Abstract
Purpose
To investigate the differences concerning post-thawing/warming follicle survival, DNA damage and apoptosis in human ovarian tissues cryopreserved by slow freezing, open, or closed vitrification methods.
Methods
A total of 50 pieces of 5 × 5 × 1 mm ovarian cortical pieces were harvested (5 donor ovaries; mean age 31 ± 6.62 years). From each donor, one cortical piece was used as baseline; the remaining were randomly assigned to slow freezing (SF), vitrification using open device (VF-open), or closed device (VF-closed) groups. After 8–10 weeks of cryostorage, tissues were evaluated 4 h after thawing/warming. Histological analysis was evaluated for follicle survival (primordial and primary follicle densities) by H&E staining. The percentages of primordial and primary follicles with DNA double-strand breaks (γH2AX) and apoptotic cell death pathway activation (AC3) were immunohistochemically assessed. Data were analysed using one-way ANOVA and LSD post hoc comparison.
Results
Compared to the baseline, primordial follicle (pdf) densities significantly declined in all cryopreserved groups (SF, VF-open, and VF-closed, P < 0.05). However, the total and non-apoptotic pdf densities were similar among SF, VF-open, and VF-closed. SF and VF with either open or closed devices did not increase the percentages of primordial or primary follicles with DNA double-strand breaks (DSBs) or apoptosis compared to the baseline or among the freezing methods in the present study.
Conclusion
Based on the intact primordial follicle survival, DNA damage, and apoptosis rates after thawing/warming, SF vs VF with either open or newly developed closed devices appear to be comparable.
Keywords: Fertility preservation, Ovarian tissue cryopreservation, Slow freezing, Vitrification, Ovarian reserve, Follicle survival
Introduction
The long-term survival rates of cancer patients have dramatically improved with the recent advances in early detection tools and treatment modalities [1]. However, treatment regimens with high-dose chemotherapy and radiotherapy in women with cancer can deplete the limited number of follicles in the ovary leading to ovarian insufficiency, infertility, and early menopause [2, 3]. Therefore, several fertility preservations options have evolved for women who undergo gonadotoxic treatments but have not completed childbearing [4, 5]. Oocyte and embryo cryopreservation are established fertility preservation methods for reproductive women who have adequate time for controlled ovarian stimulation and egg retrieval before the initiation of cancer treatment [6]. However, these options are not feasible for children and for those who require immediate start of cancer therapy. Since the first report of successful restoration of ovarian endocrine function with autologous transplantation of frozen-thawed ovarian tissue in 2000 has evolved as a promising option to preserve fertility for aforementioned patient categories [6–20]. As a result, American Society of Reproductive Medicine has recently removed ovarian tissue cryopreservation from the experimental category [21, 22], and the non-experimental potential of ovarian tissue cryopreservation was recognized by the American Society of Clinical Oncology [5].
In a recent meta-analysis, we reported that autologous transplantations of previously cryopreserved ovarian tissues result in 37.7% live birth rate, indicating clinically acceptable success rate [23]. However, not only the live birth rate can be further improved but there is also a need to extend the functional life span of ovarian auto-grafts, which averages around 26.9 months per transplant [23]. While one area of improvement may come from approaches to enhance revascularization of grafts [24], another area for improvement is to optimize the cryopreservation methods.
Currently, there are two main methods for ovarian tissue cryopreservation: slow freezing (SF) and vitrification (VF). SF method is the conventional approach that has been in practice for the last two decades, and it was the method used in almost all reported livebirths following ovarian transplantation. On the other hand, VF method is a relatively new approach for ovarian tissue freezing, and a small number of live births have been associated with this technique [25, 26].
The ultra-rapid freezing method of VF uses relatively higher concentrations of cryoprotectants and provides several practical advantages over the SF method. While the SF protocol requires an automated cryopreservation machine and about a 3–4 h process, the VF protocol can be completed in minutes without specialized equipment. There has been a major shift to VF protocol with human oocyte and embryo cryopreservation because it provides a higher survival rate on post-warming oocytes and embryos [27–31]. However, despite some promising results from bovine [32] and non-human primate ovaries [33], the efficacy of VF in human ovarian tissue cryopreservation compared to conventional SF method remains debatable [34, 35]. Another issue with vitrification is that most devices are open, resulting in potential exposure to pathogens from other specimens in the same storage tanks. Because of the concerns of disease transmission through the liquid nitrogen milieu [36], closed systems are in general preferred for long-term tissue storage.
In this study, we assessed whether the intact follicle survival after vitrification is at least equivalent to slow freezing and to determine if a newly developed closed VF device [37] is equally effective with an open VF device in human ovarian tissue cryopreservation. We analyzed the survival, extent of DNA DSBs, and apoptotic pathway activation in primordial and primary follicles in thawed/warmed human ovarian cortical tissues.
Materials and methods
Study design and human ovarian tissue harvesting
This study protocol was exempted by the Institutional Review Board (IRB) as the ovarian tissues were obtained from organ donor cadavers, not from living subjects. Ovarian tissues were harvested from organ donor cadavers (n = 50 pieces from 5 donor ovaries; mean age 31 ± 6.62 years). From each ovary, the ovarian cortex was surgically separated from medullary tissue and cut into ten 5 × 5 × 1 mm (length × width × thickness) cortical pieces. Of those 10 pieces, one cortical sample was formalin-fixed, and paraffin embedded for histological examination for baseline (fresh) assessment. The remaining nine samples were randomly assigned to the groups of SF, VF using the open (VF-open), or closed device (VF-closed).
