Abstract
Objectives:
Most transitional care initiatives to reduce rehospitalization have focused on the transition that occurs between a patient’s hospital discharge and return home. However, many patients are discharged from a skilled nursing facility (SNF) to their homes. The goal was to evaluate the effectiveness of the Mayo Clinic Care Transitions (MCCT) program (hereafter called program) among patients discharged from SNFs to their homes.
Design:
Propensity-matched control-intervention trial.
Intervention:
Patients in the intervention group received care management following nursing stay (a home visit and nursing phone calls).
Setting and Participants:
Patients enrolled after discharge from a SNF to home were matched to patients who did not receive intervention because of refusal, program capacity, or distance. Patients were age ≥60 years, at high risk for hospitalization, and discharged from a SNF.
Methods:
Program enrollees were matched through propensity score to nonenrollees on the basis of age, sex, comorbid health burden, and mortality risk score. Conditional logistic regression analysis examined 30-day hospitalization and emergency department (ED) use; Cox proportional hazards analyses examined 180-day hospital stay and ED use.
Results:
Each group comprised 160 patients (mean [SD] age, 85.4 [7.4] years). Thirty-day hospitalization and ED rates were 4.4% and 10.0% in the program group and 3.8% and 10.0% in the group with usual care (P=.76 for hospitalization; P>.99 for ED). At 180 days, hospitalization and ED rates were 30.6% and 46.3% for program patients compared with 11.3% and 25.0% in the comparison group (P<.001).
Conclusions and Implications:
We found no evidence of reduced hospitalization or ED visits by program patients vs the comparison group. Such findings are crucial because they illustrate how aggressive stabilization care within the SNF may mitigate the program role. Furthermore, we found higher ED and hospitalization rates at 180 days in program patients than the comparison group.
Keywords: care transition, emergency department, hospitalization, nursing home
Brief Summary:
Care transition after nursing home discharge is not well studied. Of propensity-matched residents discharged to home, we saw no difference in 30-day hospitalization for intervention vs usual care.
Introduction
Patients discharged from the hospital to either the community or a skilled nursing facility (SNF) are at high risk for adverse events such as rehospitalization or emergency department (ED) visits due in part to the care transition process. As patients leave the care of the hospital team, they resume treatment from their primary provider or the medical providers in a SNF. Patients discharged to a SNF have another transition in care and the potential for another high-risk adverse outcome, which highlight the strong need for direct collaborative communication.1 Those admitted to a SNF after hospitalization often have higher degrees of comorbid health conditions, functional impairment, and dementia than patients discharged directly into the community.2
Discharge from the SNF to the community continues to be an important care transition point. Among US nursing home residents, about 69% are long-stay residents, residing beyond 100 days, whereas 31% are discharged to their home and considered to be short-stay residents.3 In addition, the average nursing home resident’s age is 80 years, and women represent 67%3 within the fee-for-service Medicare beneficiary population.4
Various transitional care programs have been established to not only improve quality but also reduce care costs for patients discharged from the hospital to the community.5–7 Health care professionals often perform a face-to-face home visit, a telehealth home visit,8 or direct care through specified units.9 Patients often receive care following SNF discharge through home health care in which a registered nurse visits the home. However, the efficacy of this model is somewhat controversial. In a cohort study of 1,169 matched residents in long-term care facilities, the authors observed a 40% higher risk of hospitalization for patients who transitioned to home with coordinated home health services.10 In a study of 68,000 SNF patients with heart failure, those discharged to their respective homes with coordinated home health care had lower 30-day hospital readmission rates than patients who did not receive home health care (22.8% vs 24.5%; P<.001).11
To more fully address ongoing patient needs following hospitalization or a SNF stay, Mayo Clinic developed a face-to-face home visit care transitions program (hereafter called program). The Mayo Clinic Care Transitions (MCCT) program has successfully reduced readmissions, ED visits, and acute health care use among enrolled patients who leave the hospital to return to their communities.12 Furthermore, the program has lowered the total care costs for patients in the highest cost decile.13 The use of a dedicated care transition team to monitor and care for the patient after a SNF stay has not been evaluated systematically. We sought as our primary aim to determine the association of 30-day rehospitalization, death, and ED visits for patients discharged from a SNF who elected to enroll in the program compared with patients with SNF discharge who chose not to enroll.
Methods
Design and Setting
The study was approved by the Mayo Clinic Institutional Review Board. The present study is reported in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.14 The team conducted the study between March 1, 2016, and July 1, 2018, in the institution’s SNF practice at Mayo Clinic in Rochester, Minnesota. The Senior Services group cares for older adults across various settings, including transitions of care,12 MCCT homebound patients (Palliative Care Homebound Program),15 and residents in short- and long-term stays at 12 SNFs located within Olmsted County, Minnesota.16 This study reviewed data from ongoing clinical practice and used clinical notes from this practice.
