Abstract
Purpose
The precise timing of insemination after oocyte retrieval is sometimes challenging. In this study, we have assessed the effect of the variation in insemination timing on reproductive outcome for both conventional insemination (CI) and intracytoplasmic sperm injection (ICSI) cycles.
Methods
A single-center retrospective cohort data analysis was performed on 6559 patients (9575 oocyte retrievals) from January 2017 to July 2019. The main outcome measured was live birth rates. Secondary outcomes included fertilization rate per all oocytes retrieved, blastocyst utilization, clinical pregnancy, and miscarriage rates. The time interval between oocyte retrieval and insemination was analyzed in eight categories: 0 (0– < 0.5 h), 1 (0.5– < 1.5 h), 2 (1.5– < 2.5 h), 3 (2.5– < 3.5 h), 4 (3.5– < 4.5), 5 (4.5– < 5.5), 6 (5.5–6.5), and 7 (6.5– < 8 h). The number of retrievals in each group (0–7) was 586, 1594, 1644, 1796, 1836, 1351, 641, and 127 respectively.
Results
The mean fertilization rate for CI ranged from 54.1 to 64.9% with a significant difference between time categories 0 and 5 (p < 0.001) and 1 and 5 (p < 0.0.001). The mean fertilization rate for ICSI ranged from 52.8 to 67.3% with no significant difference between time categories. Blastocyst rate for CI and ICSI was not significantly different. Miscarriage and clinical pregnancy rates in CI and ICSI were not significantly different. Live birth rates differed significantly (p < 0.05) in CI with time categories 0 and 7 representing the lowest rates, but not in the ICSI group.
Conclusion
If performing CI or ICSI before 1.5 h and > 6.5 h, any detrimental effects are moderate on fertilization but do not affect blastocyst usage and birth rates.
Trial registration
Institutional Review Board Approval from the Beth Israel Deaconess Medical Centre [IRB Protocol #: 2015P000122].
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02299-7.
Keywords: Live birth, IVF, ICSI, Fertilization rate, Live birth rate
Introduction
The precision and timing of protocols for the in vitro fertilization (IVF) laboratory are highly influenced by the biological characteristics of the manipulated gametes. As it becomes more mainstream, the need to maintain high standards to ensure the patient receives the best results from each cycle is imperative. More people now use IVF to achieve pregnancy and this increases the laboratory and clinic workload, resulting in challenges to performing all procedures according to schedule. Data from the International Committee Monitoring Assisted Reproductive Technologies (ICMART) [1] report shows that there were nearly 2 million IVF cycles, which represented a 9.3% increase from 2015. This included a large increase in the frequency of frozen embryo transfer cycles.
With the increasing implementation of IVF, logistics have begun to play a greater role in influencing the sequence of events occurring in the IVF laboratory. Analysis of laboratory and clinical outcomes of assisted reproduction is essential to validate the use of these processes. For example, important information about individual oocytes can be obtained from observing the development of the fertilized oocyte from the 2PN stage to blastocyst, through to implantation and delivery of the live newborn. In this study, our objective was to examine how the time variation from retrieval to insemination affected the above outcomes for each retrieval; including fertilization, blastocyst development, and pregnancy outcomes.
The female gamete has a unique biological characteristic. At ovulation, the oocyte is assumed to have achieved full maturity and fertilization potential. However, in the case of ovarian hyperstimulation, this is not always true as a percentage of oocytes are still immature [2]. The nuclear maturity of the oocyte is said to have occurred when the second meiotic division occurs. It is believed that nuclear maturation occurs faster than cytoplasmic maturation hence the need for an appropriate period of incubation to allow the cytoplasm to catch up [3]. Oocyte cytoplasmic maturation includes those events that enable the oocyte to complete nuclear maturation, insemination, early embryogenesis and therefore provide a foundation for proper implantation, initiation of pregnancy, and normal fetal development[4].
Once a cumulus oocyte complex is collected, the protocol in most laboratories is to incubate the oocyte for 4–5 h before insemination [5]. This is influenced by evidence of fertilization outcomes observed by studies in the early days of IVF [6]. In most IVF laboratories, the timing of the ovulation trigger injection is usually co-ordinated such that the time of ovulation falls into a workload schedule of oocyte retrieval in the morning and inseminations by afternoon. This sequence optimizes laboratory functioning and efficiency[7].
The debate about the effect of time of insemination of the oocyte after retrieval on reproductive outcome has been ongoing from the 1980s, with publications supporting a pre-incubation period of at least 3 h using conventional insemination (CI) [5, 6]. In contrast, there are recent studies that describes minor differences in fertilization rates and others that report no differences. For example, Ho et al. [8] observed some beneficial increase in fertilization rate of oocytes pre-incubated for at least 2.5 h before insemination but not more than 8 h for both IVF and ICSI cycles. However, the study had a relatively small sample size (176 IVF cycles with 1883 eggs retrieved and 76 ICSI cycles with 802 eggs retrieved). Pujol et al. [9] also attempted to address this concern by studying how time to ICSI affects reproductive outcome using laboratory time divided into deciles, to maintain an equal category population, and showed that both biochemical and clinical pregnancy rates diminish progressively as time between oocyte pick up and ICSI increased after fresh embryo transfer. More recently, Vandenberghe et al. [10] reported that the optimal injection time window may be less stringent than previously thought, as both embryological and clinical outcome parameters were not significantly affected in relation to the early or later denudation and ICSI.
