Abstract
Mixed depression is probably different in terms of clinical course and response to treatment. Repetitive transcranial magnetic stimulation (rTMS) is well established in non-mixed depression, and theta-burst stimulation (TBS) protocol is replacing conventional protocols because of noninferiority and reduced delivery time. However, TBS has not been adequately studied in mixed states. This study was a double-blind, six-week, sham-controlled, and randomized clinical trial of bilateral TBS targeting the right and left dorsolateral prefrontal cortex, respectively. Adults with bipolar and major depressive disorder experiencing an acute mixed depression were eligible if they had not benefited from a first- or second-line treatment for acute unipolar or bipolar depression recommended by the Canadian Network for Mood and Anxiety Treatments. Out of 100 patients included, 90 composed modified intention-to-treat sample, which was patients that completed at least one week of the intervention. There were no significant differences in Montgomery-Asberg depression rating scale score changes (least squares mean difference between groups at week 3, −0.06 [95% CI, − 3.39 to 3.51; P = 0.97] in favor of sham TBS). Response and remission rates per MADRS were also not statistically different among active and sham groups (35.7% vs. 43.7%, and 28.5% vs. 37.5% respectively at week 6, ps > 0.51). No other analyses from baseline to weeks 3 or 6 revealed significant time x group interaction or mean differences among groups in the mITT sample. Bilateral TBS targeting the DLPFC is not efficacious as an add-on treatment of acute bipolar and unipolar mixed depression. ClinicalTrials.govIdentifier: NCT04123301
Subject terms: Medical research, Outcomes research
Introduction
Mood disorders are highly prevalent conditions, associated with important burden and disability [1, 2]. Certain presentations are particularly difficult to identify, manage, and treat, such as mixed depression. A recent review [3] showed that the percentage of mixed features ranges from 4.3% [4] to 58.6% [5] in bipolar disorder (BD), and from 0% [4] to 34% [5] in major depressive disorder (MDD). This variability is explained by the fact that the DSM-5 mixed features specifier excludes the symptoms of distractibility, irritability, and psychomotor agitation, also known as “DIP symptoms” [6]. While mixed manic/hypomanic episodes are relatively easier to manage [7], mixed depression has been associated with greater depression severity [8], rapid cycling [9, 10], higher comorbidities with anxiety [11], impulsivity, and substance abuse [12, 13], worst sleep outcomes [14], higher relapse rates [15], refractoriness [16], and high suicide risk [12, 17].
Repetitive transcranial magnetic stimulation (rTMS) is a non-pharmacological treatment with proven effectiveness for unipolar depression [18]. Several data support the use of high-frequency rTMS over the left dorsolateral prefrontal cortex (DLPFC), and low frequency rTMS over the right DLPFC in treatment-resistant depression [19, 20]. Recently, a new form of rTMS, theta-burst stimulation (TBS) presented noninferiority and reduced delivery time compared to standard rTMS [21–25]. Analogously to rTMS, there are two modalities of TBS: intermittent [iTBS], with excitatory effects, and continuous [cTBS], with inhibitory effects [24], which are applied over the left and right DLPFC for depression. TBS and rTMS were less investigated for bipolar and mixed depression [26, 27]. In fact, studies using rTMS for mixed states are mostly restricted to patients with mixed manic episodes [27–31].
Taking into account these issues, the main objective of this randomized, sham-controlled trial was to evaluate the efficacy and safety of TBS as adjuvant therapy in BD or MDD patients in an acute depressive episode with mixed features after at least one previous failed trial. Our primary hypothesis is that active TBS would be superior to sham as an add-on treatment in improving depressive symptoms over a three-week treatment course.
Patients and methods
Study design
We conducted a single-center, double-blind, randomized, parallel-group, sham-controlled clinical trial (Clinicaltrials.gov identifier: NCT04123301) that lasted six weeks, comprising five consecutive days a week sessions for the first three weeks and then two days a week until week 6. The protocol was approved by the Ethics Committee of the University of Sao Paulo and was conducted in accordance with the Helsinki Declaration [32].
Participants were randomized using a computer-generated list in a 1:1 ratio and block randomization was performed allowing the permutation of the order and the size of the blocks. Allocation concealment consisted of sequentially numbered cards, which determined whether the active or sham TBS coil would be used. Thus, both participants and the personnel applying the stimulation sessions were blinded to the treatment group. Raters were also blinded to allocation group status.
Participants
All participants signed an informed consent form. We enrolled adults aged 18–65 years old diagnosed with BD type I, BD type II, or MDD in a moderate or severe acute depressive episode with mixed features according to modified DSM-5 criteria, i.e., including “DIP symptoms”. The diagnosis was confirmed using a Portuguese-validated version of the structured interview of DSM-IV (Structured Clinical Interview - SCID IV)[33] modified with DSM-5 mixed features criteria and allowing “DIP symptoms”.
The main inclusion criterion was presenting a Montgomery–Åsberg Depression Rating Scale [34, 35] score >19 at baseline. Also, patients had to present a Young Mania Rating Scale (YMRS) [36] score ≥1 on three or more items, according to criteria used in the International Mood Disorders Collaborative Project [4] and consistent with definitions of mixed depression [37–39]. Patients were necessarily using an appropriate first- or second-line pharmacological treatment for an acute MDD or BD depressive episode according to the Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines [7, 40]; therefore TBS was applied as an add-on treatment in patients’ refractory to at least one adequate treatment. Pharmacotherapy remained stable during the TBS intervention, and patients were using adequate pharmacological doses for at least four weeks prior to trial onset. Exclusion criteria were provided in the study protocol published previously [41].
Interventions
TBS sessions were performed using a MagPro X100 TMS device (Magventure, Lucernemarken, Denmark). Identical butterfly coils for active and sham stimulations were used. TBS was performed over the DLPFC bilaterally and the scalp localization was obtained through anatomical measurements (F3 and F4 positions determined by the 10–20 Electroencephalographic International System).
According to the inter-hemispheric asymmetry hypothesis right and left hemispheres have opposite effects on mood control and therapeutic applications of TMS should follow the paradigm of using high-frequency stimulation to activate the left DLPFC and low-frequency to suppress the right DLPFC in depression [42]. As there were no clinical trials evaluating TBS specifically in mixed depressive episodes, our clinical protocol was based on a study [43] that evaluated TBS in treatment-resistant MDD, showing that bilateral TBS stimulation (right cTBS and left iTBS) produced greater results in treating resistant major depressive episodes compared to iTBS over the left DLPFC. As mixed states are mostly mood states that are resistant to conventional treatments, we opted for using bilateral TBS in our study. Importantly, when this study was designed, the pivotal trial of Blumberger et al. [44] was not yet available.
The optimal dose for maximal outcome for TBS or TMS is not known; however evidence from clinical studies has suggested that TBS obeys a dose-response function, that is, a greater number of stimuli administered are capable of optimizing clinical results in patients with depression[45, 46].
The TBS doses varied from 18,000 [47, 48] to 36,000 pulses [49, 50] in the studies. In our study, cTBS was applied in the right DLPFC at the dose of 1,800 pulses per session [versus 21 sessions] and iTBS in the left DLPFC at the dose of 1,800 pulses per session [versus 21 sessions] resulting in a total applied dose of 75,600 pulses, much higher than the studies previously cited. The total study time was six weeks, as most studies in the area [44, 51, 52]. We defined three weeks as the primary endpoint thinking from a clinical point of view, as mixed depression is a condition associated with great distress and therefore considering that a faster response would be particularly desirable for this population.