Intervention
The SNF practice involves frequent communication between medical provider team and SNF nursing team. A medical provider (advanced practice provider [APP] or physician) provides medical care at the facility for most days of the week. The provider visits patients in accordance with regulatory guidelines and generally within 5 business days of hospital discharge. The SNF team provides medication reconciliation, addresses acute and chronic health needs,16 and develops an SBAR document (describing situation, background, assessment, and recommendation) when new acute changes occur.17
The outpatient team determines program eligibility for each hospitalized patient through use of predefined criteria including age 60 years or older, proximity within 20 minutes of institution’s town, and an Elder Risk Assessment score of 16 or greater at hospital dismissal.12 Elder Risk Assessment score has been described previously.18 Briefly, it electronically determines key predictors of risk of readmission, including age, marital status, and comorbid health conditions, and this score is available for patients (Supplemental Table 1). Determination of eligibility occurs before hospital discharge and SNF admission.
The program following SNF discharge uses the same personnel and procedures used by patients directly discharged from the hospital. At SNF discharge, the patient is assigned an APP who works with a registered nurse to provide care. The APP provides an extensive home visit and is available for acute visits as needed while in the program. Patients maintain a relationship with their primary care provider and team. In the program, an APP evaluated a patient by coordinating a home-based face-to-face visit within 5 business days of SNF discharge. The patient was enrolled for up to 60 days in the program, with a few unusual clinical exceptions. The APP executed 5 core tasks during initial visit: 1) medication reconciliation, 2) advanced care planning, 3) education about available community resources, 4) management of chronic disease, and 5) management of acute symptoms.19 Previously, our investigators found that completion of the visit and medication reconciliation was almost 100%.20 In this model, the patient received weekly phone calls from a registered nurse and was able to receive in-home visits as needed to address acute medical changes. Specifically, the registered nurse would follow up on heart failure and address new infections. The nurse could coordinate a home visit with an APP.
Participants
Each patient in the program had been previously hospitalized before discharge to a SNF for a coordinated short-term stay. Additionally, each patient had an index SNF discharge date, which was noted in the electronic health record (EHR) and served as the index time for hospital readmission. Participants were older than 60 years and had an Elder Risk Assessment score of 16 or higher. The cohort involved local patients living within the catchment area, limited to 20-minute driving time. Participants were excluded if enrolled in another care management program (eg, hospice, dialysis, transplant, palliative care homebound). Patients were excluded if they refused EHR review.21
Participants in the comparison group met the same age and Elder Risk Assessment criteria required for program entry. These individuals were hospitalized before SNF admission, and each was discharged from the SNF into the community. In the comparison group, patients normally receive a phone call from the primary care nursing team and a posthospital office visit with a primary care provider. The comparison group was discharged home but did not receive program enrollment because of patient refusal, program capacity, or living farther than a 20-minute drive of Rochester, or a combination. Nevertheless, all patients discharged from a SNF received timely access to primary care.
Study Outcomes
The primary outcomes were rehospitalization and an ED visit within 30 days after SNF discharge. Furthermore, our team analyzed the rehospitalization, ED visits, and mortality rates at 180 days, which were considered secondary outcomes. We determined outcomes for hospitalizations and ED visits with manual abstraction from the EHR. Of note, Mayo Clinic updates mortality information in the EHR on the basis of hospital, nursing home, and locally reported deaths. In addition, the noted discharge date from the SNF served as the index date for determination of the outcomes hospitalization, ED visits or interventions, or death.
Independent Variables
Our team collected patient demographic information, including age, sex, marital status (married/partner vs other), race (White vs non-White), and ethnicity (Hispanic vs non-Hispanic). We assessed the levels of comorbid health burden and prior health care use by applying the Elder Risk Assessment score at hospital dismissal.18 We also included the total count of comorbid health conditions using the Charlson comorbidity index.22 We reported hospital length of stay and hospital dismissal service (medical vs surgical). To account for functional status, we calculated a 4-year mortality index, which included activities of daily living such as walking, bathing, managing finances, and moving objects; comorbid health conditions; age; body mass index; and smoking status.23 The information for this mortality index was manually abstracted from multiple sources in the EHR. Data collection was overseen by a trained study coordinator. Finally, the data collection methods were carefully reviewed by the research team.
