Abstract
Purpose
Increasing numbers of transgender adolescents are receiving gender-affirming treatments (GAT). Given GAT can impair reproductive function, clinical guidelines advise prior counselling regarding fertility preservation (FP). For transgender adults assigned male at birth, FP is usually achieved via a masturbatory sample and sperm cryopreservation. This is less straightforward in transgender adolescents, since they may not be developmentally ready to masturbate and/or masturbation may cause unacceptable gender dysphoria. Testicular biopsy represents an alternative method for sperm retrieval in these adolescents, but for those in early/mid puberty, it is difficult to predict whether sperm will be found. The purpose of this study was therefore to identify factors that predict successful sperm retrieval for cryopreservation via testicular biopsy.
Methods
A retrospective cohort study was undertaken at a tertiary-referral pediatric gender service. Subjects were included if they’d received a testicular biopsy in association with the commencement of GAT between 2010 and 2019. The primary outcome measure was successful sperm retrieval, and potential predictors included age, testicular volume and serum testosterone, LH and FSH levels.
Results
Of 25 subjects who received a biopsy prior to starting any GAT, 17 had successful sperm retrieval. While age, testosterone, LH and FSH levels showed minimal differences, testicular volume was significantly higher in those with successful sperm retrieval, and a threshold of ≥ 10 mL showed 92% sensitivity and 71% specificity in predicting successful retrieval. An additional 6 patients received a biopsy after starting puberty suppression and before commencement of oestrogen, and one of these individuals had sperm successfully retrieved despite > 2 years of regular puberty suppression.
Conclusion
These findings suggest that testicular volume is most useful in predicting successful sperm retrieval following testicular biopsy in transgender adolescents and are likely to be of relevance to other young people undertaking FP, including those with cancer.
Keywords: Sperm cryopreservation, Testicular biopsy, Transgender, Adolescents
Introduction
Transgender and gender diverse (henceforth trans) individuals have a gender identity different to the sex they were assigned at birth. For some, differences between their physical sex characteristics and gender identity lead to significant distress, known as gender dysphoria. In the past decade, there has been a significant increase in the number of trans young people presenting to specialist services internationally for gender-affirming treatments (GAT) [1]. GAT can involve up to three distinct stages of medical intervention, each of which may affect reproductive function and prompt trans individuals to seek fertility preservation (FP) prior to initiation of treatment [2].
One available option for medical intervention for trans young people in early to mid-puberty involves administration of gonadotropin releasing hormone agonists (GnRHa), which potently suppress pubertal development by inhibiting the hypothalamic-pituitary–gonadal axis. In doing so, GnRHa suppress the development of secondary sex characteristics which can be highly distressing for many trans individuals. The existing literature on the effect of GnRHa on reproductive function in young trans individuals is sparse, but studies on their effects in adult males as a potential contraceptive have shown a marked decline in sperm count [3]. Similar effects are expected in adolescents and, consistent with this, a decrease in testicular volume was observed in a majority of 49 transfeminine adolescents who received GnRHa (median Tanner stage 4 (range: 2–5), median age 13.6 years (range: 11.6–17.9)) [4]. Based on the use of GnRHa in adults, inhibition of sperm production appears reversible [3], but it is unclear if this is true in the context of subsequent gender affirming hormonal therapy for trans adolescents (see below).
Gender affirming hormone therapy for trans young people involves administration of either estrogen in trans females or testosterone in trans males, which promote development of secondary sex characteristics consistent with their affirmed gender identity. While some of these effects (e.g. breast development, voice deepening) are irreversible and others (e.g. fat re-distribution, amenorrhea) are not, data on the long-term effects of estrogen on sperm production are inconclusive and limited to transfeminine patients who started estrogen therapy in adulthood with no prior GnRHa use. For instance, prolonged exposure to estrogen therapy has been shown to cause testicular atrophy, absent or severely impaired spermatogenesis, and loss of Leydig cells [5, 6]. However, normal sperm parameters on semen analysis and normal spermatogenesis on testicular biopsy have also been reported in a small proportion of individuals, as has recovery of spermatogenesis upon estrogen cessation in a handful of cases [7–11].
The third possible option for GAT involves gender affirmation surgery. For trans individuals assigned male at birth, such surgery necessitates removal of the testes and leads to permanent sterility.
Given the potential for GAT to impair future fertility, international guidelines in trans health strongly recommend FP counselling prior to GAT [12, 13]. In previous studies that looked at attitudes towards FP, most adult trans females felt that FP counselling should be offered prior to GAT [14–16]. Moreover, 51% indicated that they would have considered sperm cryopreservation if this had been available and offered to them beforehand [15]. In reality, utilization of FP services among trans females varies greatly, and ranges from 9.6% to 81.8% in adults [1, 14, 17–25] and from < 5% to > 60% in adolescents [1, 19, 23, 24]. In a recent study from our own centre [25], we found that 62% of transfeminine adolescents pursued FP. In part, the low rates of FP utilization among transfeminine adolescents in previous North American studies likely reflect service barriers (e.g., cost and availability of FP procedures), given that clinics which provided FP as part of a publicly funded, multi-disciplinary program saw much higher rates of uptake [23, 25]. Another important factor that has likely influenced rates of FP utilization among this population is the type of FP offered. For instance, while most services only offered sperm cryopreservation via semen specimen, our service also offered transfeminine adolescents a testicular biopsy in order to try to extract and freeze their sperm. Although testicular biopsy involves surgery and is likely to yield fewer sperm than a semen specimen, many patients in our experience prefer testicular biopsy, viewing it as less dysphoria-inducing than masturbation which many of these young people have never attempted.
