Abstract
Although in vitro culture of human embryos is a crucial step in assisted reproduction, the lack of focused research hampers worldwide standardisation and consistent outcomes. Only 1.2% of research papers published in five leading journals in human reproduction in 2019 focused on in vitro culture conditions, creating the impression that the optimisation process has approached its limits. On the other hand, in vitro culture of mammalian embryos is based on old principles, while there is no consensus on basic issues as density, time, medium change, gas atmosphere and small technical details including the way of drop preparation. This opinion paper aims to highlight and analyse the slow advancement in this field and stimulate research for simple and affordable solutions to meet the current requirements. A possible way for advancement is discussed in detail. Selection of embryos with the highest developmental competence requires individual culture and modification of the widely used “drop under oil” approach. Current use of three-dimensional surfaces instead of large flat bottoms is restricted to time-lapse systems, but these wells are designed for optical clarity, not for the needs of embryos. The size and shape of the original microwells (Well of the Well; WOW) offer a practical and straightforward solution to combine the benefits of communal and individual incubation and improve the overall quality of cultured embryos.
Keywords: Embryo, Culture, Microwell, Well of the Well, WOW
Introduction
In 2019, the last calendar year undisturbed by the pandemic, five leading journals in reproductive biology (Human Reproduction, Fertility and Sterility, Reproductive BioMedicine Online, Biology of Reproduction, Molecular Reproduction and Development) published a total of 983 research papers. The effect of culture conditions was investigated by 12 studies (1.2%), only 4 of them dealing with the physical environment of embryos. In contrast, quality assessment and embryo selection was the subject of 19 papers (Vajta, unpublished). Although this informal pilot survey may not allow profound conclusions, it seems to be indicative. It supports the general impression that despite its crucial role in assisted reproduction, technical details of embryo culture are not in the focus of research in human IVF anymore.
The obvious question is: why? Did we do—almost—everything possible for optimisation? Were all factors scrupulously tested, all parameters fine-tuned and all interactions investigated?
To exploit all the potentials of human ART, we need to establish a culture system that provides an optimal environment for embryo development and, as a secondary goal, also meets the various practical requirements of laboratory work. In contrast to many previous opinions, including that of a recent consensus paper of prominent scientists [1], it is questionable whether the establishment of an in vivo–like situation should be the ultimate goal. Firstly, we do not know how efficient is the healthy female reproductive tract in supporting early embryo development. It may not be very impressive—rate estimates vary between 20 and 90% implanted/fertilised embryos [2], and it is impossible to calculate losses that happen during the first few days of development. Secondly, plenty of examples show that the natural way is not necessarily the best. Most therapeutic interventions of human medicine try to correct the failures of nature—and assisted reproduction is not an exception either. As emphasised earlier, mammalian embryo culture should not be regarded as an imperfect copy of the in vivo process, but an artificial procedure with its own frames, limitations and possibilities [3]. Our goal is to provide the best combination of various factors resulting in healthy embryos with excellent viability in utero and also during the subsequent one-hundred-plus years.
While contributing to the creation of human life, our responsibility is enormous. Patients, as well as laypeople, expect us to use highly optimised and standardised systems. Unfortunately, despite the impressive development achieved in the past 40 years, our embryo culture methods show wide variations, and the scientific basis is full of questions and uncertainties. There is hardly a single factor that is entirely identical in all human embryo culture systems, and there is not a single chemical or biological component that is used in all commercially available media at exactly the same concentration [3, 4]. Recent studies clearly demonstrate that there is no consensus in the most fundamental issues such as optimal pH of media, the temperature of incubation, single uninterrupted versus two-phase media systems and protein supplementation [1] [5–10]. Review articles dealing with other aspects of embryo culture also show wide variations of controversial outcomes, and systematic reviews attempting to answer a given question usually end up with the conclusion: more work is needed.
The task is rather demanding. Known quantifiable determinants influencing the outcome of in vitro culture include physical, chemical and biological ones, with tens or hundreds of different factors in each group [1]. These factors, even the most distant ones, may interrelate to each other [4]. A good example is that a simple modification of the physical position of embryos in vitro may or may not modify the outcome depending on the age of the oocyte donor [11].
Let us consider just 50 determinants (although as mentioned above there are much more) and only two variants for each, e.g. two different concentrations of any basic components in the culture medium or two different pH values (an obvious oversimplification of the real possibilities). The analysis of all possible interactions would require 250 groups, around 1014 experiments, preferably registered prospective randomised trials replicated several times by different research groups and results without any contradictions. Unfortunately, even the last requirement seems to be a utopistic goal, as proven by a recent study demonstrating that treatment practices and clinic sites may mask the effect of culture conditions [12].
This seemingly impossible task should not paralyse our research. The stakes are far too high to accept the limits of our actual systems. However, a special approach needs to be followed. In parallel with the mechanical analysis of all factors involved, creativity and intuition must also play a substantial role. Open-minded mentality, trial-and-error-type work or sometimes pure haphazard luck may lead to surprising solutions that are justified first by the outcome, and explained by basic research only retrospectively. These approaches were highly successful in the first decades of assisted reproduction, resulting in many breakthroughs in IVF, cryopreservation and stimulation protocols. Due to unfavourable changes in the financial, structural and legal environment of present-day embryo research, simple, inexpensive and easily applicable solutions exploiting new or largely (and unjustly) disregarded areas need to be explored—to find the weakest link in the chain.
The topic of this review is one of these latter areas—application of three-dimensional structures for embryo culture, more specifically the theoretical background, proper application and benefits of a static microwell system. Although microwells are now increasingly used for the fashionable time-lapse machines, their size, design and application have been mostly devised for the benefit of visual evaluation and not according to the real needs of the embryo. Summarising and evaluating data accumulated slowly during the past two decades may help to promote the broader application of microwells and to develop enhanced versions to improve our embryo culture systems and the overall efficiency of human IVF.
Communal culture
Cultured embryos are social beings and prefer to be together. An increasing number of studies have proved the beneficial effects of communal culture on blastocyst rates and subsequent in vivo development in various species, including humans [13–30]. The failure to show the benefits of group culture in some earlier reports [31] [32] may mostly be explained with the short (2 or 3 days) culture period [11] [7] [33]. The main mechanisms that supposedly support better development in group culture include accommodation, communication and protection.
Accommodation
Both meanings (“adaptation” and “shelter”) of this word are applicable to the situation. Sparse but convincing pieces of evidence prove that the solution layer immediately surrounding the developing embryo differs from the rest of the medium in certain physical and chemical parameters including pH, oxygen concentration and nutrients [39] [40]. This microenvironment may be analogous to the situation in vivo, where embryos are surrounded by a minimal, almost virtual space, especially that of the oviduct [17, 41, 42]. This may be hard to build up and easy to disturb in traditional in vitro embryo culture systems where large amounts of solutions are used, the media may be changed or the culture dishes are moved for microscopic assessments during development. The communal effort of multiple embryos may be more successful building and stabilising this environment, or reconstructing it if necessary.
Communication
Another aspect of this microenvironment is the production of bioactive ligands that may have a specific effect on the neighbouring embryos or the producer itself through paracrine or autocrine actions, respectively [14] [26, 43, 44]. These factors may be excessively diluted in vitro. Growth factors added individually or as a random mixture in serum can markedly improve embryo development [14] [38] [43]. Even replacing fetal calf serum with adult bovine serum containing approximately twice as much proteins and growth factors improves the in vitro development of cloned bovine embryos [45]. It should also be considered that embryos developing in vitro are not exposed to the multiple effects of autocrine, paracrine and endocrine factors present in the oviduct, and also produce less autocrine and paracrine ligands themselves. This difference can be partially compensated by group culture or adding hormones and growth factors to the medium [46].
Both physico-chemical and biological factors present in the microenvironment may contribute to the improved development of individually cultured embryos in embryo-conditioned media [18, 21] [29] [47]. Another indirect evidence for the benefit of this established and maintained microenvironment is the repeated (and unexpected) lack of convincing benefits of perfusion/dynamic embryo culture systems seemingly providing an optimised fresh environment for the embryos [25, 48] [49]. In static cultures, the group effect is stronger when the embryo density (embryo number/medium volume ratio) is higher, and the distance between the embryos is smaller [14] [22] [40] [50].
