Abstract
Purpose
To understand the clinical factors associated with embryo survival after vitrification in a cohort of human blastocysts screened by preimplantation genetic testing for aneuploidy (PGT-A).
Methods
Patient demographic, embryo, and cycle characteristics associated with failed euploid blastocyst survival were compared in a cohort of women (n = 6167) who underwent IVF-PGT-A.
Results
Compared to those that survived warming, vitrified euploid embryos that failed to survive after warming came from IVF cycles with significantly higher estradiol levels at time of surge (2754.8 ± 1390.2 vs. 2523.1 ± 1190.6 pg/mL, p = 0.03), number of oocytes retrieved (19.6 ± 10.7 vs. 17.5 ± 9.8, p = 0.005), and basal antral follicle count (BAFC) (15.3 ± 8.5 vs. 13.9 ± 7.2, p = 0.05). Euploid embryos were less likely to survive warming if they came from cycles before 2015 (24.6% vs. 13.2%, p < 0.001), were cryopreserved on day 7 versus day 5 or 6 (9.1% vs. 3.0%, p < 0.001), underwent two trophectoderm biopsies (6.9% vs. 2.3%, p < 0.001), had a grade C inner cell mass (15.4% vs. 7.7%, p < 0.001), or were fully hatched (41.1% vs. 12.2%, p < 0.001). In the multivariate model, which controlled for relevant confounders, the association between decreased survival and increased BAFC, year of IVF cycle, double trophectoderm biopsy, and fully hatched blastocysts remained statistically significant.
Conclusion
Euploid embryos that are fully hatched at time of vitrification, come from patients with high ovarian reserve, or require repeat trophectoderm biopsy are less likely to survive vitrification-warming. Our results provide a framework for reproductive counseling and offer realistic expectations to patients about the number of embryos needed to achieve family building goals.
Keywords: In vitro fertilization, Vitrification, Post-warming survival, Preimplantation genetic testing for aneuploidy
Introduction
Embryo cryopreservation has become an integral part of in vitro fertilization (IVF) treatment [1]. Modern assisted reproductive technology (ART) treatment has progressed from the use of slow freezing to vitrification protocols. Vitrification has several advantages over slow freezing, including being faster, more cost-effective, and less prone to ice crystal formation that could lead to mechanical and chemical damage [2]. Compared to slow-freeze protocols, vitrification has been shown to result in higher embryo survival rates and implantation and pregnancy rates, at both the cleavage stage [3, 4] and blastocyst stage [5–9].
Most vitrified embryos will survive the vitrification-warming process [8]. However, it has been shown that between 1 and 5% of vitrified blastocysts may fail vitrification-warming survival [8]. Yet, as more women rely on embryo banking for fertility preservation, identifying factors that are associated with improved vitrification and warming protocol outcomes has become increasingly more important in providing individualized, prognostic counseling to these patients.
Previous research has investigated the impact of IVF cycle characteristics on embryo vitrification and warming survival [10–16]. Lower survival rates have been demonstrated with GnRH antagonist protocols [10] and progesterone levels greater than 2 ng/mL at time of surge [11]. Many studies have also analyzed whether the day of embryo cryopreservation impacts survival [12–15]. While cryopreserved zygotes have consistently been shown to have inferior vitrification-warming survival and pregnancy outcomes [12, 13], these differences do not occur when comparing day 3 and 5 embryos that undergo slow-freeze and vitrification protocols [14, 15]. Prior studies have also shown that more favorable Gardner morphology grading, which qualifies embryos based on expansion grade (1–6), inner cell mass (ICM) grade (A–D), and trophectoderm (TE) grade (A–D), is significantly associated with improved vitrification-warming survival [16].
While previous research has identified factors such as stimulation protocol, embryo stage, and day of embryo development as significant predictors of embryo vitrification-warming survival, many of these studies are limited by the utilization of slow-freeze protocols and inclusion of unscreened embryos. These studies deserve merit for their early contribution to scientific literature; however, their findings cannot be fully applied to the current IVF treatment strategies. There is a need for updated studies that incorporate modern technologies such as PGT-A and blastocyst vitrification. Understanding the key factors associated with the inability of a cryopreserved embryo to survive the vitrification-warming process could allow for individualized patient counseling and improved clinical outcomes. The current study aimed to understand the patient, embryo, and cycle-related factors associated with euploid blastocyst vitrification and warming survival.