The true test of the normality or maturation of the oocyte is confirmed by its ability to develop to the blastocyst stage and finally the birth of a normal offspring following embryo transfer. This is because the competent oocyte is able to complete the transition from maternal to embryonic genome and proceed to implant [11]. The time to insemination gives the retrieved pre-ovulatory oocyte the chance to achieve maturation. It is thought that the reproductive capability of a human oocyte starts declining after 7 h of retrieval [12] and most studies have a peak fertilization outcome at 5–5.5 h [6, 13]. However, the majority of available literature does not include inseminations done in a broad timespan, including less than 1.5 h and after 7 h. More recent studies have also focussed largely on ICSI outcomes.
In this study, we have assessed the effect of time to insemination post-retrieval by examining: fertilization rate per egg retrieved, blastocyst rate, clinical pregnancy rate, miscarriage rate, live birth, and cumulative live birth rate in both CI and ICSI procedures at a single center using similar protocols for all cycles. Our results examined the time-span of CI or ICSI from 0 to 8 h post-retrieval by assessing outcomes of 9575 oocyte retrievals in a wide spectrum of patients.
Materials and methods
Patient population
This study population was composed of 6559 patients, who had IVF treatment with 9575 oocyte retrieval cycles at Boston IVF from January 1, 2017, to July 31, 2019. Oocyte insemination was done by either CI or ICSI. The study was conducted under an expedited Institutional Review Board Approval from the Beth Israel Deaconess Medical Centre [IRB Protocol #: 2015P000122]. All cycles with at least 1 mature oocyte retrieved in the study period were included while those with surgically retrieved sperm, frozen-thawed oocytes, and cycles with only Metaphase I or GV (Germinal Vesicle) oocytes retrieved were excluded.
Insemination criteria for ICSI and CI
Massachusetts has mandated health insurance, which covers IVF for the majority of the population working in the state. Under the IVF insurance mandate, patients with < 4 million total motile sperm per ml post preparation or with previous failed fertilization have ICSI performed. In some cases, patients with sperm numbers greater than this may do ICSI; however, the ICSI portion of the procedure is not covered by insurance.
Stimulation protocol
Patients underwent ovarian stimulation, vaginal oocyte retrieval, embryo transfer, and subsequent blastocyst vitrification using antagonist and agonist protocols at the discretion of the treating physician. These protocols included subcutaneous doses of gonadotropins; monitoring with transvaginal ultrasound, serum estradiol, luteinizing hormone, and progesterone levels; and follicle maturation with human chorionic gonadotropin (hCG), gonadotropin-releasing hormone agonist, or both, as described previously [14, 15]. Ultrasound-guided oocyte retrieval was performed 36 h after hCG administration. Embryos were transferred fresh, either on day 3 (cleavage stage) or day 5 (at the blastocyst stage) determined on a case-by-case basis. Blastocyst cryopreservation occurred on day 5 or day 6 after reaching expansion grade 3 or greater and any ICM or trophectoderm grade combination of A and B, according to the Gardner scoring criteria [16–18].
Timing of oocyte retrievals, insemination, and ICSI
The timing of laboratory events was documented by the embryologist in the Electronic Medical Record Database (eIVF, Practice Highways). Timing of HCG trigger dose was reported by the patient. Oocyte retrievals commence at 0730 and are programmed to end by 1600. If the retrieval volume exceeds this time period, a second group of retrievals will be performed in parallel. Once collected, the cumulus oocyte complexes are washed in handling media (MHM, Irvine Scientific) and placed in a culture dish of Continuous Single Culture Media (CSC) (Irvine Scientific) under oil (Vitrolife). ICSI denudation is initiated at 1030 and once denudation is completed for a patient ICSI is performed. For oocyte retrievals that occur after 1500, CI or ICSI can be initiated immediately leading to a shorter time period between retrieval and CI or ICSI. On the contrary, in some cases when there are delays in acquiring the sperm sample and the retrieval has occurred earlier, the time period can be longer.
Insemination and embryo culture
CI was performed in a culture dish in 75ul droplets using a final count of 100,000 motile sperm per drop. The COCs were placed in a group of 5 to a drop. For ICSI, denuded oocytes were assessed for nuclear maturity under the microscope and only MII oocytes were placed in the culture dish and injected. Once injected, oocytes were rinsed in CSC and placed in the post-ICSI culture dishes.
Once assessed for fertilization, embryos were cultured in drops of CSC and all embryos were cultured in bench top incubators controlled at 7%CO2, 6%O2, 87% N2, and 36.8 °C. Fertilization assessment was performed between 16 and 20 h post insemination.
