 2015). In fact, a previous study using the serotonergic neurotoxin 5,7-dihydro-xytryptamine demonstrated that raphespinal regeneration following SCI significantly contributed to functional restoration in NgR1-deficient mice (Kim et al., 2004). Similarly, our previous data showed raphespinal regeneration with functional recovery using LOTUS-overexpressing transgenic mice (Ito et al., 2018). These results supported the theory that increased raphespinal serotonergic fibers were relevant to locomotor recovery.
We transduced LOTUS into hiPSC-NS/PCs ex vivo using lentivirus and successfully improved the results gained through transplantation therapy. Zhilai et al. (2011) reported that transplantation of MSCs expressing NEP1-40 resulted in significant functional recovery following SCI, but this treatment only affected axonal regeneration in the host cells and not in the transplanted cells. On the other hand, our LOTUS-expressing NS/PCs contributed to the improved survival and increased axonal outgrowth of the transplanted cells by LOTUS expressing into themselves, and further affect host neurons by acting as a source of LOTUS secretion. How far the secreted LOTUS diffuses in the injured spinal cord remains to be verified. When soluble LOTUS was injected into the vitreous of the eye, it reached the frustrated tip of the optic nerve by diffusion (Kawakami et al., 2018), thus it is possible that LOTUS diffused into the host neuron in this study as well. In the injured spinal cord, the expression of LOTUS was halved (Hirokawa et al., 2017; Ito et al., 2018), and conversely, the expression of NgR1 and its ligands of such as Nogo were increased (Hunt et al., 2003). The delivery of LOTUS via ex vivo gene transfer with cell transplantation led to synergistic effects by replacing the lost neurons in the injured area with transplanted iPSCs and exogenously replenishing the decreased LOTUS. Furthermore, the heightened expression of neurotrophic factors strongly enhanced this synergistic effect, resulting in the advantage in the treatment of SCI. In other clinical fields, ex vivo gene therapy is expected to be applied to gene deficiency and CNS diseases (Akhtar et al., 2018). Clinical research for the treatment of β-thalassemia has already been approved using gene therapy with autologous CD34+ cells transduced with BB305 (lentivirus) vector (Thompson et al., 2018). Therefore, ex vivo gene therapy could be a clinically feasible strategy for the treatment of SCI.
In this study, we established hiPSC-NS/PCs expressing LOTUS and a system to transplant them into an SCI mouse model. On the other hand, in this experiment, we were unable to easily provide evidence that inhibition of NgR1 signaling by LOTUS expression in hiPSC-NS/PCs directly induced improved functional recovery after SCI. However, in vitro, Takei and coworkers showed that LOTUS binds to NgR1 and blocks NgR1 ligand binding to its receptor (Kurihara et al., 2012, 2014). Therefore, LOTUS suppresses NgR1-induced inhibition of axonal elongation and growth cone collapse (Kurihara et al., 2014). Similar to these results, the current in vitro analysis also showed that axon elongation was not inhibited in LOTUS-overexpressing NS/PCs, even in the presence of NgR1 ligands (Figure 2C). Therefore, based on the in vitro results, we estimated that LOTUS contributed to axonal elongation and restored locomotor function by inhibiting NgR1 signaling. In fact, in vivo effects of the blockade of NgR1 signaling in inducing the functional recovery after SCI are obvious based on the previous studies, which reported that a hemisection SCI model of NgR1 knockout mice (Kim et al., 2004) or SCI mice treated with an NgR1 antagonist showed axonal extension and motor functional recovery following injury (Li and Strittmatter, 2003). One of the possible solutions to clarify the roles of transplanted LOTUS-NS/PCs in vivo in future studies would be to use NgR1 knockout mice as a model of SCI and transplant NS/PCs. If no NgR1-independent action of LOTUS is observed because NgR1 signaling is eliminated in this mouse model, then the therapeutic effect of NS/PCs expressing LOTUS exogenously on locomotor function would be the same as that in the control-NS/PC group (without exogenous LOTUS expression). On the other hand, if the therapeutic effects of exogenous LOTUS-expressing NS/PCs are stronger than those of control-NS/PCs, then some NgR1-independent action of exogenous LOTUS expression should be detected.
