Abstract
Purpose
The aim of this review is to gather the available research focusing on female genital tract (FGT) microbiome. Research question focuses in decipher which is the role of FGT microbiota in eubiosis, assisted reproduction techniques (ARTs), and gynaecological disorders, and how microbiome could be utilised to improve reproduction outcomes and to treat fertility issues.
Methods
PubMed was searched for articles in English from January 2004 to April 2021 for “genital tract microbiota and reproduction”, “endometrial microbiome”, “microbiome and reproduction” and “microbiota and infertility”. Manual search of the references within the resulting articles was performed.
Results
Current knowledge confirms predominance of Lactobacillus species, both in vagina and endometrium, whereas higher variability of species is both found in fallopian tubes and ovaries. Microbial signature linked to different disorders such endometriosis, bacterial vaginosis, and gynaecological cancers are described. Broadly, low variability of species and Lactobacillus abundance within the FGT is associated with better reproductive and ART outcomes.
Conclusion
Further research regarding FGT microbiome configuration needs to be done in order to establish a more precise link between microbiota and eubiosis or dysbiosis. Detection of bacterial species related with poor reproductive outcomes, infertility or gynaecological diseases could shape new tools for their diagnosis and treatment, as well as resources to assess the pregnancy prognosis based on endometrial microbiota. Data available suggest future research protocols should be standardised, and it needs to include the interplay among microbiome, virome and mycobiome, and the effect of antibiotics or probiotics on the microbiome shifts.
Keywords: Vaginal microbiome, Endometrial Microbiome, Infertility, Gynaecological disorders, Human-assisted reproduction, Next-generation sequencing
Introduction: studying the female genital tract microbiome
Synergistic relationships between microorganisms and hosts are found in nearly every niche of the human body, conditioning its physiology and physiopathology [1, 2]. Likewise, the female genital tract (FGT) — vagina, cervix, endometrium, fallopian tubes, and ovaries — harbours its own microbiome, which accounts for 9% of the total bacterial amount in female body [3]. Vaginal niche was assessed within the frame of the Human Microbiome Project (HMP) in 2007 [4–6], and most recently by the integrative HMP (iHMP), studying microbiome in pregnancy and preterm birth (PTB) [7]. The cervix, uterine cavity, fallopian tubes, and ovaries (being initially considered sterile [8]), harbour their own microbiota too [9–17]. Healthy vagina is dominated by Lactobacillus species, whereas the upper genital tract (UGT) harbours a less dense but high varied amount of bacterial strains [18, 19] (Fig. 1).
Fig. 1.
Microbial composition both in upper and lower genital tract based on current knowledge. upper genital tract (UGT) comprises the cervix, the endometrium, the Fallopian tubes, and the ovaries. Lower genital tract (LGT) refers to vagina. Microbiome diversity increases from the outermost to the innermost, at the time microbial abundance decreases in the UGT compared to LGT
FGT microbiome is currently being analysed by next-generation sequencing (NGS) technologies based upon the analysis of hyper variable (V) regions of bacterial 16S ribosomal ribonucleic acid (rRNA) gene or whole metagenome sequencing (WMS). Such methods deepen at the species level [20] turning the identification of bacterial DNA in metagenomic samples much accurate compared with culture-based techniques [3]. Notwithstanding, solely the presence of bacterial DNA does not mean the existence of live bacteria. For this reason, new techniques such as metatranscriptomics analyse bacterial RNA transcription processes, which may illustrate functions being accomplished in situ by particular microbial species [21, 22]. Recent studies are steadily coming up, aiming to determine the microbiome configuration and transcriptomics in specific physiological scenarios such as the different phases of the menstrual cycle [23].
From the clinical point of view, FGT microbiome is related to reproduction outcomes, gynaecological issues, or infertility [11, 12, 14–16, 20]. Understanding the FGT microbiome and its on-purpose-modulation could be useful as a clinical tool to restore the physiological state [24–26].
This review aims to bring together several FGT microbiome studies. Descriptions of eubiotic microbiome and its shifts in response to stimuli have been addressed. In addition, the microbiome role on several gynaecological disorders, reproduction, and ART has been evaluated.
Methods
PubMed was searched for articles in English from January 2004 to April 2021 for “genital tract microbiota and reproduction”, “endometrial microbiome”, “microbiome and reproduction”, and “microbiota and infertility”. Data from studies on the FGT were gathered and manual search of the references within the resulting articles was performed.
Female genital tract microbiome
From the outermost to the innermost
Vaginal microbiome
The healthy state of vaginal microbiome was studied by several groups by amplifying the 16S rRNA gene by universal primers, therefore identifying and quantifying microbial DNA in vaginal epithelium or secretions [27, 28]. Later, within the HMP frame, vaginal microbiome was characterised in healthy volunteers through 16S rRNA gene sequencing and metagenomics [4–6]. Ravel et al. depicted five community state types (CST) in vagina from healthy women, differentiated by dominant species and pH values. L. crispatus, L. gasseri, L. iners, and L. jensenii prevail in CST I, CST II, CST III, and CST V, respectively. CST IV harbours higher ratios of strictly anaerobic genera (Prevotella, Dialister, Atopobium, Gardnerella, and Sneathia) to the detriment of lactobacilli. Depending on the Lactobacillus abundance, pH average values ranged from 4.0±0.3 (CST I) to 5.3±0.6 (CST IV) [29]. This configuration was supported by Gajer and collaborators, who described the temporal dynamics of these CST in vagina. Neither variation in the composition of bacterial communities nor high levels of species diversity appeared to be related with symptoms and health misbalance in subjects [30]. Another group performed (quantitative polymerase chain reaction) qPCR using 12 different primers for bacterial DNA amplification in swab vaginal samples taken before hysterectomy, mainly detecting Prevotella species (spp.), L. iners, and L. crispatus [9]. Part of the research conducted by Moreno et al. analysed paired vaginal samples both from the pre-receptive and receptive menstrual phases, showing total predominance of Lactobacillus in 10/13 women [11]. In 2017, Miles et al. showed predominance of Lactobacillus, Streptococcus, and Prevotella in vaginal samples, in agreement with previous findings [14]. Chen and colleagues considered L. iners and L. crispatus to be the vaginal microbial signature, being studied through 16S rRNA gene sequencing [13]. In line with these findings, Wee et al. depicted CST I and CST III predominance in 12/16 samples from healthy controls [31]. Another group found >97% Lactobacillus in healthy women (controls) [32]. Other different groups also demonstrated Lactobacillus dominated in vagina from healthy women, when it was sampled by using different devices or sequenced by different technological platforms [33–37].
According to current knowledge the vagina in healthy, non-pregnant women harbours high bacterial load, in which Lactobacillus species (spp.) prevails (Table 1).
Table 1.
Description of high throughput microbiome studies (mainly 16S rRNA gene sequencing, but also meta-transcriptomic) performed in the vagina, endometrium, cervix, fallopian tubes, and ovaries. Selected studies (except [52]) are performed in healthy women either undergoing surgery for non-infectious conditions or subjected for some Assisted Reproduction treatment. AA African American, ART assisted reproduction techniques, Av. average, CST community state types, EB endometrial biopsy, EF endometrial fluid, ET embryo transfer; f forward, ICSI intracytoplasmatic sperm injection, IVF in vitro fertilisation, LD Lactobacillus dominant, LGT lower genital tract, NLD non-Lactobacillus dominant, PS progesterone supplementation, PTB preterm birth, qPCR quantitative polymerase chain reaction, r reverse, RA Relative Abundance, RIF recurrent implantation failure, RPL recurrent pregnancy loss, TB term birth, UGT upper genital tract, VF vaginal fluid, vs. versus; V depicts the hyper variable region(s) of the 16S rRNA gene targeted for sequencing on each study, yr. years
| Publication | Study population Sample (no.) Av. age (yr.) Race/ethnicity | Diagnoses | Sampling procedure | Analysis | Top identified phyla | Topidentified taxa and/or bacterial species | Other findings | |
|---|---|---|---|---|---|---|---|---|
| Vagina | Zhou et al., 2004 | n = 5; 28–44 yr.; Caucasian | Normal premenopausal, non-pregnant, healthy women | Mid-vaginal swab frotis | Big Dye version 3, 3100 PRISM Genetic analyser, universal primers 8f and 926r | Firmicutes | Lactobacillus spp. (4/5 subjects) Atopobium spp. (1/5 subjects) | L. iners (2/4 LD women), L. crispatus (2/4 LD women) |
| Hyman et al., 2005 | n = 20; 27–44 yr.; North American | Healthy premenopausal women, not taking contraceptive steroids, without urogenital symptoms or noticeable infections | Posterior vaginal fornix epithelium was sampled with a sterile cryoloop | Big Dye terminator, 3730 DNA analyser, universal primers 8f and 1492r | Firmicutes | Lactobacillus spp. | Lactobacillus spp. (10/20 subjects); Streptococcus spp. (2/20 subjects); Gardnerella spp. (1/20 subjects); Prevotella spp. (1/20 subjects); Bifidobacterium spp. (1/20 subjects) | |
| Ravel et al., 2011 | n = 396; 30.6 yr.; 25% White, 26% Black, 24.5% Asian, 24.5% Hispanic | Non-pregnant women, regular menstruations, not antibiotic taken in the past 30 days | Mid-vaginal swab frotis | Roche 454 FLX V1-V2 regions | Firmicutes | Lactobacillus (different dominant species according to pH levels: lowest pH levels associated with CST I, CST III). | Vaginal microbiome classification in different CSTs CST I: L. crispatus CST II: L. gasseri CST III: L. iners CST V: L. jensenii CST IV: Anaerobes | |
| Gajer et al., 2012 | n = 32; reproductive age; American | Non-pregnant women | Self-collected mid-vaginal swabs and vaginal smears obtained twice-weekly for 16 weeks | 454 pyrosequencing V1-V2 regions (universal 27f and 338r) | Firmicutes | L. crispatus (CST I), L. gasseri (CST II), L. iners (CST III), CST IV | Vaginal microbiome classification according to CST types described by Ravel et al., 2011 Temporal dynamics of the CST: bacterial communities composition variation or high diversity levels do not necessarily imply dysbiosis | |
| Hyman et al., 2012 | n = 30 patients; n = 99 swabs; 28-44 yr.; Caucasian, Latin, Hispanic, Vietnamese, Chinese, White, Mediterranean, Asian, Japanese | IVF-ET patients with different stimulation protocols, with no cervical, uterine, or tubal infection | Vaginal swabs | Big Dye terminator, ABI 3730 DNA analyser, universal primers 8f and 1492r | Firmicutes | Lactobacillus spp. in 86% (85/99) swabs | 3/99 swabs deficient in Lactobacillus. 87% Lactobacillus in women who had live birth, 80% Lactobacillus in women who did not have a live birth. Lactobacillus is favourable but not sufficient for successful ET | |
| Romero et al., 2014 | n = 32 non-pregnant women, n = 22 pregnant women; Reproductive age; North American | Non-pregnant women and women undergoing normal pregnancies who delivered at term (38–43 weeks) | VF samples collected by Dacron swab every four or two visits | 454 FLX Titanium pyrosequencing V1-V2 (27f and 388r primers) | Firmicutes | Lactobacillus spp. | Bacterial lactobacilli-dominated CST might change from one to another within an individual undergoing a normal pregnancy. Normal pregnancy: higher abundance of Lactobacillus vaginalis, L. crispatus, L. gasseri, L. jensenii, plus lower abundance of other 22 phylotypes. | |