Materials and methods
This retrospective, single-center, case–control study included all vitrified-warmed euploid blastocysts from IVF-PGT-A cycles from 2010 to 2019. Donor oocyte IVF cycles were excluded. Euploid blastocysts that failed to survive after warming were compared to those that survived and were selected for transfer. Embryos that survived the vitrification-warming process were defined as those that had > 50% of the inner cell mass and trophectoderm cells intact. This study was approved by an academic Institutional Review Board with a waiver of consent for retrospective analysis of de-identified data.
Controlled ovarian stimulation (COS)
Patients underwent COS for IVF as described by Rodriguez-Purata et al. (2016) [17]. Ovarian follicular growth was measured with transvaginal ultrasound. Oocyte maturation was induced when two follicles achieved a size of 18 mm (mm). Recombinant or purified human chorionic gonadotropin (hCG) up to 10,000 international units (IU) (Ovidrel, EMD Serono; Novarel, Ferring Pharmaceuticals; or Pregnyl, Schering-Plough), leuprolide acetate 40 IU (Lupron, AbbVie Laboratories), or a combination of leuprolide acetate 40 IU (Lupron, AbbVie Laboratories) and hCG 1000 IU (Novarel, Ferring Pharmaceuticals; or Pregnyl, Schering-Plough) was used for ovulation induction. Patients then had transvaginal ultrasound-guided oocyte retrieval 36 h after luteinizing hormone surge. Metaphase II (MII) oocytes were inseminated by intracytoplasmic sperm injection (ICSI).
Embryo culture
Embryos were cultured until day 3 in Sage Quinn’s Advantage Cleavage Medium (Cooper Surgical). The embryos were then transferred to glucose-rich G-2.5 Vitrolife Blastocyst Media with 10% synthetic serum substitute (SSS) (Irvine Scientific) until day 5 to day 7 depending on the rate of embryo development. On day 0, the media solution was supplemented with 5% human serum albumin and 100 mg/mL HAS-Solution (Vitrolife). From days 1 to 7, 10% SSS (Irvine Scientific) was added to the embryo culture. Low-oxygen conditions were achieved in a Panasonic Sterisonic GxP incubator (Sanyo North America) and Nunclon 60-mm dishes under 100% paraffin oil (Ovooil™, Vitrolife) at 5% oxygen, 5.5% carbon dioxide, and 89.5% nitrogen from days 1 to 3 and 5% oxygen, 6% carbon dioxide, and 89% nitrogen from days 3 to 7. All embryos underwent assisted hatching on day 3 using the ZILOS-tk Laser (Hamilton Thorne Biosciences) with a 200–300-µs pulse to cause trophectoderm (TE) herniation via a 25–30-µm opening in the zona pellucida.
Embryo biopsy
TE biopsies were performed on days 5, 6, or 7 of embryo development. Embryos underwent TE biopsy once they reached an adequate morphologic grade ≥ 4BC (modified Gardner morphological score) [18]. The cutoff time for blastocysts to be determined suitable for biopsy and vitrification was the afternoon of day 5 or approximately 100 to 102 h post ovulatory trigger. Day 6 and day 7 embryos were determined to be suitable for biopsy and vitrification in the morning or approximately 116 to 118 h and 140 to 142 h post ovulatory trigger respectively. Embryos were placed in 10-μL drops of Enhance WG—Vitrolife HTF/HEPES and biopsied in Falcon 1006 Petri dishes (Becton Dickinson). The blastocysts were stabilized at the 3 o’clock position with a blunt glass holding pipette (internal diameter, 20–30 μm) under an Olympus IX70 microscope with Narishige micromanipulators. Four to seven trophectoderm cells were collected into the biopsy pipette (internal diameter 30 μm) using 500 µs of near-infrared pulsations and gentle traction. Trophectoderm cells were analyzed for ploidy status using quantitative real-time polymerase chain reaction (qPCR) or next-generation sequencing (NGS) [19, 20] and embryos were determined to be euploid or aneuploid. Blastocysts underwent vitrification immediately after TE biopsy (Cryotop method, Kitazato Corp.). In cases when the first biopsy was noted to non-diagnostic after vitrification, the embryos were warmed and a second biopsy was taken and analyzed for ploidy prior to re-vitrification.
