Abstract
Common fragile sites are specific chromosomal loci that show gaps, breaks, or rearrangements in metaphase chromosomes under conditions that interfere with DNA replication. The mechanism underlying the chromosomal instability at fragile sites was hypothesized to associate with late replication time. Here, we aimed to investigate the replication pattern of the common fragile site FRA7H, encompassing 160 kb on the long arm of human chromosome 7. Using in situ hybridization on interphase nuclei, we revealed that the replication of this region is initiated relatively early, before 30% of S phase is completed. However, a high fraction (∼35%) of S-phase nuclei showed allelic asynchrony, indicating that the replication of FRA7H is accomplished at different times in S phase. This allelic asynchrony is not the result of a specific replication time of each FRA7H allele. Analysis of the replication pattern of adjacent clones along FRA7H by using cell population and two-color fluorescent in situ hybridization analyses showed significant differences in the replication of adjacent clones, under normal growth condition and upon aphidicolin treatment. This pattern significantly differed from that of two nonfragile regions which showed a coordinated replication under both conditions. These results indicate that aphidicolin is enhancing an already existing difference in the replication time along the FRA7H region. Based on our replication analysis of FRA7H and on previous analysis of the common fragile site FRA3B, we suggest that delayed replication is underlying the fragility at aphidicolin-induced common fragile sites.
Fragile sites are specific chromosomal loci prone to breakage, characterized by constrictions, gaps, or breaks on chromosomes from cells exposed to specific tissue culture and chemical conditions (reviewed in reference 40). They are classified as either rare or common, depending on their frequency within the population and their mode of induction. Rare fragile sites (n = 30 in the human genome) appear in less than 5% of the human population and segregate in specific families. Common fragile sites (n = 90), on the other hand, are considered to be part of the normal chromosomal structure and are thought to present in all individuals. Most of the common fragile sites (n = 76) are induced by aphidicolin (7), an inhibitor of DNA polymerases alpha and delta (reviewed in reference 41).
Several rare fragile sites, induced by folic acid depravation, dystamycin A, or bromodeoxyuridine (BrdU) have been characterized at the molecular level (16, 19, 20, 29, 31, 48). The expression of these sites is associated with expanded CGG trinucleotide or AT-rich minisatellite repeats. Three common fragile sites (FRA3B, FRA7G, and FRA7H), all induced by aphidicolin, were identified, cloned, and sequenced (15, 28, 46). The cytogenetic expression of each of these sites appears along a region of several hundred kilobases. No expanded repeats were found in these regions.
Fragile sites were implicated in chromosomal rearrangement (8), gene amplification (4), sister chromatid exchange (9), and integration of foreign DNA (28, 32, 33, 47). This genetic instability can lead to disease manifestation (6, 16, 30) and might play a role in oncogenesis (49). Despite their instability, several common fragile sites are conserved between mouse and human (5, 8), indicating that these sites might play an important biological role.
The molecular mechanism underlying the genetic instability at fragile sites is currently not understood. The fragility inducers interfere with DNA replication, and their effect is restricted to S phase (40). Replication inhibition of Drosophila cells resulted in a morphological appearance resembling the mammalian fragile sites at the intercalary heterochromatin regions known to replicate late in S phase. All of these findings led Laird et al. to suggest that fragile sites replicate very late in the cell cycle. Upon replication stress or premature chromatin condensation, the condensation of these sequences might not be completed, and a fragile site will appear (22).
Analysis of the replication time of two rare fragile sites, FRAXA and FRAXE, showed that the normal alleles replicated very late in S phase (at S/G2), and the CGG-expanded alleles replicated even later, at G2 phase (11, 12, 39). These CGG-repeated sequences can adopt non-B-DNA structures that inhibit replication fork movement, both in vitro and in vivo (35, 43). The common fragile site FRA3B, or at least those FRA3B alleles that express fragility (45), has also been shown to replicate at the latest part of S phase (23). Following aphidicolin induction, ∼15% of the FRA3B alleles were unreplicated in the G2 phase (23). These findings support the model suggested by Laird et al. (21) and indicate that late replication may be a common feature of rare and common fragile sites (41). However, the basis for the replication delay in common fragile sites upon stress is unknown.
Here, we analyze the replication pattern of a common fragile site, FRA7H, on the long arm of human chromosome 7. Our results suggest that perturbed fork progression, which results in delayed replication along the FRA7H region, underlines the fragility of this site.
MATERIALS AND METHODS
Cells and growth conditions.
Four cell lines were used in this study: Manca (13), a human lymphoma cell line; CF33-2, a normal human lymphocyte cell line; CF33-3, a chromosome 7 isodisomic human lymphocyte cell line (44); and GM00847 (National Institute of General Medical Sciences, Camden, N.J.), a simian virus 40 (SV40)-transformed human fibroblast cell line. All cell lines were grown in RPMI medium containing 10% fetal calf serum. GM00847 cells were grown on coverslips. For fragile site induction, cells were grown for 24 h in M-199 medium in the presence of 0.4 μM aphidicolin (Sigma) and 0.5% ethanol. BrdU (10−5 M) (Sigma) was added 1 h prior to cell harvest.
DNA probes.
The cosmid clones from the FRA7H and from the paternally expressed gene 1 (PEG1) (also known as mesoderm-specific transcript [MEST]) regions were isolated from a chromosome 7-specific library (28). The control cosmid clones were derived from the cystic fibrosis transmembrane conductance regulator (CFTR) gene region (34) and from the acute myeloid leukemia 1 (AML1) gene region (http://genome.imb-jena.de).
FISH on interphase nuclei.
Nonsynchronized logarithmic cell cultures were harvested and treated with hypotonic KCl solution (0.4 M) for 20 min at 37°C, and the extracted nuclei were fixed in 3:1 methanol-acetic acid fixer. Lymphocytes were stored in suspension at −20°C, and the fibroblast coverslips were air dried and stored in sealed bags at −80°C. To ensure equal cell culture conditions, a single harvesting batch from each cell line was used in all experiments. Fluorescent in situ hybridization (FISH) was performed as described previously (25), except for the omission of the amplification steps to reduce background. Cosmid DNA was labeled with digoxigenin (DIG)-11-dUTP or biotin-16-dUTP (Boehringer Mannheim). DIG-labeled probes were detected with fluorescein isothiocyanate-conjugated sheep anti-DIG specific antibodies (Boehringer Mannheim). Biotinylated probes were detected with Texas red-conjugated avidin (Vector). S-phase nuclei were identified by using mouse monoclonal anti-BrdU immunoglobulin G antibodies (NeoMarkers), followed by aminomethylcoumarin acetic acid (AMCA)-conjugated goat anti-mouse immunoglobulin G antibodies (Jackson Laboratories).