Protection
Despite our best efforts, in vitro embryo cultures may include many harmful factors that delay or stop embryo development, or have some long-term compromising effect on developmental competence in vivo [10]. These factors include contaminated chemicals; toxic components descending from the oil overlay, dissolved from the plastic dishes or introduced by the atmosphere of the incubator, laboratories or—predominantly—supplied gas mixtures; they may be the product of embryo metabolism [34, 35], or naturally occurring inhibitors [36]. In communal culture, embryos may help each other to neutralise or minimise these factors. This rescue mechanism may certainly have its limits, as we are talking about one of the most sensitive systems in biology, where minuscule, almost undetectable changes may have a detrimental effect. On the other hand, a slight improvement caused by joint efforts of the embryos may result in a considerable difference. Analogue mechanisms were supposed to explain the supportive role of co-cultures with somatic cells in the earlier embryo production systems. However, optimisation of culture parameters eliminated the need for the complicated and unpredictable co-cultures [3] [16, 37] [38]. Admittedly, the net benefit of the collaboration between embryos to protect each other is not entirely clarified.
Individual culture as a new requirement
For decades, apart from the biological benefits, practical reasons also forced embryologist to culture embryos in groups. It was easier to prepare dishes and handle embryos, and it was less expensive, too.
However, during the past decade, more and more human IVF laboratories chose individual embryo culture. In 2010, Van Voorhis et al. found that in the USA, 9 out of the ten top-ranked IVF clinics still used group culture [51]. Four years later, according to a worldwide analysis by Christianson et al., a slight majority (55%) of labs preferred to keep embryos individually [52]. This tendency most probably continued in the past 5 years and will do so in the foreseeable future, again for practical reasons.
Most candidate methods used for the selection of embryos with the highest developmental competence require individual follow-up screenings [53] [54] [55]. Optimisation of embryo culture procedures may require sibling studies with decreased numbers of zygotes per group and the elimination of inconsistencies caused by the presence of multiple embryos. The increasing average age of patients and the decreasing intensity of stimulation in mild protocols reduce the number of zygotes to a level where group effects may not be present, creating a need for quasi- or definite individual culture systems. Finally, although relevant data are insufficient, the presence of degenerated or dead embryos may negatively affect the development of healthy ones [18, 47].
Unfortunately, the growing application of individual cultures did not stimulate a widespread effort to introduce new culture methods for the production of single embryos whose quality is similar to—or even higher than—those grown in communal cultures. Attempts to create such systems are sporadic, the applied models are diverse and hard to compare, and most reports about efficiency lack further independent confirmations. Accordingly, the overwhelming majority of laboratories still use the most traditional ways for single embryos, too.
Limitations of the current approach
But why would we need a better culture system?
Because the “drop on a flat surface covered by oil” approach has serious limitations. Before we could fully exploit the benefit of low embryo density in individual cultures, we must tackle the inconsistencies and technical problems.
According to Gardner and Lane, in humans, the minimum amount of medium should be 6.25 to 12.5 μl per embryo to avoid the depletion of nutrients and the buildup of negative factors [26]. That is consistent with the suggestion of Ebner et al., with one embryo in 6 to 10 μl medium [24]. However, these suggestions were made for communal cultures of 2 to 4 embryos, so the recommended volume of the drop is between 12 and 50 μl. An individual culture may require proportionally smaller drops.
Unfortunately, the benefits of this approach are controversial. When single human embryos were cultured in drops, in one experiment blastocyst formation was compromised if the drop volume was decreased from 25 to 7 μl [56]. Results of another publication from the same year contradicted this observation; the 7 μl drop culture was more supportive for individual human embryos than the 35 μl one [57].
This contradiction is hard to explain with the nutrient-toxic component-ligand effect circle. Both research teams used commercially available 35 mm Petri dishes, oil, two-phase media and protein supplements from different but acknowledged vendors. Variations in media composition and the related culture conditions (pH, oxygen concentration) cannot explain the contradicting outcome in 7 μl drops as early as 3 days of culture between the two studies.
We have to realise: while we deal much with minuscule differences in media composition and the possible toxic effect of practically anything in the lab including the aftershaves used by the embryologists, we seem to forget some details that may make our culture systems intrinsically handicapped even before we start to use them. Thanks to the extensive work of Swain et al. (summarised in [58]) we have now more, in some way shocking details about the osmolality issues related to the present-day culture systems. In short, Swain states that (1) despite common belief, oil does not prevent evaporation of media; (2) the level of evaporation depends on the thickness of the oil overlay, and is also determined by the shape and size of the drop (also supported by Yumoto et al.; [59]); (3) evaporation increases the osmolality and pH of the media along with the concentration of potentially harmful components; and (4) evaporation in (now widely used) dry incubators can reach dangerous levels, especially during uninterrupted culture.
The level of evaporation may be influenced by additional, partially unpredictable factors as well. Profound differences exist between seemingly identical dishes from different sources, and producers may change the surface coat without warning (De Munck, pers. comm.), resulting in different shapes of identically produced drops before overlaying it with oil. Almost all laboratories have their established way to make the drops: just put on the surface and cover with oil; or use half of the required amount, cover with oil then add the rest; or make the drop using the required amount, cover with oil then remove the medium and replace it with a fresh one, etc. Obviously, all these manipulations result in drops of different shape, height and possible osmotic characteristics even before the oil is added, and create a protective oil layer of different thickness over the drop. In addition, time is a crucial factor, and the preparation of 6, 8 or 12 drops per dish cannot happen quickly enough. The ambient temperature of the laboratory, the bench, the shape of the base of the dish, i.e. whether or not in direct contact with the heated bench, the use of air-flow boxes, even the rate of the ventilation of the laboratory, all these factors may considerably influence evaporation.
In communal cultures, with two or even four 25 to 50 μl droplets in one dish, the preparation is quicker, and the surface/volume ratio is lower. Consequently, the osmolality change is modest and may be tolerable for the embryos. However, the smaller the volume is, and the more droplets we have to prepare, evaporation becomes more drastic—and continues to increase during the whole culture period. The elevated surface/volume ratio may also result in high diffusion of lipid-soluble materials—including those required for embryo development—into the oil layer [60]. Unknown deleterious materials may also diffuse from the oil into the medium [61]. Is it possible that the “minimum required amount” was not defined by the lack of nutrients and accumulated toxic metabolic products, but (at least partially) by the inappropriate dish preparation and/or dry incubation systems accompanied by the possible negative effect of the oil overlay?
Another contradiction between two observations also suggests re-thinking of our embryo culture systems. In bovine individual embryo culture, Carolan et al. found no blastocyst development in 1 μl drops covered with oil. Compromised rates were observed in 2 or 5 μl; and 10 μl medium was required to achieve the full developmental potential in vitro [62]. In contrast, according to our experience, in a static Glass Oviduct (GO) microcapillary system (see discussed later), less than 1 μl volume was enough to support appropriate development of a single bovine embryo to the blastocyst stage, during 7 days of uninterrupted culture [63] [64]. Culture media and blastocyst rates achieved with control group cultures were similar in the two laboratories. We have to mention that mouse embryos’ requirements may be markedly different, a 2 μl drop medium may provide appropriate conditions for individual cultures and less than 1 μl is enough for two mouse zygotes to develop to blastocysts [29] [63].
Obviously, the elimination of dry incubators may help to alleviate the problem with osmolality to a certain level, but—considering the legendary conservativism of human embryology—it may take another 10 years or more. A similar change based now on rock-hard evidence that is still far from completion is the use of appropriate low oxygen gas mixtures. On the other hand, other factors partially listed above will be even more difficult to standardise. Moreover, even if we establish a highly standardised and optimised individual culture system based on the “drop under oil” principle, the suggested minimum amount of medium (7 to 10 μl) is orders of magnitude higher than the solution surrounding the embryos in vivo and may be inappropriate for the efficient manifestation of the supportive role of the microenvironment.