The use of testicular biopsy for FP was previously developed in paediatric oncology and has been used prior to gonadotoxic therapy either in pre-pubertal boys who have not yet reached spermarche or peri-pubertal boys who are not yet developmentally ready to produce a semen specimen [26]. While some of the latter may already be producing mature sperm that can be frozen, testicular tissue from pediatric oncology patients commonly contains spermatogonial stem cells but no mature sperm. In such cases, use of the frozen tissue for future reproductive purposes remains experimental.
The use of testicular biopsy for FP in transfeminine adolescents differs from its use in paediatric oncology in several important ways. Firstly, transfeminine adolescents are typically in good physical health. Secondly, there is usually less urgency in arranging testicular biopsy for trans adolescents, given the need to start GAT is usually not as pressing as chemo- or radiotherapy. Finally, use of GAT is restricted to those who have already commenced puberty, and thus testicular biopsy in such patients is more likely to yield mature sperm. However, hormonal treatments such as GnRHa and estrogen are started across a range of pubertal stages, and it would be clinically useful to be able to accurately predict whether any mature sperm is present, since this would directly assist patients, families and clinicians to decide whether or not an invasive biopsy is likely to be worthwhile. For instance, given use of frozen testicular tissue for reproductive purposes remains experimental [27] providing trans young people and their families with an estimate of the likelihood that they will be able to successfully store sperm after a testicular biopsy would help address the uncertainty involved in undertaking such a procedure (which is typically performed under institutional review board or similar governance, requires general anesthesia, and carries risks itself) [28]. Moreover, being able to forecast whether a semen specimen is likely to contain sperm would be very useful in this population, given the distress associated with masturbation [15].
At present, we do not have a reliable way of making such predictions. Spermatogenesis is known to start early in pubertal development [29, 30], in some cases even before the ability to produce an ejaculate [31]. Consistent with this, spermatozoa within the urine have been found in 5% of boys who appeared clinically pre-pubertal and in 50% of boys at Tanner stages 2 and 3 based on pubic hair pattern; in other studies, the onset of spermarche based on spermaturia was described as occurring when testicular volumes were as low as 4–5 ml (i.e. only slightly above pre-pubertal levels) [31, 32]. However, the detection of sperm from urine is of uncertain clinical value, given the time-consuming methods involved in isolating sperm and questions over the functional utility of such sperm for reproductive purposes. Several studies of adolescent males with cancer have instead analyzed semen produced either by masturbation or electroejaculation. For instance, successful sperm retrieval was observed to be as high as 90% among 26 adolescent boys aged < 16 years with the youngest being 13.5 years old [33, 34]. Similarly, a study of 106 adolescent male cancer patients from the Netherlands reported successful sperm retrieval in 68% of subjects [35], while a study of 86 adolescent male cancer patients from Denmark reported successful sperm retrieval in 93% [36]. Finally, we previously reported successful identification of sperm following testicular biopsy from 8 oncology patients as young as 12 years old, all of whom had testicular volumes > 10 ml and were Tanner stage ≥ 3 [37].
Across these various studies, successful sperm retrieval appeared to vary widely by age, Tanner stage, testicular volume and hormone levels [31, 38], with no consensus emerging with regard to clinically relevant cut off levels that might allow one to successfully predict the presence of mature sperm from an adolescent [39–42]. For example, Keene et al. concluded from their study of 180 13–17-year-old oncology patients that “cryopreservation of semen of acceptable quality for future use in assisted conception is feasible for most adolescents from age 13 years onward”, after observing a 66% rate of success in this population [43]. Meanwhile, other studies suggested alternative clinical thresholds, including eighteen months following the onset of puberty, greater than 12 years old, or the point at which a young person is able to provide a masturbatory sample [34, 44, 45]. Testicular volume was also commonly used as a potential predictor, but suggestions for predictive cut-offs above which sperm was likely to be present ranged widely from 5 ml [35, 36, 39–42] to 20 ml [32].
In light of the above, the aim of this study was to identify parameters to predict the successful isolation and cryopreservation of mature sperm from the testicular tissue of transfeminine adolescents. To achieve this goal, we undertook a retrospective analysis of testicular biopsy performed for FP among our transfeminine adolescents prior to their receiving GAT, and examined potential factors that might predict successful sperm retrieval for cryopreservation.
Materials and methods
Study setting
The Royal Children’s Hospital Gender Service (RCHGS) is a state-wide service based in Melbourne, Australia, that sees individuals up to the age of 18 years with concerns regarding their gender. Like similar clinics overseas, the RCHGS has observed a dramatic increase in referrals in recent years [46] and a formal fertility preservation service was established in 2013 [37]. Trans individuals assigned male at birth are given the option to try to store cryopreserved sperm either via a masturbatory semen sample or via surgical extraction involving testicular biopsy.