Statistical analysis
Variable determination
All analysis was conducted using time of insemination as a categorical variable in both CI and ICSI cycles. The categories for time of insemination were considered from oocyte pickup (OPU) to insemination (CI/ICSI) in timings of 0 < 0.5 h, 0.5 < 1.5 h, 1.5 < 2.5 h, 2.5 < 3.5 h, 3.5 < 4.5 h, 4.5 < 5.5 h, 5.5 < 6.5 h, and 6.5 < 8 h. As the variable of interest was time, post-retrieval cases were divided based on the above times and not distributed in groups of equal sizes. This created 8-time categories of observations stated as 0, 1, 2, 3, 4, 5, 6, and 7 respectively. Fertilization rate was defined as the number of 2PN seen on day 1 of insemination divided by the total number of retrieved oocytes in both ICSI and CI cycles, in percentage. It is important to note that ICSI fertilization rates were not based on how many MII oocytes were injected but as a percentage of all oocytes retrieved. We defined the fertilization rate using number of retrieved oocytes to ensure that we had a condition in which both the CI and ICSI cycles could be compared using the same denominator. Blastocyst rate was defined as the total number of usable blastocysts (those transferred and/or cryopreserved on day 5, 6, and 7) divided by the total number of fertilized oocytes on day 1 in both ICSI and CI cycles, in percentage.
All statistical analyses were performed using SPSS software version 22.0 (Armonk, NY, USA). A p value of < 0.05 was set as statistically significant. Graphs were plotted using MS Excel 2007 and SPSS. The categorical variables (CI or ICSI times post-retrieval) were analyzed by one-way analysis of variance (ANOVA). Post hoc analysis was determined by Tukey HSD pair-wise comparison and homogenous subset when necessary. The χ2 test was used to compare the pregnancy, miscarriage, ongoing, and live birth rates within time categories.
Results
Demographic data
We analyzed data for a total of 6559 patients with 9575 oocyte retrieval cycles from January 1, 2017, to July 31, 2019. The mean age (± SD) and mean number of oocytes (± SD) of the patients in each category are shown in Table 1. The mean oocytes inseminated across each observed time category were significantly different in the time category 0 for CI and ICSI cycles. It was significantly lower compared to time categories 1–5 in CI; and time categories 2–7 for ICSI cycles. This was largely due to smaller patient numbers in the 0 and 7 time groups.
Table 1.
Age and number of oocytes collected for patients undergoing CI and ICSI in the different time categories groups
| All cycles | CI | ICSI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time category | N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| AGE | 0 | 586 | 36.3 | 4.3 | 565 | 36.2 | 4.3 | 21 | 37.3 | 3.9 |
| 1 | 1594 | 35.7 | 4.3 | 1312 | 35.7 | 4.2 | 282 | 36.2 | 4.7 | |
| 2 | 1644 | 36.1 | 4.3 | 1099 | 36.1 | 4.3 | 545 | 36.1 | 4.3 | |
| 3 | 1796 | 35.9 | 4.3 | 905 | 36.0 | 4.2 | 891 | 36.0 | 4.4 | |
| 4 | 1836 | 36.3 | 4.2 | 643 | 36.3 | 4.3 | 1193 | 36.3 | 4.2 | |
| 5 | 1351 | 36.0 | 4.3 | 283 | 35.5 | 4.5 | 1068 | 36.1 | 4.2 | |
| 6 | 641 | 35.9 | 4.2 | 117 | 35.6 | 4.1 | 524 | 35.9 | 4.2 | |
| 7 | 127 | 36.1 | 3.6 | 31 | 36.7 | 3.6 | 96 | 36.0 | 3.6 | |
| Oocytes | 0 | 586 | 10.0 | 6.2 | 565 | 10.1* | 6.2 | 21 | 6.5** | 5.0 |
| 1 | 1594 | 12.7 | 8.8 | 1312 | 13.0 | 9.0 | 282 | 11.4 | 7.6 | |
| 2 | 1644 | 12.1 | 8.0 | 1099 | 12.2 | 8.1 | 545 | 12.0 | 7.7 | |
| 3 | 1796 | 12.6 | 8.3 | 905 | 12.6 | 8.2 | 891 | 12.6 | 8.4 | |
| 4 | 1836 | 12.3 | 8.1 | 643 | 12.6 | 8.0 | 1193 | 12.1 | 8.1 | |
| 5 | 1351 | 12.4 | 8.1 | 283 | 12.8 | 7.9 | 1068 | 12.3 | 8.2 | |
| 6 | 641 | 12.5 | 7.9 | 117 | 12.6 | 9.4 | 524 | 12.5 | 7.5 | |