In summary, the applicability of our traditional approach is highly debatable for a standardised individual embryo culture due to inconsistencies that may be difficult to eliminate or compensate. We may need another solution.
Dreams and realities
In the era of virtual reality, artificial intelligence and robotic surgery, the obvious answer should be automation based on microchip analogues. Unfortunately, in embryology, we frequently need to accept medieval-level solutions, including the absurdly primitive but most efficient vitrification techniques [65]. Our only excuse is that the controversy is not restricted to our discipline. In essence, we now experience a similar situation when the most traditional but solely efficient quarantine is applied in the fight against a contagious viral infection.
Channels and tubes
Two decades ago, microfluidics-microchannels were the great promise for the future of embryology [66]. With the passing years, the area remained extremely promising [67] [68, 69] and may remain so forever, without any practical consequences to the everyday work in a routine human IVF laboratory. To pass such an innovative approach through the financial, technical and administrative difficulties requires at least a dozen different steps, one more complicated than the other. Our impression is that the application of microchannels has not completed half of these steps yet, and the advancement seems to have slowed down due to numerous external and internal factors [70].
On the other hand, a preliminary analogue of microchannels, the GO system provided clear evidence that small narrow tubes may offer an appropriate environment to support embryo development [63]. In narrow glass microtubes, the capillary effect was used to load one-cell embryos. With manual immersion into the embryo-containing medium covered with oil, the tube picked up first an oil column, then <1 μl medium with the one-cell embryo and finally, when retracted, again some oil. The two oil plugs at the end effectively separated the medium from the gas atmosphere of incubators, and the development of embryos continued undisturbed for up to 7 days. In the case of cattle embryos, the blastocyst rate was equal to those achieved in group cultures. Expelling embryos was also easy, and without any losses [64]. Unfortunately, this “proof of concept” model for individual embryo cultures has been left unexploited due to the lack of some supporting devices, funding and—in general—interest.
Microwells: invention and achievements
Curiously, the idea to create a small impression in the bottom of the dish for individual embryo cultures has slipped the notice of embryologists for long. A similar system was described in 1993 for merging embryos with embryonic stem cells to produce aggregation chimaeras in mouse [71]. With the introduction of zona-free nuclear transfer techniques, this approach was tested with the sole purpose of keeping blastomeres of pre-compacted bovine embryos together [64] [72]. Microwells were initially prepared in four-well dishes (hence the name: Well of the Well or WOW) with pre-heated or cold metal rods and mechanical pressure. Despite efforts to avoid extensive distortion of the walls, the optical clarity was compromised, and embryo evaluation inside the microwells was difficult. However, such assessment was not needed as WOWs were used for uninterrupted culture to the blastocyst stage. On the other hand, breaking the special surface layers of some plastic wells or Petri dishes had no adverse effect on embryo development. Although preparation of the microwells with rods pre-heated over a flame required less mechanical force and resulted in smoother surfaces, the practice was later abandoned. With ambient temperature rods, the microwells were prepared when the dishes were either empty or filled with medium, then covered with oil and pre-incubated overnight. This method was very helpful to decrease the formation of gas bubbles inside the microwells.
Despite this rather primitive and drastic preparation procedure, the WOW system was found uniquely successful for the culture of single embryos of various mammalian species, of different origin and for different purposes. Regarding the original goal, zona-free embryos generated by handmade cloning in cattle, sheep, pig, horse, goat, buffalo—the total number being more than one million—were produced in the WOWs (Table 1; see also summarised in [87]). Blastocyst rates were identical to those achieved through group cultures of zona-intact, parthenogenetically activated or IVF embryos of the same species, and no significant losses or developmental abnormalities occurred after transfer. Accordingly, WOWs have successfully compensated for the lack of both the zona pellucida and the group effect by preventing the disassembly of pre-compacted embryos and maintaining high in vitro and in vivo developmental competence, respectively.
Table 1.
Selected publications describing the successful use of the WOW system for cloned zona-free embryos in domestic species
| Species and origin | Days of culture | References |
|---|---|---|
| Bovine zona-free HMC embryos | Day 0 to day 7 | 45, 72, 73, 76, 77, 79, 86, 87 |
| Ovine zona-free HMC embryos | Day 0 to day 7 | 73, 74, 76 |
| Porcine zona-free HMC embryos | Day 0 to day 5 | 75, 76, 81, 83, 84, 85 |
| Equine zona-free, cloned embryos | Day 0 to day 7 | 76, 82 |
| Buffalo zona-free HMC embryos | Day 0 to day 7 | 78 |
| Goat zona-free HMC embryos | Day 0 to day 7 | 80, 88 |
The surprisingly high developmental rates after somatic cell nuclear transfer gave the inspiration to use the system also for the individual culture of in vitro–produced bovine embryos [89]. It has been revealed that the supportive effect was independent of the number of microwells in one well, filled with 400 μl medium and 400 μl oil, and the presence of the zona pellucida did not modify the outcome. A similar supportive effect was observed in porcine, murine and human embryo cultures as well [90]. The lack of communication between embryos in two adjacent microwells was confirmed by others [91] [92]. The WOW system improved both qualitative and quantitative parameters of bovine embryos cultured in small numbers compared to those cultured in the traditional system [93] [94]. Ieda et al. found an increased number and improved quality of bovine blastocysts after individual culture in microwell vs microdrop cultures. Metabolite concentrations were also higher in WOWs than in drops [95]. Gene expression patterns of bovine embryos cultured in WOWs showed a closer resemblance to those of in vivo–derived embryos than those that were cultured on flat surfaces [96] [97]. Culture of individual bovine embryos in microwells resulted in a similar outcome as that of groups on a flat surface [98] [99]. According to Sugimura, WOW culture did not improve bovine blastocyst development rates and cell numbers compared to the traditional drop culture, but decreased apoptosis, enhanced oxygen consumption and increased pregnancy rates [100]. Tagawa et al. improved the outcome of bovine embryo bisection and monozygotic twin production by using the WOW system for culturing halves [101].
The WOW was superior to the drop for culturing in vitro–produced porcine embryos in semi-defined medium [102] and for rat embryos up to the morula stage [103]. In mouse, Dai et al. could not improve individual embryo development by using microwells [104], probably due to the inappropriate preparation and small volume (5 μl) of the covering drops. In another experiment, microwells were found to support individual mouse embryo development with an outcome similar to that of communal culture [105].
In a comparative experiment with sibling human embryos, 55 vs 37% blastocyst rates were achieved in WOWs vs traditional drop cultures [90]. A similar culture system resulted in an improvement in both in vitro and in vivo outcomes in humans [106] [107]. In a microwell system, it has been revealed that the presence of degenerated embryos in adjacent wells did not influence the development of human embryos [108]. This observation was also confirmed with bovine embryos [109]. Finally, the WOW system was also successfully used for other purposes including maturation of minke whale oocytes [110], production of embryoid bodies from mouse embryonic stem cells [111] [112] [113], human embryonic stem cell differentiation to cardiomyocytes by using embryoid bodies [114] and lipofection to produce transgenic animals [115].
Alternative technical solutions
As seen in high-speed vitrification methods, the invention of the WOW system stimulated embryologists to find alternative technical realisations for the microwell idea. The lack of industrial support for more than 10 years led to homemade ideas including wells in agar gels [73] or polydimethylsiloxane (PDMS) plates [98, 105], or produced under low pressure to reduce porosity and maintain the osmolality of the medium [99].
Microwell inserts with various shapes and arrangements were also prepared, to be placed into any culture dishes [116]. These inserts may also connect microwells through narrow microchannels permitting the exchange of soluble materials between wells, although there is no convincing evidence regarding the benefit of this approach. Another version of the microwell structure is the application of polyester mesh inserts on the bottom of culture dishes [117, 118]. Although this approach may allow more versatility to study communication between embryos, it may not offer extra benefits for routine cultures of individual embryos [119].
Eventually, for large-scale commercial application, the manual preparation with metal rods had to be replaced with the moulding of polystyrene dishes. The task was more demanding than initially expected, as it required precision instruments and considerable commercial investment. Consequently, it became possible only when a sophisticated and expensive technology needed it.