The bilateral TBS sessions were applied in the following order: cTBS over the right DLPFC followed by iTBS over the left DLPFC. The following parameters were used: for cTBS, bursts of three pulses at 50 Hz (20-ms interval between stimuli) were applied continuously for 120 sec totaling 1800 pulses in the right DLPFC; for iTBS, bursts of three pulses at 50 Hz (20-ms interval between stimuli) were applied for 2 sec, being repeated every 10 sec for a total time of 570 sec; also totaling 1800 pulses in the left DLPFC. We used a 80% intensity of the motor threshold, which was the minimum necessary stimulus to generate a visible muscular contraction in the index finger in three out of five trials, according to the safety parameters published [53].
Assessments
The primary outcome was the comparison between active and sham groups regarding the change in MADRS scores from baseline to week 3 of intervention. Secondary outcomes included the comparisons between active and sham groups regarding response rate (defined as 50% or greater reduction in MADRS scores) from baseline to weeks 3 and 6; remission rate (defined as MADRS score lower than 11) [34] in weeks 3 and 6; changes in other scales such as the Hamilton Anxiety Scale (HAM-A)[54], Global Clinical Impression of Severity (GCI-S)[55], Global Assessment of Functioning (GAF) [56], the World Health Organization questionnaire of quality of life–brief version [57] and the Barratt Impulsivity Scale [58].
Frequency of treatment-emergent manic switch (TEMS), worsening of depression and other adverse events were assessed. Blinding efficacy was assessed at the end of week 6 by asking participants and the personnel applying the stimulation sessions about their allocation group.
Clinical assessments were conducted weekly until week 3 and thereafter a final assessment was conducted at week 6. Adverse events were evaluated daily during the first week and then weekly until week 6.
Statistical analyses
Analyses were performed using the lme4 [59], mice [60] and emmeans [61] packages of R version 3.6.3 [62]. The overall significance level was set at 0.05.
The sample size calculation was based in a clinical trial of treatment-resistant unipolar depression that found a reduction of 52.5% of the depression scale for the active group and a reduction of 17.4% for the sham group (F/X2 = 6.166) [43]. Considering alfa and beta values of 0.05 and 0.1, respectively, we estimated 82 participants. A dropout rate of 20% was calculated, similar to recent studies in this field [25]. Thus, we enrolled 100 patients, 50 participants in each group.
The primary efficacy analysis was performed in the modified intention-to-treat – mITT – sample,
which were patients that completed at least one week of the intervention. Other assessments included the intention-to-treat (ITT) and per protocol (PP)samples. Continuous outcomes with more than two measurements were analyzed using 2-level linear mixed-effects models with the restricted maximum likelihood variance estimator. Continuous outcomes with two measurements were analyzed using a repeated-measures ANOVA, with group, time and their interaction as factors. The interaction between group and time is reported for these models. Frequency of TEMS and adverse events were compared among groups using Fisher’s exact test or the χ2 test. To verify blinding integrity, we asked, at week 6, for participants and the personnel applying the stimulation sessions to guess whether the allocation group was active on a 0-100 scale; guessing scores were compared using a Chi-Square test between “real group” and “guessed group” by the participant or staff member.
Results
Participants
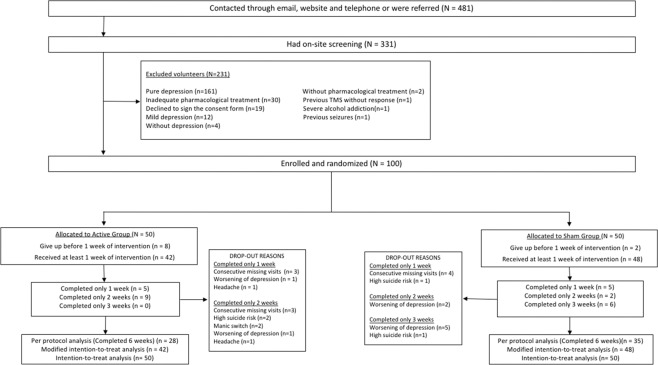
Out of 481 volunteers, 331 were screened and 231 were excluded due to several reasons. Out of 100 patients included, 90 and 63 of them composed mITT and PP samples, respectively. The dropouts that occurred before completing one trial week were eight in the active group and two in the sham group, all due to difficulties to reach the stimulation center. The dropouts that occurred before completing week three were 22 in the active group (n = 42) and nine in the sham group (n = 48) (p = 0.002). These significant unequal dropouts before week 3 were related to difficulties to reach the stimulation center. Dropouts due to adverse effects were listed in Fig. 1. The dropout rates that occurred in the whole six weeks of the study were not statistically different among active and sham groups (X-squared = 1.5444, df = 1, p value = 0.214). In terms of the number of subjects who dropped out from the study due to worsening of depression or suicide risk, there was no statistically significant difference between active (four dropouts, n = 48) and sham (three dropouts, n = 42) groups (OR = 1, CI 95% [0.17, 5.71], p = 1).
Fig. 1. Flow-chart diagram of screening, enroll and randomization.
Dropouts for each reason are provided beside each allocation group.
The groups were similar in terms of demographic characteristics, diagnosis, clinical course, scales scores, pharmacological treatments and previous electroconvulsive treatment at baseline. The whole sample consisted of 48% of patients with MDD, 33% with BD type II and 19% with BD type I. Fifty-eight patients (64%) were using at least one first-line treatment according to CANMAT guidelines and <1% had electroconvulsive therapy previously (Table 1). Unfortunately, we did not collect data regarding the duration of the current acute depressive episode. The overall prevalence of any anxiety disorder, any substance use disorder and any personality disorder were 61%, 5.5%, and 7.8%, and did not differ between groups (all ps > 0.24).
Table 1.
Baseline clinical and demographic characteristics in the mITT sample.