We accounted for socioeconomic status by including education level (4-year college degree, high school graduate, postgraduate studies, some college, or less than high school) and the housing-based socioeconomic index (HOUSES) score. The HOUSES score is a socioeconomic measure that accounts for housing characteristics of the patient based on publicly available housing information and has been described previously 24,25 We reported the HOUSES score in quartiles and with the lowest quartile associated with lower socioeconomic status.
Analysis
We compared and described the groups according to baseline factors before and after propensity matching. Patients were propensity matched on the basis of age, sex, comorbid health conditions (Elder Risk Assessment score), and the 4-year mortality index. Furthermore, our team applied a caliper, defined as 0.2 times the standard deviation of the logit of the propensity score, to serve as the maximum distance for matching cases with controls. For the groups with missing information, we reported the available data for descriptive purposes.
For the primary analysis methodology, we compared the primary events of hospitalization and ED visits at 30 days by using conditional logistic regression, which was stratified by propensity-matched pairs. The regression model was adjusted for other important factors between groups. Secondary outcomes included review of hospitalization, ED visits, and mortality rates at a 180-day period following SNF discharge. To adjust for potential differences between the 2 cohorts during the 180-day outcomes period, we used adjusted Cox proportional hazards models for a first event of hospitalization occurring after discharge from a SNF or receipt of an ED visit during this time with censoring from analysis at the time of death. Mortality rates were compared with χ2 test. P<.05 with 2-tailed analysis was considered to be statistically significant. All analyses were conducted with statistical software (SAS version 9.4; SAS Institute Inc).
Results
During the study time frame, 366 potential program patients were discharged from a SNF following hospitalization, of which 165 were enrolled in the program (Supplemental Figure 1). After matching, 160 program participants were matched with 160 patients who received usual care. Among those included in the analysis, the mean (SD) age was 85.4 (7.43) years, and 64.7% were women (Table 1). Mean (SD) Elder Risk Assessment score was 21.0 (3.60); mean (SD) 4-year mortality score was 13.8 (2.77). This mortality score indicates a potential 64% risk of death within 4 years.23 No differences were observed between cases and controls in the propensity match. Propensity matching appeared to exclude some younger men from the comparison group. In comparing cases and controls after match, we found that Charlson illness count and dementia were both significantly different between groups. No difference was discerned in marital status, educational level, or socioeconomic status (HOUSES score) (Table 2).
Table 1.
Description of the Cohort Before and After Propensity Matching Among High-Risk Patients Discharged Home From a Skilled Nursing Facility Following Hospitalization
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Controls (n=201) | Cases (n=165) | Total (N=366) | Controls (n=160) | Cases (n=160) | Total (N=320) |
| Age, y | ||||||
| Mean (SD) | 83.6 (8.46) | 85.5 (7.69) | 84.5 (8.16) | 85.2 (7.66) | 85.5 (7.21) | 85.4 (7.43) |
| Median | 84 | 86 | 85 | 86 | 86 | 86 |
| Range | 63.0-99.0 | 63.0-105.0 | 63.0-105.0 | 63.0-99.0 | 64.0-105.0 | 63.0-105.0 |
| Sex, No. (%) | ||||||
| Female | 121 (60.2) | 108 (65.5) | 229 (62.6) | 102 (63.8) | 105 (65.6) | 207 (64.7) |
| Male | 80 (39.8) | 57 (34.5) | 137 (37.4) | 58 (36.3) | 55 (34.4) | 113 (35.3) |
| ERA score | ||||||
| Mean (SD) | 20.8 (3.45) | 21.0 (3.75) | 20.9 (3.58) | 21.0 (3.51) | 20.9 (3.71) | 21.0 (3.60) |
| Median | 21 | 21 | 21 | 21 | 21 | 21 |
| Range | 16.0-31.0 | 13.0-31.0 | 13.0-31.0 | 16.0-31.0 | 13.0-31.0 | 13.0-31.0 |
| 4-y mortality riska | ||||||
| Mean (SD) | 13.5 (2.77) | 13.8 (2.84) | 13.6 (2.80) | 13.8 (2.70) | 13.8 (2.85) | 13.8 (2.77) |
| Median | 14 | 14 | 14 | 14 | 14 | 14 |
| Range | 5.0-21.0 | 7.0-23.0 | 5.0-23.0 | 5.0-21.0 | 7.0-23.0 | 5.0-23.0 |
| Discharge year, No. (%) | ||||||
| After and including 2017 | 148 (73.6) | 112 (67.9) | 260 (71.0) | 117 (73.1) | 110 (68.8) | 227 (70.9) |
| 2013-12/31/2016 | 53 (26.4) | 53 (32.1) | 106 (29.0) | 43 (26.9) | 50 (31.3) | 93 (29.1) |
Abbreviations: ERA, Elder Risk Assessment; y, year.