| Mitchell et al., 2015 | n = 58; 43 yr.; 79% White, 10% AA, 7% Hispanic | Women undergoing hysterectomy for non-cancer indications | Vagina flocked swab frotis collected before hysterectomy | qPCR 12 primers* *Flavobacterium was not tested | Bacteroidetes | Prevotella spp.(76% subjects) L. iners (61% subjects) L. crispatus (56% subjects) | ||
| Digiulio et al., 2015 | n = 49 divided in two groups (40 + 9); >18 yr.; North American | Pregnant women (11/40 delivered preterm, 4/9 delivered preterm) | Vaginal specimens sampled weekly until delivery and monthly for one year after delivery | Pyrosequencing of the V3-V5 in 40 women; Illumina-based sequencing | Firmicutes | L. crispatus in stable vaginal community. Gardnerella and Ureaplasma more abundant in CST IV. Decreased Lactobacillus plus increased anaerobes in women with post-delivery vaginal disturbance. | CST IV (poor in Lactobacillus) is related with lower gestational age at delivery time | |
| Moreno et al., 2016 | n = 13 women; reproductive age; Spanish | Fertile women 2 samples/women (both from pre-receptive and receptive phases) | Vaginal aspirates with sterile catheter | 454 pyrosequencing V3-V5 | Firmicutes | Lactobacillus spp. | 10/13 women harboured vaginal colonisation only by Lactobacillus spp. | |
| Miles et al., 2017 | n = 10; 41–57 yr.; North American | Non-pregnant, non-infected women, with no immune disease. Treated with antibiotics 30min prior surgery | Vaginal samples collected with sterile swabs before surgery | 454 pyrosequencing V1-V3 | Firmicutes | Lactobacillus spp. Streptococcus spp. Prevotella spp. | Microbial composition were highly related across the samples and across the subjects | |
| Chen et al., 2017 | n = 95; 22–48 yr.; Asian | Women intervened for conditions not involving infection, cancer, or immune diseases. Not antibiotic, hormones, of vaginal/cervical treatment within a week | Vaginal nylon flocked swabs Samples taken with no prior perturbation | Ion Torrent V5-V4 Bacterial culturing | Firmicutes | L. iners L. crispatus | Continuum microbiome changing from the vagina to the UGT | |
| Wee et al., 2018 | n = 16 (cases), 37.6 yr.; n = 16 (controls), 42.75 yr. Australian | Cases: Women with history of infertility Controls: History of pregnancies with no ART procedures, healthy, hysteroscopy indication | Sterile speculum inserted into vagina, swab | Illumina MiSeqVR V1-3 qPCR to detect Ureaplasma | Firmicutes | Lactobacillus spp. in 12/16 control samples; CST I (L. crispatus), CST III (L. iners), CST IV | Ureaplasma overrepresented in vagina of infertile women Intra-individual correlation in vagina/cervix/ endometrium | |
| Kyono et al., 2018 | n = 79 (IVF cases), 37 yr., n = 23 (non-IVF cases), 33.2 yr., n = 7 (controls), 36.6 yr.; Australian | Women enrolled for IVF treatment; infertile women not enrolled for IVF | Vaginal discharge collected with OMNIgene vaginal swab | Illumina MiSeq V4 | Firmicutes | Lactobacillus spp. | Average Lactobacillus spp.: 65.2% in IVF group, 99.4 in non-IVF group, 99.8% in controls. | |
| Brown et al., 2019 | Cases: n = 38 + n = 22 subjects with high and low risk of preterm birth, 33.3 yr.; 50% Caucasian, 25% Black, 25% Asian | Pregnant women | Vaginal swabs | V1-V2 Illumina MiSeq | Firmicutes | Prevotella, Streptococcus, Peptoniphilus, Ureaplasma, Dialister spp. Decrease in Lactobacillus spp. | Reduced Lactobacillus plus increased pathogens such Prevotella, Peptoniphilus, Streptococcus, and Dialister associated with preterm birth risk and prelabor rupture of foetal membranes | |
| Controls: n = 36 women with term birth; 33.8 yr.; 52% Caucasian, 21% Black, 27% Asian | Lactobacillus spp. L. crispatus, L. iners, L. jensenii, L. gasseri | |||||||
| Liu et al., 2019 | n = 50; 16–33 yr.; American | Cases: Patients with intrauterine adhesion | Vaginal secretions sampled by swabs | V4 Illumina HiSeq 2000 | Firmicutes (61.84%), Actinobacteria (24.37%), Bacteroidetes (8.64%), Proteobacteria (2.74%) | Cases: <97% Lactobacillus plus Gardnerella, Prevotella | Decrease in Lactobacillus abundance and overgrowth of Gardnerella and Prevotella in cases group. Intrauterine adhesions trigger an increase in vaginal diversity of case patients | |
| Controls: Healthy women | Firmicutes (92.12%), Actinobacteria (5.58%), Bacteroidetes (0.92%), Proteobacteria (0.64%) | Controls: >97% Lactobacillus | ||||||
| Koedooder et al., 2019 | n = 192; 20–44yr.; Dutch | Fresh ET, women with no emergency IVF indications | Vaginal swabs taken prior to IVF or ICSI procedure before ET | Interespace profiling (IS-pro) molecular technique | Firmicutes | Lactobacillus spp. (classification of samples in CSTs) | High Lactobacillus abundance appears to be related to IVF and ICSI success >60% L. crispatus is not advantageous | |
| Kitaya et al., 2019 | n = 28 (cases), 38.7 yr.; n = 18 (controls), 37.6 yr.; Asian | Cases: RIF patients; Controls: First attempt IVF patients | Vaginal discharge collected with OMNIgene vaginal swab | Illumina MiSeq V4 | Firmicutes | .Lactobacillus spp. Others: Streptococcus, Prevotella, Bifidobacterium, Enterococcus, Atopobium | Variation between RIF and controls: 19/28 (67.9%) LD in RIF vs. 8/18 (44.4%) LD in controls. EF and vaginal microbiome profiles are different | |
| Winters et al., 2019 | n = 25; 45 yr.; Italian | Hysterectomy intended for fibroids (n = 23) or endometrial hyperplasia (n = 2) | Vaginal sampled by Dacron swab | Illumina V4 qPCR for V1-V2 16S rRNA gene for L. iners and L. crispatus detection | Firmicutes | Lactobacillus spp., Gardnerella | ||
| Fettweis et al., 2019 | n = 135 women; 26 yr.; 78% African, 22% others | Pregnant women: n = 45 PTB (<32 weeks) + n = 90 TB (≥39 weeks) | Vaginal samples taken at 7 time points | V1-V3 Illumina Miseq | Firmicutes | Lactobacillus crispatus (in TB women) | Reduced L. crispatus plus increased Sneathia and Prevotella in PTB women | |
| Bernabeu et al., 2019 | n = 31 women; 40 yr.; Spanish | Women undergoing ICSI ET n = 17 achieved pregnancy, n = 14 did not achieve pregnancy | VF samples taken with a dry swab, just before the embryo transfer | V3-V4 Illumina MiSeq | Firmicutes | Lactobacillus (92%), Gardnerella (5%) | Greater diversity of vaginal species is found in patients who did not achieve pregnancy | |
| Xu et al., 2020 | n = 85; 24–43 yr., Asian | Infertile women due to tubal obstruction (n = 40), normal fallopian tubes (n = 45) | Vaginal sample collected by a sterile cotton swab from the posterior fornix | Illumina MiSeq V4 | Firmicutes Actinobacteria Bacteroidetes | Lactobacillus spp., Gardnerella spp., Prevotella spp., Streptococcus spp., Atopobium spp. | E. coli, S agalactiae, P. intermedia among others might be used a biomarkers for vaginal dysbiosis | |
| Riganelli et al., 2020 | n = 24; 37 yr.; Italian | Infertile women with previous ART treatment and implantation failure | VF collected by cytobrush before starting the stimulation protocol | Illumina MiSeqVR V4-5 | Firmicutes | Lactobacillus spp. L. crispatus, L. iners, L. gasseri | Lactobacillus spp. in pregnant women vs. not-pregnant cases. Vagina harbours less biodiversity and reducer number of species than endometrium | |
| Carosso et al., 2020 | n = 15 35 yr. Caucasian | Women undergoing IVF with no antibiotics/probiotics/therapy | Vaginal swabs | Illumina MiSeqVR V3-4-6 | Firmicutes | Lactobacillus spp. | Lactobacillus spp. (before PS.) Lactobacillus decrease and Prevotella, Escherichia, and Shigella spp. increase (after PS.) | |
| Cervix Cervical mucus | Miles et al., 2017 | n = 10 41–57 yr. North American | Non-pregnant, non-infected women, with no immune disease. Treated with antibiotics 30min prior surgery | Endocervical epithelium swabbed sterile after hysterectomy, salpingo-oopherectomy | 454 pyrosequencing V1-V3 | Firmicutes | Lactobacillus spp. Prevotella spp. | Microbial composition were highly related across the samples and across the subjects |
| Chen et al., 2017 | n = 95 22–48 yr. Asian | Women intervened for conditions not involved infection, cancer, or immune diseases. Not antibiotic, hormones, of vaginal/cervical treatment within a week | Nylon flocked swabs Samples taken with no prior perturbation | Ion Torrent V5-V4 Bacterial culturing | Firmicutes | Lactobacillus (RA: 97.57%) Others (RA: 2.44%) | Intra-individual continuum of bacteria that gradually changes from the outermost to the innermost | |
| Pelzer, Willner, Buttini, and Huygens, 2018 | n = 145 (cases), n = 3 (virgo intacta controls); 14–52 yr.; Australian | Women with menorrhagia or dysmenorrhea undergoing hysteroscopy and/or laparoscopy | Endocervical Samples | 454 pyrosequencing V5-V8 | Firmicutes | Lactobacillus spp. Gardnerella spp. Prevotella spp. Veillonella spp. Sneathia spp. Fusobacterium spp. | Lactobacillus spp. dominance across all endocervical groups | |
| Wee et al., 2018 | n = 10 (cases), 37.6 yr.; n = 13 (controls), 42.75 yr.; Australian | Cases: Women with history of infertility Controls: History of pregnancies with no ART procedures, healthy, hysteroscopy indication | Swabs in cervix | Illumina MiSeqVR V1-3 | Firmicutes | G. vaginalis overrepresented in cervix of infertile women L. crispatus, L. iners dominance in 12/13 control samples | Intra-individual correlation vagina/cervix/endometrium | |
| Li et al., 2018 | n = 137 women; 21–35 yr.; Chinese | Laparotomy or laparoscopy for non-infectious issues | Cervical mucus sampled by nylon flocked swabs | Illumina HiSeq | Firmicutes | Lactobacillus spp. | Intra-individual continuum of bacteria that gradually changes from the outermost to the innermost | |
| Winters et al., 2019 | n = 25; 45 yr.; Italian | Hysterectomy intended for fibroids (n = 23) or endometrial hyperplasia (n = 2) | Cervix sampled by Dacron swab | Illumina V4 qPCR for V1-V2 16S rRNA gene for L. iners and L. crispatus | Proteobacteria | Acinetobacter spp. Comamondaceae, Cloacibacterium spp., Pseudomonas spp. Lactobacillus spp. | Relative abundance data: Acinetobacter = 49%, Lactobacillus spp. = 19.24%. Similarities between cervical and endometrial microbiomes | |
| Endometrium | Mitchell et al., 2015 | n = 58; 43 yr.; 79% White, 10% AA, 7% Hispanic | Women undergoing hysterectomy for non-cancer indications | Endometrial swab frotis from the contralateral endometrium | qPCR 12 primers * *Flavobacterium was not tested | Firmicutes Bacterioidetes | L. iners (45% subjects) L. crispatus (33% subjects) Prevotella spp.(33% subjects) | 95% of women harbour UGT colonisation. Endometrial cavity is not sterile in most women |
| Franasiak et al., 2016 | n = 33; 35.9 yr.; 79% Caucasian , 15% Asian , 3% AA, 3% Hispanic | Women undergoing single embryo transfer of an euploide blastocyst | Distal part of IVF catheter tip | Ion 16S metagenomics V2-4-8, V3-6, V7-9 | Firmicutes Proteobacteria | Lactobacillus spp.: IVF Flavobacterium | No microbial profile differences between successful and unsuccessful IVF | |
| Verstraelen et al., 2016 | n = 19; 32 yr.; White Caucasian | Various reproductive conditions (RIF, RPL), with no uterine anomalies | Tao Brush TM after hysteroscopy | Illumina MiSeq V1-V2 | Bacteroidetes | B. xylanisolvens, B. thetaiotaomicron, B. fragilis, Pelomonas, | Unique microbiome dominated by Bacteroides in endometrium of non-pregnant women | |
| Moreno et al., 2016 | n = 13; reproductive age; Spanish | Fertile women 2 samples/women (both from pre-receptive and receptive phases) | EF with Wallace ET catheter | 454 pyrosequencing. V3-5 | Firmicutes | Lactobacillus spp. | 9/13 women harboured endometrial colonisation exclusively by Lactobacillus Bacterial communities different to vagina; high proportion of Atopobium, Clostridium, Gardnerella, Megasphaera, Parvimonas, Prevotella, Sphingomonas, or Sneathia | |
| n = 35 (cases), 25–40 yr.; n = 35 (controls), 18–35 yr.; Spanish | Cases: Infertile women undergoing IVF | Firmicutes Actinobacteria | Lactobacillus spp. (RA: 71.1%), Gardnerella spp. (RA: 12.6%), Bifidobacterium spp. (RA: 3.7%), Streptococcus spp. (RA: 3.2%), Prevotella spp. (RA: 0.9%) | > 90% Lactobacillus: LD; < 90% Lactobacillus: NLD. NLD associated with poor reproductive outcome | ||||