| Sham (n = 48+) | Active (n = 42) | Total (n = 90) | P value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, mean [SD] | 38.0 (10.85) | 40.8 (9.98) | 39.4 (10.4) | 0.196 |
| Gender, fem (%) | 33 (68.8) | 31 (73.8) | 64 (82.2) | 0.768 |
| Ethnicity, Caucasian (%) | 30 (62.5) | 29 (69.0) | 59 (65.5) | 0.848 |
| Marital status, married (%) | 38 (79.1) | 31 (73.8) | 69 (76.7) | 0.727 |
| Number of children, mean [SD] | 0.54 (0.99) | 0.67 (0.87) | 0.65 (0.93) | 0.529 |
| Years at school, mean [SD] | 15.33 (3.87) | 14.31 (4.18) | 14.82 (4.02) | 0.231 |
| Employment status, not employed (%) | 28 (58.3) | 23 (54.8) | 51 (56.7) | 0.898 |
| Diagnosis and clinical course | ||||
| Bipolar type I, n (%) | 10 (20.8) | 7 (16.7) | 17 (18.9) | 0.815 |
| Bipolar type II, n (%) | 19 (39.6) | 11 (26.2) | 30 (33.3) | 0.262 |
| Major depressive disorder, n (%) | 19 (39.6) | 24 (57.1) | 43 (47.8) | 0.146 |
| Recurrent depression, n (%) | 42 (87.5) | 36 (85.7) | 78 (86.7) | 0.127 |
| Melancholic depression, n (%) | 44 (91.6) | 42 (100.0) | 86 (95.5) | 0.120 |
| Atypical depression, n (%) | 3 (0.06) | 0 (0.0) | 3 (0.03) | 0.245 |
| Previous psychotic depression, n (%) | 5 (0.1) | 4 (0.09) | 9 (0.1) | 1.0 |
| Previous hospitalization due to depression, n (%) | 9 (18.8) | 10 (23.8) | 19 (21.1) | 0.108 |
| Baseline scales scores | ||||
| MADRS, mean [SD] | 34.54 (5.71) | 35.43 (6.45) | 34.98 (6.08) | 0.491 |
| YMRS, mean [SD] | 9.71 (3.02) | 10.10 (3.27) | 9.90 (3.14) | 0.561 |
| CGI-S, mean [SD] | 4.58 (0.74) | 4.60 (0.73) | 4.59 (0.73) | 0.939 |
| GAF, mean [SD] | 34.62 (10.07) | 34.19 (10.32) | 34.40 (10.19) | 0.84 |
| HAM-A, mean [SD] | 20.67 (7.16) | 21.83 (7.39) | 21.25 (7.27) | 0.449 |
| WHOQol – brief, mean [SD] | 67.88 (10.22) | 65.86 (13.83) | 66.87 (12.02) | 0.43 |
| BIS, mean [SD] | 68.38 (9.33) | 68.26 (9.48) | 68.32 (9.40) | 0.955 |
| Comorbidities | ||||
| Any anxiety disorder, n (%) | 31 (64.5) | 24 (57.1) | 55 (61.1) | 0.613 |
| Any substance use disorder, n (%) | 3 (6.2) | 2 (4.7) | 5 (5.5) | 1 |
| Any personality disorder, n (%) | 2 (4.2) | 5 (12.0) | 7 (7.8) | 0.245 |
| Pharmacological treatments | ||||
| Bipolar type I | ||||
| First line treatmentsa, n (%) | 8 (16.7) | 6 (14.3) | 14 (15.5) | 0.984 |
| Second line treatmentsb, n (%) | 3 (6.2) | 1 (2.4) | 4 (0.04) | 0.62 |
| Bipolar type II | ||||
| First line treatmentsc, n (%) | 0 (0.0) | 1 (2.4) | 1 (0.01) | 0.467 |
| Second line treatmentsd, n (%) | 19 (39.6) | 9 (21.4) | 28 (31.1) | 0.104 |
| Major depressive disorder | ||||
| First line treatmentse, n (%) | 19 (39.6) | 24 (57.1) | 43 (47.7) | 0.146 |
| Second line treatmentsf, n (%) | 0 (0.0) | 4 (9.5) | 4 (0.04) | 0.044 |
| Previous neuromodulation treatment | ||||
| Electroconvulsive therapy, n (%) | 4 (8.3) | 2 (4.8) | 6 (0.06) | 0.681 |
SD Standard deviation, MADRS Montgomery–Åsberg depression rating scale, YMRS Young mania rating scale, CGI-S Clinical global impression-severity of illness, GAF Global assessment of functioning, HAM-A Hamilton anxiety rating scale, WHOQol-brief World Health Organization quality of life questionnaire (brief version), BIS Barratt Impulsivity Scale, SSRI Selective serotonin reuptake inhibitor.
P values represent the significance of Chi-Square or Fisher exact tests for categorical and t-tests for continuous variables. SD standard deviation.
alithium or quetiapine or lamotrigine or lurasidone or lithium/divalproex + lamotrigine or lithium/divalproex + lurasidone.
bolanzapine + fluoxetine or divalproex or lithium/divalproex + SSRI or lithium/divalproex + bupropion.
cquetiapin.
dlithium or lamotrigine or bupropion or sertraline or venlafaxine.
eagomelatine or bupropion or citalopram or desvenlafaxine or duloxetine or escitalopram or fluoxetine or fluvoxamine or mirtazapine or paroxetine or sertraline or venlafaxine or vortioxetine.
ftricyclic antidepressant or trazodone or quetiapine.
Main findings
Primary outcome
Linear mixed-effect models showed no clinical superiority of active stimulation, as no significant differences were observed in MADRS scores between the two groups (Fig. 2). The least squares mean difference in MADRS scores at week 3 was −0.06, 95%CI [−3.39, 3.51], p = 0.97, in favor of sham stimulation (Table 2).
Fig. 2. Change in MADRS score over time.
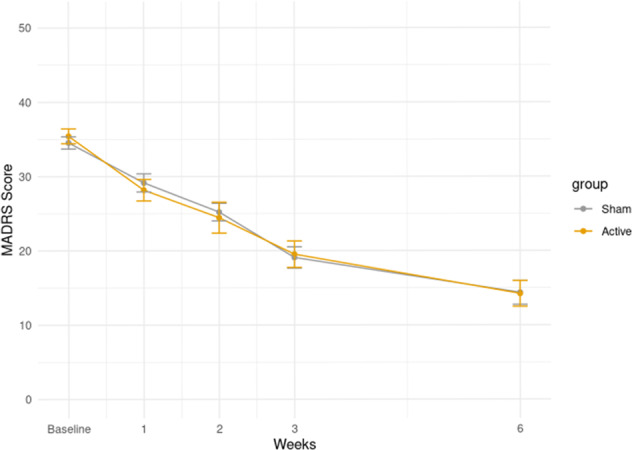
Circles represent means in each group, bars represent ±1 standard deviation from the mean. In orange, participants using active-TBS; in gray participants using sham-TBS. The y-axis indicates the values of the MADRS score in the mITT sample. On the x-axis the values at each time of the assessment are indicated (baseline, first week, second week, third week, and sixth week).
Table 2.
Linear mixed models for imputed mITT sample.