| 7 | 127 | 13.0 | 8.6 | 31 | 9.3 | 6.1 | 96 | 14.2 | 9.0 | |
*Significantly different to times 1–5
**Significantly different to times 2–7
Fertilization rate
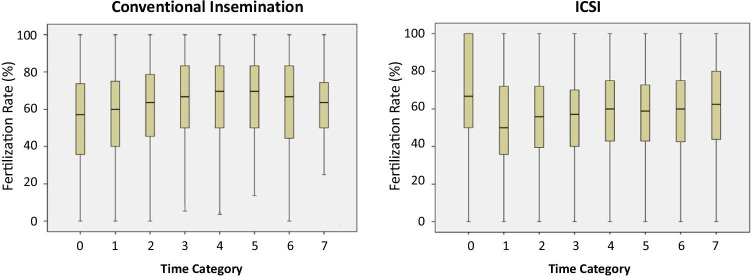
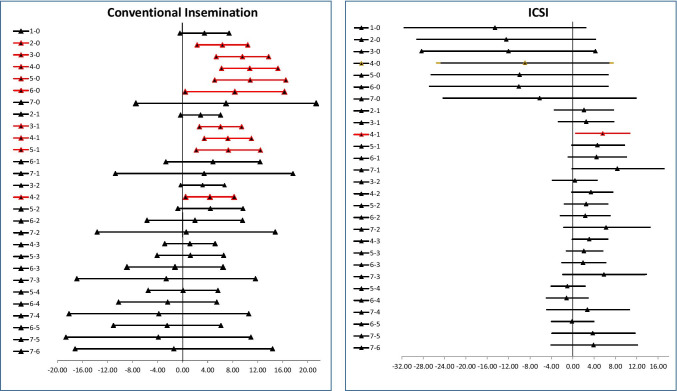
The overall mean fertilization rate was 58.6%. The modal distribution was time category 4 with a population of 1193 cycles. The fertilization rate by CI in each time category is shown in Table 2 and Fig. 1a. Analysis using ANOVA of the mean fertilization rates of the observed time categories showed a significance of p < 0.0001 indicating there were significant differences in the means of fertilization rates between some time categories. Pair-wise comparison showed that the 0 and 1 categories were significantly lower (Fig. 2a). The fertilization rate by ICSI in each time category is shown in Table 2 and Fig. 1b. Analysis using ANOVA of the mean fertilization rates of the observed time categories showed a significance of p < 0.001 indicating there were significant differences in the means of fertilization rates between some time categories. Pair-wise comparison showed that only some significant differences existed; however, the number of cases in time category 0 for ICSI was small (Fig. 2b). When examining ICSI fertilization rates per MII oocyte injected for the different time categories, no significant difference was observed (Supplementary Table 1).
Table 2.
Comparison of mean fertilization rates in cases of CI and ICSI in relation to the time of insemination after egg retrieval
| Time (Grp) | Number | Mean | SD | SE | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Min | Max | |||||
| CI | ||||||
| 0 | 565 | 54.06 | 27.54 | 1.16 | 51.78 | 56.33 |
| 1 | 1312 | 57.56 | 25.59 | 0.71 | 56.17 | 58.94 |
| 2 | 1099 | 60.43 | 25.13 | 0.76 | 58.94 | 61.92 |
| 3 | 905 | 63.62 | 25.38 | 0.84 | 61.97 | 65.28 |
| 4 | 643 | 64.79 | 26.11 | 1.03 | 62.77 | 66.81 |
| 5 | 283 | 64.87 | 25.44 | 1.51 | 61.89 | 67.85 |
| 6 | 117 | 62.41 | 26.77 | 2.47 | 57.51 | 67.31 |
| 7 | 31 | 61.00 | 26.06 | 4.68 | 51.44 | 70.56 |
| ICSI | ||||||
| 0 | 21 | 67.28 | 30.57 | 1.16 | 53.36 | 81.20 |
| 1 | 282 | 52.72 | 26.04 | 0.71 | 49.67 | 55.77 |
| 2 | 545 | 54.83 | 24.55 | 0.76 | 52.76 | 56.90 |
| 3 | 891 | 55.24 | 22.84 | 0.84 | 53.74 | 56.74 |
| 4 | 1193 | 58.34 | 23.72 | 1.03 | 56.99 | 59.69 |
| 5 | 1068 | 57.34 | 22.93 | 1.52 | 55.96 | 58.71 |
| 6 | 524 | 57.18 | 23.22 | 2.47 | 55.19 | 59.17 |
| 7 | 96 | 61.08 | 23.64 | 4.68 | 56.29 | 65.87 |
Fig. 1.
Box plots of the mean fertilization rate in the different time categories after CI and ICSI
Fig. 2.
Post hoc analysis comparison of time categories for CI and ICSI fertilization showing confidence intervals. Comparisons that are significantly different are shown in red
Blastocyst utilization rate
The blastocyst utilization rate includes blastocysts that were vitrified or transferred in fresh cycles as blastocysts. The blastocyst utilization rate for CI and ICSI is shown in Table 3. For this calculation, the day 3 freshly transferred embryos were removed. In these cases, all embryos were continued for cryopreservation to the blastocyst stage. Blastocysts derived from category 6 in CI had the highest utilization rate at 48.32% and those from category 5 had the lowest utilization rate at 42.34%. The overall blastocyst utilization rate within the observed time period was 45.26%. Blastocyst derived from category 0 in ICSI had the highest utilization rate at 48.86%; and those from category 7 had the lowest utilization rate at 38.94%. The overall blastocyst utilization rate for ICSI within the observed time period was 45.25%. Post hoc analysis of both the blastocyst utilization and overall blastocyst rate for CI and ICSI showed no significantly differences for all time categories.