Time-lapse application
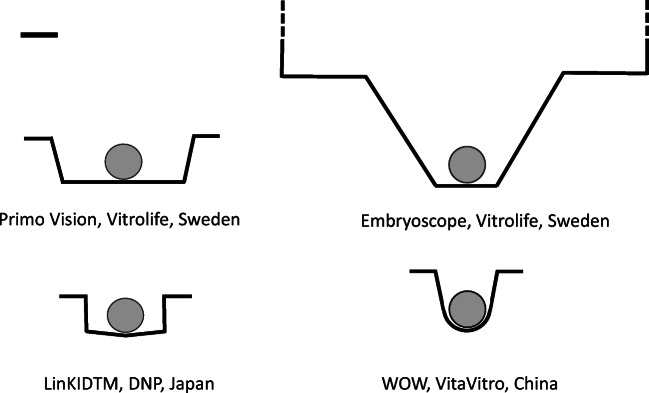
The introduction of the new generation of time-lapse machines suitable for routine use in a human IVF laboratory has suddenly increased the demand for monitoring the development of individual embryos. Analogues of the WOW system have been developed and commercially produced including the basically identical dishes of Primo Vision (Cryo-Innovation Ltd, Hungary; later: Vitrolife, Sweden) and the markedly modified Embryoscope culture plates (Unisense, Denmark; later: Vitrolife, Sweden). Subsequently, similar products were also developed for various time-lapse machines all over the world.
The common feature of these microwells is that they were produced to optimise the optical visibility and practical handling, i.e. the needs of the embryologists (Fig. 1). In some way, all of them compromised the utilisation of the original goal, i.e. to provide an optimal environment for embryo development. Accordingly, although developmental rates in these dishes may be identical to or even better than that obtained in drop cultures, the data should be interpreted with caution as they do not necessarily reflect the real possibilities and limits of a WOW system. On the other hand, the commercial production of these versatile dishes and plates also opened the way to large-scale manufacturing of ready-to-use (real) WOW dishes, fully optimised for the needs of human preimplantation embryo development in vitro.
Fig. 1.
Comparison of sizes and shapes of three commercially available microwells (Primo Vision and Embryoscope dish, Vitrolife, Sweden; and LinKIDTM culture dish, DNP, Japan) used principally for time-lapse purposes; and the original Well of the Well (WOW) dish (VitaVitro, China). Bar represents 150 μm. Globes represent average size human zona-intact one-cell embryos (150 μm diameter). Shapes and dimensions in some dishes are approximates with ±10–20% differences due to deformations and inconsistencies after moulding. Measured by ASME Y145-2018 Dimensioning and Tolerancing Test, 3D Optical Profiler, KLA Tencor Micro XAM 1200, USA
Other non-invasive methods for embryo quality assessment
The potential future application of non-invasive preimplantation genetic testing could force us to revise the “soul” of WOW, due to the necessity of keeping embryos isolated during culture in order to collect cell-free DNA from the culture medium [120]. Undoubtedly, the robustness of this genetic diagnostic tool needs to be demonstrated in the future, and the potential benefits of genetic testing versus the best care for the embryo during culture must be carefully weighed. Additionally, due to the small volume, the WOW culture system may also be useful for determining metabolic characteristics of individual embryos, including oxygen consumption [39].
Size and shape are important
From the embryo’s point of view, smaller seems to be better. A well diameter slightly larger than that of the zona pellucida appears to be sufficient for a single embryo. The shape of the bottom should probably be rounded to minimise the amount of solution surrounding the embryo. Accordingly, a semi-globe with connected straight walls—a kind of inverted sugar loaf shape—may be optimal. The actual parameters should be adjusted to the size of the embryo of the given species, which means they are almost identical for human, bovine, ovine and swine embryos, and considerably smaller in the mouse.
In the initial WOWs, the opening was much wider than the bottom, to make insertion of zygotes and especially the removal of embryos easier. However, according to the study of Feltrin et al. [119, 121], a narrower opening increases cleavage and blastocyst rates of handmade cloned bovine embryos. Similar improvement was also found in pigs (Vajta et al., unpublished), and accordingly, this narrow opening has become preferable for large-scale embryo culture in most laboratories working with handmade cloning. The minimal difference (20 to 30%) between the diameters at the bottom and the opening of the wells also helped to keep the embryos inside the wells while moving the dish. It may also prevent the embryos from floating out of the wells during an accidental hit, caused by (among others) the slamming of the door of the incubator.
The reason for improved embryo development in narrow WOWs is not entirely clear. According to the impressing calculations of Matsuura, there is an approximately two to threefold difference in the concentration of small molecules and macromolecules inside the WOW, allowing the dilution of waste materials and the concentration of autocrine factors around the embryo [122]. This calculated difference is higher in narrow wells and may explain the observed increase in developmental rates. For practical reasons, however, there is a limit to the narrowing of the diameter of the WOWs.
Main characteristics of the traditional drop under oil vs the WOW culture system are summarised in Table 2.
Table 2.
Comparison of the traditional drop under oil culture system with the WOW system. Characteristics and parameters of WOWs described in this table refer to those optimised and industrially produced for human embryo culture
| Drop under oil | Well of the Well (WOW)* | |
|---|---|---|
| Dish bottom | Flat | Inverted sugar loaf-shaped microwells |
| Total amount of medium | 7 to 50 μl | 50 μl |
| Amount of medium surrounding the oocyte or embryo | Usually between 7 and 50 μl | 6 nl in the well minus the volume of the oocyte or embryo itself, i.e. ≥3.6 nl, depending on the stage of development |
| Stability of osmolality, constituents | The larger the drop, the higher | High due to the large amount (≥50 μl) of medium covering and connecting 1 to 25 wells/embryos |
| Chance to build up a microenvironment | The smaller the drop, the higher | Very high |
| Group vs individual culture | Individual culture compromised | Both group and individual culture highly efficient |
| Individual monitoring | Only at individual culture | Appropriate with the optimal shape for embryo culture, visual evaluation slightly compromised |
| Culture system | May require medium change | No medium change required single uninterrupted culture suggested |
| Manual work | Easy, practice varies between laboratories | Easy, can be standardised |
Laboratory work with WOWs
Despite some concern, ready-to-use WOW dishes do not require more time or effort at everyday work than drop cultures. On the contrary, with practice and after following some advice, it may be even easier and more productive.
Loading of single embryos into microwells should not pose a problem even for a beginning embryologist. With appropriate wells and medium/oil cover, moving the dish to and from the incubator does not need more attention than that with drop cultures, either. Removal of the embryos from WOWs should be done carefully, although the zona pellucida provides excellent protection from any potential damage. It is recommended that the embryos should not be directly aspirated from the wells, but instead flushed out with a gentle flow of the medium after directing the pipette tip towards the side of the well. Expanded blastocysts may fill the entire space, and the embryos may get stuck in the WOWs if the walls are completely vertical (cylindrical shape). However, with smooth and slightly widening V-shaped walls connected to a round bottom, the expansion just lifts the embryos out of the well automatically. Although this kind of “hatching” may be practical and useful for the selection of the most advanced embryos, it may hamper the identification of embryos at the very last moment. A slightly increased diameter of the wells may prevent such escapes and keep expanded blastocyst inside.
When preparing ready-to-use WOW dishes for culture, embryologists may encounter their eternal enemy that hamper pipetting, scramble dishes, ruin microchannels and embitter everyday work: air bubbles. In fact, this is the only advantage of homemade preparation of WOWs with a metal rod, as using dishes pre-filled with medium and oil prevent bubble formation. Producers of ready-to-use dishes and plates suggest various solutions for the problem including pre-heating both the media and the dish before preparation, vacuum treatment of the media, mechanical removal of the bubbles by tapping the dish, pushing out bubbles with a glass rod and aspiration with a narrow polished capillary. None of these solutions is absolutely safe and efficient; moreover, seemingly bubble-free wells may also develop bubbles during the subsequent overnight incubation. In our experience, a unique, non-toxic coat applied on the surface and the proper way of filling the pre-warmed dishes with pre-warmed media is the most efficient and reliable way to get rid of this annoying problem.