| Miles et al., 2017 | n = 10; 41–57 yr.; North American | Non-pregnant, non-infected women, with no immune disease. Treated with antibiotics 30min. prior surgery | EB sampled by swabs after hysterectomy, salpingo-oopherectomy | 454 pyrosequencing V1-V3 | Firmicutes | Lactobacillus spp. Corynebacterium spp. Staphylococcus spp. Acinetobacter spp. Blautia spp. | Microbial composition were highly related across the samples and across the subjects | |
| Tao et al., 2017 | n = 70; 36.2 yr.; 61% Caucasian, 17% Asian, 14% AA, 5.6% Hispanic | IVF patients | EB with Wallace ET catheter | Illumina V4 | Firmicutes | Lactobacillus spp., Corynebacterium spp., Streptococcus, Staphylococcus spp. Bifidobacterium spp. | Lactobacillus spp. was detected in all patients | |
| Chen et al., 2017 | n = 95; 22–48 yr.; Asian | Laparotomy or laparoscopy patients for conditions not involving infection, cancer, or immune diseases | Flocked nylon swabs | Ion Torrent V5-V4 | Firmicutes | 30.6% Lactobacillus spp. 9.09% Pseudomonas spp. 9.07% Acinetobacter 7.39% Vagococcus | Endometrial microbiome cultivable 5/15 cases. Unique microbiota. Recovered biomass was 103 times lower in endometrium compared to vagina | |
| Pelzer, Willner, Buttini, and Huygens, 2018 | n = 145 (cases), n = 3 (virgo intacta controls); 14–52 yr.; Australian | Women with menorrhagia or dysmenorrhea undergoing hysteroscopy and/or laparoscopy | EB samples taking by curette | 454 pyrosequencing V5-V8 | Firmicutes | Lactobacillus spp. Gardnerella spp. Prevotella spp. Veillonella spp. Sneathia spp. Fusobacterium spp. and Jonquetella spp. in virgo intacta subjects (n = 3) | Female upper genital tract is not sterile. Fusobacterium spp. and Jonquetella spp. normally included in the bacterial vaginosis spectrum | |
| Wee et al., 2018 | n = 6 (cases), 37.6 yr.; n = 5 (controls), 42.75 yr. Australian | Cases: Women with history of infertility; Controls: History of pregnancy with no ART procedures, healthy | EB taken by endometrial curette at hysteroscopy | Illumina MiSeqVR V1-3 | Firmicutes Actinobacteria | Lactobacillus dominance in 1/6 case samples Lactobacillus spp. Bifidobacterium spp. in 4/5 control samples | Need for further exploration. Lower relative abundance of species Microbiome displays lower relative abundance compared to vagina/cervix | |
| Kyono et al., 2018 | n = 79 (IVF cases), 37 yr., n = 23 (non-IVF cases), 33.2 yr., n = 7 (controls), 36.6 yr.; Australian | Women enrolled for IVF treatment; infertile women not enrolled for IVF | EF sampled by IUI Kitazato catheter for ET | Illumina MiSeq V4 | Firmicutes | Lactobacillus spp. | Average Lactobacillus spp.: 63.9% in IVF group, 96.2% in non-IVF group, 99.8% in controls. | |
| Li et al., 2018 | n = 137; 21–35 yr.; Asian | Laparotomy or laparoscopy for non-infectious issues | Endometrial swab | Illumina HiSeq | Proteobacteria | Pseudomonas spp., Propionilbacterium spp., Streptococcus spp., Moraxella spp. | Differences between LGT and UGT (Lactobacillus does not dominate in endometrium) | |
| Liu et al., 2018 | n = 25; 35.2 yr.; Chinese | RPL patients | EF collected by ET catheter, endometrial tissue collected with Pipelle (paired samples) | Illumina MiSeq V4 | Proteobacteria | Atopobium spp., Prevotella spp., Gardnerella spp., Bifidobacterium spp., Stenotrophomas spp., Lactobacillus spp., Megasphaera spp., Staphylococcus spp., and Escherichia spp. | Authors mention EF microbiome does not reflect same configuration as tissue | |
| Kyono et al., 2019 | n = 92 (n = 47, LD; n = 45, NLD) 37 yr. Asian | Women who underwent frozen-thawed blastocyst transfer after endometrial microbiome analysis Antibiotics and prebiotic/probiotic supplementation in n = 9 NLD women | EF sampled by IUI Kitazato catheter for ET | Illumina MiSeq V4 | Firmicutes | Lactobacillus spp., Atopobium spp., Bifidobacterium spp. Gardnerella spp., Megasphaera spp., Sneathia spp., Prevotella spp. | After intervention, 9 NLD turned LD (46 LD vs. 36 NLD) LD endometrium possibly beneficial for implantation | |
| Kitaya et al., 2019 | n = 28 (cases), 38.7 yr.; n = 18 (controls), 37.6 yr.; Asian | Cases: RIF patients Controls: First attempt IVF patients | EF sampled by MedGyn Pipette IV | Illumina MiSeq V4 | Firmicutes | Lactobacillus spp., Gardnerella spp., Streptococcus spp., Prevotella spp., Burkholderia spp., and Gardnerella spp. (RIF) | Variation between RIF and controls 18/28 (64.3%) LD in RIF vs. 7/18 (38.9%) LD in controls. Burkholderia only detected in EF of RIF patients | |
| Winters et al., 2019 | n = 25; 45 yr.; Italian | Hysterectomy intended for fibroids (n = 23) or endometrial hyperplasia (n = 2) | Dacron swab middle endometrial portion after hysterectomy | Illumina V4 qPCR for V1-V2 16S rRNA gene for L. iners and L. crispatus detection | Proteobacteria | Acinetobacter spp. Comamondaceae, Cloacibacterium spp., Pseudomonas spp. <1% Lactobacillus spp. | Lactobacillus does not dominate in endometrium (rarely present, does not form the microbial Core) | |
| Younge et al., 2019 | n = 10; reproductive age; North American | Women subjected for caesarean delivery. No antibiotics 48h prior intervention | Swab from the endometrial lining | Illumina MiSeq V4 | Proteobacteria | Escherichia, Acinetobacter, Lactobacillus, Bacillus | Lactobacillus does not dominate in endometrium | |
| Garcia-Grau et al., 2019 | n = 1; 37 yr.; Spanish | Infertile RIF patient | EF sampled by Wallace ET catheter | Ion Torrent V2-4-8, V3-6,V7, V9 NextSeq500 qPCR for G. vaginalis | Actinobacteria | G. vaginalis, Lactobacillus spp., Pseudoalteromonas spp., Bifidobacterium spp., Rhodanobacter spp. | Gardnerella, Atopobium, and Bifidobacterium persisted in endometrium | |
| Hashimoto and Kyono, 2019 | n = 99; 35.3 yr.; Asian | IVF patients undergoing vitrified-warmed blastocyst transfer | EF sampled by Kitazato IUI catheter | Illumina MiSeq V4 | Actinobacteria | Gardnerella spp., Atopobium spp., Streptococcus spp., Lactobacillus spp. | IVF pregnancy rates were comparable between LD and NLD microbiome endometrial configurations (some patients achieved pregnancy with 0% Lactobacillus) | |
| Leoni et al., 2019 | n = 19; 32.3 yr.; Caucasian | Women subjected for caesarean delivery at full term of normal pregnancy | 5 × 5mm EB taken with new sterile scalpels | Illumina MiSeq V5-6 | Actinobacteria | Cutibacterium spp., Escherichia spp., Staphylococcus spp., Acinetobacter spp., Streptococcus spp., and Corynebacterium spp. (50% women) | Lactobacillus is not dominant in endometrium | |
| Moreno et al., 2020 | n = 1; 28yr.; Spanish | Patient with primary infertility diagnostic (2 yr.) and 1 failed IVF cycle | EF transcervical aspiration by ET catheter | Ion Torrent V2-4-8, V3-6,V7, V9) NextSeq500 | Firmicutes | Lactobacillus spp., Enterobacteriaceae spp., Streptococcus spp., Pseudomonas spp., Staphylococcus spp. Before the miscarriage: L. crispatus | During the successful pregnancy: Lower bacterial load plus higher lactobacilli abundance in the endometrial; L. iners was the most abundant at early pregnancy | |
| Riganelli et al., 2020 | n = 24, infertile; 37 yr.; Italian | Women with previous ART treatment and implantation failure | EB taken by Pipelle | Illumina MiSeqVR V4-5 | Firmicutes Proteobacteria Bacteroidetes Actinobacteria | Kocuria dechangensis, L. crispatus, L. iners | Kocuria dechangensis in women with implantation failure Lactobacillus spp. only in women that did not achieve pregnancy; total absence of Lactobacillus, presence of Lachnospiraceae and Enter obacteriaceae in the pregnant group. | |
| Kadogami et al., 2020 | n = 392 (n = 216, LD; n = 176), NLD); 38.6yr.;Asian | Women diagnosed from RIF. NLD patients received different combination of oral and vaginal probiotics or oral prebiotics and antibiotics | EF sampled by Pipette IV | Illumina MiSeq V4 | Firmicutes | Lactobacillus spp., Bifidobacterium spp., Gardnerella spp., Atopobium spp., Streptococcus spp., Prevotella spp., | Cure rate 78% in vaginal probiotic plus antibiotic treatments (NLD changed into LD). Lactobacillus endometrial proportion was <50% right after menstruation, gradually increasing to reach ~70% in luteal phase | |
| Carosso et al., 2020 | n = 15; 35 yr.; Caucasian | Women undergoing IVF with no antibiotics/probiotics/therapy | EB taken by Guardia® Access catheter and sterile scissors | Illumina MiSeqVR V3-4-6 | Firmicutes | Lactobacillus spp., Gardnerella spp., Prevotella spp., Propionilbacterium spp., Pseudomonas spp., Atopobium spp., Delftia spp., Pelomonas spp., Veillonella spp., Escherichia coli/Shigella | Biodiversity increases intra individually after PS | |
| Sola-Leyva et al., 2021 | n = 7; 24–31 yr.; Spanish | Women with regular menstrual cycles, no hormonal/other treatment in the last 3 months, no history of chronic disease | EBs taken by a suction curette Paired samples taken in mid-secretory and proliferative phases | RNAseq (meta- transcriptomics) IlluminaHiSeq 500 | Proteobacteria | Klebsiella pneumoniae, Clostridium botulinum, and Pasterurella multocida | Lactobacillus does not seem to dominate in the endometrium from healthy women | |
| Moreno et al., 2021 | n = 342 patients (n = 336 EF, n = 296 EB); 36 yr.; 57.3% Caucasian, 14% East Asian , 11.4% Hispanic, 17.3% Others . | Women belonging to 13 reproductive clinics undergoing IVF (≤40yr..) or patients undertaking ovum donations (≤50yr.) | EF aspirated by flexible catheter; EB taken by a cannula of cornier | Ion S5 XL, V2-4-8, V3-6, 7-9) | Firmicutes | EF and EB in patients with live birth: Lactobacillus spp. EF in patients with IVF failure: Streptococcus spp., Chryseobacterium spp., Corynebacterium spp., Haemophilus spp., Bifidobacterium spp., Staphylococcus spp., Atopobium spp., Gardnerella spp., Propionilbacterium spp. EB in patients with IVF failure: Gardnerella spp., Klebsiella spp., Atopobium spp., Finegoldia spp., Escherichia spp., Propionilbacterium spp., Haemophilus spp., Anaerococcus spp., and Bacillus spp. | Only in EF: Streptomyces, Clostridium, Chryseobacterium Only in EB: Cupriavidus, Escherichia, Klebsiella, Bacillus, Finegoldia, Micrococcus, and Tepidomonas | |
| Fallopian tubes - Follicular fluid (ovaries) | Pelzer et al., 2011 | n =71; 37 yr. (fertile), 35 yr. (infertile); Australian | Endometriosis (n = 16), PCOS (n = 14), genital tract infection (n = 9), male factor infertility (n = 18), idiopathic infertility (n = 14) | Follicular fluid collected at the time of oocyte retrieval | Culture-based methods; 16S rRNA conventional PCR | Firmicutes | L. iners, Actinomyces spp., Corynebacterium auromuosum, Fusobacterium spp., Prevotella spp., Staphylococcus spp. | Left ovary displays greater colonisation than the right ovary |
| Miles et al., 2017 | n = 10; 41–57 yr.; North American | Non-pregnant, non-infected women, with no immune disease. Treated with antibiotics 30min prior surgery | Ampullary region sampled by swabs after hysterectomy, salpingo-oopherectomy | 454 pyrosequencing V1-V3 | Firmicutes Proteobacteria Actinobacteria Bacteroidetes | Ovaries: Lactobacillus spp., Corynebacterium spp., Escherichia spp., Blaudia spp. Fallopian tubes: Bacterioides spp., Corynebacterium spp., Lactobacillus spp., Coproccocus spp., Hymenobacter spp. | Microbial composition were highly related across the samples and across the subjects | |
| Chen et al., 2017 | n = 95; 22–48 yr.; Asian | Women intervened for conditions not involving infection, cancer, or immune diseases. Not antibiotic, hormones, of vaginal/cervical treatment within a week | Laparoscopy Laparotomy | Ion Torrent V5-V4 | Proteobacteria | Acinetobacter spp. Comamonas spp. Pseudomonas spp. Dysgonomonas spp. Vagococcus spp. | Lactobacillus is only found with a RA:1.69% at fallopian tubes | |
| Pelzer, Willner, Buttini, Hafner, et al., 2018 | n =16; 34–63 yr.; Australian | Women subjected for hysterectomy or salpingectomy/oophorectomy | Fallopian tube dissection | 454 pyrosequencing V5-V8 | Firmicutes | Staphylococcus spp. Enterococcus spp. Lactobacillus spp. Pseudomonas spp. | Lactobacillus is not present in all subjects All samples harboured any kind of colonisation (positive for any bacterial species) | |
| Follicular fluid | L. iners, Actinomyces spp., C. auromuosum, Fusobacterium spp. |
Cervical microbiome
Likewise the vaginal canal, taking samples from the cervix, is a non-invasive process but the risk of contamination with vaginal species is higher. Table 1 summarises part of the research performed into cervical microbiome.