Analysis of hybridization signals.
FISH signals were visualized by conventional fluorescence microscopy. Images were captured with an intensified charge-coupled device imager (Paultek Imaging, Grass Valley, Calif.) and were digitized with a frame grabber (Imascan/MONO-D; Imagraph Corp., Chelmsford, Mass.). In each experiment, 100 to 500 (usually 200) nuclei were scored and categorized according to their replication pattern (see Results). The reproducibility of the experiments was evaluated by comparing the different categories, from groups of 50 to 100 nuclei. Only experiments with high-quality hybridization, defined as more than 85% of the nuclei showing distinct hybridization signals and less than 5% differences between the groups, were analyzed and included in the results.
RESULTS
Replication of the FRA7H region is initiated in mid-S phase.
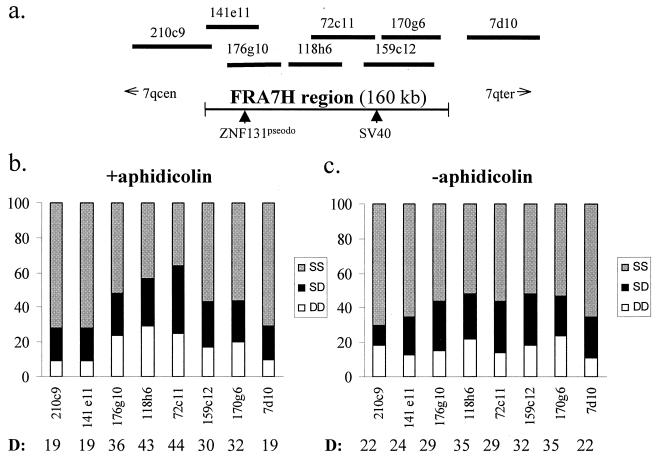
The replication pattern of the FRA7H region was analyzed by using FISH on interphase nuclei (36). In this method, unreplicated DNA is visualized as a single hybridization dot (S signal), while replicated DNA appears as a double dot (D signal). In a nonsynchronized population of dividing cells, a high percentage of D signals indicates that this particular sequence replicates relatively early in S phase, whereas a low count is obtained for sequences that replicate late in the cycle.
In a previous study, we defined 160 kb encompassing the FRA7H region, by analyzing the location of FISH signals using cosmid clones from the 7q32 region, relative to the cytogenetic expression of FRA7H (28). Here we analyze three cosmid-clones encompassing the FRA7H region, 176g10, 118h6, and 159c12, by FISH to Manca interphase nuclei. S-phase nuclei were differentiated from nuclei in G1 and G2 by detection of DNA labeled with BrdU. As shown in Table 1, similar hybridization patterns were detected along the FRA7H region. In 32 to 34% of the S-phase nuclei, the two FRA7H alleles were replicated (DD signals); in 34 to 35% of the nuclei, one allele was replicated and the other was not (SD signals); and in 31 to 33% of the nuclei, the two FRA7H alleles were unreplicated (SS signals). Thus, in ∼70% of these nuclei, at least one FRA7H allele was replicated (nuclei showing DD and SD signals), indicating that the replication of this region is initiated before 30% of the S phase is completed.
TABLE 1.
In situ hybridization pattern (%) of S-phase nuclei from Manca cell line
| Cosmid clones | SS | SD | DD |
|---|---|---|---|
| FRA7H region | |||
| 176g10 | 32 | 35 | 33 |
| 118h6 | 31 | 34 | 34 |
| 159c12 | 33 | 35 | 32 |
| Control regiona | |||
| cNH24 | 77 | 10 | 13 |
| cW44 | 73 | 12 | 15 |
The replication time of these clones was determined previously by FISH and by cell-fractionation-based methods in the Manca cell line (36).
Most sequences replicate in a fairly synchronous manner between the homologous chromosomes, with only about 10 to 20% of the nuclei showing asynchronous replication, most probably reflecting suboptimal hybridization conditions, and thus might be considered as the background level (17, 37). In contrast, in the analysis of FRA7H, ∼35% of the S-phase nuclei showed SD signals, indicating that the replication of one of the FRA7H alleles was not yet completed. We further analyzed BrdU-negative nuclei (G1 and G2 nuclei) and found that the level of SD nuclei was only 9 to 13% (data not shown). These results excluded the possibility that the high level of SD found in S-phase nuclei is due to inefficient hybridization of the FRA7H clones. Thus, the replication of FRA7H is accomplished at different times along the S phase.
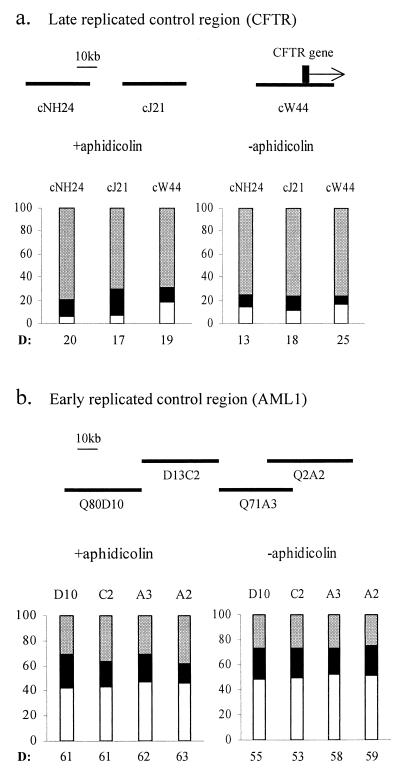
We further compared the relative replication time of the FRA7H region to that of the CFTR region, a known, relatively late-replicating control region. The CFTR region (cNH24 and cW44 cosmid clones) was found both by FISH and by cell-fractionation-based methods to replicate late in Manca cells (13 and 14% DD, respectively) (36). Our analysis revealed levels of DD signals similar to those identified by Selig et al. (13% for cNH24 and 15% for cW44) (36) (Table 1). In comparison, the cosmids encompassing the entire FRA7H region showed 32 to 34% DD signals. Thus, under normal growth conditions, most of the FRA7H alleles must complete their replication before the CFTR and other late-replicating nonfragile regions. The relatively early replication accomplishment time of FRA7H suggests that late replication initiation is not an obligatory feature of fragile sites.