The 4-year mortality risk includes age, sex, body mass index, current smoking status, difficulty bathing, difficulty with finance, difficulty walking several blocks, difficulty pulling and pushing large objects, and history of diabetes, cancer, chronic obstructive pulmonary disease, and heart failure.
Table 2.
Predictors of Hospitalization Between Case and Comparison Cohorts
| Cohorta |
||||
|---|---|---|---|---|
| Predictor | Comparison (n=160) | Case (n=160) | Total (N=320) | P value |
| Race | .10b | |||
| Non-White | 5 (3.1) | 1 (0.6) | 6 (1.9) | |
| White | 155 (96.9) | 159 (99.4) | 314 (98.1) | |
| Ethnicity | .65b | |||
| Non-Hispanic | 157 (98.1) | 158 (98.8) | 315 (98.4) | |
| Hispanic | 3 (1.9) | 2 (1.3) | 5 (1.6) | |
| Marital status | .48b | |||
| Married/partner | 59 (36.9) | 53 (33.1) | 112 (35.0) | |
| Other | 101 (63.1) | 107 (66.9) | 208 (65.0) | |
| Education level | .18b | |||
| 4-y college graduate | 6 (5.9) | 17 (14.9) | 23 (10.7) | |
| High school graduate or GED | 46 (45.5) | 40 (35.1) | 86 (40.0) | |
| Postgraduate studies | 12 (11.9) | 18 (15.8) | 30 (14.0) | |
| Some college or 2-y degree | 28 (27.7) | 28 (24.6) | 56 (26.0) | |
| Less than high school | 9 (8.9) | 11 (9.6) | 20 (9.3) | |
| Missing | 59 | 46 | 105 | |
| HOUSES quartile | .81b | |||
| 1 | 43 (46.2) | 63 (47.0) | 106 (46.7) | |
| 2 | 22 (23.7) | 35 (26.1) | 57 (25.1) | |
| 3 | 19 (20.4) | 21 (15.7) | 40 (17.6) | |
| 4 | 9 (9.7) | 15 (11.2) | 24 (10.6) | |
| Missing | 67 | 26 | 93 | |
| Charlson illness count | .04c | |||
| Mean (SD) | 4.2 (2.02) | 4.7 (2.04) | 4.4 (2.04) | |
| Median | 4 | 4.5 | 4 | |
| Range | 0.0-9.0 | 0.0-9.0 | 0.0-9.0 | |
| Dementia flag | .003b | |||
| No | 92 (57.5) | 117 (73.1) | 209 (65.3) | |
| Yes | 68 (42.5) | 43 (26.9) | 111 (34.7) | |
| Hospital LOS, d | .71c | |||
| Mean (SD) | 7.4 (3.18) | 7.7 (4.37) | 7.6 (3.82) | |
| Median | 7 | 7 | 7 | |
| Range | 2.0-20.0 | 2.0-31.0 | 2.0-31.0 | |
| Discharge clinical service | .16b | |||
| Cardiology | 12 (7.5) | 19 (11.9) | 31 (9.7) | |
| Cardiovascular surgery | 6 (3.8) | 4 (2.5) | 10 (3.1) | |
| Colon and rectal surgery | 0 (0.0) | 1 (0.6) | 1 (0.3) | |
| Family medicine | 27 (16.9) | 11 (6.9) | 38 (11.9) | |
| Gastroenterology | 1 (0.6) | 3 (1.9) | 4 (1.3) | |
| General surgery | 0 (0.0) | 1 (0.6) | 1 (0.3) | |
| Medicine service | 63 (39.4) | 73 (45.6) | 136 (42.5) | |
| Neurosurgery | 3 (1.9) | 6 (3.8) | 9 (2.8) | |
| Neurology medicine | 5 (3.1) | 7 (4.4) | 12 (3.8) | |
| Orthopedics trauma service | 14 (8.8) | 7 (4.4) | 21 (6.6) | |
| Orthopedics | 14 (8.8) | 9 (5.6) | 23 (7.2) | |
| Physical medicine | 1 (0.6) | 4 (2.5) | 5 (1.6) | |
| Pulmonary | 1 (0.6) | 2 (1.3) | 3 (0.9) | |
| Thoracic surgery | 1 (0.6) | 0 (0.0) | 1 (0.3) | |
| Trauma and critical care | 12 (7.5) | 12 (7.5) | 24 (7.5) | |
| Vascular | 0 (0.0) | 1 (0.6) | 1 (0.3) | |
Abbreviations: d, day; GED, General Educational Development; HOUSES, housing-based socioeconomic index; LOS, length of stay; y, year.