Testicular biopsy and testicular tissue processing
Testicular biopsy is offered and approved under special governance as a novel technology, and provided without charge to the patients. It is performed as a day case procedure under general anesthesia via a midline scrotal incision. A longitudinal incisional biopsy of at least 10 mm length, 3 mm width and 5 mm depth is taken from the largest testis. Biopsied testicular tissue is placed into HEPES-buffered medium containing human serum albumin. A portion of the tissue (up to 10% of total biopsy volume) is cut from the sample and dispersed using fine gauge needles, and the resulting suspension is assessed under high power microscopy for the presence of mature sperm. If mature sperm are seen, then the remaining tissue is completely dispersed and the suspension prepared for cryopreservation as previously described [47]. If no sperm are found, then the remaining tissue is cut into slices and cryopreserved as described previously [37]. If mature sperm is successfully isolated and frozen, patients incur an annual storage charge of AUD$200.
Study design
We performed a retrospective chart review of trans individuals attending the RCHGS who had a testicular biopsy for the purpose of FP in conjunction with receiving either GnRHa and/or estrogen therapy. The review period ranged from the inception of a formal fertility preservation service at the RCHGS in January 2013 until July 2019.
Measures
As well as examining whether or not any sperm was identified and frozen following testicular biopsy, we also extracted information on potential predictive measures from each patient’s electronic medical record. These measures were chosen based on their known role in puberty and reproduction and included: age, Tanner stage (based on genital development), mean testicular volume (taken as the average of left and right testicular volume), as well as serum LH, FSH and testosterone levels. For those with no Tanner stage explicitly recorded in the file, testicular volume was used to infer Tanner stage [48]. Tanner stage and testicular volume details were used so long as they were recorded within the six months prior to the testicular biopsy.
Data management and statistics
All data were recorded in Microsoft Access, and relevant statistical methods including Mann–Whitney U-tests, multiple testing correction (FDR), and Fisher’s exact test were applied using GraphPad Prism (v.7.04).
Ethics
This study was approved by the RCH Human Research Ethics Committee (#36,323).
Results
In total, 31 adolescents underwent testicular biopsy procedures during the study period. 25 of these individuals (median age/height/weight (IQR): 13.4 (1.7) years, 162 (18) cm and 54.6 (16.8) kg respectively) had their biopsies performed prior to receiving any hormonal treatment (22 prior to GnRHa, 3 prior to estrogen), and their characteristics are described in Table 1. An additional six individuals (median age: 14.5 years) had commenced GnRHa prior to biopsy, and were excluded from the primary analysis (but are considered separately later on).
Table 1.
Clinical characteristics of 25 adolescents who had testicular biopsy prior to any hormonal intervention, including comparison between those who did and did not have sperm frozen after testicular biopsy
| All patients (n = 25) |
No sperm frozen (n = 8) |
Sperm frozen (n = 17) |
|||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | FDR-corrected p-value‡ (q) | |
| Age (years) | 13.4 | 1.7 | 13.5 | 1.8 | 13.8 | 3.4 | 0.08 |
| Serum LH (IU/L)* | 1.9 | 2.2 | 1.7 | 2.2 | 2.2 | 2.6 | 0.43 |
| Serum FSH (IU/L)* | 2.7 | 2.4 | 2.8 | 2.0 | 2.7 | 3.3 | 0.43 |
| Serum testosterone (nmol/L)* | 6.7 | 9.8 | 2.5 | 6.5 | 7.8 | 9.2 | 0.05 |
| Tanner stage | 3 | 1 | 2 | 1 | 3 | 1.25 | 0.009 |
| Testicular volume (mL)† | 12 | 4 | 8.0 | 4.0 | 13.4 | 7.6 | 0.003 |
| Sperm count – total yield (n × 106) | 0.1 | 2.8 | 0 | 0 | 2.6 | 4.1 | n.c |
* LH and FSH were available for 22 of the individuals, while Testosterone was available for 23 of the individuals. The number of individuals with sperm frozen with available blood tests were limited to 14 for both LH and FSH, and 15 for testosterone; † Testicular volume was available within the allowable timeframe for 21 of the individuals, 7 of whom had no sperm frozen and 14 of whom had sperm frozen. ‡ Mann Whitney U-test was used to compare those who did and did not have sperm frozen after testicular biopsy. n.c. = not calculated
Of the 25 naïve to hormonal treatment at the time of biopsy, we observed that 17/25 (68%) were successfully able to store sperm, and had a median total sperm count of 2.6 × 106 (IQR: 0.1–2 × 106/mL) and median % motile sperm of 0 (IQR: 0–2%). Eight patients (32%) had no sperm identified and only testicular tissue was stored.
Clinical and biochemical characteristics of those who did or did not have sperm successfully extracted for cryopreservation were compared (Table 1).