The replication pattern of FRA7H region is affected by aphidicolin.
Next, we aimed to study the effect of aphidicolin on the replication pattern of the FRA7H region. Low concentration of aphidicolin (as used for fragility induction) was previously shown to slow the progression rate of cells in S phase, but not in G1 (24). Indeed, upon aphidicolin treatment, the fraction of BrdU-positive nuclei (S-phase nuclei) increased by ∼20% in Manca cells (data not shown). This partial blocking is expected to generate an accumulation of cells at the beginning of S phase, which subsequently moves as a wave throughout the phase. Hence, upon aphidicolin treatment, the fraction of nuclei with a specific replication pattern does not necessarily represent the actual replication time in S phase. It is, therefore, not appropriate to compare the replication time of a specific sequence in cells grown under normal conditions and cells grown with aphidicolin.
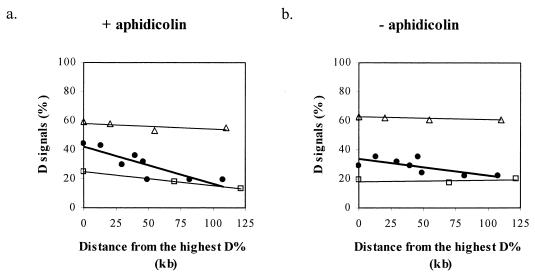
However, a similar replication time is generally expected over extensive (megabases) genomic regions until a boundary between replication zones is reached (10, 14), and sequences that replicate in the same part of S phase are expected to maintain their coordinated replication time upon aphidicolin treatment. Thus, we analyzed the effect of aphidicolin on the relative replication times of adjacent cosmid clones covering the entire FRA7H region and cosmid clones from two nonfragile site regions, the CFTR and the AML1 regions (Fig. 1 and 2). The level of replicated alleles across FRA7H demonstrated, upon aphidicolin treatment, a bipolar gradient along the entire region (Fig. 1b). This gradient could be seen in the fraction of the SS, SD, and DD nuclei. The highest level of D signals, 44%, was detected in the center of the region (clone 72c11), which gradually decreased towards the sides, reaching 19% at the edges of the region (clones 210c9, 141e11, and 7d10) (Fig. 1b). In order to evaluate the significance of this gradient, we performed a linear regression analysis. For this, the absolute distances from the center of cosmid 72c11 (showing the highest percentage of D signals [D%]) to the center of each clone (from both sides of cosmid 72c11) were used. This analysis of the replication pattern revealed a significant correlation (P = 0.006) between the D% and the distance from the FRA7H center (−0.26 D%/kb) (Fig. 3a), indicating a significant gradual decrease in the level of replicated alleles from the center of FRA7H towards both its sides. Thus, upon aphidicolin induction, the expected coordinate replication was not found across the FRA7H region. We further analyzed the replication pattern along the two nonfragile regions which are similar in size to FRA7H: the AML1, an early replicating region encompassing 140 kb and the CFTR, a late-replicating region encompassing 160 kb (Fig. 2). The analysis revealed a unipolar gradient with slight differences across each of the regions (−0.1 D%/kb in the CFTR and −0.04 D%/kb in the AML1 regions) (Fig. 3a). The regression analysis of the FRA7H slope was significantly larger than that of both the CFTR (P = 0.005) and the AML1 (P = 0.0007) slopes. Thus, upon aphidicolin treatment, the differences in the replication time along the FRA7H region are significantly higher than along control regions.
FIG. 1.
In situ hybridization pattern along the FRA7H region. (a) A cosmid map covering the FRA7H region. The SV40 and the ZNF131 pseudogene integration sites are marked by arrows. (b) Percentage of in situ hybridization signals in nuclei from the entire cell cycle, upon treatment with the fragility inducer (+aphidicolin). (c) The same analysis in nuclei grown under normal growth conditions (−aphidicolin). The percentage of alleles showing D signals (DD plus one-half SD nuclei) are indicated below each cosmid clone.
FIG. 2.
In situ hybridization pattern along nonfragile control regions. (a) Top, a cosmid map encompassed ∼160 kb from the CFTR region. The vertical bar indicates exon 1 of the CFTR gene. Bottom, percentage of in situ hybridization signals in nuclei from the entire cell cycle grown under normal growth conditions (−aphidicolin) and upon aphidicolin treatment (+aphidicolin). (b) Same analysis as in panel a of ∼140 kb from the AML1 region. The shading designations for SS, SD, and DD are the same as in Fig. 1.
FIG. 3.
Regression analysis of the correlation between the percentage of replicated alleles (D%) and the distance from the clone showing the highest level of replicated alleles along the FRA7H (●), the CFTR (□), and the AML1 (▵) regions. The distances were estimated from the center of each clone.
We subsequently analyzed the replication pattern along the studied regions under normal growth conditions. The analysis revealed a slightly higher level of D signals in the FRA7H core region (30 to 35% for clones 176g10 to 170g6) than that of both sides (22 to 24% for 7d10, 141e11, and 210c9) (Fig. 1c). The linear regression analysis (performed as explained above) revealed a significant correlation (P = 0.04) between the levels of replicated alleles and the distance from the FRA7H center region (−0.1 D%/kb) (Fig. 3b). Additionally, the regression slope is significantly larger than that of both the CFTR (P = 0.01) and the AML1 (P = 0.02) slopes. Thus, the significant difference between the replication pattern of FRA7H and that of each control region, found upon aphidicolin treatment, already exists under normal growth conditions.
In addition, our analysis revealed a significant difference between the FRA7H slopes with and without aphidicolin treatment (P = 0.02). Thus, aphidicolin treatment significantly enhanced the replication time differences along FRA7H. These results suggest that the replication of FRA7H might be particularly sensitive to aphidicolin due to an unusual replication pattern which already exists before the treatment.
The center of the FRA7H region is replicated before its sides.