Values are presented as number (percentage) of patients unless specified otherwise.
χ2 P value.
Kruskal-Wallis P value.
To obtain the primary outcome, our team determined that 4.1% of the 320 patients had rehospitalization within 30 days of SNF discharge. In the program group, 7 of 160 patients (4.4%) had a rehospitalization compared with 6 of 160 patients (3.8%) in the comparison group, which was not significantly different (Table 3). After adjustment for Charlson illness count and dementia, we found an odds ratio (OR) of 1.5 (95% CI, 0.3-9.2). In a similar manner, no differences were discerned in 30-day ED visits because 10% with ED visits were reported in both program and comparison groups (adjusted OR, 0.9 [95% CI, 0.4-2.1]).
Table 3.
Health Outcomes of 160 Patients Receiving Care Transitions Program vs 160 Patients With Usual Care Adjusted for Charlson Total Illness Count and Dementia
| Group, No. (%) |
|||||
|---|---|---|---|---|---|
| Outcome | Overall (N=320) | Program (n=160) | Comparison (n=160) | Odds ratio | P value |
| 30-d hospitalization | 13 (4.1) | 7 (4.4) | 6 (3.8) | 1.5 (0.3-9.2) | .63a |
| 30-d ED visit | 32 (10.0) | 16 (10.0) | 16 (10.0) | 0.9 (0.4-2.1) | .80a |
| 180-d hospitalization | 67 (20.9) | 49 (30.6) | 18 (11.3) | 4.1 (1.9-8.7) | <.001b |
| 180-d ED visit | 114 (35.6) | 74 (46.3) | 40 (25.0) | 2.3 (1.4-3.7) | .001b |
| 180-d death | 55 (17.2) | 25 (15.6) | 30 (18.8) | .45c | |
Abbreviations: d, day; ED, emergency department
Wald conditional logistic test.
Cox proportional hazards model.
χ2 test.
In considering the secondary outcomes of hospitalization and ED visits occurring within 180 days, we found that 49 (30.6%) of 160 program patients had hospitalization compared with 18 (11.3%) of 160 persons in the comparison group (P<.001) (Table 3). Time-to-event curves (Figure 1) show a divergence beyond 30 days. We observed a similar finding for 180-day ED visit markers occurring in the intervention group (46%) compared with the comparison group (25%). Similarly, time-to-event curves (Figure 2) show divergence in the 2 groups, with more events occurring in the care transitions group after program completion. Specifically, 55 patients died within 180 days in the cohort groups: 25 (15.6%) deaths occurred in the program group and 30 (18.8%) in the comparison group (unadjusted P=.45). Within 30 days of SNF discharge, 4 control patients died and no deaths occurred in the care transitions cohort.
Figure 1.
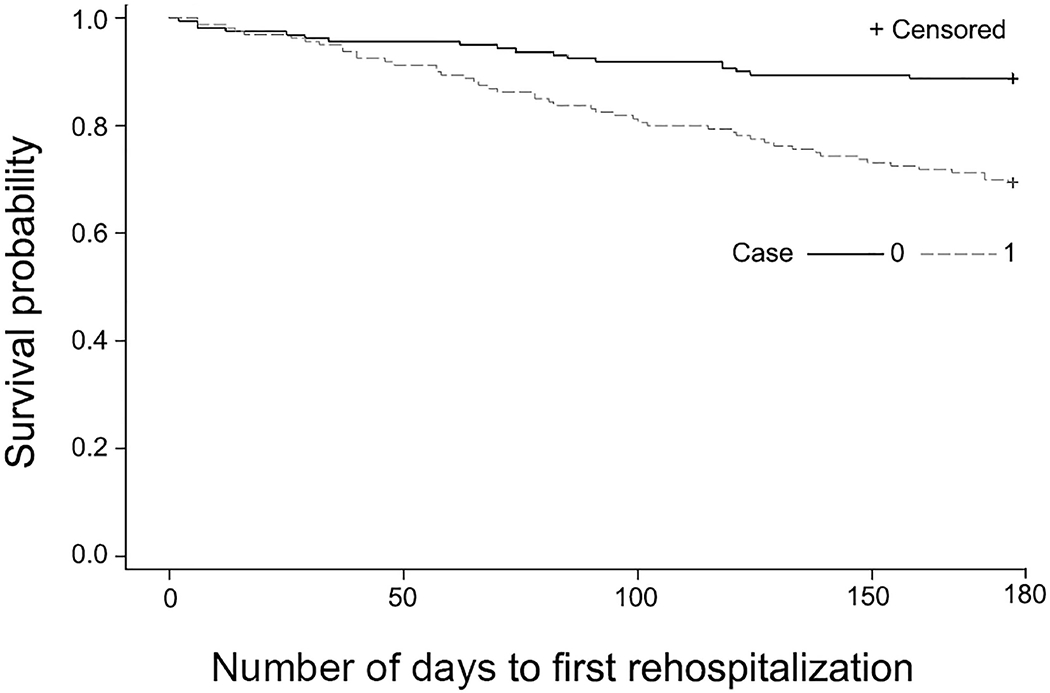
Hospitalization at 180 Days Following Nursing Home Discharge for Patients With The Mayo Clinic Care Transitions Program and Comparison Group. Control population has lower rate of hospitalization than intervention group.