Slow freezing and thawing procedure
Slow freezing of ovarian tissue was performed based on the protocol which led to the first successful case of ovarian transplantation [38]. The ovarian cortical tissues were placed into the freezing media containing 1.5 M dimethyl sulfoxide (DMSO, VWR Life Science Amresco, Solon, OH, USA) as cryoprotectant, 0.1 M sucrose (Sigma-Aldrich, St. Louis, MO, USA) in Minimum Essential Medium Eagle Alpha (MEM-alpha, Thermo Fisher Scientific, MA, USA) supplemented with 10% human serum albumin (HSA, LifeGlobal, Guilford, CT, USA). For equilibrium, cortical tissues were subjected to freezing media for 15 min on ice on a rocking table. Next, tissue pieces were placed into the cryovials containing 1.5 mL freezing media at 4 °C; then cryovials were inserted into the cooling chamber of a programmable freezer (Kryo 10 Series II, Planer Inc., Middlesex, UK). The sequential steps of freezing program were as follows: (1) cooled from 4 to 0 °C at − 1 °C/min, (2) cooled from 0 to − 9 °C at − 2 °C/min, (3) seeded manually, (4) held at − 9 °C for 10 min, (5) cooled from − 9 to − 40 °C at − 0.3 °C/min, (6) cooled from − 40 to − 140 °C at − 10 °C/min, and (7) removed cryovials immediately from cooling chamber and stored in liquid nitrogen at − 196 °C for 8–10 weeks.
On the day of thawing, cryovials were removed from the liquid nitrogen tank, immersed in warm water at 37 °C, and shaken gently until the freezing media had melted. Tissue pieces were then removed from the cryovials and placed in each thawing solutions containing gradually decreasing concentration of DMSO in a stepwise manner at room temperature: (1) 0.75 M DMSO + 0.5 M sucrose in MEM-alpha media with 10% HSA for 5 min, (2) 0.375 M DMSO + 0.25 M sucrose in MEM-alpha media with 10% HSA for 5 min, and (3) MEM-alpha with 10% HSA alone for 10 min.
Vitrification using open and closed devices, and warming procedure
Vitrification of ovarian cortical tissues was performed using the protocol previously described by Suzuki et al. with slight modifications [26]. The ovarian pieces were washed in tissue culture media (M199, Life Technologies, CA, USA) with 20% serum substitute supplement (SSS, Irvine Scientific, CA, USA) and then sequentially subjected to three different vitrification solutions at room temperature. First, tissues were equilibrated in M199 medium containing 10% ethylene glycol (EG, Wako Pure Chemical Industries, Tokyo, Japan) with 20% SSS for 5 min, then were transferred into the M199 medium containing 20% EG and 20% SSS for 5 min. As the next step, tissues were placed into the M199 medium containing 35% EG, 5% polyvinylpyrrolidone (Sigma-Aldrich, St. Louis, MO, USA), and 0.5 mol/L sucrose (EMD Millipore, Billerica, MA, USA) for 15 min. In the final step, ovarian cortical pieces were loaded onto the open VF device (OVA Cryo Device Type M, Kitazato BioPharma Co., Japan) or closed VF device (Cryosheet, Kitazato BioPharma Co., Japan) [37].
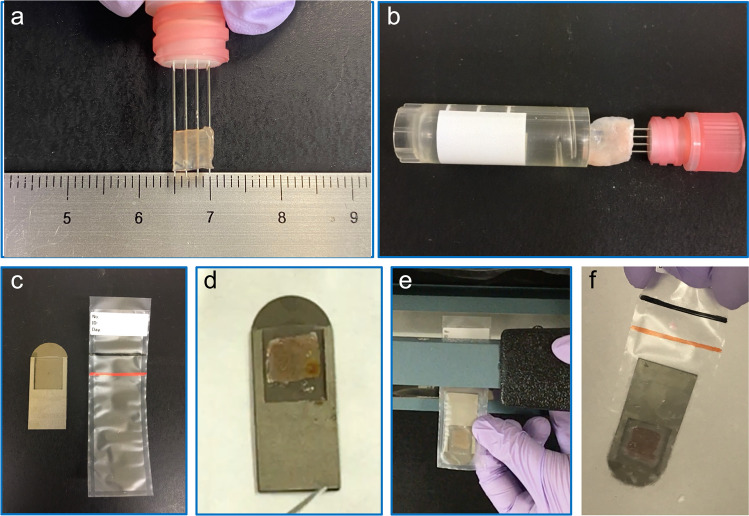
The open VF device consists of four fine stainless needles providing a metallic grid surface for loading the ovarian cortical tissue (Fig. 1a, b). After loading the ovarian tissue, the open device was directly immersed in liquid nitrogen in a vertical position and then was placed into a cryogenic vial (BD Bioscience, San Jose, CA, USA).
Fig. 1.