FET cycles
Vitrification and warming techniques were previously described [17]. Embryos were determined to have survived the vitrification-warming process based on their inner cell mass appearance and re-expansion of the blastocoel. Patients were given oral estradiol 2 mg (Estrace, Teva Pharmaceuticals) two times daily for up to 1 week and then three times daily. A transvaginal ultrasound was used to assess endometrial thickness weekly until it was greater than 7 mm. Intramuscular (IM) progesterone (progesterone injection, Watson Pharma Inc.) or oral progesterone 100 mg (Endometrin, Ferring Pharmaceuticals) and vaginal progesterone 200 mg (Prometrium, AbbVie Laboratories) twice daily were then given. The endometrial pattern was monitored and categorized as late proliferative, early secretory, or mid-late secretory, as initially described by Grunfeld et al. (1991) [21]. Embryo warming and single euploid frozen embryo transfer (FET) was performed after 5 days of progesterone supplementation and adequate endometrial preparation. Embryo transfer was performed using a Wallace catheter under transabdominal ultrasound guidance.
Outcome measures
Data was collected regarding patient baseline characteristics, including age, anti-Mullerian hormone (AMH) level, basal antral follicle count (BAFC), and body mass index (BMI). Information about cycle characteristics, including stimulation protocol; cumulative gonadotropin (GND), estradiol (E2), and progesterone (P4) levels at time of surge; day of embryo development; number of oocytes retrieved; and fertilization method, were compiled. Data on embryo characteristics, including cleavage-stage embryo cell number and percentage fragmentation, number of exposures to trophectoderm biopsy and vitrification-warming, embryo sex, and Gardner morphology grading at time of cryopreservation, were gathered. The outcome of interest was embryo survival, which was determined if > 50% of the inner cell mass and trophectoderm cells were intact after vitrification-warming.
Statistics
The dataset was stratified based on euploid blastocyst survival. A Student’s t-test, chi-squared/Fisher’s exact test, and analysis of variance (ANOVA) were used to determine whether there were significant differences between euploid blastocysts that survived or did not survive after warming. A p value < 0.05 was considered statistically significant. Likelihood of clinical outcomes was presented as odds ratios (OR) with 95% confidence intervals. The multivariate logistic regression controlled for potential confounders including age, BMI, markers of ovarian reserve (AMH, BAFC), cumulative GND dose, E2 and P4 levels at surge, and number of oocytes retrieved. Due to the presence of numerous blastocysts per patient, generalized estimating equations (GEE) were used in the linear models to account for multiple observations within the same patient. A post hoc power calculation was performed, and using a significance level of 0.05 and 80% power, it was determined that a sample size of 79 embryos in each group was required to detect a large effect size (0.35), criteria which our study met. The statistical analyses were conducted using SAS, version 9.4 and R statistical software, version 3.4.3.
Results
Baseline demographics
A total of 6167 euploid blastocysts underwent vitrification-warming, with 2.8% (n = 175) not surviving after warming. Baseline demographics of patients whose embryos survived vitrification-warming and those that did not are shown in Table 1. The two groups had patients with similar ages (35.3 ± 4.3 vs. 35.8 ± 4.0 years, p = 0.13, OR 1.00, 95% CI 0.95–1.06) and BMI (23.8 ± 4.8 vs. 23.7 ± 4.3 kg/m2, p = 0.76, OR 0.98, 95% CI 0.94–1.03). AMH levels were comparable between the two groups (4.0 ± 4.4 vs. 3.8 ± 4.2 ng/mL, p = 0.53, OR 1.02, 95% CI 0.97–1.07), but BAFC was significantly higher in patients whose embryos did not survive the vitrification-warming process (15.3 ± 8.5 vs. 13.9 ± 7.2; p = 0.05, OR 0.97, 95% CI 0.94–0.99).
Table 1.