Figure 2.
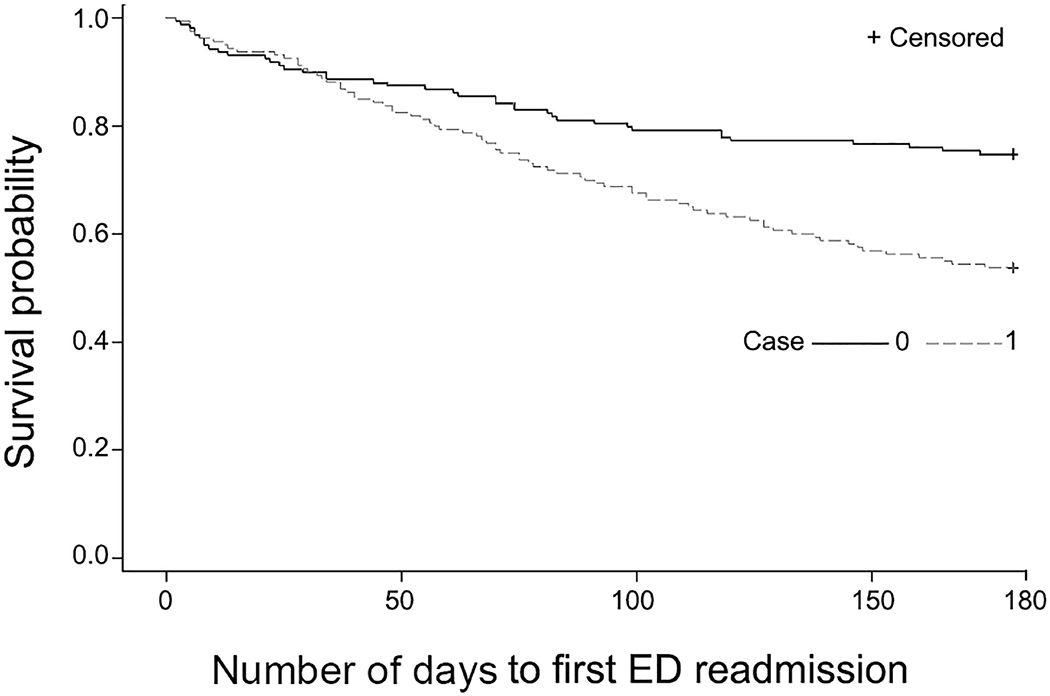
Emergency Department (ED) Visits. Control population has less ED use than the intervention group.
Discussion
In this first implementation of a program with the SNF-to-home model, we found no difference in 30-day hospitalization rates or ED visits for patients enrolled in the program compared with usual care. Novel findings were observed because program use has traditionally been implemented following dismissal from a hospital.5, 26, 27 Indeed, we have gained a broader scope of knowledge from these primary and secondary outcomes. First, the clinical practice developed the program for patients discharged directly from the hospital to the community and not from the SNF. Thus, findings of approximately 4% hospital readmission from the SNF for 30 days in the community reflect time following medical stabilization and physical therapy in the SNF. The program focuses on medication reconciliation and acute management practices that may have already been completed in the SNF; future care transitions may require a different approach when leaving the SNF. Second, risk stratification was based on patient characteristics at hospital discharge and may require unique factors involving functional status from the facility. Third, the program’s short duration likely did not provide for meeting the comprehensive needs of patients for the extended period that would be required to prevent hospitalizations or ED visits at 180 days.
Patients discharged from a SNF differ from patients discharged from the hospital, and distinctive approaches may be required. Specifically, patients receive medical, rehabilitation, and nursing care in the SNF setting. Such care may reduce the potential efficacy of the program interventions. Many hospital-related readmissions occur within 7 days of dismissal to a subacute setting.28 Previously, our team found a reduction in 30-day rehospitalization of patients who were discharged directly from the hospital into the community.12 However, this assessment accounted for the critical period of 30 days after hospital discharge. For the purpose of the present study’s intervention, the program model applied a similar approach, which may not meet the needs of patients discharged from the SNF. In the hospital practice, patients discharged directly to their respective homes often have unmet physical needs.29 The needs of the patient are not always clearly determined after a SNF discharge.