Vitrification of human ovarian tissue with an open device (Cryo Type M) (a and b) and the newly developed closed device (CryoSheet) (c–f). a The human ovarian tissue is placed on the metallic grid. b Tissue loaded metallic grid is inserted into the cryovial. c, Components of closed vitrification device including the metallic plate and the plastic pouch; d Processed ovarian tissue is placed on the closed device and covered. e Plastic pouch is sealed using electrical heat sealer. f Closed system is inserted into the liquid nitrogen
The closed VF device is a newly designed device made of titanium in rectangular shape (40 × 15 × 1 mm in length × width × thickness, respectively) with a thin plastic cover attached (Fig. 1c). The device is made of titanium because of its excellent thermal conductivity and durability. The ovarian tissue was placed onto the square pocket on the metallic surface and covered by a transparent polypropylene sheet attached to the proximal edge (Fig. 1d). Next, the closed VF device was inserted into the plastic bag made of polypropylene, and then, the bag was heat-sealed using a tabletop manual sealer (Fig. 1e). Finally, the closed VF system is immersed in liquid nitrogen (Fig. 1f). The satisfactory achievement by both VF devices was visually confirmed by the observation of the cortical tissue pieces turning transparent and be devoid of white opacities due to crystal formation. After successful vitrification, the open and closed VF devices were stored in liquid nitrogen for 8–10 weeks before they were thawed and analyzed.
On the day of warming, both tissue-loaded open and closed VF devices were immersed in 5 ml of pre-warmed M199 medium containing 20% SSS and 0.8 mol/L sucrose at 37.0 °C for 1 min. To remove cryoprotectant from the tissue, cortical pieces were placed in M199 media containing 20% SSS with 0.4 mol/L sucrose for 3 min, followed by incubation in M199 media containing 20% SSS alone for 5 min twice at room temperature. The warmed pieces were kept in modified-HTF medium supplemented with 10% SSS.
Post-thawing Process and Dilution of Cryoprotectant
After thawing/warming, the ovarian cortical pieces were cut into 1 × 1 mm small pieces, placed on Millicell Cell Culture Inserts (12 mm, 24 holes hydrophilic PTFE, 0.4 µm pore size; Millipore Sigma, Burlington, MA, USA) and transferred to the 24-well plates (Beckton Dickinson Labware, Bedford, MA, USA). Tissue pieces were covered with 100 µL of culture media containing Dulbecco’s Minimal Essential Media (DMEM, Life Global, Guilford, CT, USA), 10% HSA, 10 µg/mL insulin-transferrin-selenium (ITS-G, Thermo Fisher Scientific, MA, USA) supplement, 50 IU/mL antibiotic–antimycotic (Gibco, Thermo Fisher Scientific, MA, USA), and 0.3 IU/mL recombinant follicle stimulating hormone (Gonal-F, Merck, Kenilworth, NJ, USA). Tissues were incubated in a humidified atmosphere with 5% CO2 at 37.0 °C for 4 h. The post-thawing incubation time was set to 4 h for several reasons. First, this allowed time for the activation of DNA damage and apoptotic pathways so that the subsequent apoptotic changes and DNA damage could be morphologically assessed. Second reason was to dilute the cryoprotectant further since Nakamura et al. [39] suggested the high residual cryoprotectant level after thawing would induce apoptosis. Third, this 4-h culture period partially represented the avascular period the tissues will experience after transplantation.
Assessment of follicle survival, DNA damage, and apoptotic pathway activation
After thawing/warming and 4 h of incubation, the ovarian cortical pieces were formalin-fixed and embedded in paraffin for histological examination. The whole block of tissue (from fresh controls and each cryopreservation technique) was serially sectioned at 5 μm thickness. Approximately, a total of 50 slides, each slide with 4 serial sections, was prepared from each block. Every 10th slide was used to analyze the follicle density by hematoxylin–eosin staining, as well as DNA damage and apoptosis by immunohistochemistry. For example, slide#1, #10, #20, etc., were used to examine the follicle density; slide#2, #11, #21, etc., were used to detect DNA damage; and slide#3, #12, #22, etc., were used to detect apoptosis. From each slide, a section with the best staining quality was utilized for quantification. The other 3 serial sections were sometimes referred to when the stage or the staining of the follicles needed confirmation. An optical microscope with × 20 objective (Olympus IX73, Olympus Inc., Japan) was used, and the primordial and primary follicle densities (number of follicle/mm3) were calculated by three independent observers blinded to the group assignments. To determine DNA double-strand breaks (DSBs) in primordial and primary follicles, we used 1/500 diluted anti-γH2AX antibody (IHC-00059, Bethyl Laboratories, Texas, USA) as the first antibody. The staining was developed with HRP-conjugated anti-IgG Rabbit, Goat antibody (A120-201P, Bethyl Laboratories, Texas, USA), and a DAB substrate (SK-4100, Vector, Burlingame, CA). Finally, the sections were counterstained with hematoxylin [40]. Apoptotic pathway activation in ovarian follicles was identified by 1/800 diluted activated caspase-3 (AC3) (AF-835, R&D Systems, Minneapolis, USA) as the first antibody, then the signals were developed with HRP-conjugated anti-IgG Rabbit, Goat antibody, and a DAB substrate kit [41]. The AC3 immuno-stained tissues were counterstained with hematoxylin to visualize primordial and primary follicles.
Immunohistochemistry staining results for γH2AX and AC3 were assessed by a scoring scale as previously described [40]. Briefly, 0 score was given to no staining, 1 to faint staining, 2 to medium staining, and 3 to strong staining. Staining scores of 2 and 3 were counted as positive stained. Follicles with oocytes stained for γH2AX and AC3 antibodies were defined as γH2AX-positive and AC3-positive, respectively. The follicles with positive stained granulosa cells, but negative stained oocytes were also included in the count. The percentages of follicles positive for γH2AX and AC3 were calculated separately to represent the extent of follicle DNA damage and apoptosis, respectively. Finally, to assess the functional follicle survival, we counted the AC3-negative (non-apoptotic) follicles and compared the density of AC3-negative follicles among the groups.