Table 3.
The percentage outcome of blastocysts by time category in CI and ICSI. Utilization rate is the number of blastocysts transferred and frozen (by vitrification) per fertilized embryos, while the blastocyst rate is the number of embryos reaching the blastocyst stage (Gardner grade 3 and above) per fertilized embryo
| Time category | Number frozen | Number discarded | Number transferred | Total | Utilization rate | Average blastocyst rate |
|---|---|---|---|---|---|---|
| CI | ||||||
| 0 | 1034 | 1837 | 405 | 3276 | 43.93% | 46.7% |
| 1 | 3786 | 5532 | 882 | 10,200 | 45.75% | 53.2% |
| 2 | 3047 | 4530 | 805 | 8382 | 45.81% | 52.2% |
| 3 | 2868 | 4127 | 637 | 7632 | 45.91% | 53.5% |
| 4 | 2055 | 3098 | 396 | 5549 | 44.15% | 54.3% |
| 5 | 846 | 1441 | 212 | 2499 | 42.34% | 54.2% |
| 6 | 373 | 476 | 72 | 921 | 48.32% | 55.8% |
| 7 | 61 | 89 | 18 | 168 | 47.02% | 54.0% |
| Total | 14,070 | 21,130 | 3427 | 38,627 | 45.26% | 52.8% |
| ICSI | ||||||
| 0 | 28 | 45 | 15 | 88 | 48.86% | 49.2% |
| 1 | 570 | 912 | 172 | 1654 | 44.86% | 49.2% |
| 2 | 1294 | 2012 | 399 | 3705 | 45.70% | 50.9% |
| 3 | 2293 | 3424 | 612 | 6329 | 45.90% | 51.8% |
| 4 | 3021 | 4720 | 865 | 8606 | 45.11% | 49.6% |
| 5 | 2785 | 4253 | 803 | 7841 | 45.75% | 50.9% |
| 6 | 1331 | 2154 | 404 | 3889 | 44.51% | 49.6% |
| 7 | 252 | 497 | 65 | 814 | 38.94% | 46.7% |
| Total | 11,565 | 18,017 | 3,335 | 32,926 | 45.25% | 50.4% |
Pregnancy, miscarriage, and live birth rate
The positive pregnancy, miscarriage, and live births per transfers originating from the retrieval cycles in each time category are shown in Table 4. Cycles with transferred embryos in both fresh and frozen transfers were included in this analysis. The mean number of embryos transferred (+ SD) for both CI and ICSI in each time category did not differ significantly. For CI, the mean (+ SD) was 1.6 ± 0.9 and 1.2 ± 0.5 day 3 and day 5 fresh transfers, respectively. For ICSI, the mean (+ SD) was 1.8 ± 0.9 and 1.4 ± 0.6 for day 3 and day 5 fresh transfers, respectively. For both CI and ICSI, the mean number of embryos transferred (+ SD) was 1.1 ± 0.4 frozen blastocysts.
Table 4.
The percentage outcome of positive pregnancy, miscarriage, and live births per total transfers originating from the initial retrieval
| Time categories | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total | |
| CI | |||||||||
| Positive pregnancy | 317 | 941 | 754 | 612 | 352 | 154 | 79 | 18 | 3227 |
| 44.8% | 50.3% | 48.8% | 49.7% | 51.7% | 52.0% | 46.5% | 46.2% | 49.3% | |
| Miscarriage | 46 | 112 | 107 | 64 | 55 | 14 | 12 | 2 | 412 |
| 6.5% | 6.0% | 6.9% | 5.2% | 8.1% | 4.7% | 7.1% | 5.1% | 6.3% | |
| Live births | 199 | 638 | 488 | 405 | 232 | 105 | 46 | 9 | 2122 |
| 28.1% | 34.1% | 31.6% | 32.9% | 34.1% | 35.5% | 27.1% | 23.1% | 32.4% | |
| Day 3 fresh transfers | 119 | 217 | 182 | 132 | 75 | 32 | 22 | 3 | 782 |
| Total fresh and frozen transfers* | 708 | 1870 | 1544 | 1232 | 681 | 296 | 170 | 39 | 6540 |
| ICSI | |||||||||
| Positive pregnancy | 11 | 169 | 337 | 557 | 773 | 679 | 312 | 79 | 2917 |
| 45.8% | 48.1% | 46.9% | 47.0% | 47.3% | 47.5% | 45.9% | 51.3% | 47.2% | |
| Miscarriage | 1 | 27 | 39 | 70 | 113 | 86 | 41 | 12 | 389 |
| 4.2% | 7.7% | 5.4% | 5.9% | 6.9% | 6.0% | 6.0% | 7.8% | 6.3% | |
| Live births | 8 | 102 | 215 | 369 | 488 | 460 | 208 | 55 | 1905 |
| 33.3% | 29.1% | 29.9% | 31.1% | 29.8% | 32.2% | 30.6% | 35.7% | 30.8% | |
| Day 3 fresh transfers | 8 | 53 | 112 | 159 | 224 | 212 | 101 | 15 | |
| Total fresh and frozen transfers* | 24 | 351 | 719 | 1186 | 1635 | 1429 | 680 | 154 | 6178 |
*Includes all transfers performed (day 3 fresh and blastocyst fresh and frozen)
In both CI and ICSI, there was no significant difference across time categories for pregnancy, miscarriage, and live birth rates. The percentage of day 3 fresh transfers across all groups were not significantly different for the CI and ICSI time categories (Table 4). The percentage of PGT cycles per retrieval in the CI and ICSI groups was 32.1% (range: 27.3 to 35.5%, Chi-square = 11.5, p > 0.05) and 37.1% (range: 33.3 to 39.2%, Chi-square = 6.5, p > 0.05) respectively in both groups. Analysis across all time categories did not show any significant difference in the use of PGT-A for CI or ICSI groups.