Previous groups have described their experiences in post-SNF care. However, these models often involve home health care. In a study of 1,543 patients discharged from a SNF to home, patients received a home health visit within 1 week of dismissal, to serve as a primary means of intervention. This visit reduced the 30-day readmission rate by 40%; of note, a visit to a primary provider did not reduce hospital readmission.30 A broader study of home health care after SNF dismissals evaluated 68,000 patients who had heart failure.11 The researchers observed a reduction in 30-day hospital admission rates for patients receiving home health care (22.8%) vs those with no in-home health care (24.5%) (P<.001). Although both these studies emphasize the use of home health nursing, the impact of the provider (APP or physician) may prove greatly important in decreasing readmission rates and requires further study.
The secondary outcomes highlight further areas of growth for the clinical practice. Among 180-day outcomes, we discovered higher rates of both hospitalization and ED visits in the group receiving the program intervention compared with those who received usual care. These results occurred despite no discerned differences among patients who were enrolled in the program for 60 days, which is the program target length.31 The utilization rates in the comparison usual care group were lower than previously reported by our group.12 However, the present study was small, and the chance or variable conditions could have accounted for the differences in percentages between the 2 groups. As a second potential reason, a confounding factor is possible that was not taken into account; however, the magnitude of the protective effect seems large. Additionally, for patients enrolled in the program group, less interaction typically occurred between the primary care provider and the primary care team.
Our study has strengths, limitations, and lessons learned. It adds to the understanding of the transition occurring between SNF and home through use of an established clinical care transitions program that has been used and refined for more than 5 years. We developed a comprehensive method of collecting health outcome data and predictor information for a wide range of SNFs.
As for limitations, confounding is possible and may require further investigation about potential risks of adverse health outcomes for patients discharged from the SNF because these represent areas not routinely explored. This was a single-site study, which duly reflects a single site’s clinical practice setting for care transitions. Furthermore, the 30-day hospitalization rate that we saw among the comparison group (<4%) was lower than other published data (~25%).11 We corrected for many of the common predictors of hospitalization; however, we were not able to adjust for activities of daily living before patients left the SNF or caregiver issues beyond the spouse because of the lack of information on these predictors. We did not adjust for all potential socioeconomic issues or support within the home. We also did not adjust for use of home health following SNF discharge. Using billing data, we may have missed some outcomes of hospitalization or ED and may miss some predictors if patients received care outside our system. These patients were empaneled in our primary care practice, and we suspect that this limitation is small. The population in southeastern Minnesota has a largely northern European ancestry and can be generalized to the Upper Midwest32 but may not be generalizable to other populations.
Finally, our team has learned that a specific program design may be needed for patients discharged from a SNF—one that lasts longer than the 2-month intervention applied in the present study. We may need to modify the program to better accommodate the home health team and, potentially, community resources because patients discharged from the nursing home have different needs than patients discharged from the hospital. We also may need further social supports for post-SNF patients. These supports include community health workers, the area agency on aging, and other services for aging persons within the community outside the health system.
Conclusions and Implications
Our study team determined that use of a care transitions program did not reduce 30-day rehospitalization rates or ED visits compared with usual care. Potentially, 2 investigation areas could stem from these results. First, the need is apparent for development of a risk stratification method for SNF patients that better predicts adverse health outcomes. Second, modification is required for intervention to address the specific needs of the patient returning home from a SNF. Longer intervention with enrollment for 6 months or more may help. Third, intervention focusing on specific issues, such as enhancement of advanced care planning, may provide improved support for the patient.
Supplementary Material
Acknowledgments:
We acknowledge Mayo Clinic for providing funding and financial support for this project. We also acknowledge Kari Takahashi, MEd, for editorial support.
Financial disclosures:
National Institute on Aging grant K23AG051679 (B.T.) and National Institute of Diabetes and Digestive and Kidney Diseases grant K23 K23DK114497 (R.G.M.). Additional support was provided by the National Center for Advancing Translational Sciences grant UL1 TR000135. The interpretation and reporting of the data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Conflicts of Interest
All authors report no conflict of interest.