A study showed Lactobacillus (L. crispatus, L. iners) dominance in 12/13 cervical samples from healthy women (controls) and strong microbiome correlation among vaginal, cervical, and endometrial samples within an individual [31]. These findings are in accordance to previous research, which demonstrated microbiome is progressively changing from the lower genital tract (LGT) to the UGT, finding less Lactobacillus abundance in UGT than in LGT. In this study Lactobacilli accounted for 97.56% in cervical mucus, being L. iners and L. crispatus the most abundant species, detected through qPCR. Interestingly, intra-individual correlations between the Pouch of Douglass and cervical microbiomes were found, suggesting that cervix could be analysed to clinically draft the state of the uterus and peritoneum in the clinical realm [13]. Another publication reported that cervical mucus samples, sequenced by Illumina HiSeq (n = 25),and shotgun sequencing (n = 1), displayed Lactobacillus spp. dominance in cervix, as previously reported by the same group in 2017 [13, 38]. Endocervical samples were analysed by Miles et al. showing prevalence of Lactobacillus spp. and Prevotella spp. [14]. Pelzer and colleagues published Lactobacillus spp. was the most recovered genus in endocervical samples, followed by Gardnerella spp., Veillonella spp., Prevotella spp., Sneathia spp., or Fusobacterium spp. [39]. Another group examined cervical samples, publishing Acinetobacter was dominant (49%), followed by Pseudomonas, Cloacibacterium, and Lactobacillus [36]. In 2020, 67 cervix samples from women with different conditions and controls were analysed. 16S rRNA gene sequencing showed a proportion of almost 75% Lactobacillus, followed by >25% Gardnerella, Streptococcus, Atopobium, Prevotella, and Pseudomonas, among others [40]. As a brief remark, several studies have exhibited high diversity of species within cervical microbiome, along with specific genera (for instance, Gardnerella spp.), to be associated with human papillomavirus (HPV) risk in women [41, 42]. Such results underline the link among cervical microbiome and different gynaecological issues.
Endometrial microbiome
Endometrium harbours its unique microbiome, despite having been considered a sterile milieu for many decades (reviewed by [18, 19]). Endometrium displays a low microbial biomass, harbouring 100 to 10000 times less bacterial amount than vaginal niche [18]. Research performed on endometrium is summarised in Table 1. Several studies suggest Lactobacillus, if the most represented genus, indicate healthy uterine ambiance [10–12, 14, 31, 43]. Notwithstanding, there is still no consensus regarding the healthy bacterial microbiome configuration in endometrium, since some other groups have not reported Lactobacillus endometrial dominance [13, 17, 23, 36, 38, 44, 45].
In 2015, a cohort study assessed L. iners, Prevotella spp., and L. crispatus as the most recovered species in the UGT [9]. Franasiak and colleagues ascertained that Lactobacillus and Flavobacterium were the most abundant genera present in endometrial samples [10]. Another group concluded Proteobacteria, Firmicutes, and Bacteroidetes were dominant but Lactobacillus spp. could only be found in 4/19 endometrial samples [17]. Moreno et al. analysed 35 endometrial fluid samples taken from fertile women. Lactobacillus was the most abundant genus (71.1%), followed by Gardnerella, Bifidobacterium, Streptococcus, and Prevotella (12.6%, 3.7%, 3.2%, and 0.9%, respectively). This publication yielded a classification for samples: Lactobacillus-dominated (LD, >90% Lactobacillus spp.) and non-Lactobacillus-dominated microbiota (NLD, <90% Lactobacillus spp. plus >10% of other bacteria). This classification is valuable due to its applicability to evaluate the reproductive outcomes [25]. In 2017, Miles et al. sampled endometrial tissue from 10 subjects, concluding Lactobacillus was the most recovered genus [14]. Tao et al. targeted 16S rRNA gene from endometrial samples, concluding that 33/70 samples contained over 90% of Lactobacillus, and 50/70 samples contained over 70% of Lactobacillus [12]. These findings were consistent with the FGT Lactobacillus spp. dominance reported by Moreno et al. [11]. Conversely, Chen et al. published that Lactobacillus no longer dominated the endometrial milieu (that harboured 103 times lower biomass than vagina). Pseudomonas spp., Acinetobacter spp., Vagococcus spp., and Sphingobium spp. constituted an important fraction of the endometrial microbiota [13]. Wee and colleagues examined endometrial microbiome in a case-control study. Controls (healthy fertile women, n = 5) were mostly represented by Lactobacillus and Bifidobacterium, and endometrial microbiome had lower relative abundance compared to vagina and cervix samples [31]. Another group performed endometrial microbiome shotgun sequencing, identifying Pseudomonas, Propionilbacterium, Streptococcus, and Moraxella as the top recovered taxa [38]. Kyono et al. collected endometrial fluid samples: 47 patients were LD (>90% Lactobacillus) and 45 were NLD (<90% Lactobacillus plus >10% of other bacteria). Recovered genera were Lactobacillus, Atopobium, Bifidobacterium, Gardnerella, Megasphaera, Sheathia, and Prevotella [43]. Another study identified Lactobacillus, Pseudomonas, Streptococcus, and Atopobium in healthy women (n = 2) [40]. Winters’ group showed Acinetobacter (44%), followed by Escherichia or Pseudomonas, were dominant in endometrium, where <1% Lactobacillus was found [36]. Most recently, Sola-Leyva et al. studied samples from 7 women, in order to decipher the core microorganism composition in healthy endometrium of young women. Meta-RNA sequencing showed that Klebsiella pneumoniae, Clostridium botulinum, and Pasterurella multocida were the three most abundant bacterial species in endometrial biopsies, where Lactobacillus was not dominant [23].
Regarding UGT colonisation mechanisms, several hypotheses are put forward. UGT microbiome may derive from bacteria which ascend from the vagina, either directly or attached to the semen [46]. Communication between vagina and uterus would be facilitated or inhibited by peristaltic contractions, cervical fluid consistency, cervical folds, or the immune response [36, 47]. A publication showed that women with bacterial vaginosis (BV) had increased UGT bacterial load [48]. Conversely, other authors suggested each site displays its own community, since some vaginal species were not found in the endometrium and vice-versa [11, 16, 39]. Another hypothesis defends that UGT colonisation might arise by hematogenous spreading from gastrointestinal, airways, or oral niches [49, 50]. Finally, certain gynaecological procedures could transfer microbial load from vagina to the uterus [49].
Fallopian tubes and ovarian microbiome
Both fallopian tubes and ovaries display highly variable microbial communities among women. Lactobacillus spp. is present in lower ratio than in vagina or cervix (Fig. 1, Table 1). Fallopian tubes and ovaries harbour a variety of bacteria growing in mildly alkaline conditions, in contrast with the acidity of vaginal pH [13].
Chen’s group showed that Lactobacillus comprised only 1.69% of the median relative abundance found at fallopian tubes microbiota in their cohort of 110 women [13]. Another publication examined the composition of the microbial communities throughout fallopian tubes and ovaries of 10 women. Bacterioides, Corynebacterium, Lactobacillus, Coproccocus, or Hymenobacter were recovered from fallopian tube samples; whereas Lactobacillus, Corynebacterium, Escherichia, or Blaudia were recovered from ovary fluid, suggesting a high inter-individual variability among these anatomical sites [14]. Pelzer and colleagues reported that Lactobacillus spp. were not present all in fallopian tubes samples, but all of them were positive for at least some bacterial species [51]. Regarding microbial signature in ovarian follicular fluid, L. iners, Actinomyces spp., Corynebacterium auromuosum, Fusobacterium spp., Prevotella, or Staphylococcus spp. were found, being colonisation more prevalent in the left than in the right ovary [52]. Understanding the structure of these microbial communities might be helpful to propose alternative treatments for patients who would normally undergo salpingectomy for some pathologies [14].
Eubiosis and dysbiosis
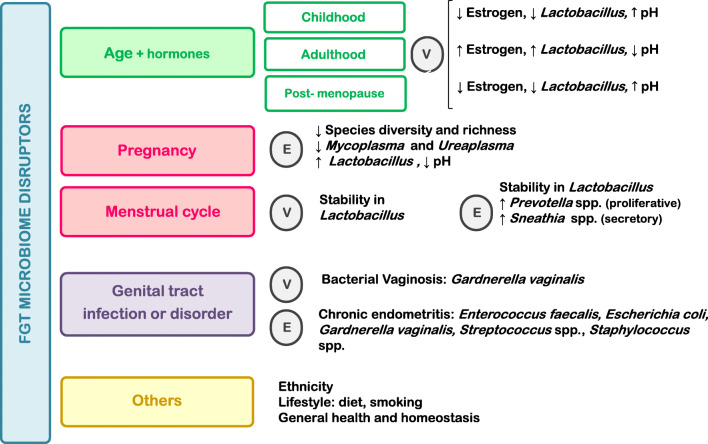
Eubiosis is the healthy and balanced microbial ecosystem, whereas dysbiosis represents the non-homeostatic state [53]. If dysbiosis is the cause or the consequence of disease is unknown, but eubiotic and dysbiotic FGT configuration matters when establishing the link among bacterial communities and gynaecological and reproductive disorders [54–56]. In general, greater microbial diversity within the FT is associated with dysbiosis, linked to higher risk of negative events in the FGT physiology [7]. Overview of the main factors conditioning eubiotic and dysbiotic FGT state is depicted in Fig. 2.
Fig. 2.
Main factors that condition eubiosis and dysbiosis in UGT (upper genital tract) and LGT (lower genital tract)
Healthy vagina harbours a structured microbial ecosystem, displaying lower alpha and beta diversity than other body sites [19, 57]. As mentioned, Lactobacillus abundance (CST I, II, III, and V) in combination with low species diversity would be associated with better reproductive outcomes. Conversely, CST IV, rich in anaerobic species such Gardnerella, Prevotella, or Atopobium, could be associated to dysbiosis [20, 29, 58]. A systematic review concluded vaginal dysbiosis is a significant risk factor for early pregnancy loss in ART patients [59].
Regarding endometrium, shifts from a LD community are linked to subfertility and dysbiosis [9, 11]. LD endometrial microbiome might predict reproductive success, being considered eubiosis, but if it harbours < 90% Lactobacillus (NLD), the prognosis of the reproductive outcome is adverse, identified as dysbiosis [11]. Other factors such the high bacterial load or the presence of certain taxa could be linked to tissue destruction and immune over-stimulation that leads to dysbiosis [60]. This is the case of the Fusobacterium spp. and Jonquetella spp. (involved in BV too) dominance in endometrium of women with menorrhagia, which might suggest a reason for their dysbiotic state [39].
FGT microbiome shifts in response to internal and external changes
FGT microbiome may undergo shifts in response to stimuli such hormonal fluctuations, pregnancy, diseases, age, or ethnic origins, as described by several authors [29, 30, 56, 61]. A summary of the main factors affecting FGT microbiota, regarding vagina and endometrium, is outlined in Fig. 3.
Fig. 3.