In conclusion, we showed that the transduction of LOTUS resulted in a significantly increased axonal extension, reduced apoptosis, and increased neurotrophic factor secretion in vitro and enhanced the survival of transplanted cells and the regrowth of raphespinal serotonergic fibers with synaptic formation integrated into the host neural circuitry. We believe that these results contribute to the recovery of motor function following SCI. Ex vivo gene therapy delivering the NgR1 antagonist LOTUS with cell transplantation can represent a therapeutic strategy for SCI.
Experimental procedures
Lentiviral vector preparation
Lentiviral vector production and infection of cells were performed as described previously (Miyoshi et al., 1998; Okada et al., 2005). Mouse LOTUS (mLOTUS) cDNA and the Venus fluorescent protein (Nagai et al., 2002; Duran-Avelar et al., 2018) gene connected by an internal ribosomal entry site (IRES) were cloned into the lentiviral vector CSII-EF1α, yielding pCSII-EF1α-mLOTUS-IRES-Venus (Figure 1A) (Iida et al., 2017). Recombinant lentiviral vector production and titer determination were performed as described previously (Hashizume et al., 2015).
Cell culture, lentivirus transduction, and neuronal differentiation analysis
The cell culture, neural induction, and neuronal differentiation analysis of hiPSCs (414C2) were performed as described previously (Itakura et al., 2015) with slight modifications. In brief, the hiPSC-NS/PCs were dissociated and infected with the mLOTUS-expressing lentivirus and a lentivirus expressing ffLuc, a fusion protein between Venus fluorescent protein and firefly luciferase (Hara-Miyauchi et al., 2012) under the control of the EF1α promoter, which were prepared as LOTUS-NS/PCs. In contrast, the control-NS/PCs were prepared by similar transduction via lentivirus expressing ffLuc. Detailed methods are presented in supplemental experimental procedures.
Western blotting
Protein isolation and western blotting were performed to determine the protein expression of the control-NS/PCs and the LOTUS-NS/PCs. Detailed methods are presented in supplemental experimental procedures.
Real-time PCR
RNA extraction and real-time PCR were performed as described previously (Nori et al., 2011), and detailed protocols are provided in supplemental experimental procedures.
Neurite outgrowth assay on NgR1 ligand-coated plates
Neurite outgrowth assay on NgR1 ligand-coated plates was performed for the evaluation of MAP2-positive neurite length in vitro. Detailed methods are presented in supplemental experimental procedures.
Apoptosis analysis
Immunostaining with anti-cleaved caspase-3 antibody was performed for the evaluation of cellular apoptosis in vitro. Detailed methods are presented in supplemental experimental procedures.
Animals
We used adult female NOD-SCID (NOD/ShiJic-scidJcl mice, 18–22 g; Oriental Yeast, Tokyo, Japan) in this study. A total of 47 mice (18 mice in the LOTUS group, 17 in the control group, and 12 in the PBS group) were used in the experiment, 9 of which died during follow-up (3 mice in the LOTUS group, 4 in the control group, and 2 in the PBS group). All experiments were approved by the ethics committee of Keio University and were in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD).
SCI and transplantation
Contusive SCI was induced at the 10th thoracic level in the spinal cords of adult female NOD-SCID mice. Nine days after the injury, LOTUS-NS/PCs or control-NS/PCs (5 × 105 cells/2 μL) were transplanted into the lesion epicenter of each mouse. Detailed methods are presented in supplemental experimental procedures.
In vivo imaging of transplanted cells
BLI was performed as described previously (Itakura et al., 2015). Detailed methods are presented in supplemental experimental procedures.
Immunohistochemistry
Immunohistochemistry (IHC) analysis was performed for the in vitro evaluation. Detailed methods are presented in supplemental experimental procedures.
Quantitative analysis of the tissue sections
Quantitative analysis of the tissue sections following SCI and transplantation was performed. Detailed methods are presented in supplemental experimental procedures.
Behavioral analysis
The locomotor function of each mouse in the control group, the LOTUS group, and the PBS group was evaluated weekly using the BMS up to 63 days after injury (Basso et al., 2006). Detailed methods are presented in supplemental experimental procedures.