Discussion
This study investigated if there were any differences in laboratory and clinical outcome in ART cycles inseminated at varying time periods after oocyte retrieval using CI or ICSI. Our findings suggest that fertilization rates for CI were significantly lower at insemination time category 0 and 1. Also, the fertilization rate for ICSI was significantly lower in the time category 1. The number of ICSI cycles in time category 0 however was low and might have affected the ability to adequately test for significant differences. Once fertilized, the embryos showed no significant decline in their ability to reach the blastocyst stage and progress to live birth in relation to the different time categories.
While we observed that fertilization might be more time sensitive, other laboratory and clinical outcomes may rely more on intrinsic embryo characteristics unrelated to timing of fertilization. It is accepted that oocyte nuclear and cytoplasmic maturation are highly co-ordinated processes and are not interdependent. Nuclear maturation encompasses the processes reversing meiotic arrest at prophase I and driving the progression of meiosis to metaphase II while cytoplasmic maturation refers to the processes that prepare the egg for activation and preimplantation development[19]. Developmentally competent oocytes are able to progress to the blastocyst stage because they are able to produce and accumulate necessary maternal mRNAs and proteins which are undoubtedly critical for successful pre-implantation development. The attrition of oocytes to live birth has previously been shown to be dramatically high [20]. It could be argued that the data in this study indicate that the event of fertilization may be the first step in weeding out incompetent oocytes. Competency therefore does not seem to be dramatically impacted by timing of insemination or ICSI indicating that intrinsic components of the oocyte may already be jeopardized prior to ovulation.
Fertilization is the first step of an ART cycle in getting to the embryo transfer stage. Previous studies on the effects of time of insemination on laboratory outcome noticed lower fertilization rates for oocytes inseminated by CI within the first 3 h [13] of oocyte retrieval, but with no significant differences after 3 h in CI and ICSI cycles [7, 21]. The initial IVF studies by Trounson et al. [6] observed optimal fertilization rates when insemination was performed at 5–5.5 h. In our own data, the only time category that impacted fertilization rates significantly was when inseminations occurred before 1.5 h after retrieval. This could be because the oocytes need some time to adjust to the laboratory environment as they are collected as pre-ovulatory oocytes. This does raise the question of whether the actual ovulation process into the fallopian tube is implicated in the final maturation step of oocytes. Allowing them to stay in contact with the cumulus cells improves their chance of attaining full maturation [22], but extended time between retrieval and insemination has little or no benefits especially in CI. The time the oocyte remains with the cumulus cells in CI is much greater so this may have other benefits. The use of ICSI may be a more rigorous examination of time of insemination post-retrieval as in that case, the cumulus cells are removed.
Several studies that investigated the optimal time of ICSI have been carried out over the years with no conclusive data. Early studies by Van de Velde et al. [23] showed that the best results are obtained if the oocytes are injected 2–6 h after retrieval. Barcena et al. [24] reported no significant difference in clinical outcomes compared to time of ICSI. In this study, however, the average patient age was 26 years and the earliest time to ICSI was 3 h after egg retrieval in both fresh and vitrified oocytes. In contrast, Pujol et al. [9] reported deteriorating implantation and pregnancy rates as incubation time increased for oocytes pre-incubated for at least 1 h before ICSI in a fresh embryo transfer cycle. They found that there was a paradoxical increase in fertilization rate as time proceeded but this had no effect on embryo quality and there was no effect on the ongoing pregnancy and live birth rates. It was proposed that oocyte aging could explain the increase in fertilization rates. Another study reported that there was improvement in MII, fertilization rates, and embryo quality after 2 h of incubation, with no difference in implantation and clinical pregnancy rates [25]. Jacobs et al. [7] also reported no difference in laboratory outcomes (fertilization and blastocyst rates) even after adjusting for cofounders in CI and ICSI cycles. However, the study did not include the outcome of inseminations done in shorter time periods. Other authors who investigated the effect of timing of oocyte denudation on ICSI [26, 27] came up with a general conclusion that supports at least 2–3 h pre-incubation to improve the competence of the oocytes. Rienzi et al. [13] showed that pre-incubation of oocytes with cumulus cell for an average of 2 h post-retrieval was important to improve the clinical outcome for ICSI cycles. In many studies, however, ICSI fertilization rates are judged by the number of MIIs injected. Our study evaluated on the basis of number of 2PN divided by all eggs retrieved. Even when examining fertilization rates of the MIIs injected, we did not see a significant difference in the time categories assessed (See supplementary Table 1). In CI cycles, we observed a statistically significant trend of increasing fertilization, blastocyst, pregnancy, ongoing pregnancy, and live birth rates as the time to insemination increased until it peaked at 4.5–5.5 h before a gradual drop in rates was seen. For ICSI cycles, we had an unexplained spike in fertilization rates for inseminations done within 30 min of retrieval, and a sudden drop between 0.5 and 1.5 h but there was no effect on subsequent embryo development. This, however, is likely due to the low number of cases performed at the earliest time point. Underlying the differences between CI and ICSI are also that ICSI has been associated with lower rates of implantation (23.0% vs 25.2%; adjusted RR, 0.93; 95% CI, 0.91–0.95) and live birth (36.5% vs 39.2%; adjusted RR, 0.95)[28].