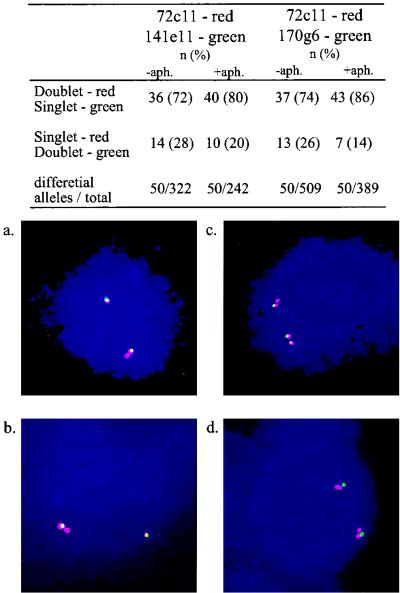
To further understand the basis for the replication pattern along the FRA7H region, we examined the replication pattern of adjacent probes at the single-cell level by using a two-color FISH analysis. We analyzed pairs of clones, each comprised of a clone from the FRA7H central region (72c11, labeled with a red fluorescent dye) and a clone from either side (141e11 or 170g6, labeled in green) (Fig. 4). In each experiment, 50 FRA7H alleles, showing S signals for one of the clones and D signals for the other, were scored. The analysis revealed that under normal growth conditions, in most of these alleles (72% for one pair and 74% for the other), the FRA7H central region (cosmid 72c11) was already replicated, while the adjacent ∼40-kb region at both sides (cosmids 141e11 and 170g6) were not (Fig. 4, upper panel). Upon aphidicolin treatment, the fraction of alleles showing this pattern was higher (80 and 86%). This pattern is significantly deviating from the 1:1 ratio expected to result from inefficient hybridization. This single-cell analysis indicates that the center of the FRA7H is replicated before its sides upon aphidicolin treatment, as well as under normal growth conditions.
FIG. 4.
Two-color FISH analysis of the replication pattern of adjacent clones along the FRA7H region. Upper panel, number and percentage of FRA7H alleles showing differential FISH signals between cosmid clone 72c11 (red) and either 141e11 or 170g6 (green). The number of alleles that were scored until reaching 50 alleles showing differential signals is also indicated. Bottom panel, examples of nuclei showing differential in situ hybridization signals between clones 72c11 and 141e11 (a and b) and between clones 72c11 and 170g6 (c and d).
In order to investigate the significance of these results, we performed a similar analysis by using pairs of clones from the CFTR (n = 1) and the AML1 (n = 5) nonfragile site regions, residing 35 to 95 kb apart. In all the pairs, the fraction of alleles showing S signal for one probe and D signal for the other was about the same as that of the opposite combination (mean, 56% ± 1.3%), both under normal conditions and upon aphidicolin treatment. This ratio is significantly different from that found for pairs of clones from the FRA7H region, under normal growth conditions and upon aphidicolin treatment (P = ∼0). In addition, the ability to clearly detect the actual replication directions above the 1:1 background in FRA7H but not in the controls probably reflects larger differences in replication time along FRA7H. Furthermore, this larger difference exists both under normal growth conditions and upon aphidicolin treatment, as was found by using the cell population analysis (Fig. 3).
FRA7H alleles from both parental origins have the same replication pattern.
Next, we aimed to study the nature of the allelic asynchrony (high SD level) in the replication pattern of the FRA7H region. Such a high level of asynchrony was previously found for large regions (hundreds of kilobases) harboring monoallelic-expressed genes, including parental imprinted genes (17). A paternally specific expressed gene, PEG1, was recently mapped ∼750 kb centromeric to FRA7H (28). Therefore, we aimed to investigate whether the high level of allelic asynchrony of the FRA7H region reflected imprinted replication time of the PEG1 region or of another imprinted region, which may include the fragile region. For this, we analyzed the replication pattern of cosmid clones from the PEG1 gene, from the FRA7H region, and from a control region in cells from a child with a maternal isodisomy of the entire chromosome 7 (CF33-3) (44) and from his normal mother (CF33-2) (Table 2). The PEG1 cosmid, 53g3, showed an asynchronous replication pattern (31% SD signals) in the normal CF33-2 cell line and a synchronous late replication pattern in the isodisomic CF33-3 cell line (7% SD and 88% SS signals) (Table 2). The replication pattern of the PEG1 cosmid was also studied in Manca cell line and showed 31% SD signals (data not shown), indicating allelic asynchrony. However, clones from the FRA7H region (both from the central region, 72c11, and from the side, 7d10), showed replication asynchrony in the isodisomic cell line, as in the normal heterodisomic mother and in Manca cell lines (Table 1 and Table 2). These results suggest that the FRA7H alleles, of both parental origins, have the same replication pattern, indicating that the replication time of FRA7H is not parentally imprinted.
TABLE 2.
In situ hybridization pattern of nuclei from a child with a chromosome-7 maternal isodisomy (CF33-3) and from his normal mother (CF33-2)
| Cell line | PEG1 53g3 | FRA7H
|
Control cNH24 | |
|---|---|---|---|---|
| 72c11 | 7d10 | |||
| CF33-2 | ||||
| SS | 62 | 63 | 66 | 83 |
| SD | 31 | 26 | 25 | 11 |
| DD | 7 | 11 | 9 | 6 |
| CF33-3 | ||||
| SS | 88 | 52 | 67 | 81 |
| SD | 7 | 29 | 22 | 13 |
| DD | 5 | 19 | 11 | 6 |
FRA7H replication time is not allele specific.
High SD levels could also arise from a non-parental imprinted mechanism, as in the case of X-inactivated and olfactory receptor genes (2, 3). Thus, we extended the replication asynchrony analysis to another cell line (GM00847) containing marked chromosomes 7, which was previously used for the cloning of the FRA7H region. In this cell line, SV40 DNA was integrated into one of the FRA7H alleles, telomeric to cosmid 72c11 (Fig. 1a) (28). A subsequent duplication event of the two entire chromosomes 7 resulted in four copies of chromosome 7, two of which are marked by the SV40 DNA. We applied two-color FISH analysis, using the FRA7H clone 72c11 and SV40 DNA. First, we analyzed the FRA7H replication pattern in each of the duplicated chromosome pairs. The analysis revealed a similar pattern with high SD fraction in each pair: 53% SS, 25% SD, and 22% DD for the SV40 marked alleles, and 56% SS, 26% SD, and 18% DD signals for the nonmarked alleles. These results are similar to those found in the isodisomy CF33-3 and in the normal Manca cell lines (Table 1 and Table 2) for cosmid 72c11. Thus, allelic asynchrony may be found between FRA7H identical alleles (as in CF33-3 and GM00847) as well as between different alleles (as in Manca and CF33-2), suggesting that sequence polymorphism between FRA7H alleles is not the basis for the allelic asynchrony.