Baseline demographics
| Did not survive warming (n = 175) | Survived warming (n = 5992) | p value | |
|---|---|---|---|
| Age (years) | 35.3 ± 4.3 | 35.8 ± 4.0 | 0.13 |
| AMH (ng/mL) | 4.0 ± 4.4 | 3.8 ± 4.2 | 0.53 |
| BAFC | 15.3 ± 8.5 | 13.9 ± 7.2 | 0.05 |
| BMI (kg/m2) | 23.8 ± 4.8 | 23.7 ± 4.3 | 0.76 |
Cycle characteristics
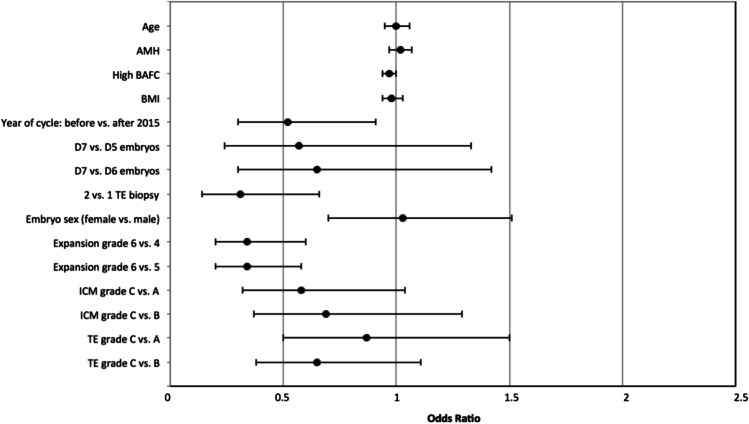
Cycle characteristics of blastocysts that survived warming and those that did not are summarized in Table 2. Ovarian stimulation protocol, total GND dose, and fertilization method were not associated with survival outcomes. Embryos cryopreserved before 2015 were less likely to survive warming than those cryopreserved after 2015 (OR 0.52, 95% CI 0.30–0.91). Embryos that failed to survive vitrification-warming came from women with significantly higher E2 levels at time of surge (2754.8 ± 1390.2 pg/mL, p = 0.03) and number of oocytes retrieved (19.6 ± 10.7, p = 0.005). Embryo survival outcomes were not significantly impacted by P4 levels at time of surge (1.0 ± 0.9 vs. 0.9 ± 0.6 ng/mL, p = 0.15). Cell number at cleavage stage (8.8 ± 2.1 vs. 8.7 ± 1.8, p = 0.45) and percentage fragmentation (2.0 ± 4.2 vs. 2.7 ± 5.4, p = 0.09) at cleavage stage were similar between groups. Embryos cryopreserved on day 7 were less likely to survive after warming (8.2%) than embryos cryopreserved on day 5 (1.9%) or day 6 (3.9%, Table 3), and this difference was most pronounced when comparing day 7 and day 5 embryos (p < 0.001). Embryos that underwent two trophectoderm biopsies had significantly lower odds of survival (OR 0.31, 95% CI 0.14–0.66, p = 0.003) than embryos that had a single biopsy. Repeated vitrification and warming itself was not associated with embryo survival outcomes (p = 0.19). Male and female embryos had comparable survival rates (p = 0.74). Fully hatched embryos had significantly decreased odds of survival than embryos with expansion grade 4 (OR 0.34, 95% CI 0.20–0.60) and grade 5 (OR 0.20, 95% CI 0.20–0.58). Embryos with ICM grade C were less likely to survive vitrification-warming, compared to those with ICM grade A (5.5% vs. 2.6%, p < 0.001) and grade B (5.5% vs. 2.6%, p < 0.001). Trophectoderm grade at time of cryopreservation was not associated with embryo survival. In the multivariate model, which controlled for relevant confounders, the association between decreased embryo survival and increased BAFC, year of IVF cycle, double trophectoderm biopsy, and fully hatched blastocysts remained statistically significant (Fig. 1).
Table 2.