There are 2 competing factors that govern the timing of insemination. The first is the biology of the oocyte and the second is the logistics of performing an IVF cycle. Timing of maturation is presumed to take place after HCG trigger which in our clinic is 36 h prior to oocyte retrieval. The maturation times can then be extrapolated to post trigger by adding 36 h, even though this could be subject to some patient variation. The overall competence of an oocyte largely determines reproductive outcomes of an ART cycle. The ovulated oocyte is assumed to complete cytoplasmic maturation during transportation into the ampulla. In in vitro models, retrieved oocytes are mostly pre-ovulatory and they maintain the nuclear/cytoplasmic asynchrony [29]. Assuming the viability of the oocyte is similar to in-vivo conditions, we can say that the oocyte might have a window of fertilization and not just a specific time where fertilization is most likely to occur. This time window is easier to accommodate in CI but is effectively overridden in ICSI cycles. Interestingly, timing of ICSI when comparing only fertilization rates of MII oocytes showed no differences.
When we consider laboratory logistics, an IVF cycle has to be performed to achieve the best results within the available time period. For example, a busy laboratory performing oocyte retrievals late in the afternoon may not have the capacity to perform ICSI or CI 4–5 h later. The data presented in this study indicate that overall, early ICSI or CI is not detrimental to cycle outcome. Most laboratories with low patient load are able to function according to the biology of the oocyte; however, in a very busy laboratory with high cycle turnover, a balance may need to be achieved between oocyte biology and logistics. The retrieved pre-ovulatory oocytes can be inseminated after at least 1.5 h of incubation without any detriment to fertilization, blastocyst utilization rate, or pregnancy and live birth rates.
This study had several limitations. First is that it was a retrospective study hence we could not exclude the effect of some unknown variables. Secondly, timing of events was dependent on the both the patient and embryologist. This limitation was compensated for by rounding off the time spread into categories covering a unique time range; however, this created unevenness in case numbers between groups. Even with these limitations, our study had the strength of a large sample size, even though early times had smaller numbers in ICSI. This time spread was considered to be representative of the challenges faced in a busy laboratory. Our main focus was on the mean rate of cycle outcomes in each time category considered and this helped to reduce the effect of outliers in our data. We were able to consider the case of rapid inseminations done within the first 30 min in both CI and ICSI cycles as this was not studied in majority of the available literature [7, 9, 13]. The number of cycles in the 0 and 7 time categories for both ICSI and CI was however lower. A further limitation is that in some cases, not all frozen embryos are accounted for and that some day 3 fresh embryo transfers were performed which could impact blastocyst utilization rates. Importantly, our own data also represented the outcome of ICSI from all oocytes and not just MII oocytes injected. The population of our study is also representative of the average population that seek ART treatment with the exception of cycles with surgical sperm and other extreme semen parameter related cases. Massachusetts has mandated health insurance covering IVF; hence, it is more inclusive in the representation of the ART patients.