| Sham | Active | Least squares mean difference (95% CI) | Group and time interaction | P value | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean [SD] | N | Mean [SD] | |||||
| Primary outcome | ||||||||
| MADRS, baseline to week 3 | ||||||||
| MADRS, baseline | 48 | 34.54 (5.71) | 42 | 35.43 (6.45) | 0.0629 (−3.3867 to 3.5125) | F1,15.24 = 0.002120649 | 0.9715 | |
| MADRS, week 3 | 41 | 17.98 (10.04) | 28 | 19.36 (11.16) | ||||
| Secondary outcomes | ||||||||
| MADRS, baseline to week 3 (stratified for MDD) | ||||||||
| MADRS, baseline | 19 | 33.95 (6.48) | 24 | 33.88 (7.37) | −0.101 (−4.8834 to 4.6814) | F1,33.24 = 1.146865 | 0.9673 | |
| MADRS, week 3 | 16 | 16.44 (7.94) | 19 | 20.11 (11.15) | ||||
| MADRS, baseline to week 3 (stratified for BD) | ||||||||
| MADRS, baseline | 29 | 34.93 (5.22) | 18 | 37.5 (4.37) | 0.531 (−4.3102 to 5.3722) | F1,17.84 = 0.6010476 | 0.8308 | |
| MADRS, week 3 | 25 | 18.96 (11.22) | 9 | 17.78 (11.68) | ||||
| MADRS, baseline to week 6 | ||||||||
| MADRS, week 6 | 36 | 14.11 (11.83) | 28 | 16.5 (11.98) | 0.332 (−3.1764 to 3.8404) | F1,37.54 = 0.2181534 | 0.8532 | |
| YMRS, baseline to week 3 and 6 | ||||||||
| YMRS, baseline | 48 | 9.71 (3.02) | 42 | 10.1 (3.27) | 0.282 (−0.9038 to 1.4678) | F1,116.59 = 0.2275535 | 0.6426 | |
| YMRS, week 3 | 41 | 4.15 (3.42) | 28 | 4.54 (3.52) | ||||
| YMRS, week 6 | 36 | 3.53 (4.18) | 28 | 3.57 (3.65) | 0.231 (−0.9254 to 1.3874) | F1,111.35 = 0.001526855 | 0.6965 | |
| HAM-A, baseline to week 3 and 6 | ||||||||
| HAM-A, baseline | 48 | 20.67 (7.16) | 42 | 21.83 (7.39) | 1.45 (−0.9804 to 3.8804) | F1,58.06 = 0.0146615 | 0.245 | |
| HAM-A, week 3 | 41 | 9.9 (7.4) | 28 | 12.61 (8.71) | ||||
| HAM-A, week 6 | 36 | 9.06 (8.22) | 28 | 11.43 (7.99) | 1.46 (−1.0292 to 3.9492) | F1,93.45 = 0.09531674 | 0.2548 | |
| CGI-S, baseline to week 3 and 6 | ||||||||
| CGI-S, baseline | 48 | 4.58 (0.74) | 42 | 4.6 (0.73) | −0.0126 (−0.2987 to 0.2735) | F1,121.38 = 0.09647006 | 0.9311 | |
| CGI-S, week 3 | 41 | 3.63 (1.04) | 28 | 3.71 (1.08) | ||||
| CGI-S, week 6 | 36 | 2.78 (1.33) | 28 | 3.25 (1.29) | −1.37 (−5.0548 to 2.3148) | F1,127.03 = 0.08313419 | 0.4702 | |
| GAF, baseline to week 3 and 6 | ||||||||
| GAF, baseline | 48 | 34.63 (10.07) | 42 | 34.19 (10.32) | −1.46 (−5.0076 to 2.0876) | F1,96.52 = 0.9209387 | 0.4222 | |
| GAF, week 3 | 41 | 52.29 (13.14) | 28 | 47.79 (15.41) | ||||
| GAF, week 6 | 36 | 61.47 (17.08) | 28 | 57.36 (18.51) | −1.37 (−5.0548 to 2.3148) | F1,127.03 = 0.08313419 | 0.4702 | |
| WHOQol, baseline to week 6 | ||||||||
| WHOQol, baseline | 48 | 67.88 (10.22) | 42 | 65.86 (13.83) | −1.94 (−7.1732 to 3.2932) | F1,25.95 = 0.000878861 | 0.4688 | |
| WHOQol, week 6 | 36 | 75.56 (14.06) | 28 | 74.93 (19.91) | ||||
| BIS, baseline to week 6 | ||||||||
| BIS, baseline | 48 | 68.38 (9.33) | 42 | 68.26 (9.48) | −1.94 (−7.1732 to 3.2932) | F1,169.09 = 0.13873 | 0.6528 | |
| BIS, week 6 | 36 | 64.14 (10.92) | 28 | 63 (8.69) | ||||
SD Standard Deviation, MADRS Montgomery–Åsberg depression rating scale, YMRS Young mania rating scale, CGI-S Clinical global impression-severity of illness, GAF Global assessment of functioning, HAM-A Hamilton anxiety rating scale, WHOQol-brief World Health Organization quality of life questionnaire (brief version), BIS Barratt Impulsivity Scale. P values represent the significance of Chi-Square or Fisher exact tests for categorical and t-tests for continuous variables. SD standard deviation.
Secondary outcomes
Response and remission rates per MADRS were also not statistically different among active and sham groups (35.7% vs. 43.7%, and 28.5% vs. 37.5% respectively at week 6, ps > 0.51). No other analyses from baseline to weeks 3 or 6 revealed significant time x group interaction or mean differences among groups in the mITT sample, including changes in YMRS score, CGI-S, GAF score, HAM-A score, WHOQol score, BIS score, and response and remission rates at week 3 (Tables 2 and 3). Results in ITT and PP samples are provided in Supplementary material.
Table 3.
MADRS response and remission in mITT sample.
| Sham | Active | χ2 | P value | |
|---|---|---|---|---|
| MADRS, baseline to week 3 | ||||
| Response | 21/48 | 10/42 | 3.1108 | 0.0777 |
| Remission | 14/48 | 7/42 | 1.3202 | 0.2506 |
| MADRS, baseline to week 6 | ||||
| Response | 21/48 | 15/42 | 0.31436 | 0.575 |
| Remission | 18/48 | 12/42 | 0.452 | 0.5014 |
MADRS Montgomery–Åsberg depression rating scale, mITT modified intention-to-treat sample, ITT Intention-to-treat sample, PP per protocol sample.
P values represent the significance of Chi-Square test. χ2, Chi-Square.
The blinding integrity assessed through Pearson’s Chi-squared test with Yates’ continuity correction was guaranteed in relation to the participants (X-squared = 4.7661e-31, df = 1, p value = 1), but it was not preserved in relation to the staff (X-squared = 5.5815, df = 1, p value = 0.01815). TEMS was not statistically different among active and sham groups (p = 0.5). TMS side effects were not different significant among active and sham groups.
Discussion
To our knowledge, this is the first randomized, double-blind, sham-controlled clinical trial assessing the efficacy, safety, and tolerability of bilateral TBS for the treatment of major depressive episodes with mixed features of BD and MDD. Our primary hypothesis that active TBS would be superior to sham TBS was not demonstrated. Moreover, secondary outcomes as response and remission rates and changes in other scales were not different among active and sham groups from baseline to weeks 3 or 6. Active and sham TBS were similar in the rate of adverse events. Our results were similar for both BD type I, BD type II, and MDD patients.
We enrolled a real-world sample without excluding other comorbidities and using at least one first or second-line treatment according to CANMAT guidelines for BD or MDD. We included patients who had failed at least one treatment trial, although most had >3 depressive episodes throughout their lives. Although it is not recommended the use of antidepressants for bipolar mixed depression, they are widely used for treatment-resistant MDD, many of them are mixed states, so that we allowed MDD patients to receive conventional antidepressants, as there is only one trial [63] assessing the treatment of MDD with mixed features. Blinding integrity was guaranteed in relation to the study participants, but it was not preserved in relation to the personnel. The personnel guessed the group in which the patients were due to physical characteristics observed during stimulation, such as the noise of the active and sham coils. The personnel did not have access to the clinical evaluations performed and, therefore, were not aware of whether the patients were improving or worsening with the treatment, they only applied the stimulation sessions. Therefore, this finding does not affect the internal validity of the study since the clinical evaluations of the primary and secondary outcomes were carried out by an independent team.