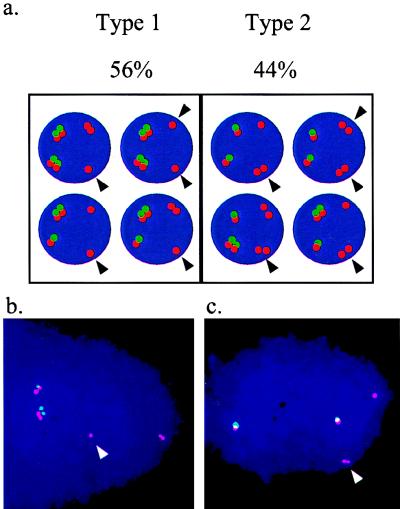
Next, we studied the replication pattern of marked and nonmarked FRA7H alleles. For this, we searched for two types of nuclei, type 1 including nuclei in which at least one nonmarked FRA7H allele replicated later (S signals) than at least one of the SV40-marked FRA7H alleles (D signals) and type 2 including nuclei in which at least one nonmarked FRA7H allele preceded the replication of at least one marked allele (Fig. 5). Out of 43 such nuclei analyzed, 24 (56%) were of type 1 and 19 (44%) were of type 2. The similar distribution of these two types indicated that in each cycle each of the FRA7H alleles could be replicated relatively late. Hence, the allelic asynchrony found in the FRA7H region was not the result of a specific replication time of each FRA7H allele.
FIG. 5.
Replication pattern of FRA7H alleles in GM00847 cell line, carrying a duplicated SV40-marked chromosome 7. (a) Illustrations of the two possible type of nuclei: type 1, nuclei in which at least one nonmarked FRA7H allele, marked by an arrow, replicated later than at least one of the SV40-marked alleles; type 2, nuclei in which at least one nonmarked FRA7H allele, marked by an arrow, preceded the replication of at least one of the marked alleles. The percentage of nuclei of each type is indicated. (b) Examples of hybridization signals of type 1 nuclei. (c) Examples of hybridization signals of type 2 nuclei.
DISCUSSION
The analysis of the FRA7H region revealed that the replication of this region is initiated at mid-S phase, earlier than many nonfragile regions (Table 1). Laird et al. predicted that fragile sites replicate very late in the cycle, and our results are not inconsistent with this model. This model suggested that upon replication stress or premature chromatin condensation, late replicating regions would fail to complete their condensation and express chromosomal fragility (22, 26). This does not exclude the possibility that regions that initiate (and usually complete) the replication relatively early might complete the replication late and express fragility as a result of specific sensitivity to elongation stress. This sensitivity might result from perturbed fork progression which leads to delayed replication along the region. Assuming normal regulation of initiation time together with a delayed replication along the region, the following might be expected: (i) high levels of allele-nonspecific asynchronous replication; (ii) large differences in the replication time between adjacent sequences; (iii) a specific sensitivity to elongation stress enhanced by the inducer, leading to a further replication delay; (iv) a subset of the alleles (that were extremely delayed) might express fragility. All these features have been noted for the FRA7H region.
The analysis of the FRA7H replication time revealed a high level of allelic asynchrony (>30%) along the fragile region. Usually, an asynchronous replication pattern is found only in 10 to 20% of the nuclei (17, 37). In contrast, high levels of SD signals (30 to 40%) were found for monoallelic-expressed genes such as those subject to parental imprinting or X inactivation or the olfactory receptor genes (2, 3, 17). In these cases, each allele is regulated to initiate its replication at a specific time in S phase. It is interesting to note that the maternal allele of the PEG1 gene was found in our analysis to replicate later than the paternal allele, as was previously found for other imprinted zones (17).
The results of the FRA7H analysis identified a novel type of allelic asynchrony, which is not the outcome of allele-specific replication time. This became apparent from three lines of evidence: (i) high levels of allelic asynchrony between two maternal copies of chromosome 7 (Table 2), (ii) high levels of allelic asynchrony between identical duplicated chromosome 7 (Table 2 and Fig. 5), and (iii) equal probability of each of the FRA7H alleles to be delayed in each cycle (Fig. 5). All these results excluded the possibility that genetic or epigenetic mechanisms predetermine the specific replication time of each of the FRA7H alleles. Thus, the FRA7H alleles could replicate at different times along the cycle, independently of each other.
Common fragile sites are often expressed on both homologues in the same cell (1). An analysis of 5,600 metaphases from different individuals revealed that the frequencies of homozygous expression in eight aphidicolin-induced common fragile sites (including FRA7H and FRA3B) were close and even higher than the expected frequencies if each homologue is equally likely to express fragility (1). Our results, showing an equal probability of each FRA7H allele to be delayed (Fig. 5), are consistent with this analysis. Allele-specific late replication time and fragility were recently reported for the common fragile site FRA3B (45), but the reason for this was unclear and might represent a phenomenon unique to the particular analyzed cell line.
The genome of animal cells is organized as distinct replication time zones typically made up of multiple (10 to 40) adjacent replicons, each encompassing 50 to 300 kb, that are coordinately regulated to undergo replication at the same time in S phase (10, 14). Thus, a similar replication time pattern is generally expected over extensive (megabases) genomic regions until a boundary between replication zones is reached (38). Upon aphidicolin treatment, this regulation is not expected to change. Indeed, under normal growth conditions, two control regions, one early replicating (AML1) and one late replicating (CFTR), showed a coordinated replication of adjacent clones (slopes = −0.017 and 0.006 D%/kb, respectively) (Fig. 3). In contrast, in the FRA7H region, a bimodal gradient was revealed with a high difference in replication time between adjacent clones. This gradient significantly correlated with the distance from the central region (slope = −0.11 D%/kb, P = 0.04) and significantly differed from each of the control regions (P = 0.02 and 0.01, respectively) (Fig. 3). Furthermore, this bipolar gradient encompassed a region that is well correlated with the 160-kb FRA7H region that shows gaps and constrictions on metaphase chromosomes (28). However, it will be important to analyze the replication pattern and fragility expression of additional clones flanking the analyzed FRA7H region since the analyzed 160 kb of the FRA7H region might not contain all fragile sequences at 7q32 (28). In summary, the uncoordinated replication of FRA7H is unusual and might reflect intrinsic features which affect the replication.
Upon aphidicolin treatment, a complete coordinated replication pattern (slope = −0.04 D%/kb) was found along the AML1 region, and only slight differences were found in the CFTR region (slope = −0.1 D%/kb). In contrast, the FRA7H showed a highly uncoordinated replication pattern (slope = −0.26 D%/kb) which significantly differed from both the AML1 and CFTR regions (P = 0.0007 and 0.005, respectively). Therefore, the replication pattern of the fragile region significantly differs from nonfragile regions, both under normal growth conditions and upon aphidicolin treatment. This difference is also found upon separate analyses of each side of the bipolar FRA7H gradient (data not shown).