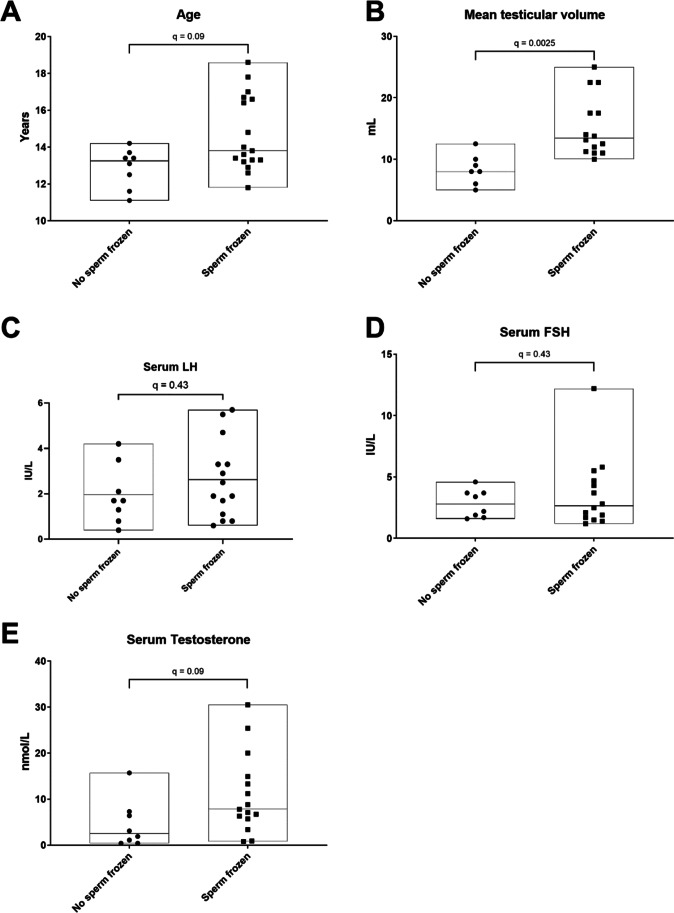
The ages of those who had sperm frozen (median: 13.8 years; IQR: 3.4 years) were similar to that of those who did not (median: 13.5 years; IQR: 1.8 years) (FDR-corrected p-value: 0.09) (Fig. 1A).
Fig. 1.
Comparison of (A) age, (B) testicular volume, (C) serum LH, (D) serum FSH and (E) serum testosterone in adolescents who did and did not have sperm frozen after testicular biopsy. Median and IQR values are indicated, and FDR-corrected p-values (q) are also displayed
Tanner stage and mean testicular volume were available for 14 of the 17 individuals who had sperm frozen and 7 of the 8 who did not have sperm frozen. At Tanner stage 2, 0 of 4 patients had sperm frozen. At Tanner stage 3, 8 of 11 (73%) patients had sperm frozen. At Tanner stages 4 and 5, 6 of 6 patients (n = 3 per stage) had sperm frozen. Consistent with these findings, mean testicular volumes were observed to be significantly greater in the group that successfully had sperm dissected and frozen (median: 13.4 mL; IQR: 7.6 mL) compared to those who did not (median: 8.0 mL; IQR 4 mL; FDR-corrected p-value: < 0.01) (Fig. 1B).
Hormonal data were available for 14–15 of the 17 individuals who had sperm frozen and all 8 of those who did not have sperm frozen. No differences in serum LH or FSH levels were observed between the two groups (Fig. 1C–D). A higher serum testosterone (median: 7.8 nmol/L; IQR: 9.2) was observed in those who had sperm successfully cryopreserved compared to those who did not (median: 2.5 nmoL/L; IQR:6.5; FDR-corrected p-value: 0.09), but as with age, there was significant overlap across the two groups (Fig. 1E), with even relatively a high level > 15 nmoL/L not associated with sperm cryopreservation in one individual.
The ability of different age, testicular volume and testosterone thresholds to predict successful retrieval of sperm for cryopreservation was assessed (Table 2). These data indicate that a testicular volume cut-off of 10–12 mL allows optimal prediction in terms of sensitivity and specificity.
Table 2.
Using Testicular volume, age and serum testosterone to predict successful retrieval of sperm for freezing following testicular biopsy
| % with sperm frozen | Sensitivity | Specificity | p–value | |
|---|---|---|---|---|
| Mean testicular volume (mL) | ||||
| ≥ 6 | 70.0 | 1.00 | 0.14 | 0.33 |
| ≥ 8 | 73.7 | 1.00 | 0.29 | 0.10 |
| ≥ 10 | 86.7 | 0.92 | 0.71 | 0.006 |
| ≥ 12 | 91.6 | 0.79 | 0.86 | 0.02 |
| ≥ 14 | 100 | 0.43 | 1 | 0.11 |
| ≥ 16 | 100 | 0.29 | 1.00 | 0.27 |
| Age (years) | ||||
| ≥ 11 | 68.0 | 1.00 | 0.00 | > 0.99 |
| ≥ 12 | 72.7 | 0.94 | 0.25 | 0.23 |
| ≥ 13 | 73.7 | 0.82 | 0.38 | 0.34 |
| ≥ 14 | 88.9 | 0.47 | 0.88 | 0.18 |
| ≥ 15 | 100 | 0.35 | 1.00 | 0.13 |
| ≥ 16 | 100 | 0.35 | 1.00 | 0.13 |
| Serum testosterone (nmol/L) | ||||
| ≥ 4 | 78.6 | 0.79 | 0.63 | 0.08 |
| ≥ 8 | 85.7 | 0.43 | 0.88 | 0.19 |
| ≥ 12 | 83.3 | 0.36 | 0.88 | 0.35 |
| ≥ 16 | 100 | 0.21 | 1.00 | 0.27 |
| ≥ 20 | 100 | 0.21 | 1.00 | 0.27 |
Finally, we also considered those six individuals who had testicular biopsies after commencing GnRHa (Table 3). At the time of biopsy, the median age of this group was 14.5 years (range: 13.4–17.1) and the median treatment duration on GnRHa was 1.5 years (range 0.4–3.7). Three patients had received Leuprorelin 30 mg IM, one had been on Goserelin 10.8 mg SC before switching to Leuprorelin 30 mg IM, and two others had been on Goserelin 10.8 mg SC before switching to Triptorelin 22.5 mg. Notably, one of these patients had small numbers of mature, non-motile spermatozoa (< 1 × 105) successfully isolated and frozen at the age of 15 years. This was despite having been on GnRHa regularly for > 2 years — initially Goserelin every 2–3 months for the first 21 months and then Leuprorelin every 3 months thereafter (the most recent injection of which was 7 weeks before the biopsy) — and having shown both biochemical and clinical evidence of effective pubertal blockade. While this patient’s Tanner stage and testicular volumes prior to starting GnRHa were unfortunately unavailable (due to their refusal of a genital examination), history suggested that they were at Tanner stage 1–2 12 months before commencement of GnRHa and baseline serum testosterone just prior to starting treatment was relatively high (9.2 nmol/L).