Conclusion
This large retrospective study indicated that both CI and ICSI are optimal when performed between 1.5 and 6.5 h after oocyte pickup for consistent outcomes. If performing CI or ICSI prior to 1.5 h, any detrimental effects are moderate on fertilization but do not impact the final blastocyst usage and pregnancy rate. Further studies on fertilization outcome related to time of insemination are warranted, in particular when considering factors that may impact the oocyte such as the specific cause of infertility and stimulation protocols.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adamson GD, de Mouzon J, Chambers G, Zegers-Hochschild F, Mansour R, Ishihara O, et al. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2016 https://secureservercdn.net/198.71.233.206/3nz.654.myftpupload.com/wp-content/uploads/ICMART-ESHRE-WR2016-FINAL-20200901.pdf. Accessed July 7, 2021. [DOI] [PubMed]
- 2.The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. 10.1093/humrep/der037. [DOI] [PubMed]
- 3.Kidder BL, Zhao K. Efficient library preparation for next-generation sequencing analysis of genome-wide epigenetic and transcriptional landscapes in embryonic stem cells. Methods Mol Biol. 2014;1150:3–20. doi: 10.1007/978-1-4939-0512-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Watson AJ. Oocyte cytoplasmic maturation: a key mediator of oocyte and embryo developmental competence. J Anim Sci. 2007;85:E1–3. doi: 10.2527/jas.2006-432. [DOI] [PubMed] [Google Scholar]
- 5.Harrison KL, Wilson LM, Breen TM, Pope AK, Cummins JM, Hennessey JF. Fertilization of human oocytes in relation to varying delay before insemination. Fertil Steril. 1988;50:294–297. doi: 10.1016/s0015-0282(16)60076-6. [DOI] [PubMed] [Google Scholar]
- 6.Trounson AO, Mohr LR, Wood C, Leeton JF. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J Reprod Fertil. 1982;64:285–294. doi: 10.1530/jrf.0.0640285. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs M, Stolwijk AM, Wetzels AM. The effect of insemination/injection time on the results of IVF and ICSI. Hum Reprod. 2001;16:1708–1713. doi: 10.1093/humrep/16.8.1708. [DOI] [PubMed] [Google Scholar]
- 8.Ho JY, Chen MJ, Yi YC, Guu HF, Ho ES. The effect of preincubation period of oocytes on nuclear maturity, fertilization rate, embryo quality, and pregnancy outcome in IVF and ICSI. J Assist Reprod Genet. 2003;20:358–364. doi: 10.1023/a:1025476910771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujol A, García D, Obradors A, Rodríguez A, Vassena R. Is there a relation between the time to ICSI and the reproductive outcomes? Hum Reprod. 2018;33:797–806. doi: 10.1093/humrep/dey067. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberghe LTM, Santos-Ribeiro S, De Munck N, Desmet B, Meul W, De Vos A, et al. Expanding the time interval between ovulation triggering and oocyte injection: does it affect the embryological and clinical outcome? Hum Reprod. 2021;36:614–623. doi: 10.1093/humrep/deaa338. [DOI] [PubMed] [Google Scholar]
- 11.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 12.Falcone P, Gambera L, Pisoni M, Lofiego V, De Leo V, Mencaglia L, et al. Correlation between oocyte preincubation time and pregnancy rate after intracytoplasmic sperm injection. Gynecol Endocrinol. 2008;24:295–299. doi: 10.1080/09513590802095613. [DOI] [PubMed] [Google Scholar]
- 13.Rienzi L, Ubaldi F, Anniballo R, Cerulo G, Greco E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:1014–1019. doi: 10.1093/humrep/13.4.1014. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan DA, Leung A, Resetkova N, Ruthazer R, Penzias AS, Sakkas D, et al. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil Steril. 2017;107(397–404):e3. doi: 10.1016/j.fertnstert.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 15.The Boston IVF Handbook of Infertility. 4th ed. Boca Rotan: CRC Press; 2018.
- 16.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sakkas D, Gardner DK. Evaluation of embryo quality: analysis of morphology and physiology. Boca Raton: CRC Press; 2017. [Google Scholar]
- 18.Shear MA, Vaughan DA, Modest AM, Seidler EA, Leung AQ, Hacker MR, et al. Blasts from the past: is morphology useful in PGT-A tested and untested frozen embryo transfers? Reprod Biomed Online. 2020;41:981–989. doi: 10.1016/j.rbmo.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- 20.Patrizio P, Sakkas D. From oocyte to baby: a clinical evaluation of the biological efficiency of in vitro fertilization. Fertil Steril. 2009;91:1061–1066. doi: 10.1016/j.fertnstert.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–2079. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- 22.Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15(5):573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- 23.Van de Velde H, De Vos A, Joris H, Nagy ZP, Van Steirteghem AC. Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3160–3164. doi: 10.1093/humrep/13.11.3160. [DOI] [PubMed] [Google Scholar]
- 24.Bárcena P, Rodríguez M, Obradors A, Vernaeve V, Vassena R. Should we worry about the clock? Relationship between time to ICSI and reproductive outcomes in cycles with fresh and vitrified oocytes. Hum Reprod. 2016;31:1182–1191. doi: 10.1093/humrep/dew070. [DOI] [PubMed] [Google Scholar]
- 25.Isiklar A, Mercan R, Balaban B, Alatas C, Aksoy S, Urman B. Impact of oocyte pre-incubation time on fertilization, embryo quality and pregnancy rate after intracytoplasmic sperm injection. Reprod Biomed Online. 2004;8:682–686. doi: 10.1016/s1472-6483(10)61649-5. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho M, Leal F, Mota S, Aguiar A, Sousa S, Nunes J, et al. The effect of denudation and injection timing in the reproductive outcomes of ICSI cycles: new insights into the risk of in vitro oocyte ageing. Hum Reprod. 2020;35:2226–2236. doi: 10.1093/humrep/deaa211. [DOI] [PubMed] [Google Scholar]
- 27.Patrat C, Kaffel A, Delaroche L, Guibert J, Jouannet P, Epelboin S, et al. Optimal timing for oocyte denudation and intracytoplasmic sperm injection. Obstet Gynecol Int. 2012;2012:403531. doi: 10.1155/2012/403531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313:255–263. doi: 10.1001/jama.2014.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27:27–47. doi: 10.1093/humupd/dmaa043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.