Cycle characteristics
| Did not survive warming (n = 175) | Survived warming (n = 5992) | p value | |
|---|---|---|---|
| Ovarian stimulation protocol | 0.38 | ||
| GnRH antagonist | 90.3% (158/175) | 86.7% (5195/5992) | |
| GnRH agonist downregulation | 2.3% (4/175) | 2.4% (146/5992) | |
| Microdose GnRH agonist | 7.4% (13/175) | 10.9% (651/5992) | |
| Year of cycle | < 0.001 | ||
| Before 2015 | 24.6% (43/175) | 13.2% (790/5992) | |
| 2015 and after | 75.4% (132/175) | 86.8% (5202/5992) | |
| Cumulative gonadotropin dose (IU) | 3365.8 ± 1274.6 | 3465.2 ± 1290.2 | 0.32 |
| Estradiol at surge (pg/mL) | 2754.8 ± 1390.2 | 2523.1 ± 1190.6 | 0.03 |
| Progesterone at surge (ng/mL) | 1.0 ± 0.9 | 0.9 ± 0.6 | 0.15 |
| Oocytes retrieved | 19.6 ± 10.7 | 17.5 ± 9.8 | 0.005 |
| Fertilization method | 0.12 | ||
| Conventional | 0.0% (0/175) | 1.6% (94/5976) | |
| ICSI | 100.0% (175/175) | 98.4% (5882/5976) | |
| Cleavage stage—cell number | 8.8 ± 2.1 | 8.7 ± 1.8 | 0.45 |
| Cleavage stage—% fragmentation | 2.0 ± 4.2 | 2.7 ± 5.4 | 0.09 |
| Day of embryo development at cryopreservation | < 0.001 | ||
| 5 | 37.7% (66/175) | 58.3% (3494/5992) | |
| 6 | 53.1% (93/175) | 38.7% (2319/5992) | |
| 7 | 9.1% (16/175) | 3.0% (179/5992) | |
| Number of trophectoderm biopsies | < 0.001 | ||
| 1 | 93.1% (163/175) | 97.7% (5857/5992) | |
| 2 | 6.9% (12/175) | 2.3% (135/5992) | |
| Number of times vitrified and warmed | 0.19 | ||
| 1 | 99.4% (174/175) | 97.6% (5850/5992) | |
| 2 | 0.6% (1/175) | 2.4% (142/5992) | |
| Embryo sex | 0.74 | ||
| Male | 47.7% (83/174) | 49.3% (2941/5969) | |
| Female | 52.3% (91/174) | 50.7% (3028/5969) | |
| Expansion grade | < 0.001 | ||
| 2 | 0.0% (0/175) | 0.0% (1/5992) | |
| 3 | 0.0% (0/175) | 0.2% (10/5992) | |
| 4 | 29.1% (51/175) | 49.7% (2980/5992) | |
| 5 | 29.7% (52/175) | 37.9% (2269/5992) | |
| 6 | 41.1% (72/175) | 12.2% (732/5992) | |
| ICM grade | < 0.001 | ||
| A | 59.4% (104/175) | 65.0% (3892/5992) | |
| B | 25.1% (44/175) | 27.3% (1635/5992) | |
| C | 15.4% (27/175) | 7.7% (460/5992) | |
| D | 0.0% (0/0) | 0.001% (3/5992) | |
| Trophectoderm grade | 0.02 | ||
| A | 49.1% (86/175) | 39.3% (2350/5981) | |
| B | 32.6% (57/175) | 42.4% (2535/5981) | |
| C | 18.3% (32/175) | 18.3% (1096/5981) | |
| D | 0.0% (0/0) | 0.002% (10/5981) |
Table 3.
Multivariate analysis: odds of embryo survival
| Did not survive warming | Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|---|
| Year of cycle | ||||
|
Before 2015 2015 and after |
5.2% (43/833) 2.5% (132/5334) |
Before 2015 vs. after 2015: 0.52 | 0.30–0.91 | 0.02 |
| Day of embryo development | ||||
|
5 6 7 |
1.9% (66/3560) 3.9% (93/2412) 8.2% (16/195) |
D7 vs. D5: 0.57 D7 vs. D6: 0.65 |
0.24–1.33 0.30–1.42 |
0.19 0.28 |
| Number of TE biopsy | ||||
|
1 2 |
2.7% (163/6020) 8.2% (12/147) |
2 vs. 1: 0.31 | 0.14–0.66 | 0.003 |
| Expansion grade | ||||
|
4 5 6 |
1.7% (51/3031) 2.2% (52/2321) 9.0% (72/804) |
6 vs. 4: 0.34 6 vs. 5: 0.34 |
0.20–0.60 0.20–0.58 |
< 0.001 < 0.001 |
| ICM grade | ||||
|
A B C |
2.6% (104/3996) 2.6% (44/1679) 5.5% (27/487) |
C vs. A: 0.58 C vs. B: 0.69 |
0.32–1.04 0.37–1.29 |
0.07 0.24 |
Fig. 1.