Statistical analysis
All data are presented as the mean ± SEM. The Mann-Whitney U test for single comparisons was used to detect any significant differences between groups with respect to the RT-PCR analyses and the IHC results. One-way ANOVA followed by the Tukey-Kramer test for multiple comparisons was used to evaluate the differences in the neurite outgrowth assay, apoptosis analyses, H&E staining, IHC results, rotarod test, and DigiGait results. Repeated-measures two-way ANOVA followed by the Tukey-Kramer test was used for the BMS analyses and IVIS photon counts. Differences were considered significant at p < 0.05 or p < 0.01. IBM SPSS Statistics (v.24) was used for all calculations. In the Gardner-Altman plots, we used DABEST (data analysis with bootstrap-coupled estimation): open-source libraries for MATLAB, Python, and R (https://www.estimationstats.com) (Ho et al., 2019).
Author contributions
S.I. designed the project, performed most of the experiments, collected and interpreted the data, and wrote the manuscript, with technical assistance from N.N. Y.K., K.K., S.N., M.M., and K.T. provided experimental support and ideas for the project. M.N. and H.O. designed the studies, supervised the overall project, and prepared the final manuscript.
Acknowledgments
We thank S. Yamanaka at CiRA (Kyoto University) for the human iPSCs (414C2). We are grateful for the assistance of H. Miyoshi, T. Okubo, Y. Hoshino, Y. Tanimoto, R. Shibata, K. Kajikawa, K. Ago, T. Kitagawa, and M. Kawai, who are all members of the spinal cord research team at the Department of Orthopedic Surgery and Physiology, Keio University School of Medicine, Tokyo, Japan.We also thank T. Harada, K. Yasutake, and M. Akizawa for their assistance with the experiments and animal care. This work was supported by the Japan Agency for Medical Research and Development (AMED) grant JP JJ P21bm0204001 (to H.O. and M.N.) and partly by The General Insurance Association of Japan Medical Research Grant on traffic accidents 16-1-20.
Published: October 14, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.09.006.
Contributor Information
Masaya Nakamura, Email: masa@keio.jp.
Hideyuki Okano, Email: hidokano@a2.keio.jp.
Supplemental information
References
- Akhtar A.A., Gowing G., Kobritz N., Savinoff S.E., Garcia L., Saxon D., Cho N., Kim G., Tom C.M., Park H., et al. Inducible expression of GDNF in transplanted iPSC-derived neural progenitor cells. Stem Cell Reports. 2018;10:1696–1704. doi: 10.1016/j.stemcr.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal J.K. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008 doi: 10.1126/science.1161151. 10.1126/science.1161151 [DOI] [PubMed] [Google Scholar]
- Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., McTigue D.M., Popovich P.G. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Chen M.S., Huber A.B., van der Haar M.E., Frank M., Schnell L., Spillmann A.A., Christ F., Schwab M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Dergham P., Ellezam B., Essagian C., Avedissian H., Lubell W.D., McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher T.L., Baldwin K.T., Mironova Y.A., Koriyama Y., Raiker S.J., Askew K.L., Wood A., Geoffroy C.G., Zheng B., Liepmann C.D., et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil C.I., Winton M.J., McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J. Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Avelar M.J., Vazquez-Reyes A., Gonzalez-Mercado A.L., Zambrano-Zaragoza J.F., Ayon-Perez M.F., Agraz-Cibrian J.M., Gutierrez-Franco J., Vibanco-Perez N. pirA- and pirB-like gene identification in Micrococcus luteus strains in Mexico. J. Fish Dis. 2018;41:1667–1673. doi: 10.1111/jfd.12874. [DOI] [PubMed] [Google Scholar]
- Forgione N., Fehlings M.G. Rho-ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014;82:e535–e539. doi: 10.1016/j.wneu.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Fournier A.E., Takizawa B.T., Strittmatter S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y., Abematsu M., Falk A., Tsujimura K., Sanosaka T., Juliandi B., Semi K., Namihira M., Komiya S., Smith A., Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012;30:1163–1173. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- GrandPre T., Li S., Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- GrandPre T., Nakamura F., Vartanian T., Strittmatter S.M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Hara-Miyauchi C., Tsuji O., Hanyu A., Okada S., Yasuda A., Fukano T., Akazawa C., Nakamura M., Imamura T., Matsuzaki Y., et al. Bioluminescent system for dynamic imaging of cell and animal behavior. Biochem. Biophys. Res. Commun. 2012;419:188–193. doi: 10.1016/j.bbrc.2012.01.141. [DOI] [PubMed] [Google Scholar]