Statistical analysis
Data were analyzed using JMP Pro ver13.2. (Statistical Discovery software). For each donor ovary, ovarian primordial and primary follicle densities, and γH2AX and AC3 immunohistochemistry staining percentages between each group were compared using one-way-ANOVA and LSD Method for post hoc multiple comparison analysis. Differences were considered significant when P < 0.05.
Results
Follicle survival with slow freezing versus vitrification
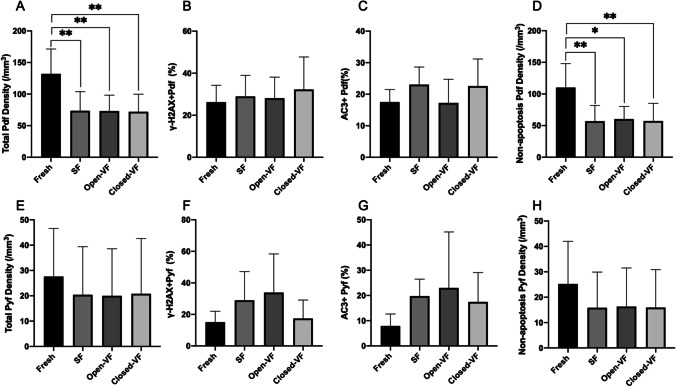
Consistent with previous studies that ovarian cortex predominantly contains primordial follicles, our results showed a higher density of primordial follicles than that of primary follicles in the baseline controls. Compared to the baseline primordial follicle density in fresh fixed cortical tissue of same ovaries, the primordial follicle densities were reduced significantly with all cryopreservation methods (Table 1; Fig. 3a; P < 0.05). However, the primary follicle density differences were not statistically significant after cryopreservation with any method (SF, VF-open, and VF-closed) compared to the fresh fixed baseline (Table 2; Fig. 3e).
Table 1.
Impact of slow freezing and vitrification (with conventional open device and novel closed device) on primordial follicles survival and DNA damage. a Fresh, b SF (slow freezing), c VF-open (vitrification with open device), d VF-closed (vitrification with closed device)
| Variables | Fresha (n = 5) | SFb (n = 5) | Open-VFc (n = 5) | Closed-VFd (n = 5) | P values |
|---|---|---|---|---|---|
| Total Pdf density (/mm3) | 132.0 ± 39.4 | 73.6 ± 30.2 | 73.2 ± 25.0 | 72.0 ± 27.9 | a vs b = 0.009** |
| a vs c = 0.009** | |||||
| a vs d = 0.008** | |||||
| b vs c = 0.984 | |||||
| b vs d = 0.936 | |||||
| d vs d = 0.952 | |||||
| γH2AX + Pdf (%) | 26.2 ± 8.0 | 28.9 ± 10.0 | 28.1 ± 10.0 | 32.2 ± 15.4 | a vs b = 0.707 |
| a vs c = 0.797 | |||||
| a vs d = 0.409 | |||||
| b vs c = 0.905 | |||||
| b vs d = 0.649 | |||||
| c vs d = 0.566 | |||||
| AC3 + Pdf (%) | 17.5 ± 4.0 | 23.1 ± 5.6 | 17.2 ± 7.5 | 22.6 ± 8.6 | a vs b = 0.207 |
| a vs c = 0.948 | |||||
| a vs d = 0.247 | |||||
| b vs c = 0.186 | |||||
| b vs d = 0.911 | |||||
| d vs d = 0.223 | |||||
| Pdf survival density (/mm3) | 110.1 ± 37.8 | 57.0 ± 24.8 | 60.2 ± 19.8 | 58.0 ± 27.9 | a vs b = 0.009** |
| a vs c = 0.014* | |||||
| a vs d = 0.009** | |||||
| b vs c = 0.855 | |||||
| b vs d = 0.992 | |||||
| c vs d = 0.862 |
Data are presented as mean ± sd or %. p < 0.05 considered as statistically significant. One-way ANOVA with post hoc comparison (lsd) was used to compare primordial follicle density, DNA damage rate, apoptotic rate, and non-apoptotic density among fresh, SF, open-VF, and closed-VF groups. SF slow freezing, VF vitrification (with conventional open device and novel closed device), pdf primordial follicle
Fig. 3.
Efficacy of slow freezing and vitrification with conventional open and closed devices. a Primordial follicle density(/mm3). b The percentage of γH2AX-positive primordial follicles. c The percentage of AC3-positive primordial follicles. d Non-apoptotic primordial follicle density(/mm3). e Primary follicle density(/mm3). f The percentage of γH2AX-positive primary follicles. g The percentage of AC3-positive primary follicles. h Non-apoptotic of primordial follicle density(/mm3). Data are presented as mean ± SE or %. One-way ANOVA with LSD post hoc analysis was used for multiple group comparisons. VF-open, vitrification using open device; VF-closed, vitrification using the closed device; Pdf, primordial follicle; Pyf, primary follicle. *p < 0.05, compared to fresh; **p < 0.01, compared to fresh
Table 2.