According to the interhemispheric asymmetry theory, right and left hemispheres have opposite effects on mood control and therapeutic applications of TMS should follow this paradigm [64]. The few neuroimaging studies in mixed states to date support the hypothesis of lateralization of brain abnormalities in relation to depressive and manic symptoms, suggesting that neurofunctional abnormalities, preferentially located in the frontal and limbic areas of the right hemisphere, may be associated with the depressive component whereas abnormalities in similar regions on the left would be associated with the manic component [65]. An open and uncontrolled study demonstrated that rTMS over the right DLPFC over three weeks led to a response rate of 46% and 15% in depressive and manic scales, respectively, in BD type I mixed state [27]. On the other hand, a double-blind and sham-controlled clinical trial demonstrated that bilateral TBS (right cTBS and left iTBS) produced greater results in treatment-resistant major depressive episodes than unilateral stimulation [49]. As a result, we opted to use bilateral TBS (right cTBS and left iTBS) protocol in mixed depression treatment.
Mixed states are difficult to treat and are often hidden in treatment-resistant samples [66–71]. In particular, the treatment of depressive symptoms in mixed depression represents a clinical dilemma, mainly because conventional antidepressant medications commonly worsen instability and intra-episodic mood changes [72–74]. Most rTMS clinical trials were carried on in treatment-resistant depression samples and different protocols have been reported as effective and safe, with a low risk of (hypo)manic switches, suggesting a likely mood-stabilizing effect [75]. Thus, we hypothesized that mixed depressions would be adequately treated with rTMS. However, our results did not corroborate this hypothesis. Furthermore, the findings in the complete ITT analysis (see Supplementary material) suggested that active TBS could be inferior compared to sham TBS in treating mixed depression, i.e., active TBS could worsen mixed depression as conventional antidepressants do. Moreover, although TEMS did not differ among groups, the two manic switches during the trial occurred in the active group, suggesting that TBS could actually worsen manic symptoms.
Earlier studies had already indicated that patients with mixed depression presentation were similar to BD patients in terms of early-onset, recurrence, positive family history of bipolar disorder and refractoriness [76, 77]. Although mixed states were commonly described in BD, recent research proved that mixed depression could also occur in MDD [78–80]. Several systematic reviews [20, 81–84] evaluated the efficacy of TMS in the treatment of major depressive episodes of mixed samples of BD and MDD patients and TMS is approved by the FDA for the treatment of major depressive episodes regardless of primary diagnosis. Nevertheless, rTMS randomized and sham-controlled clinical trials exclusively in bipolar samples are scarce. Although there is preliminary evidence of the efficacy of high-frequency rTMS [85, 86] and high-frequency deep TMS [87] in BD, the data in regard to novel protocols such as TBS have been inconclusive [88]. Indeed, there is a risk of extending data from MDD dominant samples to BD when novel protocols such as TBS have evidence of efficacy only in MDD. In consonance of growing evidence that TBS is less effective in bipolar depression compared to MDD [89–91], our data indicate that TBS is also less effective in mixed depression compared to no-mixed depressive episodes treatments.
TMS trials in manic episodes have been done with high-frequency rTMS in the right prefrontal cortex; however, the definitive evidence of the effectiveness of TMS in the treatment of mania is not yet available [92–97]. Another feasible way to treat manic symptoms could be iTBS delivery in the right DLPFC. Nevertheless, it is unknown whether stimulating the right DLPFC in a mixed depression could improve manic symptoms but worsen depression. Further studies are needed to elucidate these issues.
One first potential limitation of this trial is the absence of use of MRI-guided neuronavigation in every session - an approach that is not feasible or cost-efficient for most rTMS clinics. However, it has been previously demonstrated that the DLPFC target used in this trial can be accurately localized without MRI via a scalp-based measurement known as BeamF3 [98]. Thus, the present findings can be generalized more broadly to rTMS clinics where MRIguidance is unavailable. A second limitation is the short duration of this clinical trial that comprised a six-week intervention. Despite this, the most recent researches with TBS had two-week follow-up [47–50, 99, 100]. Further studies can confirm whether our findings can be generalized for longer follow-up clinical trials. Here, we opted to describe the main results of our study according to pre-established outcomes reported previously (e.g., clinicaltrials.gov). Notwithstanding, it is indeed important to further explore, in post-hoc approaches, possible predictors associated with our findings. This will be examined in future studies.
In conclusion, we have found that active bilateral TBS was not superior compared to sham as an effective add-on treatment in moderate to severe mixed depression of BD I, BD II or MDD. Our findings show that TBS does not have a mood-stabilizing effect and may even worsen mood stability in mixed depression which can guide clinicians’ decision with regard to the best TMS protocols in mixed depressed samples.
Funding
This study received funding from the Research foundation support agency of the state of Sao Paulo (FAPESP): 2017/19237-1. Period: July, 2018 to June, 2020.
Supplementary information
Author contributions
Conceived and designed the clinical trial: DFT, ARB, RAM. Contributed to the acquisition, analysis, or interpretation of data for the work: DFT, PS, CGRS, DHM, LCLV, IK, LB, PMF, ARB, RAM. Wrote the first draft of the manuscript: DFT. Contributed to the writing of the manuscript: ARB, RAM.
Competing interests
DFT worked as a speaker and produced scientific source during the last two years for the following pharmaceutical companies: Cristália, Aché, Torrent, Abbott, Lundbeck. ARB reports grants from SaoPaulo Research State Foundation (FAPESP, 2017/50223-6, 2018/10861-7, 2019/06009-6), Newton Advanced Fellowship (12/1010), Brazilian National Council of Scientific Development Productivity Support (PQ-1B) and University of Sao Paulo Medical School Productivity Support (PIPA-A). . RAM reports grants from Sao Paulo Research State Foundation (FAPESP) and from the National Research Council (CNPq). Other authors declare no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01080-9.