Comparison of the replication pattern of FRA7H with and without aphidicolin revealed a significant difference (P = 0.02). The same comparison of the CFTR region revealed no difference along the AML1 (slope = −0.02 D%/kb) region and some degree of uncoordinated replication along the CFTR region (slope = −0.1 D%/kb) (Fig. 3). Thus, the difference in the effect of aphidicolin between the fragile region and the control regions might be a quantitative rather than a qualitative difference. In fact, the slope of the CFTR region upon aphidicolin treatment is not significantly different from the slope of the fragile region without the treatment. Thus, the replication pattern of the CFTR region upon treatment resembles the pattern of the FRA7H under normal conditions.
The differences in the replication pattern along FRA7H, found already under normal growth conditions, suggest that the region has intrinsic features that might delay the replication. The exposure of the region to aphidicolin only enhances this delay to a level that might confer fragility. Thus, it is expected that the specific effect of aphidicolin on fragile regions is not due to a specific interaction with the aphidicolin but rather relates to the fragile site specific features. Indeed, Glover et al. showed that fragility could be induced at common fragile sites (including FRA7H) by interrupting the replication either by inhibition of DNA polymerases (aphidicolin) or by perturbation of the deoxynucleotide triphosphate pools (thymidylate stress). In contrast, the rare fragile site FRAXA was induced only by thymidylate stress and not by aphidicolin (7). Thus, common fragile sites appear to be sensitive to a general inhibition of replication progression, rather than to an interaction with a specific inducer. Our findings are consistent with this hypothesis and suggest that the intrinsic delayed replication along the region contributes to the molecular basis of the site-specific sensitivity of common fragile sites. Furthermore, adjacent cosmid clones along 800 kb of the FRAXA region revealed a coordinated replication pattern in the normal and the expanded alleles (39), supporting the hypothesis that separate but overlapping mechanisms account for the appearance of the FRAXA and the common fragile sites (7).
We further analyzed FRA7H alleles showing a differential replication pattern between adjacent clones along the FRA7H region (Fig. 4). The analysis revealed that the FRA7H central region was replicated before its sides, which may suggest that the replication of this region initiates from one bidirectional origin of replication located at the center of the region. Importantly, the ability to detect the replication direction along the FRA7H but not along the control regions might indicate a low rate of elongation. Alternatively, these results might indicate different initiation times of adjacent small replicons.
Extended analysis of the FRA7H region revealed clusters of sequences with a potential for high flexibility and low stability and sequences with a potential to form triple helixes and to function as matrix-scaffold attachment regions (28). These non-B-DNA structures might be randomly formed and resolved, leading to irregular progression of replication forks. This might explain the random accomplishment time of replication, resulting in allelic asynchrony. The probability for such interruptions is expected to accumulate as the replication forks progress along the region, passing through additional sequences with a potential to form non-B-DNA structures. Assuming replication initiation from an origin located at the FRA7H central region and interrupted elongation along the entire FRA7H region, sequences flanking the replication origin are expected to replicate early and asynchronously, while regions at the periphery are expected to replicate later and asynchronously. This might explain why, in some of the FRA7H alleles, the peripheries of the FRA7H region accomplished their replication much later than the center. According to this hypothesis, a reduction of the DNA polymerization rate by aphidicolin (24) is expected to increase the probability for interruptions along the fragile region. Thus, some of the FRA7H alleles might fail to accomplish replication and condensation in time, and thus form a fragile site.
As of today, it is still unclear whether the expression of fragility at metaphase results from unreplicated or just uncondensed DNA. The analysis of the replication status of FRA7H alleles during G2 might shed light on this unresolved question.
Two additional aphidicolin-induced fragile sites, FRA3B and FRA7G, have been analyzed at the molecular level. The cytogenetic expression of these sites appears along a region of several hundred kilobases which also harbors clusters of sequences with potentially high flexibility (27, 28). Replication time analysis of ∼900 kb from the FRA3B region revealed that markers along this region replicated at different times throughout the cell cycle. The reason for this unusual phenomenon remains unclear (45). However, we suggest that the replication pattern identified in this region might reflect four adjacent distinct bipolar gradients of ∼150 kb each (see Fig. 4 in reference 45). In addition, an independent replication time analysis of ∼300 kb from the FRA3B region could also be interpreted as showing two similar bipolar gradients, between the proximal aphidicolin-induced breakpoint cluster and exon 5 of the FHIT gene (see Fig. 4 in reference 23). Thus, FRA3B might comprise several adjacent regions which resemble the genomic structure of the FRA7H region.
Common fragile sites are thought to be part of normal chromosome structure; however, the fragility is expressed in less than 5% of the metaphase chromosomes at most of the sites, including FRA7H. The most inducible fragile site in the human genome is FRA3B, expressing fragility in 10 to 15% of the chromosomes (42). The reason for this partial expression is unknown. Our results suggest that a subset of alleles, in which an extreme elongation delay occurred, would express the fragility. Thus, the high inducibility of FRA3B might result either from the clustering of regions resembling the genomic structure of FRA7H, from the late replication time identified for this site (23), or from the combination of both.
Taken together, the analyses of FRA7H and FRA3B indicate that the basis for fragility at aphidicolin-induced fragile sites might be associated with delayed replication along the fragile regions, which in turn might confer a specific sensitivity to replication stress. This delay might reflect an irregular rate of elongation or an unusual initiation of regulation. The irregular elongation hypothesis could explain both the random replication time between alleles and the ordered replication time along each allele of the FRA7H region. This hypothesis is also consistent with the observations that all the inducers of the different fragile sites interrupt replication elongation by nucleotide depletion, DNA polymerase inhibition, or DNA intercalation (7, 41).
Alternatively, the results of this study might reflect an unusual organization of replication time zones along FRA7H in which several small adjacent replicons initiate their replication at different times along the cycle. This possibility is inconsistent with the known mammalian genome organization and does not provide a simple explanation for the coexistence of random replication times between alleles and ordered replication along each allele. However, such an unusual organization of replication time zones might be an intrinsic feature of common fragile sites. Analysis of the replication directions along common fragile sites should distinguish between these possibilities and thus deepen our understanding of the mechanism underlying fragility at common fragile sites.
ACKNOWLEDGMENTS
We thank R. Ofir and S. Selig for assistance in the FISH analysis, A. Rosenthal and M. Schilhabel for providing the AML1 cosmid clones, and H. Cedar for helpful discussions.