Impact of slow freezing and vitrification (with conventional open device and novel closed device) on primary follicles survival and DNA damage. a Fresh, b SF (slow freezing), c VF-open (vitrification with open device), d VF-closed (vitrification with closed device)
| Variables | Fresha (n = 5) | SFb (n = 5) | Open-VFc (n = 5) | Closed-VFd (n = 5) | P values |
|---|---|---|---|---|---|
| Total Pyf density (/mm3) | 27.6 ± 19.0 | 20.4 ± 19.0 | 20.0 ± 18.6 | 20.8 ± 21.8 | a vs b = 0.570 |
| a vs c = 0.549 | |||||
| a vs d = 0.592 | |||||
| b vs c = 0.975 | |||||
| b vs d = 0.975 | |||||
| c vs d = 0.949 | |||||
| γH2AX + Pyf (%) | 15.1 ± 6.9 | 29.0 ± 18.2 | 33.9 ± 24.5 | 29.9 ± 22.1 | a vs b = 0.207 |
| a vs c = 0.094 | |||||
| a vs d = 0.826 | |||||
| b vs c = 0.649 | |||||
| b vs d = 0.291 | |||||
| c vs d = 0.140 | |||||
| AC3 + Pyf (%) | 7.9 ± 4.7 | 19.7 ± 6.8 | 23.0 ± 22.2 | 17.4 ± 11.7 | a vs b = 0.176 |
| a vs c = 0.089 | |||||
| a vs d = 0.271 | |||||
| b vs c = 0.701 | |||||
| b vs d = 0.786 | |||||
| c vs d = 0.515 | |||||
| Pyf survival density (/mm3) | 25.2 ± 16.8 | 15.9 ± 14.0 | 16.3 ± 15.2 | 15.9 ± 14.9 | a vs b = 0.349 |
| a vs c = 0.371 | |||||
| a vs d = 0.351 | |||||
| b vs c = 0.964 | |||||
| b vs d = 0.996 | |||||
| c vs d = 0.968 |
Data are presented as mean ± sd or %. p < 0.05 considered as statistically significant. one-way ANOVA with post hoc comparison (lsd) was used to compare primary follicle density, DNA damage rate, apoptotic rate, and non-apoptotic density among fresh, SF, open-VF, and closed-VF groups. SF slow freezing, VF vitrification (with conventional open device and closed device), pyf primary follicle
Primordial and primary follicle DNA damage after slow freezing versus vitrification
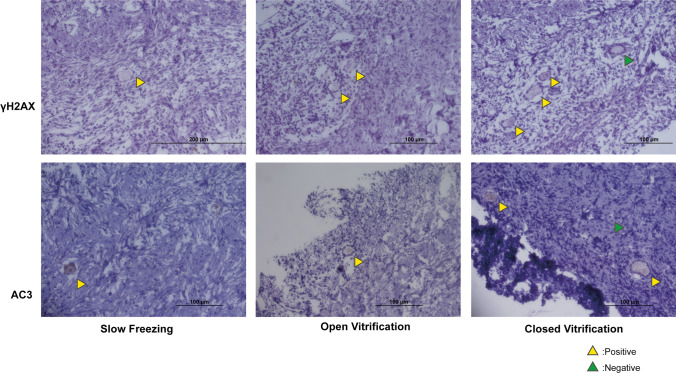
When we compared all three freezing methods to the baseline, we found similar percentage of primordial or primary follicles staining for γH2AX. We also found no differences in the percentages of γH2AX-positive primordial or primary follicles among the three cryopreservation methods (Tables 1 and 2; Fig. 2; Fig. 3 b and f). These data suggest that neither the cryopreservation process in general nor the method of freezing cause a detectable differential effect on DNA integrity in human primordial or primary follicles.
Fig. 2.
Representative histological sections that were immunohistochemically stained for γH2AX (DNA damage) and AC3 (follicle apoptosis) from cryopreserved human ovaries using slow freezing (SF), open vitrification (OVF), and closed vitrification (CVF) protocols
Primordial and primary follicle apoptosis after slow freezing versus vitrification
Similarly, none of the cryopreservation methods induced significant differences on the percentages of primordial and primary follicles with apoptosis (AC3) in comparison to fresh fixed baseline and among the three methods of freezing in the present study. These findings suggested that the method of freezing did not cause a detectable difference on apoptosis in the early stage of human follicles (Tables 1 and 2; Fig. 2; Fig. 3 c and g).
The comparison of AC3-negative (non-apoptotic) primordial or follicle densities also did not reveal any differences among the groups, indicating that none of the methods is superior to the other in intact follicle survival rate (Tables 1 and 2; Fig. 3 d and h).
Follicle survival, DNA damage and apoptosis in vitrified tissues: open versus closed VF device
To determine whether there is a difference in intact follicle survival rate between the open and closed vitrification methods, we performed a subgroup comparison between the VF-open and VF-closed. We found similar total and non-apoptotic primordial or primary follicle densities in the VF-open and VF-closed groups. Likewise, the percentages of apoptotic (AC3-positive) and DNA-damaged (γH2AX positive) in primordial and primary follicles did not differ significantly between the VF-open and VF-closed groups (Tables 1 and 2; Fig. 2; Fig. 3 b, c, f, and g). These results suggest that VF-closed system could provide a follicle survival efficacy similar to the currently used VF-open system.