Table 3.
Characteristics of 6 patients who had testicular biopsy after commencing GnRHa
| Median (range) | |
|---|---|
| Age at biopsy (years) | 14.4 (13.4–17.1) |
| Height (cm) | 165 (1.56–1.76) |
| Weight (kg) | 51.4 (40.5–65) |
| Tanner stage* | 2 (2–5) |
| Testicular volume (mL)† | 5.5 (4–11.5) |
| Serum LH on GnRHa (IU/L)* | 0.4 (< 0.2–1) |
| Serum FSH (IU/L) on GnRHa (IU/L)* | 0.8 (0.1–4) |
| Serum testosterone on GnRHa (nmol/L)* | < 0.5 (< 0.4–1.8) |
| Duration of GnRHa treatment at biopsy (months) | 19.8 (5.4–44.1) |
*Data available for only 5 individuals
† Data available for only 3 individuals; for the 2 individuals with the smaller testicular volumes, these were assessed prior to GnRHa commencement; for the other individual, these were assessed 2 years after GnRHa were started
Discussion
Fertility counselling is a crucial aspect in the care of trans adolescents considering hormonal interventions [12, 13, 49]. For trans adolescents assigned male at birth, a clinical challenge that commonly arises during such counselling is whether or not a young person is likely to have any sperm to cryopreserve. Knowing the likely answer to this question directly influences the advice and recommendations that a clinician will make and hence the decision-making of patients and their families.
Previously, age has been suggested as one potential determinant in deciding whether or not to pursue sperm cryopreservation. For instance, one study suggested that cryopreservation of semen for future use in assisted conception is feasible for most adolescents from age 13 years [43], while another that similarly examined masturbatory semen samples suggested all patients aged > 12 years should be offered sperm freezing [44]. Consistent with these previous suggestions from the study of adolescent oncology patients, we observed that several individuals aged as young as 11–12 years were able to successfully freeze sperm (Fig. 1). However, 4 of 8 (50%) patients who had no sperm identified from testicular biopsy were already aged 13–14 years, and using these ages as a threshold to predict successful retrieval of sperm for cryopreservation showed poor sensitivity and specificity (Table 2).
Serum sex hormone levels are often measured as part of routine clinical care of trans adolescents considering hormone treatment, and so in theory might represent a convenient metric to guide fertility counselling in this population. Previously, however, Van Casteren et al. showed no significant difference in serum testosterone, LH and FSH levels among 80 13–18-year-old adolescent cancer patients with or without successful retrieval of sperm for cryopreservation from semen [50]. While our results were similar in terms of serum LH and FSH, we did observe higher serum testosterone levels in patients who successfully froze sperm. However, as with age, we found that various thresholds to predict successful sperm cryopreservation based on testosterone levels tended to show poor sensitivity and/or specificity.
Testicular volume has previously been suggested to be a useful predictor of successful fertility preservation during adolescence. For example, Hagenas et al. found testicular volume correlated with successful semen collection in their cohort of 86 12–17-year-old cancer patients and suggested that any individual with testicular volumes of ≥ 5 mL should be offered semen banking [36]. However, another study suggested a much higher testicular volume threshold of ≥ 20 mL, but this observation was based on spermaturia, which is known to be highly variable [32]. Our results suggest a cut-off of ≥ 5 mL may be too low, since we failed to observe sperm in anyone with less than 10 mL testicular volume. Similarly, a threshold of ≥ 20 mL appears too high based on our data, since many young people with testicular volumes less than 20 mL were able to successfully freeze sperm. Instead, we found that a testicular volume in between these previous recommendations (i.e. ≥ 10–12 mL) is likely to be a useful clinical cut-off in assisting with fertility counselling based on sensitivity and specificity analyses.
Taken together, our results are likely to be useful for trans adolescents considering testicular biopsy for FP. Indeed, based on these data, we have adapted our fertility counselling practices to recommend that adolescents wait until their testicular volumes are ≥ 10 mL if they wish to attempt FP via testicular biopsy. As a result, many of our patients now delay commencement of GnRHa until this threshold is reached. However, delaying GnRHa treatment in this way must be balanced against the risk of unwanted masculinization (e.g., voice changes) and ongoing psychological distress that accompanies pubertal progression in these adolescents. Our findings may also be useful in providing counselling to trans adolescents willing to give a semen specimen, although it remains unclear what the correlation is between finding sperm on testicular biopsy and in semen. Similarly, our data may be helpful in determining the likelihood of being able to successfully retrieve sperm for freezing in pediatric oncology patients who undergo testicular biopsies while in early puberty. Having said that, our patients were physically well, and it is possible that spermatogenesis in our cohort and those recently diagnosed with malignancy may be different. Nevertheless, our findings are broadly consistent with our own observations from a small cohort of oncology patients, 8 of whom had sperm identified following testicular biopsy and had testicular volumes > 10 mL [37].