References
- 1.Ferrari AJ, Stockings E, Khoo JP, Erskine HE, Degenhardt L, Vos T, et al. The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18:440–50. doi: 10.1111/bdi.12423. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdolini N, Agius M, Ferranti L, Moretti P, Piselli M, Quartesan R. The state of the art of the DSM-5 “with mixed features” specifier. The Scientific World Journal. 2015;2015::757258. [DOI] [PMC free article] [PubMed]
- 4.McIntyre R, Tohen M, Berk M, Zhao J, Weiller E. DSM-5 mixed specifier for manic episodes: evaluating the effect of depressive features on severity and treatment outcome using asenapine clinical trial data. J Affect Disord. 2013;150:378–83. doi: 10.1016/j.jad.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Hergueta T, Weiller E. Evaluating depressive symptoms in hypomanic and manic episodes using a structured diagnostic tool: validation of a new Mini International Neuropsychiatric Interview (MINI) module for the DSM-5’With Mixed Features’ specifier. Int J bipolar Disord. 2013;1:1–10. doi: 10.1186/2194-7511-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeshima M, Oka T. DSM‐5‐defined ‘mixed features’ and B enazzi’s mixed depression: which is practically useful to discriminate bipolar disorder from unipolar depression in patients with depression? Psychiatry Clin Neurosci. 2015;69:109–16. doi: 10.1111/pcn.12213. [DOI] [PubMed] [Google Scholar]
- 7.Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntyre RS, Soczynska JK, Cha DS, Woldeyohannes HO, Dale RS, Alsuwaidan MT, et al. The prevalence and illness characteristics of DSM-5-defined “mixed feature specifier” in adults with major depressive disorder and bipolar disorder: results from the International Mood Disorders Collaborative Project. J Affect Disord. 2015;172:259–64. doi: 10.1016/j.jad.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann P, Brückl T, Nocon A, Pfister H, Lieb R, Wittchen H-U, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subthreshold bipolarity. Arch Gen psychiatry. 2009;66:1341–52. doi: 10.1001/archgenpsychiatry.2009.158. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg JF, Perlis RH, Bowden CL, Thase ME, Miklowitz DJ, Marangell LB, et al. Manic symptoms during depressive episodes in 1380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166:173–81. doi: 10.1176/appi.ajp.2008.08050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perugi G, Angst J, Azorin J-M, Bowden CL, Mosolov S, Reis J, et al. Mixed features in patients with a major depressive episode: the BRIDGE-II-MIX study. The. J Clin psychiatry. 2015;76:351–58. doi: 10.4088/JCP.14m09092. [DOI] [PubMed] [Google Scholar]
- 12.Popovic D, Vieta E, Azorin JM, Angst J, Bowden CL, Mosolov S, et al. Suicide attempts in major depressive episode: evidence from the BRIDGE‐II‐Mix study. Bipolar Disord. 2015;17:795–803. doi: 10.1111/bdi.12338. [DOI] [PubMed] [Google Scholar]
- 13.Tavormina G. Bipolar disorders and bipolarity: the notion of the “mixity”. Psychiatr Danubina. 2019;31:434–7. [PubMed] [Google Scholar]
- 14.Talih F, Gebara NY, Andary FS, Mondello S, Kobeissy F, Ferri R. Delayed sleep phase syndrome and bipolar disorder: pathogenesis and available common biomarkers. Sleep Med Rev. 2018;41:133–40. doi: 10.1016/j.smrv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Shim IH, Woo YS, Bahk W-M. Prevalence rates and clinical implications of bipolar disorder “with mixed features” as defined by DSM-5. J Affect Disord. 2015;173:120–25. doi: 10.1016/j.jad.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Verdolini N, Hidalgo‐Mazzei D, Murru A, Pacchiarotti I, Samalin L, Young A, et al. Mixed states in bipolar and major depressive disorders: systematic review and quality appraisal of guidelines. Acta Psychiatr Scandinavica. 2018;138:196–222. doi: 10.1111/acps.12896. [DOI] [PubMed] [Google Scholar]
- 17.Seo H-J, Wang H-R, Jun T-Y, Woo YS, Bahk W-M. Factors related to suicidal behavior in patients with bipolar disorder: the effect of mixed features on suicidality. Gen hospital psychiatry. 2016;39:91–96. doi: 10.1016/j.genhosppsych.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Berlim M, Van den Eynde F, Tovar-Perdomo S, Daskalakis Z. Response, remission and dropout rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychological Med. 2014;44:225. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 19.Teng S, Guo Z, Peng H, Xing G, Chen H, He B, et al. High-frequency repetitive transcranial magnetic stimulation over the left DLPFC for major depression: session-dependent efficacy: a meta-analysis. Eur Psychiatry. 2017;41:75–84. doi: 10.1016/j.eurpsy.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Brunoni AR, Chaimani A, Moffa AH, Razza LB, Gattaz WF, Daskalakis ZJ, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA psychiatry. 2017;74:143–52. doi: 10.1001/jamapsychiatry.2016.3644. [DOI] [PubMed] [Google Scholar]
- 21.Schwippel T, Schroeder PA, Fallgatter AJ, Plewnia C. Clinical review: The therapeutic use of theta-burst stimulation in mental disorders and tinnitus. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;92:285–300. doi: 10.1016/j.pnpbp.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 23.Cao X, Deng C, Su X, Guo Y. Response and remission rates following high-frequency vs. low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): a meta-analysis of randomized, double-blind trials. Front psychiatry. 2018;9:413. doi: 10.3389/fpsyt.2018.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C-T, Chen M-H, Juan C-H, Liu R-S, Lin W-C, Bai Y-M, et al. Effects of prefrontal theta-burst stimulation on brain function in treatment-resistant depression: a randomized sham-controlled neuroimaging study. Brain stimulation. 2018;11:1054–62. doi: 10.1016/j.brs.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald PB, Hoy KE, Elliot D, McQueen RS, Wambeek LE, Daskalakis ZJ. Accelerated repetitive transcranial magnetic stimulation in the treatment of depression. Neuropsychopharmacology. 2018;43:1565–72. doi: 10.1038/s41386-018-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefaucheur J-P, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clinical neurophysiology. 2020;131:474-528. [DOI] [PubMed]
- 27.Pallanti S, Grassi G, Antonini S, Quercioli L, Salvadori E, Hollander E. rTMS in resistant mixed states: an exploratory study. J Affect Disord. 2014;157:66–71. doi: 10.1016/j.jad.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Zeeuws D, De Rycker K, De Raedt R, De Beyne M, Baeken C, Vanderbruggen N. Intensive high-frequency repetitive transcranial magnetic stimulation treatment in an electroconvulsive shock therapy-resistant bipolar I patient with mixed episode. Brain stimulation. 2011;4:46–49. doi: 10.1016/j.brs.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Praharaj SK, Ram D, Arora M. Efficacy of high frequency (rapid) suprathreshold repetitive transcranial magnetic stimulation of right prefrontal cortex in bipolar mania: a randomized sham controlled study. J Affect Disord. 2009;117:146–50. doi: 10.1016/j.jad.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Saba G, Rocamora JF, Kalalou K, Benadhira R, Plaze M, Lipski H, et al. Repetitive transcranial magnetic stimulation as an add-on therapy in the treatment of mania: a case series of eight patients. Psychiatry Res. 2004;128:199–202. doi: 10.1016/j.psychres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord. 2004;78:253–57. doi: 10.1016/S0165-0327(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 32.Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ. 2008;86:650–52. doi: 10.2471/BLT.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del-Ben C, Vilela J, de S, Crippa JA, Hallak JEC, Labate CM, et al. Confiabilidade de” Entrevista Clınica Estruturada para o DSM-IV–Versao Clınica” traduzida para o português./Reliability of the Structured Clinical Interview for DSM-IV—Clinical Version translated into Portuguese. Rev Bras Psiquiatr. 2010;23:156–59. [Google Scholar]