Discussion
Although long considered as an experimental procedure by most, cryopreservation of human ovarian tissue with subsequent auto-transplantation has become an efficient fertility preservation with live birth rate at 25.4–30.6% [42] and 37.7% [23] since the first successful report of ovarian transplantation with cryopreserved tissue [7] and subsequent initial livebirths associated with ovarian transplantation [43]. As a result, ASRM has recently declared ovarian tissue freezing and transplantation as an established procedure for young cancer patients desiring fertility preservation [21]. Currently, there are two cryopreservation techniques used for reproductive tissue freezing: SF and VF [11, 13, 15, 16, 19, 25, 26, 42, 44]. The success of SF method has been demonstrated by long-term experience and over 200 live births after transplantation of frozen-thawed ovarian tissues [42]. On the other hand, there have been few live births after transplantation of vitrified-warmed ovarian tissue [25, 26, 44]. Studies have shown that both methods are associated with a significant loss of primordial follicles due to cryoinjury [45, 46]. To reduce cryoinjury in ovarian cortical tissues, researchers investigated several cryopreservation protocols using various cryoprotectants in different combination and concentrations [47], antifreeze proteins [48], processing timing and temperatures [49], cryo-devices [50], and freezing systems [51, 52] using animal and human ovarian tissues. From our experience, the thickness of the ovarian tissue should be as little as 1 mm to avoid ice formation during cryopreservation [33]. In addition, it is important to confirm the satisfactory cryopreservation visually. The cortical tissue pieces should turn transparent and be devoid of white opacities which represent crystal formation.
In this study, we found that the primordial densities significantly declined in both SF group and VF groups (open and closed systems) due to cryoinjury, consistent with previous reports [45, 46]. However, the primary follicles were found to be more resistant to cryoinjury since all cryopreservation methods (SF, VF-open, and VF-closed) showed comparable primary follicle densities with the fresh baseline controls. None of the cryopreservation methods (SF, VF-open, and VF-closed) caused a detectable increase in DNA damage and apoptosis compared to fresh baseline control. Since neither DNA damage nor apoptosis is increased in surviving follicles with any of the cryopreservation methods compared to fresh, a non-apoptotic mechanism or lysis of oocytes and granulosa cells may be involved to induce the large primordial follicle loss caused by the freezing processes. Some may be concerned that the ischemic damage may have also induced the loss of primordial follicles in the 4-h incubation post-thawing/warming. First, the media contained a HEPES buffer to reduce oxidative stress and all groups were exposed to same culture conditions and period. Secondly, this 4-h culture period may partially represent the avascular period the tissues will experience after transplantation. For the latter reason, we believe our culture model increases the clinical applicability of our findings.
Although numerous studies have been conducted to compare SF and VF methods, it is still unclear whether vitrification or slow freezing method provides better results for primordial follicle survival in human ovarian tissue. Indeed, Amorim suggested the inconsistent results were due to inconsistent methodology, such as the differences in concentrations of cryoprotectants, immersing time, media, and evaluation criteria [53]. Slow freezing still might preserve the early-stage follicles better than vitrification [54]. Slow freezing for ovarian tissue cryopreservation is superior to vitrification in terms of follicle survival and growth after xenotransplantation [55]. Vitrification and slow freezing produce equivalent results with respect to preservation of intact primordial follicles in the cryopreserved human ovarian tissue. However, these studies varied in the cryopreservation protocols used [56]. A recent meta-analysis suggested that vitrification may be more effective than slow freezing, with less primordial follicular DSBs and better preservation of stromal cells [57]. However, a few recent studies contradicted each other regarding the effect of cryopreservation procedure on genomic integrity in ovarian follicles [53, 58–60]. Hence, whether VF or SF causes less impact on oocyte DNA integrity remains to be determined.
There has been a concern regarding the risk of viral cross-contamination in currently used VF-open systems [36]. In VF-open systems, ovarian tissue is ultra-rapidly frozen and stored in direct contact with the liquid nitrogen, which can harbour viruses. We developed a new VF-closed system [37, 52], in which the cortical tissues are flash-frozen using the supreme heat conductivity of titanium and stored in a sealed bag to eliminate the direct contact with liquid nitrogen. We have previously tested our VF-closed system using mouse and monkey ovarian tissues [37, 52]. This is the first study to compare these systems in a human model. The results of present study using human ovarian tissues suggest that the VF with closed device provides similar efficacy compared to it with open device.
The major strength of our study is its paired design to compare SF and VF methods using human ovarian tissues. In addition, we for the first time investigated the efficacy of the newly developed closed-VF device.
A limitation of our study was that we tested follicle survival and DNA integrity in cryopreserved human ovarian cortical tissues on the day of thawing/warming only, after 4 h of culture. Although we showed that follicle survival and DNA integrity were similarly preserved with VF as in SF, further functional studies such as with the rates of follicle activation, follicle grading, chromatin patterns of follicles, and ultimately xenografting are needed. If our findings are confirmed in human ovarian xenografting models, clinical trials may be performed to test the success of ovarian transplantation with vitrified tissues in patients. Our novel findings provide the critical baseline data for these future translational studies.
Based on the intact primordial follicle survival, DNA damage, and apoptosis rates after thawing/warming, SF vs VF with either open or newly developed closed methods appear to be comparable. In addition, this closed VF system can be used with similar efficacy compared to the currently used open VF system. Our findings, when confirmed with in vivo studies, would lend credence to initiate the clinical trials for ovarian cryopreservation using the newly developed closed-VF devices.
Acknowledgements
We thank Dr. Tomoe Koizumi from St. Marianna University for her support in the biostatistical analysis.