One surprising outcome of our study was the observation of mature sperm in one of six patients who underwent a biopsy after commencing GnRHa. This suggests that spermatogenesis can continue even in the presence of ongoing GnRHa treatment, which in this patient’s case had begun at 13.5 years and then been given regularly every 2–3 months for > 2 years thereafter. However, we cannot rule out the possibility that their GnRHa activity had diminished at the time of their biopsy, which occurred 7 weeks after their previous depot injection. Similarly, we do not know whether this individual was already producing sperm at the time they started their GnRHa, and thus cannot tell whether spermarche had occurred prior to or during GnRHa treatment.
Of course, our study is not without other limitations. Firstly, although this study represents, to our knowledge, the largest reported cohort of non-oncology adolescents undertaking testicular biopsies for the purposes of FP, the overall number of participants remains small. Secondly, there was usually a short delay between clinical assessment of testicular volume, Tanner stage and serum hormone levels and the testicular biopsy actually being performed (median delay was 1.1 months (IQR: 0.9–2.5)), which arose due to differences in timing between their paediatric assessment and date of surgery. Thus, when considering these data for the purposes of providing fertility counselling, clinicians should be aware that the actual testicular volumes, Tanner stages and hormone levels at the time of biopsy (and sperm assessment) may have been slightly higher than those upon which our analysis was based. As such, it is possible that using our recommended 10–12 mL testicular volume threshold might overpredict the likelihood of sperm being present, although given the relatively short interval between assessment and biopsy the overall effect of this delay is likely to have been minor. Finally, we were missing data on testicular volume, Tanner stage, and serum hormone levels for a small number of patients, and we are unsure how these might have affected our results. Looking ahead, as the use of testicular biopsies for FP becomes more common in pediatric practice, it will therefore be important to further examine the question of what clinical features best predict successful retrieval of sperm for cryopreservation in adolescents.
Acknowledgements
KCP is supported by the Hugh Williamson Foundation Trust and the Royal Children’s Hospital Foundation.
Data availability
Data will be available upon reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
This study was conducted with the approval of the RCH Human Research Ethics Committee (#36323). As a retrospective audit of clinical files, consent was not required. However, consent was obtained from the individual who had sperm identified despite being on GnRHa, given the information shared about their case made them potentially identifiable.
Consent for publication
All named authors consent to the publication of this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angus Peri and Astrid Ahler contributed equally to this paper.
References
- 1.Chen M, Fuqua J, Eugster EA. Characteristics of referrals for gender dysphoria over a 13-year period. J Adolesc Health. 2016 [cited 2020 Jul 19];58(3):369–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054139X1500676X. [DOI] [PMC free article] [PubMed]
- 2.Lai TC, McDougall R, Feldman D, Elder CV, Pang KC. Fertility counseling for transgender adolescents: a review. J Adolesc Health. 2020 [cited 2020 Jul 19];66(6):658–65. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054139X20300343. [DOI] [PubMed]
- 3.Linde R, Doelle GC, Alexander N, Kirchner F, Vale W, Rivier J, et al. Reversible inhibition of testicular steroidogenesis and spermatogenesis by a potent gonadotropin-releasing hormone agonist in normal men: an approach toward the development of a male contraceptive. N Engl J Med. 1981 [cited 2020 Jul 19];305(12):663–7. Available from: 10.1056/NEJM198109173051203. [DOI] [PubMed]
- 4.Schagen SEE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. 2016 [cited 2020 Jul 19];13(7):1125–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1743609516302193. [DOI] [PubMed]
- 5.Schulze C. Response of the human testis to long-term estrogen treatment: morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res. 1988;251(1):31–43. doi: 10.1007/BF00215444. [DOI] [PubMed] [Google Scholar]
- 6.Leavy M, Trottmann M, Liedl B, Reese S, Stief C, Freitag B, et al. Effects of elevated β-estradiol levels on the functional morphology of the testis — new insights. Sci Rep. 2017 [cited 2020 Jul 19];7(1):39931. Available from: http://www.nature.com/articles/srep39931. [DOI] [PMC free article] [PubMed]
- 7.Thiagaraj D, Gunasegaram R, Loganath A, Peh KL, Kottegoda SR, Ratnam SS. Histopathology of the testes from male transsexuals on oestrogen therapy. Ann Acad Med Singap. 1987;16(2):347–348. [PubMed] [Google Scholar]
- 8.Venizelos ID, Paradinas FJ. Testicular atrophy after oestrogen therapy. Histopathology. 1988 [cited 2020 Jul 19];12(4):451–4. Available from: 10.1111/j.1365-2559.1988.tb01961.x. [DOI] [PubMed]
- 9.Lübbert H, Leo-Roßberg I, Hammerstein J. Effects of ethinyl estradiol on semen quality and various hormonal parameters in a eugonadal male. Fertil Steril. 1992 [cited 2020 Jul 19];58(3):603–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0015028216552716. [DOI] [PubMed]
- 10.Adeleye AJ, Reid G, Kao C-N, Mok-Lin E, Smith JF. Semen parameters among transgender women with a history of hormonal treatment. Urology. 2019 [cited 2020 Jul 19];124:136–41. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0090429518310872. [DOI] [PubMed]
- 11.Schneider F, Neuhaus N, Wistuba J, Zitzmann M, Heß J, Mahler D, et al. Testicular functions and clinical characterization of patients with gender dysphoria (GD) undergoing Sex Reassignment Surgery (SRS). J Sex Med. 2015 [cited 2020 Jul 19];12(11):2190–200. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1743609515344489. [DOI] [PubMed]
- 12.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, Version 7. Int J Transgenderism. 2012 [cited 2020 Jul 19];13(4):165–232. Available from: 10.1080/15532739.2011.700873.