During our extensive work with manually prepared WOWs, we did not experience any positive or negative effect of using more or fewer wells (from 1 to 50) covered with various amounts of medium (8 to 400 μl for one WOW/embryo, single uninterrupted culture to the blastocyst stage). The distance between the wells was determined empirically, to make preparation, loading, evaluation and removal trouble-free. Using various distances did not influence developmental rates [89] (Vajta, unpublished) (Chen, unpublished). This observation was also confirmed by others [27, 91].
Conclusion
Although embryo culture is a crucial part of human-assisted reproduction, establishment and widespread application of techniques meeting new demands have been slowed down after the millennium. A simple modification of the culture dish offers a solution to compensate for the disadvantages of individual embryo culture and eliminate the inconsistencies related to osmotic changes during dish preparation and incubation. By culturing embryos in microwells of appropriate size and shape, the established microenvironment ensures quantitative and qualitative improvement in developmentally competent blastocyst development and helps to standardise embryo culture conditions between different laboratories.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gábor Vajta, Email: gabor.vajta@hotmail.com.
Lodovico Parmegiani, Email: drlparmegiani@hotmail.com.
Zoltan Machaty, Email: zmachaty@purdue.edu.
Wen Bin Chen, Email: chenwb@vitavitro.com.
Sergey Yakovenko, Email: 7909018@mail.ru.
References
- 1.Cairo Consensus Group ‘There is only one thing that is truly important in an IVF laboratory: everything’ Cairo Consensus Guidelines on IVF Culture Conditions. Reprod BioMed Online. 2020;40:33–60. doi: 10.1016/j.rbmo.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis GE. Early embryo mortality in natural human reproduction: what the data say. F1000Research. 2016;5:2765. doi: 10.12688/f1000research.8937.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vajta G, Rienzi L, Cobo A, Yovich J. Embryo culture: can we perform better than nature? Reprod Biomed. Online. 2010:453–69. [DOI] [PubMed]
- 4.Pool TB, Schoolfield J, Han D. Human embryo culture media comparisons. Methods Mol Biol. 2012;912:376–386. doi: 10.1007/978-1-61779-971-6_21. [DOI] [PubMed] [Google Scholar]
- 5.Gatimel N, Moreau J, Parinaud J, Léandri RD. Need for choosing the ideal pH value for IVF culture media. J Assist Reprod Genet. 2020. 10.1007/s10815-020-01726-5. [DOI] [PMC free article] [PubMed]
- 6.Baak NA, Cantineau AE, Farquhar C, Brison DR. Temperature of embryo culture for assisted reproduction. Cochrane Database Syst Rev. 2019;9:CD012192. doi: 10.1002/14651858.CD012192.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain JE. Optimal human embryo culture. Semin Reprod Med. 2015;33:103–117. doi: 10.1055/s-0035-1546423. [DOI] [PubMed] [Google Scholar]
- 8.Sfontouris IA, Martins WP, Nastri CO, Viana IGR, Navarro PA, Raine-Fenning N, van der Poel S, Rienzi L, Racowsky C. Blastocyst culture using single versus sequential media in clinical IVF: a systematic review and meta-analysis of randomised controlled trials. J Assist Reprod Genet. 2016;33:1261–1272. doi: 10.1007/s10815-016-0774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed ML, Hamic A, Thompson DJ, Caperton CL. Challenging traditional embryo culture techniques with a simplified continuous single medium protocol. J Clin Embryol. 2009;13:33–41. [Google Scholar]
- 10.Krisher RL, Schlenker T. Culture of human preimplantation embryos in a clinical ART setting. Methods Mol Biol. 2006;2019:355–371. doi: 10.1007/978-1-4939-9566-0_24. [DOI] [PubMed] [Google Scholar]
- 11.Rebollar-Lazaro I, Matson P. The culture of human cleavage stage embryos alone or in groups: effect upon blastocyst utilisation rates and implantation. Reprod Biol. 2010;10:227–234. [PubMed] [Google Scholar]
- 12.Castillo CM, Harper J, Roberts SA, O’Neill HC, Johnstone ED, Brison DR. The impact of selected embryo culture conditions on ART treatment cycle outcomes: a UK national study. Hum Reprod Open. 2020;2020:1–13. doi: 10.1093/hropen/hoz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiley L, Yamami S, Van Muyden D. Effect of potassium concentration, type of protein supplement, and embryo density on mouse preimplantation development in vitro. Fertil Steril. 1986;45:111–119. doi: 10.1016/s0015-0282(16)49107-7. [DOI] [PubMed] [Google Scholar]
- 14.Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci U S A. 1990;87:4756–4760. doi: 10.1073/pnas.87.12.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieger D. Culture systems: Physiological and environmental factors that can affect the outcome of human ART. Methods Mol Biol. 2012;912:333–354. doi: 10.1007/978-1-61779-971-6_19. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DK, Lane M, Spitzer A, Batt PA. Enhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells : amino acids , vitamins , and culturing embryos in groups stimulate development. Biol Reprod. 1994;400:390–400. doi: 10.1095/biolreprod50.2.390. [DOI] [PubMed] [Google Scholar]
- 17.Moessner J, Dodson WC. The quality of human embryo growth is improved when embryos are cultured in groups rather than separately. Fertil Steril. 1995;64:1034–1035. doi: 10.1016/s0015-0282(16)57925-4. [DOI] [PubMed] [Google Scholar]
- 18.Salahuddin S, Ookutsu S, Goto K, Nakanishi Y, Nagata Y. Fertilization and early embryology: effects of embryo density and co-culture of unfertilised oocytes on embryonic development of in-vitro fertilised mouse embryos. Hum Reprod. 1995;10:2382–2385. doi: 10.1093/oxfordjournals.humrep.a136303. [DOI] [PubMed] [Google Scholar]
- 19.Almagor M, Bejar C, Kafka I, Yaffe H. Pregnancy rates after communal growth of preimplantation human embryos in vitro. Fertil Steril. 1996. [DOI] [PubMed]
- 20.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor α1. Biol Reprod. 1997;56:1088–1096. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- 21.Spindler RE, Wildt DE. Quality and age of companion felid embryos modulate enhanced development by group culture. Biol Reprod. 2002;173:167–173. doi: 10.1095/biolreprod66.1.167. [DOI] [PubMed] [Google Scholar]
- 22.Stokes PJ, Abeydeera LR, Leese HJ. Development of porcine embryos in vivo and in vitro ; evidence for embryo F cross talk in vitro. Dev Biol. 2005;284:62–71. doi: 10.1016/j.ydbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Nagao Y, Iijima R, Saeki K. Interaction between embryos and culture conditions during in vitro development of bovine early embryos. Zygote. 2008;16:127–133. doi: 10.1017/S0967199408004644. [DOI] [PubMed] [Google Scholar]
- 24.Ebner T, Shebl O, Moser M, Mayer RB, Arzt W, Tews G. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod BioMed Online. 2010;21:762–768. doi: 10.1016/j.rbmo.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Swain JE, Smith GD. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update. 2011;17:541–557. doi: 10.1093/humupd/dmr006. [DOI] [PubMed] [Google Scholar]
- 26.Reed M. Chapter 16 Culture systems : embryo density. Embryo Cult Methods Mol Biol: Methods Protoc. 2012;912:273–312. doi: 10.1007/978-1-61779-971-6_16. [DOI] [PubMed] [Google Scholar]