Forest plot of the multivariate analysis: odds of embryo survival
Discussion
To our knowledge, this study is the first to determine predictors of vitrification-warming survival among a cohort of vitrified euploid blastocysts. Our research demonstrates that embryos from women with high ovarian reserve and those that undergo more than one trophectoderm biopsy for PGT-A or are hatched prior to vitrification have significantly lower odds of survival.
Patients with markers of high ovarian reserve, specifically high follicle counts, had reduced odds of embryo survival in our study population. We propose that higher BAFC may be associated with an underlying polycystic ovary syndrome (PCOS) diagnosis among a high proportion of patients. This finding might lend credence to the long-theorized link between PCOS and poor oocyte and embryo quality [22]. Future research examining the relationship between embryo survival and specific infertility diagnoses would further clarify this clinical consideration. While Medved et al. (2006) found that GnRH agonist protocols had more favorable outcomes than GnRH antagonist protocols, our study did not demonstrate any association between stimulation protocol and embryo survival [10]. These conflicting results may be explained by differences in cryopreservation techniques, as Medved et al. (2006) utilized slow-freeze methods. Based on our results, providers can individualize protocols to best optimize a patient’s response to IVF, as variation in stimulation protocols do not appear to affect embryo survival outcomes with vitrification. Pavone et al.’s (2011) finding that estradiol levels at time of surge are not associated with survival of vitrified embryos corroborates the findings of our study [13]. That study also demonstrated a drop-off in embryo survival rates when serum progesterone levels on day of surge exceeded 2 ng/dL, which was not supported by our results. The discrepancy may be explained by an average progesterone level of approximately 1 ng/mL in our study. However, a recent analysis by our group failed to demonstrate a relationship between progesterone elevation, up to 2.0 ng/mL, at time of surge in the fresh IVF cycles and pregnancy outcomes in subsequent single euploid FET cycles [23]. While poor outcomes in high responders have often been attributed to stimulation protocols and elevated hormones levels at time of surge, the lack of association between embryo survival and these factors in our study further implicates factors, such as high ovarian reserve and response, often seen with PCOS, for reduced odds of embryo survival.
As more women undergo freeze-all cycles, the synchronization of uterine receptivity and embryo development has become less of a concern. Thus, embryos, that traditionally were only cryopreserved if they reached maturation by day 3, can now be cryopreserved up until day 7 of development. The clinical implications of vitrifying these slow-growing embryos and the association between day of vitrification and warming survival have been studied with conflicting results. Both Han et al. (2012) and Pavone et al. (2011) found similar survival rates in day 3 and 5 embryos [13, 15]. Similarly, El Toukhy et al. (2011) and Cimadomo et al. (2018) determined that embryos cryopreserved on day 5 and day 6 had comparable rates of blastocyst survival after warming [14, 16]. However, Cimadomo et al. (2018) demonstrated lower rates of survival in embryos that were not ready for vitrification until day 7 [16]. Our study echoed these findings in cycles utilizing vitrification and PGT-A, demonstrating that 8.2% of embryos vitrified on day 7 failed to survive after warming, compared to only 1.9% and 3.9% of those vitrified on day 5 and day 6, respectively. Hernandez-Nieto et al. (2019) further showed that embryos vitrified on day 7 had poorer clinical outcomes than those vitrified on day 5 and day 6, with higher aneuploidy rates and lower implantation rates, clinical pregnancy rates, and live birth rates [24]. Time-lapse studies have similarly shown that faster developing blastocysts are associated with higher euploidy rates [25, 26]. While vitrification may be delayed up until day 7 in order to maximize the number of embryos cryopreserved from a given cycle, patients should be counseled that these slow-growing embryos are less likely to survive warming, likely due to poorer quality and reproductive potential.