- 13.Telfer MM, Tollit MA, Pace CC, Pang KC. Australian standards of care and treatment guidelines for transgender and gender diverse children and adolescents. Med J Aust. 2018;209(3):132–136. doi: 10.5694/mja17.01044. [DOI] [PubMed] [Google Scholar]
- 14.Wierckx K, Van Caenegem E, Pennings G, Elaut E, Dedecker D, Van de Peer F, et al. Reproductive wish in transsexual men. Hum Reprod. 2012 [cited 2020 Jul 19];27(2):483–7. Available from: 10.1093/humrep/der406. [DOI] [PubMed]
- 15.De Sutter P, Verschoor A, Hotimsky A, Kira K. The desire to have children and the preservation of fertility in transsexual women: A survey. Int J Transgend. 2002;6(3):215–221.
- 16.von Doussa H, Power J, Riggs D. Imagining parenthood: the possibilities and experiences of parenthood among transgender people. Cult Health Sex. 2015 [cited 2020 Jul 19];17(9):1119–31. Available from: 10.1080/13691058.2015.1042919. [DOI] [PubMed]
- 17.Jones CA, Reiter L, Greenblatt E. Fertility preservation in transgender patients. Int J Transgenderism. 2016;17:76–82. doi: 10.1080/15532739.2016.1153992. [DOI] [Google Scholar]
- 18.Armuand G, Dhejne C, Olofsson JI, Rodriguez-Wallberg KA. Transgender men’s experiences of fertility preservation: a qualitative study. Hum Reprod. 2017;32(2):383–390. doi: 10.1093/humrep/dew323. [DOI] [PubMed] [Google Scholar]
- 19.Nahata L, Tishelman AC, Caltabellotta NM, Quinn GP. Low fertility preservation utilization among transgender youth. J Adolesc Health. 2017 [cited 2020 Jul 19];61(1):40–4. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054139X16309582. [DOI] [PubMed]
- 20.Auer MK, Fuss J, Nieder TO, Briken P, Biedermann SV, Stalla GK, et al. Desire to have children among transgender people in Germany: a cross-sectional multi-center study. J Sex Med. 2018;15(5):757–767. doi: 10.1016/j.jsxm.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 21.Kyweluk MA, Sajwani A, Chen D. Freezing for the future: transgender youth respond to medical fertility preservation. Int J Transgenderism. 2018;19:401–16. doi: 10.1080/15532739.2018.1505575. [DOI] [Google Scholar]
- 22.Riggs DW, Bartholomaeus C. Fertility preservation decision making amongst Australian transgender and non-binary adults. Reprod Health. 2018;15(1):181. doi: 10.1186/s12978-018-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brik T, Vrouenraets LJJJ, Schagen SEE, Meissner A, de Vries MC, Hannema SE. Use of fertility preservation among a cohort of transgirls in the Netherlands. J Adolesc Health. 2019;64(5):589–593. doi: 10.1016/j.jadohealth.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Chiniara LN, Viner C, Palmert M, Bonifacio H. Perspectives on fertility preservation and parenthood among transgender youth and their parents. Arch Dis Child. 2019;104(8):739–744. doi: 10.1136/archdischild-2018-316080. [DOI] [PubMed] [Google Scholar]
- 25.Pang KC, Peri A, Chung HE, Telfer M, Elder CV, Grover S, Jayasinghe Y. Rates of fertility preservation use among transgender adolescents. JAMA Pediatr. 2020;174(9):890–891. 10.1001/jamapediatrics.2020.0264. [DOI] [PMC free article] [PubMed]
- 26.Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015 [cited 2020 Jul 19];30(11):2463–75. Available from: 10.1093/humrep/dev190. [DOI] [PubMed]
- 27.Jahnukainen K, Stukenborg J-B. Clinical review: present and future prospects of male fertility preservation for children and adolescents. J Clin Endocrinol Metab. 2012;97(12):4341–4351. doi: 10.1210/jc.2012-3065. [DOI] [PubMed] [Google Scholar]
- 28.Wallace SA, Blough KL, Kondapalli LA. Fertility preservation in the transgender patient: expanding oncofertility care beyond cancer. Gynecol Endocrinol. 2014;30(12):868–871. doi: 10.3109/09513590.2014.920005. [DOI] [PubMed] [Google Scholar]
- 29.Müller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int J Androl. 1983;6(2):143–156. doi: 10.1111/j.1365-2605.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 30.Hovatta O. Cryopreservation of testicular tissue in young cancer patients. Hum Reprod Update. 2001;7(4):378–383. doi: 10.1093/humupd/7.4.378. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen CT, Skakkebaek NE, Richardson DW, Darling JA, Hunter WM, Jørgensen M, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab. 1986;62(3):532–535. doi: 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer F, Marr J, Seidel C, Tilgen W, Schärer K. Assessment of gonadal maturation by evaluation of spermaturia. Arch Dis Child. 1990;65(11):1205–1207. doi: 10.1136/adc.65.11.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliesch S, Behre HM, Jürgens H, Nieschlag E. Cryopreservation of semen from adolescent patients with malignancies. Med Pediatr Oncol. 1996;26(1):20–27. doi: 10.1002/(SICI)1096-911X(199601)26:1<20::AID-MPO3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 34.Kamischke A, Jürgens H, Hertle L, Berdel WE, Nieschlag E. Cryopreservation of sperm from adolescents and adults with malignancies. J Androl. 2004;25(4):586–592. doi: 10.1002/j.1939-4640.2004.tb02829.x. [DOI] [PubMed] [Google Scholar]