The study was supported by a grant from the Israel Foundation for Sciences and Humanities to B.K.
REFERENCES
- 1.Austin M J, Collins J M, Corey L A, Nance W E, Neale M C, Schieken R M, Brown J A. Aphidicolin-inducible common fragile-site expression: results from a population survey of twins. Am J Hum Genet. 1992;50:76–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Boggs B A, Chinault A C. Analysis of replication timing properties of human X-chromosomal loci by fluorescence in situ hybridization. Proc Natl Acad Sci USA. 1994;91:6083–6087. doi: 10.1073/pnas.91.13.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- 4.Coquelle A, Pipiras E, Toledo F, Buttin G, Debatisse M. Expression of fragile sites triggers intrachromosomal mammalian gene amplification and sets boundaries to early amplicons. Cell. 1997;89:215–225. doi: 10.1016/s0092-8674(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 5.Djalali M, Adolph S, Steinbach P, Winking H, Hameister H. A comparative mapping study of fragile sites in the human and murine genomes. Hum Genet. 1987;77:157–162. doi: 10.1007/BF00272384. [DOI] [PubMed] [Google Scholar]
- 6.Gecz J, Gedeon A K, Sutherland G R, Mulley J C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat Genet. 1996;13:105–108. doi: 10.1038/ng0596-105. [DOI] [PubMed] [Google Scholar]
- 7.Glover T W, Berger C, Coyle J, Echo B. DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet. 1984;67:136–142. doi: 10.1007/BF00272988. [DOI] [PubMed] [Google Scholar]
- 8.Glover T W, Hoge A W, Miller D E, Ascara-Wilke J E, Adam A N, Dagenasis S L, Wilke C M, Dierice H A, Beer D G. The murine Fhit gene is highly similar to its human orthologue and maps to a common fragile site region. Cancer Res. 1998;58:3409–3414. [PubMed] [Google Scholar]
- 9.Glover T W, Stein C K. Induction of sister chromatid exchanges at common fragile sites. Am J Hum Genet. 1987;41:882–890. [PMC free article] [PubMed] [Google Scholar]
- 10.Hand R. Regulation of DNA replication on subchromosomal units of mammalian cells. Cell Biol. 1975;64:89–97. doi: 10.1083/jcb.64.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen R S, Canfield T K, Fjeld A D, Mumm S, Laird C D, Gartler S M. A variable domain of delayed replication in FRAXA fragile X chromosomes: X inactivation-like spread of late replication. Proc Natl Acad Sci USA. 1997;94:4587–4592. doi: 10.1073/pnas.94.9.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen R S, Canfield T K, Lamb M M, Gartler S M, Laird C D. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993;73:1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- 13.Hayday A C, Gillies S D, Saito H, Wood C, Wiman K, Hayward W S, Tonegawa S. Activation of a translocated human c-myc gene by an enhancer in the immunoglobulin heavy-chain locus. Nature. 1984;307:334–340. doi: 10.1038/307334a0. [DOI] [PubMed] [Google Scholar]
- 14.Holmquist G P. Chromosome bands, their chromatin flavors, and their functional features. Am J Hum Genet. 1992;51:17–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Qian C, Jenkins R B, Smith D I. Fish mapping of YAC clones at human chromosomal band 7q31.2: identification of YACS spanning FRA7G within the common region of LOH in breast and prostate cancer. Genes Chromosomes Cancer. 1998;21:152–159. doi: 10.1002/(sici)1098-2264(199802)21:2<152::aid-gcc11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.Jones C, Penny L, Mattina T, Yu S, Baker E, Voullaire L, Langdon W, Sutherland G, Richards R, Tunnacliffe A. Association of a chromosome deletion syndrome with a fragile site within the proto-oncogene CBL2. Nature. 1995;376:145–149. doi: 10.1038/376145a0. [DOI] [PubMed] [Google Scholar]
- 17.Kitsberg D, Selig S, Brandeis M, Simon I, Keshet I, Driscoll D J, Nicholls R D, Cedar H. Allele-specific replication timing of imprinted gene regions. Nature. 1993;364:459–463. doi: 10.1038/364459a0. [DOI] [PubMed] [Google Scholar]
- 18.Kitsberg D, Selig S, Keshet I, Cedar H. Replication structure of the human beta-globin gene domain. Nature. 1993;366:588–590. doi: 10.1038/366588a0. [DOI] [PubMed] [Google Scholar]
- 19.Knight S J, Flannery A V, Hirst M C, Campbell L, Christodoulou Z, Phelps S R, Pointon J, Middleton-Price H R, Barnicoat A, Pembrey M E, et al. Trinucleotide repeat amplification and hypermethylation of a CpG island in FRAXE mental retardation. Cell. 1993;74:127–134. doi: 10.1016/0092-8674(93)90300-f. [DOI] [PubMed] [Google Scholar]
- 20.Kremer E J, Pritchard M, Lynch M, Yu S, Holman K, Baker E, Warren S T, Schlessinger D, Sutherland G R, Richards R I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991;252:1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- 21.Laird C D, Hansen R S, Canfield T K, Lamb M M, Gartler S M. Chromosomal fragile sites: molecular test of the delayed-replication model. Cold Spring Harbor Symp Quant Biol. 1993;58:633–635. doi: 10.1101/sqb.1993.058.01.070. [DOI] [PubMed] [Google Scholar]
- 22.Laird C E, Jaffe G, Karpen M, Lamb M, Nelson R. Fragile sites in human chromosomes as regions of late-replicating DNA. Trends Genet. 1987;3:274. [Google Scholar]
- 23.Le Beau M M, Rassool F V, Neilly M E, Espinosa III R, Glover T W, Smith D I, McKeithan T W. Replication of a common fragile site, FRA3B, occurs late in S phase and is delayed further upon induction: implications for the mechanism of fragile site induction. Hum Mol Genet. 1998;7:755–761. doi: 10.1093/hmg/7.4.755. [DOI] [PubMed] [Google Scholar]
- 24.Levenson V, Hamlin J L. A general protocol for evaluating the specific effects of DNA replication inhibitors. Nucleic Acids Res. 1993;21:3997–4004. doi: 10.1093/nar/21.17.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichter P, Cremer T, Borden J, Manuelidis L, Ward D C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum Genet. 1988;80:224–234. doi: 10.1007/BF01790090. [DOI] [PubMed] [Google Scholar]