- 27.Sugimura S, Akai T, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, et al. Effect of embryo density on in vitro development and gene expression in bovine in vitro-fertilized embryos cultured in a microwell system. J Reprod Dev. 2013;59:115–122. doi: 10.1262/jrd.2012-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ergin EG, Caliskanan E, Yalcinkaya E, Oztel Z, Cokelez K, Ozay A, et al. Frequency of embryo multinucleation detected by time-lapse system and its impact on pregnancy outcome. Fertil Steril. 2014;102:1029–1033. doi: 10.1016/j.fertnstert.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Kelley RL, Gardner DK. In vitro culture of individual mouse preimplantation embryos: the role of embryo density, microwells, oxygen, timing and conditioned media. Reprod BioMed Online. 2017;34:441–454. doi: 10.1016/j.rbmo.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Ruíz M, Santamaría-López E, Blasco V, Hernáez MJ, Caligara C, Pellicer A, Fernández-Sánchez M, Prados N. Effect of group embryo culture under low-oxygen tension in benchtop incubators on human embryo culture: prospective, randomised, controlled trial. Reprod Sci. 2020;27:1522–1533. doi: 10.1007/s43032-020-00150-5. [DOI] [PubMed] [Google Scholar]
- 31.Spyropoulou I, Karamalegos C, Bolton VN. A prospective randomised study comparing the outcome of in-vitro fertilisation and embryo transfer following culture of human embryos individually or in groups before embryo transfer on day 2. Hum Reprod. 1999;14:76–79. doi: 10.1093/humrep/14.1.76. [DOI] [PubMed] [Google Scholar]
- 32.Rijnders PM, Jansen CAM. Influence of group culture and culture volume on the formation of human blastocysts: a prospective randomised study. Hum Reprod. 1999;14:2333–2337. doi: 10.1093/humrep/14.9.2333. [DOI] [PubMed] [Google Scholar]
- 33.Alhelou Y, Adenan NAM, Ali J. Embryo culture conditions are significantly improved during uninterrupted incubation : a randomised controlled trial. Reprod Biol. 2018;18:40–45. doi: 10.1016/j.repbio.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod. 1993;385:377–385. doi: 10.1095/biolreprod48.2.377. [DOI] [PubMed] [Google Scholar]
- 35.Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? BioEssays. 1994;16:31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- 36.Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update. 1995;1:91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- 37.Bavister BD. Co-culture for embryo development: is it really necessary? Hum Reprod. 1992;7:1339–1341. doi: 10.1093/oxfordjournals.humrep.a137569. [DOI] [PubMed] [Google Scholar]
- 38.Holm P, Booth PJ, Schmidt MH, Greve T, Callesen H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology. 1999;52:683–700. doi: 10.1016/S0093-691X(99)00162-4. [DOI] [PubMed] [Google Scholar]
- 39.Lopes AS, Greve T, Callesen H. Quantification of embryo quality by respirometry. Theriogenology. 2007;67:21–31. doi: 10.1016/j.theriogenology.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction. 2006;131:269–277. doi: 10.1530/rep.1.00677. [DOI] [PubMed] [Google Scholar]
- 41.Kane MT, Carney EW, Ellington JE. The role of nutrients, peptide growth factors and co-culture cells in development of preimplantation embryos in vitro. Theriogenology. 1992;38:297–313. doi: 10.1016/0093-691x(92)90237-l. [DOI] [PubMed] [Google Scholar]
- 42.Gandolfi F. Autocrine, paracrine and environmental factors influencing embryonic development from zygote to blastocyst. Theriogenology. 1994;41:95–100. [Google Scholar]
- 43.O’Neill C. The potential roles for embryotrophic ligands in preimplantation embryo development. Hum Reprod Update. 2008;14:275–288. doi: 10.1093/humupd/dmn002. [DOI] [PubMed] [Google Scholar]
- 44.O’Neill C. Autocrine mediators are required to act on the embryo by the 2-cell stage to promote normal development and survival of mouse preimplantation embryos in vitro. Biol Reprod. 1998;58:1303–1309. doi: 10.1095/biolreprod58.5.1303. [DOI] [PubMed] [Google Scholar]
- 45.Vajta G, Lewis I, Trounson A, Purup S, Maddox-Hyttel P, Schmidt M, et al. Handmade somatic cell cloning in cattle: analysis of factors contributing to high efficiency in vitro. Biol Reprod. 2003;68:571–578. doi: 10.1095/biolreprod.102.008771. [DOI] [PubMed] [Google Scholar]
- 46.Stojanov T, Alechna S, O’Neill C. In-vitro fertilisation and culture of mouse embryos in vitro significantly retards the onset of insulin-like growth factor II expression from the zygotic genome. Mol Hum Reprod. 1999;5:116–124. doi: 10.1093/molehr/5.2.116. [DOI] [PubMed] [Google Scholar]
- 47.Tao T, Robichaud A, Mercier J, Ouellette R. Influence of group embryo culture strategies on the blastocyst development and pregnancy outcome. J Assist Reprod Genet. 2013;30:63–68. doi: 10.1007/s10815-012-9892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deter RL. Quantitative morphological analysis of early mouse embryogenesis in vitro. I. Perfusion culture system, tissue preparation, sampling. J Embryol Exp Morpholog. 1977;40:91–100. [PubMed] [Google Scholar]
- 49.Thompson JG. Culture without the petri-dish. Theriogenology. 2007;67:16–20. doi: 10.1016/j.theriogenology.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Lane M, Gardner DK. Effect of incubation volume and embryo density on the development and viability of mouse embryos in vitro. Hum Reprod. 1992;7:558–562. doi: 10.1093/oxfordjournals.humrep.a137690. [DOI] [PubMed] [Google Scholar]
- 51.Van Voorhis BJ, Thomas M, Surrey ES, Sparks A, Ph D. What do consistently high-performing in vitro fertilisation programs in the U.S. do? Fertil Steril. 2010;94:1346–1349. doi: 10.1016/j.fertnstert.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 52.Christianson MS, Zhao Y, Shoham G, Granot I, Safran A, Khafagy A, Leong M, Shoham Z. Embryo catheter loading and embryo culture techniques: results of a worldwide web-based survey. J Assist Reprod Genet. 2014;31:1029–1036. doi: 10.1007/s10815-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pribenszky C, Losonczi E, Molnar M, Lang Z, Matyas S, Rajczy K, et al. Prediction of in-vitro developmental competence of early cleavage-stage mouse embryos with compact time-lapse equipment. Reprod BioMed Online. 2010;20:371–379. doi: 10.1016/j.rbmo.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–1463. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 55.Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, Baker VL, Adamson GD, Abusief ME, Gvakharia M, Loewke KE, Shen S. Improving embryo selection using a computer-automated time-lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412–419. doi: 10.1016/j.fertnstert.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 56.De Munck N, Santos-Ribeiro S, Mateizel I, Verheyen G. Reduced blastocyst formation in reduced culture volume. J Assist Reprod Genet. 2015;32:1365–1370. doi: 10.1007/s10815-015-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minasi MG, Fabozzi G, Casciani V, Lobascio AM, Colasante A, Scarselli F, Greco E. Improved blastocyst formation with reduced culture volume: comparison of three different culture conditions on 1128 sibling human zygotes. J Assist Reprod Genet. 2015;32:215–220. doi: 10.1007/s10815-014-0399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swain JE. Controversies in ART: considerations and risks for uninterrupted embryo culture. Reprod BioMed Online. 2019;39:19–26. doi: 10.1016/j.rbmo.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Yumoto K, Iwata K, Sugishima M, Yamauchi J, Nakaoka M, Tsuneto M. Unstable osmolality of microdrops cultured in non-humidified incubators. J Assist Reprod Genet. 2019;36:1571–1577. doi: 10.1007/s10815-019-01515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu KP. Studies on the bovine follicular oocyte maturation and fertilisation in vitro. Copenhagen, Denmark: The Royal Veterinary and Agricultural University; 1987. [Google Scholar]