- 35.Adank MC, van Dorp W, Smit M, van Casteren NJ, Laven JSE, Pieters R, et al. Electroejaculation as a method of fertility preservation in boys diagnosed with cancer: a single-center experience and review of the literature. Fertil Steril. 2014 [cited 2020 Jul 20];102(1):199–205.e1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0015028214002933. [DOI] [PubMed]
- 36.Hagenas I, Jorgensen N, Rechnitzer C, Sommer P, Holm M, Schmiegelow K, et al. Clinical and biochemical correlates of successful semen collection for cryopreservation from 12–18-year-old patients: a single-center study of 86 adolescents. Hum Reprod. 2010 [cited 2020 Jul 20];25(8):2031–8. Available from: 10.1093/humrep/deq147. [DOI] [PubMed]
- 37.Ho WLC, Bourne H, Gook D, Clarke G, Kemertzis M, Stern K, et al. A short report on current fertility preservation strategies for boys. Clin Endocrinol. 2017 [cited 2020 Dec 28];87(3):279–85. 10.1111/cen.13377. [DOI] [PubMed]
- 38.Ji C-Y, Ohsawa S. Onset of the release of spermatozoa (spermarche) in Chinese male youth. Am J Hum Biol. 2000;12(5):577–587. doi: 10.1002/1520-6300(200009/10)12:5<577::AID-AJHB1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Zachmann M, Prader A, Kind HP, Häfliger H, Budliger H. Testicular volume during adolescence Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29(1):61–72. [PubMed] [Google Scholar]
- 40.Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127(1):100–102. doi: 10.1016/S0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 41.Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. Eur J Endocrinol. 2004;151(6):747–757. doi: 10.1530/eje.0.1510747. [DOI] [PubMed] [Google Scholar]
- 42.Wu FC, Brown DC, Butler GE, Stirling HF, Kelnar CJ. Early morning plasma testosterone is an accurate predictor of imminent pubertal development in prepubertal boys. J Clin Endocrinol Metab. 1993;76(1):26–31. doi: 10.1210/jcem.76.1.8421096. [DOI] [PubMed] [Google Scholar]
- 43.Keene DJB, Sajjad Y, Makin G, Cervellione RM. Sperm banking in the United Kingdom is feasible in patients 13 years old or older with cancer. J Urol. 2012;188(2):594–597. doi: 10.1016/j.juro.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Bahadur G, Ling KLE, Hart R, Ralph D, Wafa R, Ashraf A, et al. Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod. 2002;17(12):3157–3161. doi: 10.1093/humrep/17.12.3157. [DOI] [PubMed] [Google Scholar]
- 45.Dabaja AA, Wosnitzer MS, Bolyakov A, Schlegel PN, Paduch DA. When to ask male adolescents to provide semen sample for fertility preservation? Transl Androl Urol. 2014;3(1):2–8. doi: 10.3978/j.issn.2223-4683.2014.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang KC, de Graaf NM, Chew D, Hoq M, Keith DR, Carmichael P, et al. Association of media coverage of transgender and gender diverse issues with rates of referral of transgender children and adolescents to specialist gender clinics in the UK and Australia. JAMA Netw Open. 2020 [cited 2020 Dec 28];3(7):e2011161. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2768726. [DOI] [PMC free article] [PubMed]
- 47.Bourne H, Archer J, Edgar DH, Baker HWG. Sperm preparation techniques. In: Textbook of assisted reproductive techniques. Taylor & Francis: London; 2012. p. 61–74.
- 48.Emmanuel M, Bokor BR. Tanner Stages. In: StatPearls. Treasure Island: StatPearls Publishing; 2020 [cited 2021 Jan 8]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK470280/.
- 49.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, et al. Endocrine treatment of transsexual persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009 [cited 2020 Jul 19];94(9):3132–54. Available from: https://academic.oup.com/jcem/article/94/9/3132/2596324. [DOI] [PubMed]
- 50.van Casteren NJ, Dohle GR, Romijn JC, de Muinck K-S, Weber RFA, van den Heuvel-Eibrink MM. Semen cryopreservation in pubertal boys before gonadotoxic treatment and the role of endocrinologic evaluation in predicting sperm yield. Fertil Steril. 2008;90(4):1119–1125. doi: 10.1016/j.fertnstert.2007.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon reasonable request.
Not applicable.