- Hashizume O., Ohnishi S., Mito T., Shimizu A., Ishikawa K., Nakada K., Soda M., Mano H., Togayachi S., Miyoshi H., et al. Corrigendum: epigenetic regulation of the nuclear-coded GCAT and SHMT2 genes confers human age-associated mitochondrial respiration defects. Sci. Rep. 2015;5:14591. doi: 10.1038/srep14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa T., Zou Y., Kurihara Y., Jiang Z., Sakakibara Y., Ito H., Funakoshi K., Kawahara N., Goshima Y., Strittmatter S.M., Takei K. Regulation of axonal regeneration by the level of function of the endogenous Nogo receptor antagonist LOTUS. Sci. Rep. 2017;7:12119. doi: 10.1038/s41598-017-12449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J., Tumkaya T., Aryal S., Choi H., Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat. Methods. 2019;16:565–566. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- Hunt D., Coffin R.S., Prinjha R.K., Campbell G., Anderson P.N. Nogo-A expression in the intact and injured nervous system. Mol. Cell Neurosci. 2003;24:1083–1102. doi: 10.1016/j.mcn.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Iida T., Iwanami A., Sanosaka T., Kohyama J., Miyoshi H., Nagoshi N., Kashiwagi R., Toyama Y., Matsumoto M., Nakamura M., Okano H. Whole-genome DNA methylation analyses revealed epigenetic instability in tumorigenic human iPS cell-derived neural stem/progenitor cells. Stem Cells. 2017;35:1316–1327. doi: 10.1002/stem.2581. [DOI] [PubMed] [Google Scholar]
- Itakura G., Kobayashi Y., Nishimura S., Iwai H., Takano M., Iwanami A., Toyama Y., Okano H., Nakamura M. Controlling immune rejection is a fail-safe system against potential tumorigenicity after human iPSC-derived neural stem cell transplantation. PLoS One. 2015;10:e0116413. doi: 10.1371/journal.pone.0116413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Nagoshi N., Tsuji O., Shibata S., Shinozaki M., Kawabata S., Kojima K., Yasutake K., Hirokawa T., Matsumoto M., et al. LOTUS inhibits neuronal apoptosis and promotes tract regeneration in contusive spinal cord injury model mice. eNeuro. 2018;5 doi: 10.1523/ENEURO.0303-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N., Tanaka N., Oishi Y., Hamasaki T., Nakanishi K., Sakai N., Ochi M. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine. 2007;32:1272–1278. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Iwanami A., Nakamura M., Kishino A., Kikuchi K., Shibata S., Okano H.J., Ikegami T., Moriya A., Konishi O., et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Kurihara Y., Saito Y., Fujita Y., Yamashita T., Takei K. The soluble form of LOTUS inhibits nogo receptor-mediated signaling by interfering with the interaction between nogo receptor type 1 and p75 neurotrophin receptor. J. Neurosci. 2018;38:2589–2604. doi: 10.1523/JNEUROSCI.0953-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Liu B.P., Park J.H., Strittmatter S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kojima K., Miyoshi H., Nagoshi N., Kohyama J., Itakura G., Kawabata S., Ozaki M., Iida T., Sugai K., Ito S., et al. Selective ablation of tumorigenic cells following human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in spinal cord injury. Stem Cells Transl. Med. 2019;8:260–270. doi: 10.1002/sctm.18-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y., Takai T., Takei K. Nogo receptor antagonist LOTUS exerts suppression on axonal growth-inhibiting receptor PIR-B. J. Neurochem. 2020;155:285–299. doi: 10.1111/jnc.15013. [DOI] [PubMed] [Google Scholar]
- Kurihara Y., Iketani M., Ito H., Nishiyama K., Sakakibara Y., Goshima Y., Takei K. LOTUS suppresses axon growth inhibition by blocking interaction between Nogo receptor-1 and all four types of its ligand. Mol. Cell Neurosci. 2014;61:211–218. doi: 10.1016/j.mcn.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Kurihara Y., Arie Y., Iketani M., Ito H., Nishiyama K., Sato Y., Nakamura F., Mizuki N., Goshima Y., Takei K. The carboxyl-terminal region of Crtac1B/LOTUS acts as a functional domain in endogenous antagonism to Nogo receptor-1. Biochem. Biophys. Res. Commun. 2012;418:390–395. doi: 10.1016/j.bbrc.