Main variables involved in vaginal and endometrial microbiome shifts. Predominant taxonomy associated to each condition is depicted. (E, endometrium; V, vagina)
Age and hormonal shifts through women lifespan
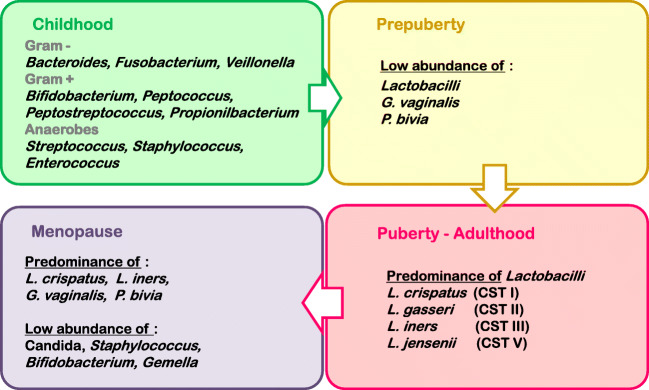
Vaginal microbiota fluctuations triggered by age are driven by hormonal shifts [30, 61, 62]. During childhood, vaginal pH is neutral, and anaerobes and E. coli predominate in the vaginal niche [63]. At puberty, vaginal epithelial cells, stimulated by oestrogen, start to produce glycogen, leading to the lactobacilli dominance that prevails in healthy vagina during reproductive years [63]. Lactobacillus spp. produce lactic acid, hydrogen peroxide, and bacteriocins, factors leading to a pH decrease, lactobacilli own adhesion to epithelial cells, and pathogenic bacteria detriment [64–66]. As oestrogen levels decrease in menopause, glycogen does too, triggering a descent in lactobacilli population [67]. Notwithstanding, there is still a predominance of L. crispatus and L. iners, as well as Gardnerella or Prevotella [68]. Figure 4 summarises vaginal microbial signature across the female life stages.
Fig. 4.
Outline of the most predominant microorganisms in the vaginal niche throughout the female lifecycle
Menstrual cycle
There is a current debate about whether the microbiome remains stable during the menstrual cycle or, oppositely, if it changes throughout different phases. Gajer et al. observed high levels of species turnover and microbiota dynamism triggered by menstruation that did not dramatically affect community function [30]. Moreno et al. showed that the endometrial bacterial community did not significantly varied within the pre-receptive and receptive endometrial phases [11]. Another study analysed both intra and inter cycle fluctuations in vaginal and endometrial microbiome, showing high stability in the lactobacilli abundance [35]. On the contrary, other groups have suggested microbial communities differ between menstrual cycles. Chen et al. reported differences in the uterine microbiome between menstrual phases (P. acnes was more abundant in the secretory phase) [13]. Another group reported Prevotella spp. and Sneathia spp. increased, respectively, in the proliferative and secretory phases (women were suffering menorrhagia and dysmenorrhea, suggesting these bacteria might have a function such pathologies) [39]. Kadogami et al. recently found that Lactobacillus endometrial proportion ranged from <50% (after menstruation) to ~70% (luteal phase) [69]. Since changes in oestrogen levels trigger changes in vaginal [70, 71] and presumably in endometrial microbiome, Carosso et al. studied putative microbiome shifts after controlled ovarian stimulation and progesterone supplementation. In vagina, Lactobacillus decreased whereas Prevotella, Escherichia, and Shigella increased; in endometrium, Lactobacillus slightly decreased with an increase in Prevotella and Atopobium [34]. A recent publication reported endometrial microbiome to be more transcriptionally active in the mid-secretory compared to the proliferative phase, which suggest bacterial functions are regulated in a cycle-dependent way [23].
Pregnancy
Pregnancy is a condition that naturally changes the vaginal microbial signature, leading to a microbiome shift compared to the non-pregnant state. Whereas non-pregnant women might show variation in the Lactobacillus spp. abundance, this genus is invariable maintained, along with a reduced species diversity, during pregnancy progression in vagina [72–74]. According to Zheng and colleagues, L. iners abundance in vagina decreased in second and third trimesters whereas L. crispatus increased in the second trimester [75]. Romero et al. reported that in spite of such lactobacilli stability and dominance, the individual CST may change from one lactobacilli-dominated CST to a different one [29, 72]. Related to this, Aagaard’s group stated that vaginal microbiome harboured by women who were close to the pregnancy term kept similarities to the non-pregnant vaginal microbiome configuration. This suggest that, at some point near the delivery time, vaginal microbiome of pregnant women could return to the configuration it displayed before the gestation [74]. Freitas and collaborators reported lower Mycoplasma and Ureaplasma concentration and higher Lactobacillus spp. load in healthy pregnant women compared to non-pregnant cohort [76]. Endometrium was sampled at the time of caesarean delivery in ten women, finding Escherichia, Acinetobacter, Lactobacillus¸ and Bacillus as the most recovered taxa [45]. Other group found Cutibacterium, Escherichia, Staphylococcus, Acinetobacter, Streptococcus, and Corynebacterium in more than 50% of the endometrial samples (n = 19), taken from women at caesarean deliveries [44]. Acinetobacter and Escherichia were the most recovered genera in both studies [44, 45]. Interestingly, Moreno et al. sampled endometrial fluid within a successful pregnancy. According to their findings, this patient, who had suffered a spontaneous miscarriage, harboured lower bacterial load and higher lactobacilli abundance in the endometrial fluid sample taken during a successful pregnancy (sampled at pregnancy week 4) in comparison to samples taken before the miscarriage. L. crispatus and other species were detected before the miscarriage, whereas L. iners was the most abundant in the endometrium during the early pregnancy [77]. In mid stages of pregnancy, anti-inflammatory uterine milieu and microbial species harboured by the placenta (such as Proteobacterium, Actinobacterium, Firmicutes, Bacteroidetes, and Temericutes) may help to maintain healthy and low-risk pregnancies. This was depicted by several studies and by the iHMP, in which 1527 pregnancies were longitudinally monitored [7, 19, 78, 79]. Anti-inflammatory ambiance changes to pro-inflammatory before the onset of labour [7].
Regarding delivery and PTB, it has been suggested that high bacterial diversity (CST IV plus Lactobacillus depletion, and/or increased levels of Sneathia spp., Prevotella spp., among others) in vagina during the first semester of pregnancy is associated with higher risk of PTB [73, 80–82]. These hypotheses have been also supported the iHMP: women experiencing PTB were less likely to harbour L. crispatus dominance in vaginal microbiome [7]. Another group found Lactobacillus and Bifidobacterium richness in vaginal communities might be associated with decreased risk of early PTB [83]. An important clinical implication is the possibility of monitoring pregnancy progression using microbiome and their functions to discern between good and bad pregnancy prognosis.
Role of the female genital tract microbiome in reproduction, gynaecology and obstetrics
Microbiome and gynaecological disorders
Bacterial vaginosis
BV is usually typified by a detriment in Lactobacillus dominance, linked to Gardnerella, Mycoplasma, and Prevotella increase, being somehow comparable to CST IV configuration [84, 85]. Anaerobic bacteria dominance during BV can also be related with Gardnerella biofilms development [48]. Ceccarani et al. reported L. crispatus (present in controls, n = 21) was replaced by L. iners, present along with Gardnerella, Prevotella, Megaspheaera, Roseburia, and Atopobium in BV samples (n = 20) [86]. However, it is still unclear whether either these are pathogens that cause BV or they are just opportunistic bacteria that take advantage of the higher pH and colonise vagina [87, 88]. Antibiotics are usually the first-line treatment to face BV [26], but novel approaches such vaginal microbiome transplants are considered to be feasible in antibiotics-nonresponsive and recurrent patients, as shown by a case series performed in 2019 [89]. A recent systematic review and meta-analysis addressed that BV was associated with early spontaneous abortion in in vitro fertilisation (IVF) patients [90].
Chronic endometritis
Chronic endometritis (CE) is the persistent inflammatory state of the endometrium, raised by an imbalance between bacterial species and female immune system [25, 91]
The endometrial microbiome in women with CE harboured Lactobacillus, Enterobacter, Pseudomonas, and Gardnerella (33.21%, 7.17%, 7.32%, and 6.91%, respectively). Gardnerella was not detected in control samples from healthy women [16]. Similarly, Liu et al. concluded than infertile women with CE harboured Bifidobacterium, Prevotella, and Gardnerella, which did not occur in non-CE infertile women [92]. Another study showed Gardnerella, Klebsiella, and Streptococcus were significantly increased both in endometrial fluid and biopsy samples from women who did not achieve pregnancy [93]. Since CE might go along with recurrent implantation failure (RIF) [94, 95], the development of new diagnosis tools and its treatment with antibiotics could improve pregnancy outcomes [96–98]. CE is to date the gynaecological issue which undergo the greatest improvement if treated with antibiotics, since the bacterial infection is a clear risk in this case [99]. Main bacteria triggering CE are E. faecalis, Enterobacteriaceae, Streptococcus spp., Staphylococcus spp., G. vaginalis, Mycoplasma spp., among others [98].
Epithelial ovarian cancer
Epithelial ovarian cancer (EOC) is one of the most lethal malignancies affecting women [100]. In 2017, Banerjee and colleagues identified the microbiome uniquely present in EOC, composed by Proteobacteria (52%), Firmicutes (21%), Bacteroidetes, Chlamydiae, Spirochaetes, and Temericutes. Authors suggested microbiome could influence both induction and progression of the EOC. However, it may have occurred that the tumour had generate a microenvironment in which these specific bacteria are able to persist [101]. Matching with these results, Zhou et al. analysed high-grade serous EOC samples, isolating Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria as the most represented taxa [102]. Since there is emerging evidence of the active role of microbiota in the development or metastasis of malignancies [103], establishing an unique EOC microbiome could provide useful biomarkers for EOC diagnosis or prevention [101].
Endometriosis
Endometriosis is the growth of endometrial tissue outside the uterine cavity, causing pelvic pain or infertility [104]. The pathophysiology of endometriosis is not yet fully elucidated [105], and FGT microbiome has been studied to decipher its putative involvement in endometriosis risk.
Gardnerella, Enterococcus, Streptococcus, and Staphylococcus were found among the pathogenic species isolated in endometriotic samples compared to controls, where Lactobacillus spp. was predominant [106]. Streptococcaceae and Moraxellaceae significantly increased in endometriotic samples [15]. Cregger and colleagues found Lactobacillus, Barnesiella, Flavobacterium, and Pseudomonas to be the most predominant genera in samples from women with and without endometrial lesions [107]. Pseudomonas, along with Acinetobacter, Vagococcus, and Sphingobium, were found in uterine endometriotic lesions samples [13]. Another study showed Atopobium was absent both in cervical and vaginal microbiota, whereas Gardnerella increased in cervical microbiota in the endometriosis group, in comparison with controls [57]. In 2019, Akiyama and collaborators examined cervical samples collected women with endometriosis (n = 30). Despite Lactobacillus being predominant, Enterobacteriaceae and Streptococcus were increased in the endometriosis group compared to controls [108]. Hernandes et al. showed different bacterial profiles in deep endometriotic lesions: reduced predominance of Lactobacillus and more abundance of Alishewanella, Enterococcus, and Pseudomonas, compared to eutopic endometrial controls [109]. Since endometrial infection triggers uterine contractility, this could explain the retrograde seeding of endometrial cells outside the uterus, allowing endometriosis progression [110, 111]. In 2018, Khan proposed the “bacterial contamination hypothesis”: as the levels of E. coli lipopolysaccharide are increased in endometriosis, endometrial bacteria binding Toll-like receptors could elicit the immune response in endometriosis [112]. If confirmed, microbiome could be considered as a non-invasive tool in the diagnosis of endometriosis. Such is the case of Streptococcus or Atopobium [15, 40, 113], or the increase in diversity along with lactobacilli detriment in the cervical mucus and endometrial microbiomes, which have been proposed to be markers for endometriosis diagnosis [109, 114].
Endometrial cancer
It is estimated that 15% of tumours are related to infectious agents [115]. The role of the uterine microbiota in endometrial cancer (EC) was first assessed by Walther-António, concluding that Atopobium vaginae and Porphyromonas somerae were associated with EC. The inflammatory role of bacteria (triggering immune response) was considered in the onset this pathology [116]. Later, P. somerae was established as a predictive biomarker of EC [117]. Another group described Acinetobacter, Pseudomonas, Cloacibacterium, and Escherichia to be predominant in EC [36]. Most recently, Lu and collaborators have published a study showing Micrococcus to be more abundant in the EC group (n = 25) compared to controls (n = 25, benign uterine lesions), suggesting dysbiosis in EC might be associated with this genus in particular [118]. However, the microbial configuration of EC is not fully unravelled yet.
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is the most common cause of female endocrine-related infertility, being its prevalence among 5–20% in reproductive age women [119]. FGT microbiome is under study, although the core microbial composition that might trigger the PCOS onset has not been defined yet [120].