- 61.Roh S, Choi YJ, Min BM. A novel microtube culture system that enhances the in vitro development of parthenogenetic murine embryos. Theriogenology. 2008;69:262–267. doi: 10.1016/j.theriogenology.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Carolan C, Lonergan P, Khatir H, Mermillod P. In vitro production of bovine embryos using individual oocytes. Mol Reprod Dev. 1996;150:145–150. doi: 10.1002/(SICI)1098-2795(199610)45:2<145::AID-MRD6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 63.Thouas GA, Jones GM, Trounson AO. The “GO” system - A novel method of microculture for in vitro development of mouse zygotes to the blastocyst stage. Reproduction. 2003;126:161–169. doi: 10.1530/rep.0.1260161. [DOI] [PubMed] [Google Scholar]
- 64.Vajta G, Lewis IM, Hyttel P, Thouas GA, Trounson AO. Somatic cell cloning without micromanipulators. Cloning. 2001;3:89–95. doi: 10.1089/15204550152475590. [DOI] [PubMed] [Google Scholar]
- 65.Vajta G. Vitrification in ART: past, present and future. Theriogenology. 2020. 10.1016/j.theriogenology.2020.01.057. [DOI] [PubMed]
- 66.Pool TB. Recent advances in the production of viable human embryos in vitro *. Reprod BioMed Online. 2002;4:294–302. doi: 10.1016/s1472-6483(10)61820-2. [DOI] [PubMed] [Google Scholar]
- 67.Smith GD, Takayama S. Gamete and embryo isolation and culture with microfluidics. Theriogenology. 2007;68:190–195. doi: 10.1016/j.theriogenology.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 68.Smith GD, Swain JE, Bormann CL. Microfluidics for gametes, embryos, and embryonic stem cells. Semin Reprod Med. 2011;29:5–14. doi: 10.1055/s-0030-1268699. [DOI] [PubMed] [Google Scholar]
- 69.Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod. 2017;23:257–268. doi: 10.1093/molehr/gaw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vajta G. The global mission of mammalian embryology : are we as good as supposed ? Curr Trends Clin Embryol. 2015;2:127–134. [Google Scholar]
- 71.Wood SA, Allen ND, Rossant J, Auerbach A, Nagy A. Nonüinjection methods for the production of embryonic stem cell-embryo chimaeras. Nature. 1993;365:87–89. doi: 10.1038/365087a0. [DOI] [PubMed] [Google Scholar]
- 72.Booth PJ, Tan SJ, Reipurth R, Holm P, Callesen H. Simplification of bovine somatic cell nuclear transfer by application of a zona-free manipulation technique. Cloning Stem Cells. 2001;3:139–150. doi: 10.1089/153623001753205098. [DOI] [PubMed] [Google Scholar]
- 73.Peura TT, Vajta G. A comparison of established and new approaches in ovine and bovine nuclear transfer. Cloning Stem Cells. 2003;5:257–277. doi: 10.1089/153623003772032772. [DOI] [PubMed] [Google Scholar]
- 74.Zhang P, Liu P, Dou H, Chen L, Chen L, Lin L, Tan P, Vajta G, Gao J, du Y, Ma RZ. Handmade cloned transgenic sheep rich in Omega-3 fatty acids. PLoS One. 2013;8:e55941. doi: 10.1371/journal.pone.0055941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du Y, Kragh PM, Zhang Y, Li J, Schmidt M, Bøgh IB, et al. Piglets born from handmade cloning, an innovative cloning method without micromanipulation. Theriogenology. 2007;68:1104–1110. doi: 10.1016/j.theriogenology.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 76.Lagutina I, Lazzari G, Duchi R, Turini P, Tessaro I, Brunetti D, Colleoni S, Crotti G, Galli C. Comparative aspects of somatic cell nuclear transfer with conventional and zona-free method in cattle, horse, pig and sheep. Theriogenology. 2007;67:90–98. doi: 10.1016/j.theriogenology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Rodríguez L, Navarrete FI, Tovar H, Cox JF, Castro FO. High developmental potential in vitro and in vivo of cattle embryos cloned without micromanipulators. J Assist Reprod Genet. 2008;25:13–16. doi: 10.1007/s10815-007-9194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah RA, George A, Singh MK, Kumar D, Chauhan MS, Manik R, Palta P, Singla SK. Hand-made cloned buffalo ( Bubalus bubalis ) embryos: comparison of different media and culture systems. Cloning Stem Cells. 2008;10:435–442. doi: 10.1089/clo.2008.0033. [DOI] [PubMed] [Google Scholar]
- 79.Ribeiro EDS, Gerger RPDC, Ohlweiler LU, Ortigari I, Mezzalira JC, Forell F, et al. Developmental potential of bovine hand-made clone embryos reconstructed by aggregation or fusion with distinct cytoplasmic volumes. Cloning Stem Cells. 2009;11:377–386. doi: 10.1089/clo.2009.0022. [DOI] [PubMed] [Google Scholar]
- 80.Akshey YS, Malakar D, De AK, Jena MK, Garg S, Dutta R, et al. Hand-made cloned goat ( Capra hircus ) embryos—a comparison of different donor cells and culture systems. Cell Reprog. 2010;12:581–588. doi: 10.1089/cell.2009.0120. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Østrup O, Li J, Vajta G, Kragh PM, Purup S, Callesen H. Cell colony formation induced by Xenopus egg extract as a marker for improvement of cloned blastocyst formation in the pig. Cell Rep. 2011;13:521–526. doi: 10.1089/cell.2011.0029. [DOI] [PubMed] [Google Scholar]
- 82.Gambini A, De Stefano A, Bevacqua RJ, Karlanian F, Salamone DF. The aggregation of four reconstructed zygotes is the limit to improve the developmental competence of cloned equine embryos. PLoS One. 2014;9:e110998. doi: 10.1371/journal.pone.0110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Callesen H, Liu Y, Pedersen HS, Li R, Schmidt M. Increasing efficiency in production of cloned piglets. Cell Rep. 2014;16:2–5. doi: 10.1089/cell.2014.0053. [DOI] [PubMed] [Google Scholar]
- 84.Liu T, Dou H, Xiang X, Li L, Li Y, Lin L, Pang X, Zhang Y, Chen Y, Luan J, Xu Y, Yang Z, Yang W, Liu H, Li F, Wang H, Yang H, Bolund L, Vajta G, du Y. Factors determining the efficiency of porcine somatic cell nuclear transfer: data analysis with over 200,000 reconstructed embryos. Cell Rep. 2015;17:463–471. doi: 10.1089/cell.2015.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buemo CP, Gambini A, Moro LN, Hiriart MI, Fernández-Martín R, Collas P, et al. Embryo aggregation in pig improves cloning efficiency and embryo quality. PLoS One. 2016;11:1–14. doi: 10.1371/journal.pone.0146390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cortez J, Vajta G, Valderrama N, Portocarrero G, Quintana J. High pregnancy and calving rates with a limited number of transferred handmade cloned bovine embryos. Cell Rep. 2018;20:4–8. doi: 10.1089/cell.2017.0024. [DOI] [PubMed] [Google Scholar]
- 87.Vajta G. A Sleeping Beauty Awaiting the kiss? Opinion piece: cloning. Cell Reprog. 2018;20:145–156. doi: 10.1089/cell.2017.0058. [DOI] [PubMed] [Google Scholar]
- 88.Bhat MH, Yaqoob SH, Khan FA, Khan HM, Fazili R, Ganai NA, et al. Live birth of a Pashmina goat kid after transfer of handmade cloned embryos. J Reprod Dev [Internet]. 2019;e-pub. 10.1262/jrd.2018-126. [DOI] [PubMed]
- 89.Vajta G, Peura TT, Holm P, Páldi A, Greve T, Trounson AO, et al. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev. 2000;55:256–264. doi: 10.1002/(SICI)1098-2795(200003)55:3<256::AID-MRD3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 90.Vajta G, Korösi T, Du Y, Nakata K, Ieda S, Kuwayama M, et al. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod BioMed Online. 2008;17:73–81. doi: 10.1016/s1472-6483(10)60296-9. [DOI] [PubMed] [Google Scholar]
- 91.Kang SS, Ofuji S, Imai K, Huang W, Koyama K, Yanagawa Y, et al. The efficacy of the well of the well (WOW) culture system on development of bovine embryos in a small group and the effect of number of adjacent embryos on their development. Zygote. 2014;23:412–415. doi: 10.1017/S096719941400001X. [DOI] [PubMed] [Google Scholar]
- 92.Matoba S, Fair T, Lonergan P. Maturation, fertilisation and culture of bovine oocytes and embryos in an individually identifiable manner: a tool for studying oocyte developmental competence. Reprod Fertil Dev. 2010;22:839–851. doi: 10.1071/RD09277. [DOI] [PubMed] [Google Scholar]