Author contribution
Yodo Sugishita and Kutluk Oktay contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yodo Sugishita, Nao Suzuki, Enes Taylan, Tai Kawahara, Bunyad Shahmurzada, and Kutluk Oktay. The first draft of the manuscript was written by Yodo Sugishita, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Kutluk Oktay is supported by NIH’s NICHD and NCI (R01HD053112). Nao Suzuki is supported by AMED (Japan Agency for Medical Research and Development and No. 16gk0110014h0001).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
This study protocol was exempted by the IRB as the ovarian tissues were obtained from organ donor cadavers, not from living subjects.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yodo Sugishita and Enes Taylan contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011;3(8):782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10(3):251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 4.Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100(5):1224–31. 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed]
- 5.Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. doi: 10.1200/jco.2018.78.1914. [DOI] [PubMed] [Google Scholar]
- 6.Taylan E, Oktay KH. Current state and controversies in fertility preservation in women with breast cancer. World J Clin Oncol. 2017;8(3):241–248. doi: 10.5306/wjco.v8.i3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919. doi: 10.1056/nejm200006223422516. [DOI] [PubMed] [Google Scholar]
- 8.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94.e1–9. doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oktay K, Taylan E, Sugishita Y, Goldberg GM. Robot-assisted laparoscopic transplantation of frozen-thawed ovarian tissue. J Minim Invasive Gynecol. 2017;24(6):897–898. doi: 10.1016/j.jmig.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Taylan E, Oktay K. Application of Decellularized Tissue Scaffolds in Ovarian Tissue Transplantation. Methods Mol Biol. 2018;1577:177–181. doi: 10.1007/7651_2017_35. [DOI] [PubMed] [Google Scholar]
- 11.Marin L, Bedoschi G, Kawahara T, Oktay KH. History, evolution and current state of ovarian tissue auto-transplantation with cryopreserved tissue: a successful translational research journey from 1999 to 2020. Reprod Sci. 2020;27(4):955–962. doi: 10.1007/s43032-019-00066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 13.Gellert SE, Pors SE, Kristensen SG, Bay-Bjørn AM, Ernst E, Yding AC. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;35(4):561–570. doi: 10.1007/s10815-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oktay KH, Marin L, Petrikovsky B, Terrani M, Babayev SN. Delaying reproductive aging by ovarian tissue cryopreservation and transplantation: is it prime time? Trends Mol Med. 2021 doi: 10.1016/j.molmed.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozen G, Agresta F, Gook D, Braat D, Stern CJ. Success and challenges in fertility preservation after ovarian tissue grafting. Lancet. 2015;385(9981):1947. doi: 10.1016/s0140-6736(15)60959-x. [DOI] [PubMed] [Google Scholar]
- 16.von Wolff M, Germeyer A, Liebenthron J, Korell M, Nawroth F. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part II: fertility preservation techniques. Arch Gynecol Obstet. 2018;297(1):257–267. doi: 10.1007/s00404-017-4595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gook DA, Hale L, Edgar DH. Live birth following transfer of a cryopreserved embryo generated from a cryopreserved oocyte and a cryopreserved sperm: case report. J Assist Reprod Genet. 2007;24(1):43–45. doi: 10.1007/s10815-006-9093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotz L, Dittrich R, Hoffmann I, Beckmann MW. Ovarian tissue transplantation: experience from germany and worldwide efficacy. Clin Med Insights Reprod Health. 2019;13:1179558119867357. doi: 10.1177/1179558119867357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meirow D, Ra'anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112(6):1022–33. 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed]
- 22.Martinez F. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108(3):407–15.e11. doi: 10.1016/j.fertnstert.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci. 2017;24(8):1111–1120. doi: 10.1177/1933719117702251. [DOI] [PubMed] [Google Scholar]
- 24.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS ONE. 2011;6(4):e19475. doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 27.Huang JY, Chian RC, Gilbert L, Fleiszer D, Holzer H, Dermitas E, et al. Retrieval of immature oocytes from unstimulated ovaries followed by in vitro maturation and vitrification: a novel strategy of fertility preservation for breast cancer patients. Am J Surg. 2010;200(1):177–183. doi: 10.1016/j.amjsurg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Fasano G, Moffa F, Dechène J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol Endocrinol. 2011;9:150. doi: 10.1186/1477-7827-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edgar DH, Gook DA. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum Reprod Update. 2012;18(5):536–554. doi: 10.1093/humupd/dms016. [DOI] [PubMed] [Google Scholar]
- 30.Roy TK, Brandi S, Tappe NM, Bradley CK, Vom E, Henderson C, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29(11):2431–2438. doi: 10.1093/humrep/deu214. [DOI] [PubMed] [Google Scholar]