- 34.Hawley C, Gale T, Sivakumaran T, group HNR. Defining remission by cut off score on the MADRS: selecting the optimal value. J Affect Disord. 2002;72:177–84. doi: 10.1016/s0165-0327(01)00451-7. [DOI] [PubMed] [Google Scholar]
- 35.Duarte-Guerra LS, Gorenstein C, Paiva-Medeiros PF, Santo MA, Neto FL, Wang Y-P. Clinical utility of the Montgomery-Åsberg Depression Rating Scale for the detection of depression among bariatric surgery candidates. BMC psychiatry. 2016;16:1–8. doi: 10.1186/s12888-016-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilela J, Crippa J, Del-Ben C, Loureiro S. Reliability and validity of a Portuguese version of the Young Mania Rating Scale. Braz J Med Biol Res. 2005;38:1429–39. doi: 10.1590/s0100-879x2005000900019. [DOI] [PubMed] [Google Scholar]
- 37.Swann AC, Lafer B, Perugi G, Frye MA, Bauer M, Bahk W-M, et al. Bipolar mixed states: an international society for bipolar disorders task force report of symptom structure, course of illness, and diagnosis. Am J Psychiatry. 2013;170:31–42. doi: 10.1176/appi.ajp.2012.12030301. [DOI] [PubMed] [Google Scholar]
- 38.Tohen M, Kanba S, McIntyre RS, Fujikoshi S, Katagiri H. Efficacy of olanzapine monotherapy in the treatment of bipolar depression with mixed features. J Affect Disord. 2014;164:57–62. doi: 10.1016/j.jad.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Azorin J-M, Kaladjian A, Adida M, Fakra E, Belzeaux R, Hantouche E, et al. Self-assessment and characteristics of mixed depression in the French national EPIDEP study. J Affect Disord. 2012;143:109–17. doi: 10.1016/j.jad.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540–60. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavares DF, Dos Santos CGR, Valiengo L, Klein I, Borrione L, Forte PM, et al. Efficacy, safety, and tolerability of theta-burst stimulation in mixed depression: design, rationale, and objectives of a randomized, double-blinded, sham-controlled trial. Front psychiatry. 2020;11:435. doi: 10.3389/fpsyt.2020.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunoni AR, Sampaio-Junior B, Moffa AH, Aparício LV, Gordon P, Klein I, et al. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry. 2019;41:70–81. doi: 10.1590/1516-4446-2017-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li C-T, Chen M-H, Juan C-H, Huang H-H, Chen L-F, Hsieh J-C, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137:2088–98. doi: 10.1093/brain/awu109. [DOI] [PubMed] [Google Scholar]
- 44.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92. doi: 10.1016/S0140-6736(18)30295-2. [DOI] [PubMed] [Google Scholar]
- 45.Chistyakov AV, Rubicsek O, Kaplan B, Zaaroor M, Klein E. Safety, tolerability and preliminary evidence for antidepressant efficacy of theta-burst transcranial magnetic stimulation in patients with major depression. Int J Neuropsychopharmacol. 2010;13:387–93. doi: 10.1017/S1461145710000027. [DOI] [PubMed] [Google Scholar]
- 46.Nettekoven C, Volz LJ, Kutscha M, Pool EM, Rehme AK, Eickhoff SB, et al. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–59. doi: 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plewnia C, Pasqualetti P, Große S, Schlipf S, Wasserka B, Zwissler B, et al. Treatment of major depression with bilateral theta burst stimulation: a randomized controlled pilot trial. J Affect Disord. 2014;156:219–23. doi: 10.1016/j.jad.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Li CT, Cheng CM, Chen MH, Juan CH, Tu PC, Bai YM, et al. Antidepressant efficacy of prolonged intermittent theta burst stimulation monotherapy for recurrent depression and comparison of methods for coil positioning: a randomized, double-blind, sham-controlled study. Biol Psychiatry. 2020;87:443–50. doi: 10.1016/j.biopsych.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Li CT, Chen MH, Juan CH, Huang HH, Chen LF, Hsieh JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137:2088–98. doi: 10.1093/brain/awu109. [DOI] [PubMed] [Google Scholar]
- 50.Chistyakov AV, Kreinin B, Marmor S, Kaplan B, Khatib A, Darawsheh N, et al. Preliminary assessment of the therapeutic efficacy of continuous theta-burst magnetic stimulation (cTBS) in major depression: a double-blind sham-controlled study. J Affect Disord. 2015;170:225–9. doi: 10.1016/j.jad.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 51.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 52.Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. 2015;14:64–73. doi: 10.1002/wps.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oberman L, Edwards D, Eldaief M, Pascual-Leone A. Safety of theta burst transcranial magnetic stimulation: a systematic review of the literature. J Clin Neurophysiol. 2011;28:67. doi: 10.1097/WNP.0b013e318205135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freire MÁ, Figueiredo VLMD, Gomide A, Jansen K, Silva RAD, Magalhães PVDS, et al. Escala Hamilton: estudo das características psicométricas em uma amostra do sul do Brasil. J Brasileiro de Psiquiatria. 2014;63:281–89. [Google Scholar]
- 55.Guy W. ECDEU Assessment Manual for Psychopharmacology-Revised. Rockville, MD: National Institute of Mental Health. Psychopharmacol Res Branch. 1976:217–22.
- 56.Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale. Br J Psychiatry. 1995;166:654–59. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- 57.Fleck M, Louzada S, Xavier M, Chachamovich E, Vieira G, Santos L, et al. Aplicação da versão em português do instrumento abreviado de avaliação da qualidade de vida” WHOQOL-bref”. Rev de saúde pública. 2000;34:178–83. doi: 10.1590/s0034-89102000000200012. [DOI] [PubMed] [Google Scholar]
- 58.Malloy-Diniz LF, Mattos P, Leite WB, Abreu N, Coutinho G, Paula JJD, et al. Tradução e adaptação cultural da Barratt Impulsiveness Scale (BIS-11) para aplicação em adultos brasileiros. J Brasileiro de Psiquiatria. 2010;59:99–105. [Google Scholar]
- 59.Bates D, Mächler M, Bolker B, Walker S Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823. 2014.
- 60.Buuren SV, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J of statistical software. 2010;45:1–68.
- 61.Lenth R, Singmann H, Love J, Buerkner P, Herve M. Emmeans: Estimated marginal means, aka least-squares means. R package version. 2018;1:3. [Google Scholar]
- 62.Team RC (Vienna, Austria: R Foundation for Statistical Computing. Retrieved from, 2017).
- 63.Suppes T, Silva R, Cucchiaro J, Mao Y, Targum S, Streicher C, et al. Lurasidone for the Treatment of Major Depressive Disorder With Mixed Features: A Randomized, Double-Blind, Placebo-Controlled Study. Am J Psychiatry. 2016;173:400–7. doi: 10.1176/appi.ajp.2015.15060770. [DOI] [PubMed] [Google Scholar]
- 64.Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic Evid Arch Neurol. 1982;39:210–8. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- 65.Kaladjian A, Belzeaux R, Micoulaud-Franchi JA, Cermolacce M, Fakra E, Azorin JM. [Mixed states and neuroimaging] Encephale. 2013;39:S162–6. doi: 10.1016/S0013-7006(13)70116-7. [DOI] [PubMed] [Google Scholar]
- 66.Sani G, Napoletano F, Vöhringer PA, Sullivan M, Simonetti A, Koukopoulos A, et al. Mixed depression: clinical features and predictors of its onset associated with antidepressant use. Psychother Psychosom. 2014;83:213–21. doi: 10.1159/000358808. [DOI] [PubMed] [Google Scholar]
- 67.Fornaro M, Stubbs B, De Berardis D, Perna G, Valchera A, Veronese N, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17:241. doi: 10.3390/ijms17020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pacchiarotti I, Kotzalidis GD, Murru A, Mazzarini L, Rapinesi C, Valentí M, et al. Mixed features in depression: the unmet needs of diagnostic and statistical manual of mental disorders fifth edition. Psychiatr Clin North Am. 2020;43:59–68. doi: 10.1016/j.psc.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Cuomo A, Nikolova VL, Yalin N, Arnone D, Fagiolini A, Young AH. Pharmacological treatment of mixed states. CNS Spectr. 2017;22:186–95. doi: 10.1017/S1092852917000013. [DOI] [PubMed] [Google Scholar]