Our analysis, which included a large sample size of PGT-A tested euploid embryos, found that a second trophectoderm biopsy, but not repeated vitrification-warming cycles, negatively impacted blastocyst survival. Taylor et al. (2014) found similar outcomes and corroborated that repeat trophectoderm biopsies decreased embryo survival [27]. The generalizability of that study is limited by its small sample size, with twelve studied in the repeat vitrification-warming and two patients in the repeat trophectoderm biopsy groups. Those findings are in contrast to the study published by Ciamdomo et al. (2018), who found no effect of trophectoderm biopsy on vitrified embryo survival [16]. However, Ciamdomo et al. (2018) compared embryos that were biopsied to those that did not undergo biopsy or PGT-A. While Ciamdomo et al. (2018) found that embryos with average or poor morphologic quality were less likely to survive warming, our study was the first to individually examine the factors contributing to a final morphologic grade. In our study, we observed that fully hatched embryos had lower odds of survival than embryos with an expansion grade 4 or 5. A possible explanation for this finding is that repeat trophectoderm biopsy and increased exposure of cryoprotectants in fully hatched embryo could introduce duress and reduce tolerance to vitrification and warming. However, it is unclear whether these negative effects persist once an embryo survives vitrification-warming and is transferred. Some studies have shown that fully hatched embryos had similar clinical pregnancy rates as those that were not fully hatched, whereas others showed improved clinical pregnancy rates with fully hatched embryos [28, 29]. Aluko et al. (2020) also examined obstetric outcomes among embryo that underwent repeat cryopreservation and found that it had a negative impact on clinical pregnancy and live birth rates [30].
The current study was limited by its retrospective design and is subject to some level of inherent confounding bias. Given the rarity of the study outcome, there was a limited sample size of cases, with only 2.8% of the vitrified blastocysts in the study not surviving after warming. Despite the use of a case–control study design to address this, our study may have been underpowered to uncover certain associations. There also may have been changes in laboratory and clinical protocols that confounded the results of our study, given the period of time over which the data was collected.
While previous studies have looked at conventional slow-freeze techniques and embryo survival, this study is the first to report on the association in euploid blastocysts that were both screened with PGT-A and cryopreserved via vitrification. Our statistical analysis also bolstered the clinical implications of our results. The use of a GEE model enabled us to account for the inclusion of multiple embryos from the same patient. We also controlled for relevant clinical confounders in the multivariate regression.
This study investigated the clinical factors associated with vitrification-warming survival outcomes and found that euploid embryos that are fully hatched, underwent multiple trophectoderm biopsies, or came from patients with high ovarian reserves were less likely to survive after warming. These results have important clinical implications as providers can utilize this data to better counsel patients regarding their prognosis and realistic expectations about embryo survival. Undoubtedly, there will be a growing number of patients who pursue embryo banking. Our study may offer pertinent information about embryo vitrification-warming outcomes and could assist patients in their decision-making process about how many embryos to bank in order to achieve their family building goals. Future studies that focus on whether there are underlying genomic, proteomic, and metabolic factors that drive suboptimal embryo survival would ultimately improve IVF outcomes.
Author contribution
MO, CB, DG, JAL, ABC, and LS contributed to the design and implementation of the research, the analysis of the results, and the writing of the manuscript.
Data availability
Data is available upon request.
Code availability
Not applicable.
Declarations
Ethics approval
This study was approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board with a waiver of consent for retrospective analysis of de-identified data.
Conflict of interest
ABC is the Chief Medical Officer of Sema4 and Medical Director of Progyny. MO, CB, DG, JAL, and LS have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagy ZP, Shapiro D, Ching-Chien C. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril. 2020;113:241–247. doi: 10.1016/j.fertnstert.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Strauss JF, Barbieri RL. Yen & Jaffe’s reproductive endocrinology: pathophysiology, and clinical management. 8th ed. Philadelphia: Elsevier; 2019.