Colonising microorganisms in ovarian follicular fluid affected fertilisation rates in women with PCOS (n = 14), probably due to oocyte in vivo damage after being exposed to bacterial species or their metabolites [52]. Subsequent research by the same group in 49 women diagnosed of PCOS demonstrated 29% and 71% of the subjects displayed colonised or contaminated follicular fluid, respectively, which was related with a negative IVF outcome. Among the microorganisms, Propionilbacterium spp., Streptococcus spp., Actinomyces spp., Staphylococcus spp., and Bifidobacterium spp. were found [121].
The role of the LGT in PCOS has also been studied [120]. Vaginal and cervical biopsies from 47 PCOS patients and 50 healthy patients were analysed in a study conducted by Tu et al. Results exhibited increased abundance of pathogens such as Gardnerella vaginalis, Mycoplasma hominis, and Prevotella, as well as reduced Lactobacillus both in vaginal and cervix in PCOS group compared to controls [122]. Similar results showed increased Mycoplasma and Prevotella, and decreased L. crispatus in 39 PCOS cases compared 40 healthy controls [123], indicating Mycoplasma could be proposed as a PCOS marker, according to these publications [122, 123].
Microbiome in reproduction and assisted reproduction techniques
Vaginal and endometrial microbiome have been studied in subfertile patients, due to the fact it might constrain pregnancy achievement and ART results [60, 124].
In the peri-implantation period, bacteria and bacterial derived fragments can elicit inflammatory immune responses (mediated by pro-inflammatory cytokines), as it also occurs in the endometrial receptivity acquisition [125, 126]. Lactic acid might benefit and support endometrial receptivity and blastocyst invasion as well, meaning lactobacilli abundance in FGT matters to achieve pregnancy [11, 127]. Regarding vaginal microbiome, if exclusively composed by Lactobacillus, it conforms the best scenario for a successful embryo transfer outcomes, according to several studies and experts [25, 128].
Another group ascertained that the lower Lactobacillus in vagina, the lower likelihood to undergo successful embryo implantation. However, >60% of L. crispatus was unfavourable to achieve pregnancy, which suggest the acidity is linked the implantation outcome [129]. Apart from greater lactobacilli abundance, limited diversity of species also triggered better results after embryo transfer, as concluded by another group [130]. A review and meta-analysis by Singer et al. has concluded that women with dysbiotic vaginal microbiota have 1.4 times less chance of becoming pregnant after an IVF treatment, compared with women harbouring eubiotic configuration [131].
Looking at the link between microbiome and implantation window, reproductive success was predicted based on the endometrial abundance of Lactobacillus spp. in IVF patients. Displaced implantation window and therefore embryo transfer failure might occur due to FGT microbiome shifts due to ovarian stimulation prior to IFV, which suggest considering transferring a frozen embryo [34], or trying to modulate FGT microbiome (see the “FGT microbiome modulation in clinical activity might help to improve reproductive results” section). >90% of lactobacilli (LD) was significantly linked to better embryo implantation results, on-going pregnancy, etc. <90% of Lactobacillus spp. in combination with >10% of other genera (NLD) was linked to poor reproductive outcomes in such women [11]. This was questioned by another group, which showed Lactobacillus abundance did not significantly correlated with the IVF result, since Flavobacterium was one of the most recovered taxa in endometrium [10]. Kyono and collaborators analysed the endometrial fluid from patients undergoing IVF. 15/79 patients achieved pregnancy after embryo transfer, and Lactobacillus average was >96% in pregnant women, according to LD endometrium condition [11, 35]. Subsequently, a pilot study analysed endometrial samples from infertile women. A total of 47 women harboured LD endometrium, whereas 45 harboured NLD, from which 9 changed to LD after antibiotics and prebiotic/probiotic treatments. Although results showed pregnancy rates did not significantly differed between LD and NLD samples, authors remarked the usefulness in deciphering Lactobacillus dominance before IVF [43]. Contrary to what has been previously shown, Hashimoto and Kyono concluded NLD endometrium and taxa such Atopobium, Gardnerella, and Streptococcus did not impede implantation after blastocyst transfer, observing comparable pregnancy rates between LD and NLD [132]. Wee and collaborators analysed vaginal, cervical, and endometrial samples comparing fertile and infertile women. Results showed a trend by which Ureaplasma and G. vaginalis were overrepresented in vagina and cervix of infertile women, respectively [31]. Another publication depicted Kocuria dechangensis was present in endometrial tissue before ovarian stimulation in women with implantation failure [33]. A multicentre prospective observational study has recently sequenced endometrial microbiome from fluid and biopsy samples taken from 342 ART patients at the time of endometrial receptivity analysis (ERA [133]). Lactobacillus and commensal bacteria (Cupriavia, Finegoldia, Microbacterium, and Tepidomonas) were present in higher proportion in patients having live birth. Women with adverse results (clinical miscarriage or no pregnancy achievement) harboured Bifidobacterium, Chryseobacterium, Haemophilus, Klebsiella, Neisseria, Staphylococcus, Streptococcus, Gardnerella, and Atopobium, being these last two species strongly associated with BV [93]. These studies suggest the potential value of microbial species as biomarkers to predict successful reproductive outcome before embryo transfer, avoiding losing blastocysts [33, 93]
Uterine microbiome might have a role in cases of RIF or recurrent pregnancy loss (RPL) [134]. A study examined paired endometrial fluid and tissue samples from 25 patients who had suffered RPL. Results displayed Atopobium, Prevotella, Gardnerella, Bifidobacterium, Stenotrophomas, Lactobacillus, Megasphaera, Staphylococcus, and Escherichia as the most recovered taxa, albeit results from fluid and tissue did not fully matched [135]. Kitaya et al. studied paired vaginal and endometrial fluid samples from women with RIF, who harboured increased Gardnerella compared to controls, and Burkholderia spp. (not found in controls) [95]. These findings are consistent with a longitudinal study that showed Gardnerella, Atopobium, and Bifidobacterium persisted in the endometrium of a patient suffering from RIF [136].
Gathering all this knowledge, microbiome of women undergoing ART should be screened in advance, in order to improve results prior starting the treatment [25].
FGT microbiome modulation in clinical activity might help to improve reproductive results
The main and last objective of ART is to achieve healthy pregnancies and live birth in their patients. As FGT microbiome, and in particular vaginal and endometrial microbiome might condition embryo implantation and the course of pregnancy as well as the occurrence or non-occurrence of gynaecological diseases, its intended modification may be decisive in achieving the purpose of ART under some circumstances [25, 26].
In particular situations, such BV or CE, the use of antibiotics, probiotics, and/or prebiotics could be considered. Albeit antibiotic use is still controversial, and should not be used routinely to change vaginal microbial configuration, it has been suggested that their prescription could be relevant for certain patients, as it is the case of women undergoing RIF [25, 137]. Recently, the effect of different combinations of oral and vaginal probiotics or oral prebiotics and antibiotics in 176 with RIF was categorised as NLD (~25.2% Lactobacillus and Bifidobacterium, and higher concentration of Gardnerella, Atopobium, Streptococcus¸ and Prevotella). After treatment, microbiome was evaluated and there was cure rate of 78% with the combination of vaginal probiotic suppository plus antibiotics [69].
New promising strategies to improve ART outcomes or to manage gynaecological disorders related to FGT microbiome, such as vaginal microbiome transplantation [89, 138] and algorithm design for FGT microbial profile stratification [129] are currently under development.
However, microbiome configuration differs among individuals [139, 140], and samples are usually taken in women with previous medical conditions during surgical interventions, which might account for microbiome oscillations from what is considered as “normal microbiome” [141]. Moreover, sampling uterus, ovaries, and Fallopian tubes is invasive, there is risk of vaginal and cervical bacteria contamination, and their analysis is usually arduous due to the low microbial biomass harboured by the UGT [23, 26]. Due to these reasons, FGT characterisation, although needed for a preliminary diagnostic in reproduction, is still challenging. It requires a profound experimental design, a neat and standardised execution (detailing nucleic acid extraction methods, describing controls and reporting contamination, and maintaining the protocol during the whole study), and results must be interpreted with caution [142–145].
Conclusions
Microbiome impact has recently gained importance due to its role not only in pathology but also in host physiology. This review assesses current research about FGT microbiome and its implication in female health and reproduction.
General consensus depicts microbial communities change progressively from the vagina to the ovary, decreasing in abundance and increasing in diversity, from the outermost to the innermost. Rich in Lactobacillus vaginal and endometrial microbial profiles are linked to better reproductive outcomes. Addressing FGT microbiome provides new clinical diagnostic tool for previously misunderstood fertility issues and new approaches for ART in women. Moreover, this could be used to design novel treatments (such probiotics or microbiome transplantations) to improve endometrial receptivity, to achieve healthier pregnancies, and to treat gynaecological disorders such endometriosis or cancer.
However, the main question still remains in unravelling if there is either a “microbial taxa core” or a “functional core” (understood as the pool of metabolic functions performed by microbiome) that leads to a eubiotic state. Bearing in mind that this configuration can vary among individuals, there is a limitation to establish a comparison among bacterial communities, and also in deciphering whether any specific configuration is eubiotic or dysbiotic. In this regard, the unification of research protocols and the standardisation of NGS techniques must be considered as a key for future research in the reproductive microbiome field.
Following steps point to FGT virome, mycobiome, and microbiome interrelation, to fully describe physiology in this body site. By pooling all this knowledge, clinical implications of microbiome in FGT could be assessed, eventually achieving a better gynaecological and reproductive health in such patients. Although the clinical recommendations for profiling and modulation FGT microbiome are yet to be fully elucidated, a new horizon in the field of personalised medicine is currently open.
Acknowledgements
The authors are very grateful to Irene Bosch for the original drawing on Figure 1 and Figure 2.
Author contribution
P.P. contributed to the data collection and interpretation, E.L. revised the article and approved the final version. The first and last authors significantly contributed to the study conception and design, performed statistical analyses, and data interpretation and drafted the article.
Declarations
Conflict of interest
P.P declares no conflicts of interest. E.L. received a grant from Ferring in 2020, has provided consultancy services for MSD and Ferring Pharmaceuticals, and is part of the Ferring Pharmaceuticals LIFE program and Merck Global program for Fertility Innovation Leaders. During the past 12 months, she has received honoraria from Angelini/IBSA, Merck, MSD, and Ferring Pharmaceuticals for lecturing.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies J. In a Map for human life, count the microbes, too. Science (80-). 2001;291:2316b – 2316. 10.1126/science.291.5512.2316b [DOI] [PubMed]
- 2.Franasiak JM, Scott RT. Reproductive tract microbiome in assisted reproductive technologies. Fertil Steril. 2015;104:1364–1371. doi: 10.1016/j.fertnstert.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Moreno I, Simon C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod Med Biol. 2019;18:40–50. doi: 10.1002/rmb2.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, et al. The NIH human microbiome project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proctor LM, Creasy HH, Fettweis JM, Lloyd-Price J, Mahurkar A, Zhou W, et al. The Integrative human microbiome project. Nature. 2019;569:641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tissier H. Recherches sur la flore intestinale des nourrissons : état normal et pathologique. 1st ed. Paris:G. Carre and C. Naud;1900.