- 93.Pereira DC, Dode MAN, Rumpf R. Evaluation of different culture systems on the in vitro production of bovine embryos. Theriogenology. 2005;63:1131–1141. doi: 10.1016/j.theriogenology.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 94.Cebrian-Serrano A, Salvador I, Silvestre MA. Beneficial effect of two culture systems with small groups of embryos on the development and quality of in vitro-produced bovine embryos. J Vet Med Ser C Anat Histol Embryol. 2014;43:22–30. doi: 10.1111/ahe.12043. [DOI] [PubMed] [Google Scholar]
- 95.Ieda S, Akai T, Sakaguchi Y, Shimamura S, Sugawara A, Kaneda M, Matoba S, Kagota M, Sugimura S, Kaijima H. A microwell culture system that allows group culture and is compatible with human single media. J Assist Reprod Genet. 2018;35:1869–1880. doi: 10.1007/s10815-018-1252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoelker M, Rings F, Lund Q, Ghanem N, Phatsara C, Griese J, Schellander K, Tesfaye D. Effect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitro. Reproduction. 2009;137:415–425. doi: 10.1530/REP-08-0370. [DOI] [PubMed] [Google Scholar]
- 97.Ghanem N, Salilew-Wondim D, Gad A, Tesfaye D, Phatsara C, Tholen E, Looft C, Schellander K, Hoelker M. Bovine blastocysts with developmental competence to term share similar expression of developmentally important genes although derived from different culture environments. Reproduction. 2011;142:551–564. doi: 10.1530/REP-10-0476. [DOI] [PubMed] [Google Scholar]
- 98.Akagi S, Hosoe M, Matsukawa K, Ichikawa A, Tanikawa T, Takahashi S. Culture of bovine embryos on a polydimethylsiloxane (PDMS) microwell plate. J Reprod Dev. 2010;56:475–479. doi: 10.1262/jrd.09-213h. [DOI] [PubMed] [Google Scholar]
- 99.Iwamoto D, Kato N, Taniguchi S, Taguchi Y, Kishi M, Saeki K. In vitro culture of single bovine embryos with microwell plates made of poly(dimethylsiloxane) cured under low pressure. Int J Biomater. 2018;2018. 10.1155/2018/7546986. [DOI] [PMC free article] [PubMed]
- 100.Sugimura S, Akai T, Somfai T, Hirayama M, Aikawa Y, Ohtake M, Hattori H, Kobayashi S, Hashiyada Y, Konishi K, Imai K. Time-lapse cinematography-compatible polystyrene-based microwell culture system: a novel tool for tracking the development of individual bovine embryos. Biol Reprod. 2010;83:970–978. doi: 10.1095/biolreprod.110.085522. [DOI] [PubMed] [Google Scholar]
- 101.Tagawa M, Matoba S, Narita M, Saito N, Nagai T, Imai K. Production of monozygotic twin calves using the blastomere separation technique and Well of the Well culture system. Theriogenology. 2008;69:574–582. doi: 10.1016/j.theriogenology.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Kamiya C, Kobayashi M, Fukui Y. In vitro culture conditions using chemically defined media for in vitro matured and intracytoplasmically inseminated porcine oocytes. J Reprod Dev. 2006;52:625–632. doi: 10.1262/jrd.18025. [DOI] [PubMed] [Google Scholar]
- 103.Popova E, Bader M, Krivokharchenko A. Effect of culture conditions on viability of mouse and rat embryos developed in vitro. Genes (Basel) 2011;2:332–344. doi: 10.3390/genes2020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dai SJ, Xu CL, Wang J, Sun YP, Chian RC. Effect of culture medium volume and embryo density on early mouse embryonic development: tracking the development of the individual embryo. J Assist Reprod Genet. 2012;29:617–623. doi: 10.1007/s10815-012-9744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung YH, Hsiao YH, Kao WL, Hsu CH, Yao DJ, Chen C. Microwells support high-resolution time-lapse imaging and development of preimplanted mouse embryos. Biomicrofluidics. 2015;9. 10.1063/1.4918642. [DOI] [PMC free article] [PubMed]
- 106.Iwayama H, Korekane M, Hara T, Hirai Y, Tokonami K, Yamashita M. Clinical application of a microwell system to in vitro culture of human preimplantation embryos. In Vitro. 2008;25:167–171. [Google Scholar]
- 107.Kida Y, Yamada S, Kawakita N, Yoshimura T, Fukunaga N, Asada Y. The effect of modification of the embryo culture environment on human embryo development. Fertil Steril. 2018;110:e365–e366. [Google Scholar]
- 108.Watanabe H, Kitasaka H, Yoshimura T, Kojima M, Fukunaga N, Asada Y. Effect of degenerated embryos on group cultured embryos in a well of the well culture system. Fertil Steril. 2018;110:e52. [Google Scholar]
- 109.Wydooghe E, Vaele L, Piepers S, Dewulf J, Van Abbeel ED, De Sutter P, et al. Individual commitment to a group effect: strengths and weaknesses of bovine embryo group culture. Reproduction. 2014;148:519–529. doi: 10.1530/REP-14-0213. [DOI] [PubMed] [Google Scholar]
- 110.Fukui Y, Iwayama H, Matsuoka T, Nagai H, Koma N, Mogoe T, et al. Attempt at intracytoplasmic sperm injection of in vitro matured oocytes in common minke whales (Balaenoptera acutorostrata) captured during the Kushiro Coast Survey. J Reprod Dev. 2007;53:945–952. doi: 10.1262/jrd.18182. [DOI] [PubMed] [Google Scholar]
- 111.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 112.Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim JH, Lee SH. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010;31:4296–4303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]
- 113.Jeong GS, Song JH, Kang AR, Jun Y, Kim JH, Chang JY, Lee SH. Surface tension-mediated, concave-microwell arrays for large-scale, simultaneous production of homogeneously sized embryoid bodies. Adv Healthc Mater. 2013;2:119–125. doi: 10.1002/adhm.201200070. [DOI] [PubMed] [Google Scholar]
- 114.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomater. 2010;31:1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ikeda S, Sugimoto M, Kume S. Lipofection of siRNA into bovine 8-16-cell stage embryos using zona removal and the well-of-the-well culture system. J Reprod Dev. 2018;64:199–202. doi: 10.1262/jrd.2017-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krisher RL, Wheeler MB. Towards the use of microfluidics for individual embryo culture. Reprod Fertil Dev. 2010;32:9. doi: 10.1071/RD09219. [DOI] [PubMed] [Google Scholar]
- 117.Booth PJ, Watson TJ, Leese HJ. Prediction of porcine blastocyst formation using morphological, kinetic, and amino acid depletion and appearance criteria determined during the early cleavage of in vitro-produced embryos. BiolReprod. 2007;77:765–779. doi: 10.1095/biolreprod.107.062802. [DOI] [PubMed] [Google Scholar]
- 118.Somfai T, Inaba Y, Aikawa Y, Ohtake M, Kobayashi S, Akai T, et al. Culture of bovine embryos in polyester mesh sections : the effect of pore size and oxygen tension on in vitro development. Reprod Domest Anim. 2010;1109:1104–1109. doi: 10.1111/j.1439-0531.2009.01502.x. [DOI] [PubMed] [Google Scholar]
- 119.Feltrin C, Forell F, Machado M, Queiroz LM, Peixer M, Malard P, et al. Effectiveness of microwell-based in vitro culture systems for zona-free cloned bovine embryos. Acta Sci Vet. 2015;43:1298. [Google Scholar]
- 120.Brouillet S, Martinez G, Coutton C, Hamamah S. Is cell-free DNA in spent embryo culture medium an alternative to embryo biopsy for preimplantation genetic testing? A systematic review. [Internet]. Reprod BioMed Online. Pre-Proof online publication. 2020. 10.1016/j.rbmo.2020.02.002. [DOI] [PubMed]
- 121.Feltrin C, Forell F, dos Santos L, Rodrigues JL. In vitro bovine embryo development after nuclear transfer by handmade cloning using a modified WOW culture system. Reprod Fertil Dev. 2006;18:126. [Google Scholar]
- 122.Matsuura K. Numerical calculations for diffusion effects in the well-of-the-well culture system for mammalian embryos. Reprod Fertil Dev. 2014;26:742–751. doi: 10.1071/RD13025. [DOI] [PubMed] [Google Scholar]