References
- 1.Campbell Britton M, Petersen-Pickett J, Hodshon B, et al. Mapping the care transition from hospital to skilled nursing facility. J Eval Clin Pract 2020;26(3):786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marengoni A, Aguero-Torres H, Timpini A, et al. Rehabilitation and nursing home admission after hospitalization in acute geriatric patients. J Am Med Dir Assoc 2008;9(4):265–270. [DOI] [PubMed] [Google Scholar]
- 3.Fashaw SA, Thomas KS, McCreedy E, et al. Thirty-Year Trends in Nursing Home Composition and Quality Since the Passage of the Omnibus Reconciliation Act. J Am Med Dir Assoc 2020;21(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima JC, Ogarek J, Mor V Untapped Potential: Using the HRS-Medicare-Linked Files to Study the Changing Nursing Home Population. Med Care 2018;56(3):216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman EA, Parry C, Chalmers S, et al. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006;166(17):1822–1828. [DOI] [PubMed] [Google Scholar]
- 6.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009;150(3):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naylor M, Brooten D, Jones R, et al. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med 1994;120(12):999–1006. [DOI] [PubMed] [Google Scholar]
- 8.McWilliams A, Roberge J, Anderson WE, et al. Aiming to Improve Readmissions Through InteGrated Hospital Transitions (AIRTIGHT): a Pragmatic Randomized Controlled Trial. J Gen Intern Med 2019;34(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liss DT, Ackermann RT, Cooper A, et al. Effects of a Transitional Care Practice for a Vulnerable Population: a Pragmatic, Randomized Comparative Effectiveness Trial. J Gen Intern Med 2019;34(9):1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wysocki A, Kane RL, Dowd B, et al. Hospitalization of elderly Medicaid long-term care users who transition from nursing homes. J Am Geriatr Soc 2014;62(1):71–78. [DOI] [PubMed] [Google Scholar]
- 11.Weerahandi H, Bao H, Herrin J, et al. Home Health Care After Skilled Nursing Facility Discharge Following Heart Failure Hospitalization. J Am Geriatr Soc 2020;68(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi PY, Naessens JM, Peterson SM, et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthc (Amst) 2016;4(1):30–35. [DOI] [PubMed] [Google Scholar]
- 13.Hanson GJ, Borah BJ, Moriarty JP, et al. Cost-Effectiveness of a Care Transitions Program in a Multimorbid Older Adult Cohort. J Am Geriatr Soc 2018;66(2):297–301. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med 2007;45(4):247–251. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, Thorsteinsdottir B, Cha SS, et al. Health care outcomes and advance care planning in older adults who receive home-based palliative care: a pilot cohort study. J Palliat Med 2015; 18(1):38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra A, Rahman PA, Sneve A, et al. Risk of 30-Day Hospital Readmission Among Patients Discharged to Skilled Nursing Facilities: Development and Validation of a Risk-Prediction Model. J Am Med Dir Assoc 2019;20(4):444–450 e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan MC. Using SBAR communications in efforts to prevent patient rehospitalizations. Home Healthc Nurse 2013;31(9):504–515; quiz 515-507. [DOI] [PubMed] [Google Scholar]
- 18.Crane SJ, Tung EE, Hanson GJ, et al. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res 2010;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borkenhagen LS, McCoy RG, Havyer RD, et al. Symptoms Reported by Frail Elderly Adults Independently Predict 30-Day Hospital Readmission or Emergency Department Care. J Am Geriatr Soc 2018;66(2):321–326. [DOI] [PubMed] [Google Scholar]
- 20.Thorsteinsdottir B, Peterson SM, Naessens JM, et al. Care Transitions Program for High-Risk Frail Older Adults is Most Beneficial for Patients with Cognitive Impairment. J Hosp Med 2019;14(6):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca WA, Grossardt BR, Brue SM, et al. Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol 2018;47(2):368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47(11): 1245–1251. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 2006;295(7):801–808. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health 2016;70(3):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med 2014;174(7):1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor MD, Brooten DA, Campbell RL, et al. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004;52(5):675–684. [DOI] [PubMed] [Google Scholar]
- 28.Ouslander JG, Naharci I, Engstrom G, et al. Hospital Transfers of Skilled Nursing Facility (SNF) Patients Within 48 Hours and 30 Days After SNF Admission. J Am Med Dir Assoc 2016;17(9):839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland DE, Mistiaen P, Bowles KH. Problems and unmet needs of patients discharged “home to self-care”. Prof Case Manag 2011;16(5):240–250; quiz 251-242. [DOI] [PubMed] [Google Scholar]
- 30.Carnahan JL, Slaven JE, Callahan CM, et al. Transitions From Skilled Nursing Facility to Home: The Relationship of Early Outpatient Care to Hospital Readmission. J Am Med Dir Assoc 2017;18(10):853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi PY, Haas LR, Quigg SM, et al. 30-day hospital readmission of older adults using care transitions after hospitalization: a pilot prospective cohort study. Clin Interv Aging 2013;8:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