- 26.McManus J, Perry P, Sumner A T, Wright D M, Thomson E J, Allshire R C, Hastie N D, Bickmore W A. Unusual chromosome structure of fission yeast DNA in mouse cells. J Cell Sci. 1994;107:469–486. doi: 10.1242/jcs.107.3.469. [DOI] [PubMed] [Google Scholar]
- 27.Mimori K, Druck T, Inoue H, Alder H, Berk L, Mori M, Huebner K, Croce C M. Cancer-specific chromosome alterations in the constitutive fragile region FRA3B. Proc Natl Acad Sci USA. 1999;96:7456–7461. doi: 10.1073/pnas.96.13.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishmar D, Rahat A, Scherer S W, Nyakatura G, Hinzmann B, Kohwi Y, Mandel-Gutfroind Y, Lee J R, Drescher B, Sas D E, Margalit H, Platzer M, Weiss A, Tsui L-C, Rosenthal A, Kerem B. Molecular characterization of a common fragile site (FRA7H) on human chromosome 7 by the cloning of an SV40 integration site. Proc Natl Acad Sci USA. 1998;95:8141–8146. doi: 10.1073/pnas.95.14.8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nancarrow J, Kremer E, Holman K, Eyre H, Doggett N, Le Paslier D, Callen D, Sutherland G, Richards R. Implications of FRA16A structure for the mechanism of chromosomal fragile site genesis. Science. 1994;264:1938–1941. doi: 10.1126/science.8009225. [DOI] [PubMed] [Google Scholar]
- 30.Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas M F, Mandel J L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 31.Parrish J E, Oostra B A, Verkerk A J, Richards C S, Reynolds J, Spikes A S, Shaffer L G, Nelson D L. Isolation of a GCC repeat showing expansion in FRAXF, a fragile site distal to FRAXA and FRAXE. Nat Genet. 1994;8:229–235. doi: 10.1038/ng1194-229. [DOI] [PubMed] [Google Scholar]
- 32.Popescu N, Zimonjic D, DiPaolo J. Viral integration, fragile sites, and proto-oncogen in human neoplasia. Hum Genet. 1990;84:383–386. doi: 10.1007/BF00195804. [DOI] [PubMed] [Google Scholar]
- 33.Rassool F V, McKeithan T W, Neilly M E, van Melle E, Espinosa III R, Le Beau M M. Preferential integration of marker DNA into the chromosomal fragile site at 3p14: an approach to cloning fragile sites. Proc Natl Acad Sci USA. 1991;88:6657–6661. doi: 10.1073/pnas.88.15.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rommens J M, Iannuzzi M C, Kerem B, Drumm M L, Melmer G, Dean M, Rozmahel R, Cole J L, Kennedy D, Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 35.Samadashwily G M, Raca R, Mirkin S M. Trinucleotide repeats affect DNA replication in vivo. Nat Genet. 1997;17:298–304. doi: 10.1038/ng1197-298. [DOI] [PubMed] [Google Scholar]
- 36.Selig S, Okumura K, Ward D C, Cedar H. Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J. 1992;11:1217–1225. doi: 10.1002/j.1460-2075.1992.tb05162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon I, Tenzen T, Reubinoff B E, Hillman D, McCarrey J R, Cedar H. Asynchronous replication of imprinted genes is established in the gametes and maintained during development. Nature. 1999;401:929–932. doi: 10.1038/44866. [DOI] [PubMed] [Google Scholar]
- 38.Strehl S, LaSalle J M, Lalande M. High-resolution analysis of DNA replication domain organization across an R/G-band boundary. Mol Cell Biol. 1997;17:6157–6166. doi: 10.1128/mcb.17.10.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian P S, Nelson D L, Chinault A C. Large domains of apparent delayed replication timing associated with triplet repeat expansion at FRAXA and FRAXE. Am J Hum Genet. 1996;59:407–416. [PMC free article] [PubMed] [Google Scholar]
- 40.Sutherland G. Heritable fragile sites on human chromosomes. I. Factors affecting expression in lymphocyte culture. Am J Hum Genet. 1979;31:125–135. [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland G R, Baker E, Richards R I. Fragile sites still breaking. Trends Genet. 1998;14:501–506. doi: 10.1016/s0168-9525(98)01628-x. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland G R, Richards R I. Fragile sites-cytogenetic similarity with molecular diversity. Am J Hum Genet. 1999;64:354–359. doi: 10.1086/302267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usdin K, Woodford K J. CGG repeats associated with DNA instability and chromosome fragility form structures that block DNA synthesis in vitro. Nucleic Acids Res. 1995;23:4202–4209. doi: 10.1093/nar/23.20.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voss R, Ben-Simon E, Avital A, Godfrey S, Zlotogora J, Dagan J, Tikochinski Y, Hillel J. Isodisomy of chromosome 7 in a patient with cystic fibrosis: could uniparental disomy be common in humans? Am J Hum Genet. 1989;45:373–380. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Darling J, Zhang J S, Huang H, Liu W, Smith D I. Allele-specific late replication and fragility of the most active common fragile site, FRA3B. Hum Mol Genet. 1999;8:431–437. doi: 10.1093/hmg/8.3.431. [DOI] [PubMed] [Google Scholar]
- 46.Wilke C M, Guo S W, Hall B K, Boldog F, Gemmill R M, Chandrasekharappa S C, Barcroft C L, Drabkin H A, Glover T W. Multicolor FISH mapping of YAC clones in 3p14 and identification of YAC spanning + both FRA3B and the t(3;8) associated with hereditary renal cell carcinoma. Genomics. 1994;22:319–326. doi: 10.1006/geno.1994.1390. [DOI] [PubMed] [Google Scholar]
- 47.Wilke C M, Hall B K, Hoge A, Pardee W, Smith D I, Glover T W. FRA3B extends over a broad region and contains a spontaneous HPV16 integration site: direct evidence for the coincidence of viral integration sites and fragile sites. Hum Mol Genet. 1996;5:187–195. doi: 10.1093/hmg/5.2.187. [DOI] [PubMed] [Google Scholar]
- 48.Yu S, Mangelsdorf M, Hewett D, Hobson L, Baker E, Eyre H J, Lapsys N, Le Paslier D, Doggett N A, Sutherland G R, Richards R I. Human chromosomal fragile site FRA16B is an amplified AT-rich minisatellite repeat. Cell. 1997;88:367–374. doi: 10.1016/s0092-8674(00)81875-9. [DOI] [PubMed] [Google Scholar]
- 49.Yunis J J, Soreng A. Constitutive fragile sites and cancer. Science. 1984;226:1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]