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Li S., Strittmatter S.M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Wang S., Francis R., Whan R., Watson C., Paxinos G. Distribution of raphespinal fibers in the mouse spinal cord. Mol. Pain. 2015;11:42. doi: 10.1186/s12990-015-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Jones L., Tuszynski M. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp. Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Lu P., Woodruff G., Wang Y., Graham L., Hunt M., Wu D., Boehle E., Ahmad R., Poplawski G., Brock J., et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu. Rev. Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- McKerracher L., David S., Jackson D.L., Kottis V., Dunn R.J., Braun P.E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Blomer U., Takahashi M., Gage F.H., Verma I.M. Development of a self-inactivating lentivirus vector. J. Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G., Doherty P., Walsh F.S., Crocker P.R., Filbin M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nagai T., Ibata K., Park E.S., Kubota M., Mikoshiba K., Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Niederost B., Oertle T., Fritsche J., McKinney R.A., Bandtlow C.E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. U S A. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., Ishii K., Yamane J., Iwanami A., Ikegami T., Katoh H., Iwamoto Y., Nakamura M., Miyoshi H., Okano H.J., et al. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005;19:1839–1841. doi: 10.1096/fj.05-4082fje. [DOI] [PubMed] [Google Scholar]
- Prinjha R., Moore S.E., Vinson M., Blake S., Morrow R., Christie G., Michalovich D., Simmons D.L., Walsh F.S. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Radtke C., Tan A.M., Zhao P., Hamada H., Houkin K., Honmou O., Kocsis J.D. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Iketani M., Kurihara Y., Yamaguchi M., Yamashita N., Nakamura F., Arie Y., Kawasaki T., Hirata T., Abe T., et al. Cartilage acidic protein-1B (LOTUS), an endogenous Nogo receptor antagonist for axon tract formation. Science. 2011;333:769–773. doi: 10.1126/science.1204144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savio T., Schwab M.E. Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc. Natl. Acad. Sci. U S A. 1990;87:4130–4133. doi: 10.1073/pnas.87.11.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.-A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M., et al. Gene therapy in patients with transfusion-dependent beta-thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- Uezono N., Zhu Y., Fujimoto Y., Yasui T., Matsuda T., Nakajo M., Abematsu M., Setoguchi T., Mori S., Takahashi H.K., et al. Prior treatment with anti-high mobility group box-1 antibody boosts human neural stem cell transplantation-mediated functional recovery after spinal cord injury. Stem Cells. 2018;36:737–750. doi: 10.1002/stem.2802. [DOI] [PubMed] [Google Scholar]
- Wang K.C., Koprivica V., Kim J.A., Sivasankaran R., Guo Y., Neve R.L., He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wang Q., Gou X., Xiong L., Jin W., Chen S., Hou L., Xu L. Trans-activator of transcription-mediated delivery of NEP1-40 protein into brain has a neuroprotective effect against focal cerebral ischemic injury via inhibition of neuronal apoptosis. Anesthesiology. 2008;108:1071–1080. doi: 10.1097/ALN.0b013e318173f66b. [DOI] [PubMed] [Google Scholar]
- Wu X., Xu X.M. RhoA/Rho kinase in spinal cord injury. Neural Regen. Res. 2016;11:23–27. doi: 10.4103/1673-5374.169601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zheng S., Wu H., Wu Y., Liu S., Fan M., Zhang J. Identification of BLyS (B lymphocyte stimulator), a non-myelin-associated protein, as a functional ligand for Nogo-66 receptor. J. Neurosci. 2009;29:6348–6352. doi: 10.1523/JNEUROSCI.5040-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gu X., Yuan X. Phenylalanine activates the mitochondria-mediated apoptosis through the RhoA/Rho-associated kinase pathway in cortical neurons. Eur. J. Neurosci. 2007;25:1341–1348. doi: 10.1111/j.1460-9568.2007.05404.x. [DOI] [PubMed] [Google Scholar]
- Zhilai Z., Hui Z., Yinhai C., Zhong C., Shaoxiong M., Bo Y., Anmin J. Combination of NEP 1-40 infusion and bone marrow-derived neurospheres transplantation inhibit glial scar formation and promote functional recovery after rat spinal cord injury. Neurol. India. 2011;59:579–585. doi: 10.4103/0028-3886.84341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.