- 31.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online. 2009;18(4):568–577. doi: 10.1016/s1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto S, Suzuki N, Yamanaka M, Hosoi Y, Ishizuka B, Morimoto Y. Effects of vitrification solutions and equilibration times on the morphology of cynomolgus ovarian tissues. Reprod Biomed Online. 2010;21(4):501–509. doi: 10.1016/j.rbmo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Ting AY, Yeoman RR, Lawson MS, Zelinski MB. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26(9):2461–2472. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;28(5):1267–1279. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielanski A. A review of the risk of contamination of semen and embryos during cryopreservation and measures to limit cross-contamination during banking to prevent disease transmission in ET practices. Theriogenology. 2012;77(3):467–482. doi: 10.1016/j.theriogenology.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Sugishita Y, Okamoto N, Uekawa A, Yamochi T, Nakajima M, Namba C, et al. Oocyte retrieval after heterotopic transplantation of ovarian tissue cryopreserved by closed vitrification protocol. J Assist Reprod Genet. 2018;35(11):2037–2048. doi: 10.1007/s10815-018-1298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286(12):1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Obata R, Okuyama N, Aono N, Hashimoto T, Kyono K. Residual ethylene glycol and dimethyl sulphoxide concentration in human ovarian tissue during warming/thawing steps following cryopreservation. Reprod Biomed Online. 2017;35(3):311–313. doi: 10.1016/j.rbmo.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, et al. Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology. 2001;142(6):2468–2480. doi: 10.1210/endo.142.6.8078. [DOI] [PubMed] [Google Scholar]
- 42.Dolmans MM, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: a review of five leading European centers. Fertil Steril. 2021;115(5):1102–1115. doi: 10.1016/j.fertnstert.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Shapira M, Dolmans MM, Silber S, Meirow D. Evaluation of ovarian tissue transplantation: results from three clinical centers. Fertil Steril. 2020;114(2):388–397. doi: 10.1016/j.fertnstert.2020.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Silber SJ, DeRosa M, Goldsmith S, Fan Y, Castleman L, Melnick J. Cryopreservation and transplantation of ovarian tissue: results from one center in the USA. J Assist Reprod Genet. 2018;35(12):2205–2213. doi: 10.1007/s10815-018-1315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 46.Kim GA, Kim HY, Kim JW, Lee G, Lee E, Ahn JY, et al. Effectiveness of slow freezing and vitrification for long-term preservation of mouse ovarian tissue. Theriogenology. 2011;75(6):1045–1051. doi: 10.1016/j.theriogenology.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Youm HW, Lee JR, Lee J, Jee BC, Suh CS, Kim SH. Optimal vitrification protocol for mouse ovarian tissue cryopreservation: effect of cryoprotective agents and in vitro culture on vitrified-warmed ovarian tissue survival. Hum Reprod. 2014;29(4):720–730. doi: 10.1093/humrep/det449. [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Kim SK, Youm HW, Kim HJ, Lee JR, Suh CS, et al. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS ONE. 2015;10(5):e0126252. doi: 10.1371/journal.pone.0126252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bos-Mikich A, Marques L, Rodrigues JL, Lothhammer N, Frantz N. The use of a metal container for vitrification of mouse ovaries, as a clinical grade model for human ovarian tissue cryopreservation, after different times and temperatures of transport. J Assist Reprod Genet. 2012;29(11):1267–1271. doi: 10.1007/s10815-012-9867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herraiz S, Novella-Maestre E, Rodríguez B, Díaz C, Sánchez-Serrano M, Mirabet V, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101(3):775–784. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Dittrich R, Maltaris T. A simple freezing protocol for the use of an open freezing system for cryopreservation of ovarian tissue. Cryobiology. 2006;52(1):166. doi: 10.1016/j.cryobiol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto N, Nakajima M, Sugishita Y, Suzuki N. Effect of mouse ovarian tissue cryopreservation by vitrification with Rapid-i closed system. J Assist Reprod Genet. 2018;35(4):607–613. doi: 10.1007/s10815-018-1121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amorim CA, David A, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification of human ovarian tissue: effect of different solutions and procedures. Fertil Steril. 2011;95(3):1094–1097. doi: 10.1016/j.fertnstert.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 54.Dalman A, DeheshkarGoonehFarahani NS, Totonchi M, Pirjani R, Ebrahimi B, Rezazadeh Valojerdi M. Slow freezing versus vitrification technique for human ovarian tissue cryopreservation: an evaluation of histological changes, WNT signaling pathway and apoptotic genes expression. Cryobiology. 2017;79:29–36. doi: 10.1016/j.cryobiol.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Ryu KJ, Kim B, Kang D, Kim YY, Kim T. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int J Mol Sci. 2019;20(13). 10.3390/ijms20133346. [DOI] [PMC free article] [PubMed]
- 56.Zhou XH, Zhang D, Shi J, Wu YJ. Comparison of vitrification and conventional slow freezing for cryopreservation of ovarian tissue with respect to the number of intact primordial follicles: a meta-analysis. Medicine (Baltimore) 2016;95(39):e4095. doi: 10.1097/md.0000000000004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-analysis. Sci Rep. 2017;7(1):8538. doi: 10.1038/s41598-017-09005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao Z, Wang Y, Li LL, Li SW. In vitro culture thawed human ovarian tissue: NIV versus slow freezing method. Cryo Letters. 2013;34(5):520–526. [PubMed] [Google Scholar]
- 59.Chang HJ, Moon JH, Lee JR, Jee BC, Suh CS, Kim SH. Optimal condition of vitrification method for cryopreservation of human ovarian cortical tissues. J Obstet Gynaecol Res. 2011;37(8):1092–1101. doi: 10.1111/j.1447-0756.2010.01496.x. [DOI] [PubMed] [Google Scholar]
- 60.Leonel ECR, Corral A, Risco R, Camboni A, Taboga SR, Kilbride P, et al. Stepped vitrification technique for human ovarian tissue cryopreservation. Sci Rep. 2019;9(1):20008. doi: 10.1038/s41598-019-56585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.