- 9.Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212:611.e1–611.e9. doi: 10.1016/j.ajog.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franasiak JM, Werner MD, Juneau CR, Tao X, Landis J, Zhan Y, et al. Endometrial microbiome at the time of embryo transfer: next-generation sequencing of the 16S ribosomal subunit. J Assist Reprod Genet. 2016;33:129–136. doi: 10.1007/s10815-015-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazán J, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215:684–703. doi: 10.1016/j.ajog.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Tao X, Franasiak JM, Zhan Y, Scott RT, Rajchel J, Bedard J, et al. Characterizing the endometrial microbiome by analyzing the ultra-low bacteria from embryo transfer catheter tips in IVF cycles: next generation sequencing (NGS) analysis of the 16S ribosomal gene. Hum Microbiome J. 2017;3:15–21. doi: 10.1016/j.humic.2017.01.004. [DOI] [Google Scholar]
- 13.Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8. 10.1038/s41467-017-00901-0. [DOI] [PMC free article] [PubMed]
- 14.Miles SM, Hardy BL, Merrell DS. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil Steril. 2017;107:813–820.e1. doi: 10.1016/j.fertnstert.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Khan KN, Fujishita A, Masumoto H, Muto H, Kitajima M, Masuzaki H, et al. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2016;199:69–75. doi: 10.1016/j.ejogrb.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Fang RL, Chen LX, Shu WS, Yao SZ, Wang SW, Chen YQ. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am J Transl Res. 2016;8:1581–1592. [PMC free article] [PubMed] [Google Scholar]
- 17.Verstraelen H, Vilchez-Vargas R, Desimpel F, Jauregui R, Vankeirsbilck N, Weyers S, et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ. 2016;4. 10.7717/peerj.1602. [DOI] [PMC free article] [PubMed]
- 18.Baker JM, Chase DM, Herbst-Kralovetz MM. Uterine Microbiota: residents, tourists, or invaders? Front Immunol. 2018;9:1–16. doi: 10.3389/fimmu.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno I, Franasiak JM. Endometrial microbiota—new player in town. Fertil Steril. 2017;108:32–39. doi: 10.1016/j.fertnstert.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Franasiak JM, Scott RT. Endometrial microbiome. Curr Opin Obstet Gynecol. 2017;29:146–152. doi: 10.1097/GCO.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 21.Bashiardes S, Zilberman-Schapira G, Elinav E. Use of metatranscriptomics in microbiome research. Bioinform Biol Insights. 2016;10:19–25. doi: 10.4137/BBI.S34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, et al. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16:410–422. doi: 10.1038/s41579-018-0029-9. [DOI] [PubMed] [Google Scholar]
- 23.Sola-Leyva A, Andrés-León E, Molina NM, Terron-Camero LC, Plaza-Díaz J, S-L MJ, et al. Mapping the entire functionally active endometrial microbiota. Hum Reprod. 2021;00:1–11. doi: 10.1093/humrep/deaa372. [DOI] [PubMed] [Google Scholar]
- 24.Ravel J, Brotman RM. Translating the vaginal microbiome: gaps and challenges. Genome Med. 2016;8:2–4. doi: 10.1186/s13073-016-0291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Velasco JA, Budding D, Campe H, Malfertheiner SF, Hamamah S, Santjohanser C, et al. The reproductive microbiome – clinical practice recommendations for fertility specialists. Reprod BioMed Online. 2020;41:443–453. doi: 10.1016/j.rbmo.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Molina NM, Sola-Leyva A, Jose Saez-Lara M, Plaza-Diaz J, Tubic-Pavlovic A, Romero B, et al. New opportunities for endometrial health by modifying uterine microbial composition: present or future? Biomolecules. 2020;10. 10.3390/biom10040593. [DOI] [PMC free article] [PubMed]
- 27.Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW. Microbes on the human vaginal epithelium. Proc Natl Acad Sci U S A. 2005;102:7952–7957. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ, et al. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150:2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 29.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, Mcculle SL, et al. Vaginal microbiome of reproductive-age women. PNAS. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4. 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed]
- 31.Wee BA, Thomas M, Sweeney EL, Frentiu FD, Samios M, Ravel J, et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust New Zeal J Obstet Gynaecol. 2018;58:341–348. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Kong Y, Gao Y, Ren Y, Zheng C, Deng X, et al. Revealing the interaction between intrauterine adhesion and vaginal microbiota using high-throughput sequencing. Mol Med Rep. 2019;49:4167–4174. doi: 10.3892/mmr.2019.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riganelli L, Iebba V, Piccioni M, Illuminati I, Bonfiglio G, Neroni B, et al. Structural variations of vaginal and endometrial microbiota: hints on female infertility. Front Cell Infect Microbiol. 2020;10:350. doi: 10.3389/fcimb.2020.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carosso A, Revelli A, Gennarelli G, Canosa S, Cosma S, Borella F, et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: a pilot study. J Assist Reprod Genet. 2020;37:2315–2326. doi: 10.1007/s10815-020-01878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyono K, Hashimoto T, Nagai Y, Sakuraba Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: a single-center pilot study. Reprod Med Biol. 2018;17:297–306. doi: 10.1002/rmb2.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winters AD, Romero R, Gervasi MT, Gomez-Lopez N, Tran MR, Garcia-Flores V, et al. Does the endometrial cavity have a molecular microbial signature? Sci Rep. 2019;9:1–17. doi: 10.1038/s41598-019-46173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Bian G, Zheng M, Lu G, Chan WY, Li W, et al. Fertility factors affect the vaginal microbiome in women of reproductive age. Am J Reprod Immunol. 2020;83:1–10. doi: 10.1111/aji.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Chen C, Wei W, Wang Z, Dai J, Hao L, et al. The metagenome of the female upper reproductive tract. Gigascience. 2018;7:1–8. doi: 10.1093/gigascience/giy107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelzer ES, Willner D, Buttini M, Huygens F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2018;111:933–943. doi: 10.1007/s10482-017-0992-6. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Gu Z, Zhang W, Jia S, Wu Y, Zheng P, et al. Microbiome of the lower genital tract in Chinese women with endometriosis by 16s-rRNA sequencing technique: a pilot study. Ann Transl Med. 2020; 8:1440. 10.21037/atm-20-1309. [DOI] [PMC free article] [PubMed]
- 41.Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 2020;16:1–20. doi: 10.1371/journal.ppat.1008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20. 10.1186/s12879-020-05324-9. [DOI] [PMC free article] [PubMed]
- 43.Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: an analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol. 2019;18:72–82. doi: 10.1002/rmb2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leoni C, Ceci O, Manzari C, Fosso B, Volpicella M, Ferrari A, et al. Human Endometrial microbiota at term of normal pregnancies. Genes (Basel) 2019;10:1–11. doi: 10.3390/genes10120971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Younge N, McCann JR, Ballard J, Plunkett C, Akhtar S, Araújo-Pérez F, et al. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight. 2019;4. 10.1172/jci.insight.127806. [DOI] [PMC free article] [PubMed]
- 46.Svenstrup HF, Fedder J, Abraham-Peskir J, Birkelund S, Christiansen G. Mycoplasma genitalium attaches to human spermatozoa. Hum Reprod. 2003;18:2103–2109. doi: 10.1093/humrep/deg392. [DOI] [PubMed] [Google Scholar]
- 47.Hansen LK. Becher N, Bastholm S, Glavind J, Ramsing M, J. Kim C, et al. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obs. Gynecol Scand. 2014;93:102–108. doi: 10.1038/s41598-019-39414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swidsinski A, Verstraelen H, Loening-Baucke V, Swidsinski S, Mendling W, Halwani Z. Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS One. 2013;8:1–6. doi: 10.1371/journal.pone.0053997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Grau I, Simon C, Moreno I. Uterine microbiome-low biomass and high expectations. Biol Reprod. 2019;101:1102–1114. doi: 10.1093/biolre/ioy257. [DOI] [PubMed] [Google Scholar]
- 50.Solt I. The human microbiome and the great obstetrical syndromes: a new frontier in maternal-fetal medicine. Best Pract Res Clin Obstet Gynaecol. 2015;29:165–175. doi: 10.1016/j.bpobgyn.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Pelzer ES, Willner D, Buttini M, Hafner LM, Theodoropoulos C, Huygens F. The fallopian tube microbiome: implications for reproductive health. Oncotarget. 2018; 9:21541–51. 10.18632/oncotarget.25059. [DOI] [PMC free article] [PubMed]
- 52.Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, et al. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod. 2011;26:1799–1812. doi: 10.1093/humrep/der108. [DOI] [PubMed] [Google Scholar]
- 53.Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, Trancassini M, et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39:1–12. [PubMed] [Google Scholar]
- 54.Fischbach MA. Microbiome: Focus on Causation and Mechanism. Cell. 2018;174:785–790. doi: 10.1016/j.cell.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green KA, Zarek SM, Catherino WH. Gynecologic health and disease in relation to the microbiome of the female reproductive tract. Fertil Steril. 2015;104:1351–1357. doi: 10.1016/j.fertnstert.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Diop K, Dufour JC, Levasseur A, Fenollar F. Exhaustive repertoire of human vaginal microbiota. Hum Microbiome J. 2019;11. 10.1016/j.humic.2018.11.002.
- 57.Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A, et al. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-39700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8:1–11. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skafte-holm A, Humaidan P, Bernabeu A, Lledo B, Jensen JS, Haahr T. The association between vaginal dysbiosis and reproductive outcomes in sub-fertile women undergoing ivf-treatment: a systematic prisma review and meta-analysis. Pathogens. 2021;10:1–17. doi: 10.3390/pathogens10030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Einenkel R, Zygmunt M, Muzzio DO. Microorganisms in the healthy upper reproductive tract: from denial to beneficial assignments for reproductive biology. Reprod Biol. 2019;19:113–118. doi: 10.1016/j.repbio.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Greenbaum S, Greenbaum G, Moran-Gilad J, Weintruab AY. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol. 2019;220:324–335. doi: 10.1016/j.ajog.2018.11.1089. [DOI] [PubMed] [Google Scholar]
- 62.Leyva-Gómez G, Del Prado-Audelo ML, Ortega-Peña S, Mendoza-Muñoz N, Urbán-Morlán Z, González-Torres M, et al. Modifications in vaginal microbiota and their influence on drug release: challenges and opportunities. Pharmaceutics. 2019;11:1–18. doi: 10.3390/pharmaceutics11050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang B, Fettweis JM, Brooks JP, Jefferson KK, Buck GA. The Changing landscape of the vaginal microbiome. Clin Lab Med. 2014;34:747–761. doi: 10.1016/j.cll.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoyancheva G, Marzotto M, Dellaglio F, Torriani S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch Microbiol. 2014;196:645–653. doi: 10.1007/s00203-014-1003-1. [DOI] [PubMed] [Google Scholar]
- 65.Tachedjian G, O’Hanlon DE, Ravel J. The implausible “in vivo” role of hydrogen peroxide as an antimicrobial factor produced by vaginal microbiota. Microbiome. 2018;6. 10.1186/s40168-018-0418-3. [DOI] [PMC free article] [PubMed]
- 66.Witkin SS, Mendes-Soares M. LI, Jayaram Aswathi, J LW, Forney Larry J. Influence of vaginal bacteria and d- and l-lactic acid isomers on vaginal extracellular matrix metal.pdf. MBio. 2013;4:1–7. doi: 10.1128/mBio.00460-13.Editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gupta S, Kumar N, Singhal N, Kaur R, Manektala U. Vaginal microflora in postmenopausal women on hormone replacement therapy. Indian J Pathol Microbiol. 2006;49:457–461. [PubMed] [Google Scholar]
- 68.Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric. 2009;12:91–105. doi: 10.1080/13697130802585576. [DOI] [PubMed] [Google Scholar]
- 69.Kadogami D, Nakaoka Y, Morimoto Y. Use of a vaginal probiotic suppository and antibiotics to influence the composition of the endometrial microbiota. Reprod Biol. 2020;20:307–314. doi: 10.1016/j.repbio.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42–50. doi: 10.1016/j.maturitas.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 71.Power ML, Quaglieri C, Schulkin J. Reproductive microbiomes: a new thread in the microbial network. Reprod Sci. 2017;24:1482–1492. doi: 10.1177/1933719117698577. [DOI] [PubMed] [Google Scholar]
- 72.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:1–19. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Digiulio DB, Callahan BJ, Mcmurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. PNAS. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7:1–13. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng N, Guo R, Yao Y, Jin M, Cheng Y, Ling Z. Lactobacillus iners is associated with vaginal dysbiosis in healthy pregnant women: a preliminary study. Biomed Res Int. 2019;2019. 10.1155/2019/6079734. [DOI] [PMC free article] [PubMed]
- 76.Freitas AC, Chaban B, Bocking A, Rocco M, Yang S, Hill JE, et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci Rep. 2017;7:1–16. doi: 10.1038/s41598-017-07790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moreno I, Garcia-Grau I, Bau D, Perez-Villaroya D, Gonzalez-Monfort M, Vilella F, et al. The first glimpse of the endometrial microbiota in early pregnancy. Am J Obstet Gynecol. 2020;222:296–305. doi: 10.1016/j.ajog.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:1–11. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wylie KM, Wylie T, Cahill AG, Macones GA, Tuuli MG, Stout MJ. The Vaginal Eukaryotic DNA Virome and Preterm Birth. Am J Obstet Gynecol. 2018;219:189.e1–189.e12. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brown RG, Al-Memar M, Marchesi JR, Lee YS, Smith A, Chan D, et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl Res. 2019;207:30–43. doi: 10.1016/j.trsl.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, et al. The vaginal microbiome and preterm birth. Nat Med. 2019;25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tabatabaei N, Eren AM, Barreiro LB, Yotova V, Dumaine A, Allard C, et al. Vaginal microbiome in early pregnancy and subsequent risk of spontaneous preterm birth: a case–control study. BJOG An Int J Obstet Gynaecol. 2019;126:349–358. doi: 10.1111/1471-0528.15299. [DOI] [PubMed] [Google Scholar]
- 84.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 85.Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451–463. doi: 10.1113/JP271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ceccarani C, Foschi C, Parolin C, D’Antuono A, Gaspari V, Consolandi C, et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci Rep. 2019;9. 10.1038/s41598-019-50410-x. [DOI] [PMC free article] [PubMed]
- 87.Vazquez F, Fernández-Blázquez A, García B. Vaginosis Vaginal microbiota. Enferm Infecc Microbiol Clin. 2019;37:592–601. doi: 10.1016/j.eimc.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 88.Coudray MS, Madhivanan P. Bacterial vaginosis—a brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020;245:143–148. doi: 10.1016/j.ejogrb.2019.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25:1500–1504. doi: 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]
- 90.Haahr T, Zacho J, Bräuner M, Shathmigha K, Skov Jensen J, Humaidan P. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2019;126:200–207. doi: 10.1111/1471-0528.15178. [DOI] [PubMed] [Google Scholar]
- 91.Cicinelli E, De Ziegler D, Nicoletti R, Colafiglio G, Saliani N, Resta L, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89:677–684. doi: 10.1016/j.fertnstert.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 92.Liu Y, Ko EY-L, Wong KK-W, Chen X, Cheung W-C, Law TS-M, et al. Endometrial microbiota in infertile women with and without chronic endometritis as diagnosed using a quantitative and reference range-based method. Fertil Steril. 2019;112:707–717.e1. doi: 10.1016/j.fertnstert.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 93.Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahçeci M, Barrionuevo MJ, et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients medRxiv. 2021. 10.1101/2021.02.05.21251207. [DOI] [PMC free article] [PubMed]
- 94.Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93:437–441. doi: 10.1016/j.fertnstert.2008.12.131. [DOI] [PubMed] [Google Scholar]
- 95.Kitaya K, Nagai Y, Arai W, Sakuraba Y, Ishikawa T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediat Inflamm. 2019;2019:1–10. doi: 10.1155/2019/4893437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cicinelli E, Matteo M, Tinelli R, Pinto V, Marinaccio M, Indraccolo U, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. 2014;21:640–647. doi: 10.1177/1933719113508817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cicinelli E, Matteo M, Trojano G, Mitola PC, Tinelli R, Vitagliano A, et al. Chronic endometritis in patients with unexplained infertility: prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. 2018:79. 10.1111/aji.12782. [DOI] [PubMed]
- 98.Moreno I, Cicinelli E, Garcia-Grau I, Gonzalez-Monfort M, Bau D, Vilella F, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218:602.e1–602.e16. doi: 10.1016/j.ajog.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 99.Kitaya K, Takeuchi T, Mizuta S, Matsubayashi H, Ishikawa T. Endometritis: new time, new concepts. Fertil Steril. 2018;110:344–350. doi: 10.1016/j.fertnstert.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 100.American Cancer Society | Information and Resources about for Cancer[Internet; available at https://www.cancer.org/]. (accessed 2021 Jan 9).