- 70.Suppes T, Ostacher M. Mixed features in major depressive disorder: diagnoses and treatments. CNS Spectr. 2017;22:155–60. doi: 10.1017/S1092852917000256. [DOI] [PubMed] [Google Scholar]
- 71.Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Azorin JM, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: acute and long-term treatment of mixed states in bipolar disorder. World J Biol Psychiatry. 2018;19:2–58. doi: 10.1080/15622975.2017.1384850. [DOI] [PubMed] [Google Scholar]
- 72.Patel R, Reiss P, Shetty H, Broadbent M, Stewart R, McGuire P, et al. Do antidepressants increase the risk of mania and bipolar disorder in people with depression? A retrospective electronic case register cohort study. BMJ Open. 2015;5:e008341. doi: 10.1136/bmjopen-2015-008341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjørklund L, Horsdal HT, Mors O, Østergaard SD, Gasse C. Trends in the psychopharmacological treatment of bipolar disorder: a nationwide register-based study. Acta Neuropsychiatr. 2016;28:75–84. doi: 10.1017/neu.2015.52. [DOI] [PubMed] [Google Scholar]
- 74.Stahl SM, Morrissette DA, Faedda G, Fava M, Goldberg JF, Keck PE, et al. Guidelines for the recognition and management of mixed depression. CNS Spectr. 2017;22:203–19. doi: 10.1017/S1092852917000165. [DOI] [PubMed] [Google Scholar]
- 75.Tee MMK, Au CH. A systematic review and meta-analysis of randomized sham-controlled trials of repetitive transcranial magnetic stimulation for bipolar disorder. Psychiatr Q. 2020;91:1225–47. doi: 10.1007/s11126-020-09822-6. [DOI] [PubMed] [Google Scholar]
- 76.Benazzi F, Akiskal HS. Delineating bipolar II mixed states in the Ravenna-San Diego collaborative study: the relative prevalence and diagnostic significance of hypomanic features during major depressive episodes. J Affect Disord. 2001;67:115–22. doi: 10.1016/s0165-0327(01)00444-x. [DOI] [PubMed] [Google Scholar]
- 77.Akiskal HS, Benazzi F, Perugi G, Rihmer Z. Agitated “unipolar” depression re-conceptualized as a depressive mixed state: implications for the antidepressant-suicide controversy. J Affect Disord. 2005;85:245–58. doi: 10.1016/j.jad.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, Gamma A, et al. Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode: the BRIDGE study. Arch Gen Psychiatry. 2011;68:791–8. doi: 10.1001/archgenpsychiatry.2011.87. [DOI] [PubMed] [Google Scholar]
- 79.Judd LL, Schettler PJ, Akiskal H, Coryell W, Fawcett J, Fiedorowicz JG, et al. Prevalence and clinical significance of subsyndromal manic symptoms, including irritability and psychomotor agitation, during bipolar major depressive episodes. J Affect Disord. 2012;138:440–8. doi: 10.1016/j.jad.2011.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pae CU, Vöhringer PA, Holtzman NS, Thommi SB, Patkar A, Gilmer W, et al. Mixed depression: a study of its phenomenology and relation to treatment response. J Affect Disord. 2012;136:1059–61. doi: 10.1016/j.jad.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 81.Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response remission and dropout rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–39. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 82.Zhang YQ, Zhu D, Zhou XY, Liu YY, Qin B, Ren GP, et al. Bilateral repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Braz J Med Biol Res. 2015;48:198–206. doi: 10.1590/1414-431X20144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CHY, Young AH. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. Bmj. 2019;364:l1079. doi: 10.1136/bmj.l1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mutz J, Edgcumbe DR, Brunoni AR, Fu CHY. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: A systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev. 2018;92:291–303. doi: 10.1016/j.neubiorev.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 85.McGirr A, Karmani S, Arsappa R, Berlim MT, Thirthalli J, Muralidharan K, et al. Clinical efficacy and safety of repetitive transcranial magnetic stimulation in acute bipolar depression. World Psychiatry. 2016;15:85–6. doi: 10.1002/wps.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nguyen TD, Hieronymus F, Lorentzen R, McGirr A, Østergaard SD. The efficacy of repetitive transcranial magnetic stimulation (rTMS) for bipolar depression: A systematic review and meta-analysis. J Affect Disord. 2021;279:250–55. doi: 10.1016/j.jad.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, et al. Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42:2593–601. doi: 10.1038/npp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bulteau S, Beynel L, Marendaz C, Dall’Igna G, Peré M, Harquel S, et al. Twice-daily neuronavigated intermittent theta burst stimulation for bipolar depression: A Randomized Sham-Controlled Pilot Study. Neurophysiol Clin. 2019;49:371–75. doi: 10.1016/j.neucli.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Rostami R, Kazemi R, Nitsche MA, Gholipour F, Salehinejad MA. Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin Neurophysiol. 2017;128:1961–70. doi: 10.1016/j.clinph.2017.07.395. [DOI] [PubMed] [Google Scholar]
- 90.Yang YB, Chan P, Rayani K, McGirr A. Comparative effectiveness of repetitive transcranial magnetic stimulation in unipolar and bipolar depression. Can J Psychiatry. 2021;66:313–15. doi: 10.1177/0706743720950938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGirr A, Vila-Rodriguez F, Cole J, Torres IJ, Arumugham SS, Keramatian K, et al. Efficacy of active vs sham intermittent theta burst transcranial magnetic stimulation for patients with bipolar depression: a randomized clinical trial. JAMA Netw Open. 2021;4:e210963. doi: 10.1001/jamanetworkopen.2021.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grisaru N, Chudakov B, Yaroslavsky Y, Belmaker RH. Transcranial magnetic stimulation in mania: a controlled study. Am J Psychiatry. 1998;155:1608–10. doi: 10.1176/ajp.155.11.1608. [DOI] [PubMed] [Google Scholar]
- 93.Kaptsan A, Yaroslavsky Y, Applebaum J, Belmaker RH, Grisaru N. Right prefrontal TMS versus sham treatment of mania: a controlled study. Bipolar Disord. 2003;5:36–9. doi: 10.1034/j.1399-5618.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 94.Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Disord. 2004;78:253–7. doi: 10.1016/S0165-0327(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 95.Saba G, Rocamora JF, Kalalou K, Benadhira R, Plaze M, Lipski H, et al. Repetitive transcranial magnetic stimulation as an add-on therapy in the treatment of mania: a case series of eight patients. Psychiatry Res. 2004;128:199–202. doi: 10.1016/j.psychres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 96.Praharaj SK, Ram D, Arora M. Efficacy of high frequency (rapid) suprathreshold repetitive transcranial magnetic stimulation of right prefrontal cortex in bipolar mania: a randomized sham controlled study. J Affect Disord. 2009;117:146–50. doi: 10.1016/j.jad.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 97.Pathak V, Sinha VK, Praharaj SK. Efficacy of adjunctive high frequency repetitive transcranial magnetic stimulation of right prefrontal cortex in adolescent mania: a randomized sham-controlled study. Clin Psychopharmacol Neurosci. 2015;13:245–9. doi: 10.9758/cpn.2015.13.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, et al. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul. 2015;8:965–73. doi: 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prasser J, Schecklmann M, Poeppl TB, Frank E, Kreuzer PM, Hajak G, et al. Bilateral prefrontal rTMS and theta burst TMS as an add-on treatment for depression: a randomized placebo controlled trial. World J Biol Psychiatry. 2015;16:57–65. doi: 10.3109/15622975.2014.964768. [DOI] [PubMed] [Google Scholar]
- 100.Duprat R, Desmyter S, Rudi de R, van Heeringen K, Van den Abbeele D, Tandt H, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission? J Affect Disord. 2016;200:6–14. doi: 10.1016/j.jad.2016.04.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.