- 3.Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pado G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90:186–193. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet. 2009;26:347–354. doi: 10.1007/s10815-009-9318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol. 2009;21:270–274. doi: 10.1097/GCO.0b013e3283297dd6. [DOI] [PubMed] [Google Scholar]
- 6.AbdelHafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online. 2010;20:209–222. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Keskintepe L, Sher G, Machnicka A, Tortoriella D, Bayrak A, Fisch J, et al. Vitrification of human embryos subjected to blastomere biopsy for pre-implantation genetic screening produces higher survival and pregnancy rates than slow freezing. J Assist Reprod Genet. 2009;26:629–635. doi: 10.1007/s10815-009-9369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29:2794–2801. doi: 10.1093/humrep/deu246. [DOI] [PubMed] [Google Scholar]
- 10.Medved R, Virant-Klun I, Meden-Vrtovec H, Tomazevic T. Outcome of frozen-thawed blastocysts derived from gonadotropin releasing hormone agonist or antagonist cycles. J Assist Reprod Genet. 2006;23:275–279. doi: 10.1007/s10815-006-9051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal L, Kovacs P, Witt B, Jindal S, Santoro N, Barad D. Postthaw blastomere survival is predictive of the success of frozen-thawed embryo transfer cycles. Fertil Steril. 2004;82:821–826. doi: 10.1016/j.fertnstert.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 12.Surrey E, Keller J, Stevens J, Gustofson R, Minjarez D, Schoolcraft W. Freeze-all: enhanced outcomes with cryopreservation at the blastocyst stage versus pronuclear stage using slow-freeze techniques. Reprod Biomed Online. 2010;21:411–417. doi: 10.1016/j.rbmo.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Pavone ME, Innes J, Hirshfeld-Cytron J, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Hum Reprod Sci. 2011;4:23–28. doi: 10.4103/0974-1208.82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Toukhy T, Wharf E, Walavalkar R, Singh A, Bolton V, Khalaf Y, et al. Delayed blastocyst development does not influence the outcome of frozen-thawed transfer cycles. BJOG. 2011;118:1551–1556. doi: 10.1111/j.1471-0528.2011.03101.x. [DOI] [PubMed] [Google Scholar]
- 15.Han AR, Park CW, Lee HS, Yang KM, Song IO, Koong MK. Blastocyst transfer in frozen-thawed cycles. Clin Exp Reprod Med. 2012;39:114–117. doi: 10.5653/cerm.2012.39.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimadomo D, Capalbo A, Levi-Setti PE, Soscia D, Orlando G, Albani E, et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum Reprod. 2018;33:1992–2001. doi: 10.1093/humrep/dey291. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Purata J, Lee J, Whitehouse M, Duke M, Grunfeld L, Sandler B, et al. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet. 2016;33:401–412. doi: 10.1007/s10815-016-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner DK, Stevens J, Sheehan CB, Schoolcraft W. Analysis of blastocyst morphology. In: Elder K, Jacques C, eds. Human preimplantation embryo selection. London: Informa Healthcare, 2007;79–87.
- 19.Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT., Jr Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012;97:819–824. doi: 10.1016/j.fertnstert.2012.01.115. [DOI] [PubMed] [Google Scholar]
- 20.Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, et al. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil Steril. 2014;101:1375–1382. doi: 10.1016/j.fertnstert.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Grunfeld L, Walker B, Bergh PA, Sandler B, Hofmann G, Navot D. High-resolution endovaginal ultrasonography of the endometrium: a noninvasive test for endometrial adequacy. Obstet Gynecol. 1991;78:200–204. [PubMed] [Google Scholar]
- 22.Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17:17–33. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, et al. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod. 2020;35:1889–1899. doi: 10.1093/humrep/deaa123. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34:1697–1706. doi: 10.1093/humrep/dez129. [DOI] [PubMed] [Google Scholar]
- 25.Desai N, Goldberg J, Austin C, Falcone T. Are cleavage anomalies, multinucleation or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy? Fertil Steril. 2018;109:665–674. doi: 10.1016/j.fertnstert.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod. 2016;31:2245–2254. doi: 10.1093/humrep/dew183. [DOI] [PubMed] [Google Scholar]
- 27.Taylor TH, Patrick JL, Gitlin SA, Michael Wilson J, Crain JL, Griffin DK. Outcomes of blastocysts biopsied and vitrified once versus those cryopreserved twice for euploid blastocyst transfer. Reprod Biomed Online. 2014;29:59–64. doi: 10.1016/j.rbmo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106:1370–1378. doi: 10.1016/j.fertnstert.2016.07.1095. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Purata J, Gingold J, Lee J, Whitehouse M, Slifkin R, Briton-Jones C, et al. Hatching status before embryo transfer is not correlated with implantation rate in chromosomally screened blastocysts. Hum Reprod. 2016;31:2458–2470. doi: 10.1093/humrep/dew205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aluko A, Vaughan DA, Modest AM, Penzias AS, Hacker MR, Thorton K, Sakas D. Multiple cryopreservation-warming cycles, coupled with blastocyst biopsy, negatively affect IVF outcomes. Reprod Biomed Online. 2021;42:572–578. doi: 10.1016/j.rbmo.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.
Not applicable.