- 101.Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Coukos G, et al. The ovarian cancer oncobiome. Oncotarget. 2017;8:36225–36245. doi: 10.18632/oncotarget.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou B, Sun C, Huang J, Xia M, Guo E, Li N, et al. The biodiversity Composition of microbiome in ovarian carcinoma patients. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-018-38031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science (80-) 2020;368:973–980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giudice LC. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vargas E, Aghajanova L, Gemzell-Danielsson K, Altmäe S, Esteban FJ. Cross-disorder analysis of endometriosis and its comorbid diseases reveals shared genes and molecular pathways and proposes putative biomarkers of endometriosis. Reprod BioMed Online. 2020;40:305–318. doi: 10.1016/j.rbmo.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Khan KN, Fujishita A, Kitajima M, Hiraki K, Nakashima M, Masuzaki H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis. Hum Reprod. 2014;29:2446–2456. doi: 10.1093/humrep/deu222. [DOI] [PubMed] [Google Scholar]
- 107.Cregger MA, Lenz K, Leary E, Leach R, Fazleabas A, White B, et al. Reproductive microbiomes: using the microbiome as a novel diagnostic tool for endometriosis. Reprod. Immunol Open Access. 2017;02. 10.21767/2476-1974.100036.
- 108.Akiyama K, Nishioka K, Khan KN, Tanaka Y, Mori T, Nakaya T, et al. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am J Reprod Immunol. 2019;82:1–9. doi: 10.1111/aji.13147. [DOI] [PubMed] [Google Scholar]
- 109.Hernandes C, Silveira P, Rodrigues Sereia AF, Christoff AP, Mendes H, Valter de Oliveira LF, et al. Microbiome profile of deep endometriosis patients: comparison of vaginal fluid, endometrium and lesion. Diagnostics. 2020:10–163. 10.3390/diagnostics10030163. [DOI] [PMC free article] [PubMed]
- 110.Takebayashi A, Kimura F, Kishi Y, Ishida M, Takahashi A, Yamanaka A, et al. The association between endometriosis and chronic endometritis. PLoS One. 2014;9. 10.1371/journal.pone.0088354. [DOI] [PMC free article] [PubMed]
- 111.Pinto V, Matteo M, Tinelli R, Mitola PC, De Ziegler D, Cicinelli E. Altered uterine contractility in women with chronic endometritis. Fertil Steril. 2015;103:1049–1052. doi: 10.1016/j.fertnstert.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. 2018;17:125–133. doi: 10.1002/rmb2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G. Endometriosis and the microbiome: a systematic review. BJOG An Int J Obstet Gynaecol. 2019:1–11. 10.1111/1471-0528.15916. [DOI] [PubMed]
- 114.Wei W, Zhang X, Tang H, Zeng L, Wu R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann Clin Microbiol Antimicrob. 2020;19. 10.1186/s12941-020-00356-0. [DOI] [PMC free article] [PubMed]
- 115.Shahanavaj K, Gil-Bazo I, Castiglia M, Bronte G, Passiglia F, Carreca AP, et al. Cancer and the microbiome: potential applications as new tumor biomarker. Expert Rev Anticancer Ther. 2015;15:317–330. doi: 10.1586/14737140.2015.992785. [DOI] [PubMed] [Google Scholar]
- 116.Walther-António MRS, Chen J, Multinu F, Hokenstad A, Distad TJ, Cheek EH, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016;8. 10.1186/s13073-016-0368-y. [DOI] [PMC free article] [PubMed]
- 117.Walsh DM, Hokenstad AN, Chen J, Sung J, Jenkins GD, Chia N, et al. Postmenopause as a key factor in the composition of the endometrial cancer microbiome (ECbiome) Sci Rep. 2019;9:1–16. doi: 10.1038/s41598-019-55720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu W, He F, Lin Z, Liu S, Tang L, Huang Y, et al. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int J Cancer. 2021;148:1708–1716. doi: 10.1002/ijc.33428. [DOI] [PubMed] [Google Scholar]
- 119.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JSE, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. 2016;2. 10.1038/nrdp.2016.57. [DOI] [PubMed]
- 120.Giampaolino P, Foreste V, Di Filippo C, Gallo A, Mercorio A, Serafino P, et al. Microbiome and PCOS: state-of-art and future aspects. Int J Mol Sci. 2021;22:1–16. doi: 10.3390/ijms22042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pelzer ES, Allan JA, Waterhouse MA, Ross T, Beagley KW, Knox CL. Microorganisms within human follicular fluid: effects on IVF. PLoS One. 2013;8:e59062. doi: 10.1371/journal.pone.0059062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tu Y, Zheng G, Ding G, Wu Y, Xi J, Ge Y, et al. Comparative analysis of lower genital tract microbiome between PCOS and Healthy women. Front Physiol. 2020;11:1–10. doi: 10.3389/fphys.2020.01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hong X, Qin P, Huang K, Ding X, Ma J, Xuan Y, et al. Association between polycystic ovary syndrome and the vaginal microbiome: a case-control study. Clin Endocrinol. 2020;93:52–60. doi: 10.1111/cen.14198. [DOI] [PubMed] [Google Scholar]
- 124.Moreno I, Simon C. Relevance of assessing the uterine microbiota in infertility. Fertil Steril. 2018;110:337–343. doi: 10.1016/j.fertnstert.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 125.Gnainsky Y, Granot I, Aldo P, Barash A, Or Y, Mor G, et al. Biopsy-induced inflammatory conditions improve endometrial receptivity: the mechanism of action. Reproduction. 2015;149:75–85. doi: 10.1530/REP-14-0395. [DOI] [PubMed] [Google Scholar]
- 126.Maier E, Anderson RC, Roy NC. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients. 2015;7:45–73. doi: 10.3390/nu7010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schoenmakers S, Laven J. The vaginal microbiome as a tool to predict IVF success. Curr Opin Obstet Gynecol. 2020;32:169–178. doi: 10.1097/GCO.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 128.Hyman RW, Herndon CN, Jiang H, Palm C, Fukushima M, Bernstein D, et al. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2012;29:105–115. doi: 10.1007/s10815-011-9694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, De Jonge JD, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod. 2019;34:1042–1054. doi: 10.1093/humrep/dez065. [DOI] [PubMed] [Google Scholar]
- 130.Bernabeu A, Lledo B, Díaz MC, Lozano FM, Ruiz V, Fuentes A, et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet. 2019;36:2111–2119. doi: 10.1007/s10815-019-01564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Singer M, Borg M, Ouburg S, Morré SA. The relation of the vaginal microbiota to early pregnancy development during in vitro fertilization treatment—a meta-analysis. J Gynecol Obstet Hum Reprod. 2019;48:223–229. doi: 10.1016/j.jogoh.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 132.Hashimoto T, Kyono K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J Assist Reprod Genet. 2019;36:2471–2478. doi: 10.1007/s10815-019-01630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed]
- 134.Benner M, Ferwerda G, Joosten I, van der Molen RG. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum Reprod Update. 2018;24:393–415. doi: 10.1093/humupd/dmy012. [DOI] [PubMed] [Google Scholar]
- 135.Liu Y, Wong KKW, Ko EYL, Chen X, Huang J, Tsui SKW, et al. Systematic comparison of bacterial colonization of endometrial tissue and fluid samples in recurrent miscarriage patients: implications for future endometrial microbiome studies. Clin Chem. 2018;64:1743–1752. doi: 10.1373/clinchem.2018.289306. [DOI] [PubMed] [Google Scholar]
- 136.Garcia-Grau I, Perez-Villaroya D, Bau D, Gonzalez-Monfort M, Vilella F, Moreno I, et al. Taxonomical and functional assessment of the endometrial microbiota in a context of recurrent reproductive failure: A case report. Pathogens. 2019;8:4–6. doi: 10.3390/pathogens8040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chenoll E, Moreno I, Sánchez M, Garcia-Grau I, Silva Á, González-Monfort M, et al. Selection of new probiotics for endometrial health. Front Cell Infect Microbiol. 2019;9. 10.3389/fcimb.2019.00114. [DOI] [PMC free article] [PubMed]
- 138.DeLong K, Zulfiqar F, Hoffmann DE, Tarzian AJ, Ensign LM. Vaginal Microbiota transplantation: the next frontier. J. Law, Med. Ethics. 2019;47:555–567. doi: 10.1177/1073110519897731. [DOI] [PubMed] [Google Scholar]
- 139.Koedooder R, Mackens S, Budding A, Fares D, Blockeel C, Laven J, et al. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum Reprod Update. 2019;25:298–325. doi: 10.1093/humupd/dmy048. [DOI] [PubMed] [Google Scholar]
- 140.Shafquat A, Joice R, Simmons SL, Huttenhower C. Functional and phylogenetic assembly of microbial communities in the human microbiome. Trends Microbiol. 2014;22:261–266. doi: 10.1016/j.tim.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peric A, Weiss J, Vulliemoz N, Baud D, Stojanov M. Bacterial colonization of the female upper genital tract. Int J Mol Sci. 2019;20:3405. doi: 10.3390/ijms20143405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O’Callaghan JL, Turner R, Dekker Nitert M, Barrett HL, Clifton V, Pelzer ES. Re-assessing microbiomes in the low-biomass reproductive niche. BJOG An Int J Obstet Gynaecol. 2020;127:147–158. doi: 10.1111/1471-0528.15974. [DOI] [PubMed] [Google Scholar]
- 143.Weyrich LS, Farrer AG, Eisenhofer R, Arriola LA, Young J, Selway CA, et al. Laboratory contamination over time during low-biomass sample analysis. Mol Ecol Resour. 2019;19:982–996. doi: 10.1111/1755-0998.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Molina NM, Sola-Leyva A, Haahr T, Aghajanova L, Laudanski P, Castilla JA, et al. Analysing endometrial microbiome: methodological considerations and recommendations for good practice. Hum Reprod. 2021;00:1–21. doi: 10.1093/humrep/deab009. [DOI] [PubMed] [Google Scholar]
- 145.Leigh Greathouse K, Sinha R, Vogtmann E. DNA extraction for human microbiome studies: the issue of standardization. Genome Biol. 2019;20:212. doi: 10.1186/s13059-019-1843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]