Abstract
Background
Women with polycystic ovary syndrome (PCOS) are often infertile and opt for artificial reproductive techniques (ART) to conceive. Disrupted pro-/antioxidant balance in oocyte microenvironment may contribute towards sub-optimal oocyte/embryo quality and poor ART outcome in them.
Methods
Activities/levels of redox markers and their transcript expression were investigated in follicular fluid and granulosa cells respectively, in women with PCOS (n = 71) and controls (n = 50) undergoing in vitro fertilization (IVF). Correlation analysis of redox markers and IVF parameters was performed.
Results
Activities of superoxide dismutase, glutathione reductase, glutathione peroxidase, and paraoxonase1 were significantly lower in follicular fluid of PCOS women than in controls. Levels of lipid peroxidation, oxidative protein modification, and oxidized glutathione were higher, whereas those of total antioxidant capacity, total thiols, and reduced glutathione were lower in follicular fluid of PCOS women than in controls. Further, comparison of redox markers based on insulin resistance and BMI status of study participants showed similar trends, indicating that PCOS pathophysiology is a significant contributor to oxidative stress irrespective of insulin resistance and BMI. Transcript levels of antioxidant enzymes were lower in granulosa cells from PCOS women than in controls, and they accorded with their activities in follicular fluid. Moreover, few redox markers showed significant correlations with oocyte/embryo quality and pregnancy outcome.
Conclusion
Our data indicates disrupted redox homeostasis in follicular environment in PCOS which may negatively influence oocyte/embryo quality. Further, granulosa cells may play crucial role in maintaining follicular redox homeostasis. Glutathione system and paraoxonase1 could be explored further as surrogates for IVF prognosis/outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02241-x.
Keywords: PCOS, Oocyte/embryo quality, Oxidative stress, Follicular fluid, Granulosa cells, IVF
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive age, with global prevalence of 6–15%. It is typically characterized by oligo/anovulation, hyperandrogenemia and polycystic ovaries, but its etiology remains elusive. These women are susceptible to other co-morbidities like obesity, insulin resistance, type 2 diabetes mellitus (T2DM), cardiovascular disease, and endometrial cancer [1]. PCOS may also lead to considerable economic, psychological, and quality of life implications. Combination of endocrine, gynecological and metabolic anomalies can lead to ovarian dysfunction and subsequent infertility in these women, who often require assisted reproductive techniques (ART) to conceive [2].
PCOS is associated with sub-optimal oocyte quality and poor outcomes of ART treatment [3]. The follicular microenvironment consists of follicular fluid (FF) and granulosa cells (GCs). FF is a uniquely tailored milieu of essential nutrients and hormones to nourish oocytes and serves as medium for cross-talk between oocyte and follicular cells. GCs are closely attached to oocyte, involved in crucial functions like metabolism, transporting small molecules to oocyte via gap junctions, and protecting oocyte from insults of external environment [2]. The oocyte-granulosa regulatory loop and the composition of follicular microenvironment are critical for the orchestrated conduct of these events, and any alterations in molecular makeup of FF/GCs may affect oocyte quality and can serve as ideal potential surrogates for that [3]. Earlier proteomics study has shown downregulation of critical antioxidants in FF from women with PCOS indicating altered redox status in oocyte microenvironment of PCOS women [4].
Folliculogenesis is an energy demanding process with high metabolic activity; hence, ROS generation is inevitable [5]. However, they need to be neutralized once their function has been completed, as they are highly unstable and can snatch electrons from lipids, proteins, and nucleic acids leading to oxidative damage to these macromolecules, malfunction, or even loss of their function. Thus, a robust, tightly regulated antioxidant system is required to maintain pro-antioxidant balance. Role of ROS in female reproductive system has been described elaborately by several groups and they have been shown to be indispensable for folliculogenesis with some of them taking part in signaling cascades like NF-kB and VEGF essential for follicular growth [5–7]. Excess ROS can lead to compromised oocyte competence due to mitochondrial dysfunction, reduced ATP production, oocyte aging, and oocyte/embryonic aneuploidy [2]. Particularly in PCOS women, increased androgen level generates excess ROS due to high activity of P450 SCC enzyme. Additionally, elevated level of LH which can function as H2O2 can contribute towards skewed redox balance in them [6]. Concomitantly, around 50–70% of women with PCOS are insulin resistant which may contribute towards increased oxidative stress (OS) via hyperglycemia and higher levels of free fatty acids which in turn produce ROS via hyper-activated electron transport chain [8].
Few studies have discerned levels/activity of pro-/antioxidant markers including lipid peroxidation, total antioxidant capacity (TAC), superoxide dismutase (SOD) activity, and thiols in FF of women with PCOS and other infertility disorders undergoing in vitro fertilization (IVF), but data remains inconsistent warranting their further inspection. The multifactorial nature of PCOS and key role of OS in its pathophysiology rationalize the investigation of redox markers in FF as it is a niche for growing follicle.
The aim of our study is to investigate the redox markers in the FF, ones which have not been explored much along with a few common ones in very well-characterized groups of women with PCOS and controls. Also, to know the potential indicators of IVF outcome, we have studied the association between redox markers and IVF parameters. Further, considering the relationship of PCOS with insulin resistance and obesity, we sub-grouped our study population based on obesity and insulin resistance status and investigated redox markers in FF which has not been explored yet to the best of our knowledge. The transcript expression of antioxidant enzymes was also examined to understand their contribution in maintenance of redox status of follicular nexus.
Materials and methods
Study design, participants, and sample collection
This study was approved by Ethics committees of P. D. Hinduja National Hospital and Medical Research Centre, Mumbai and ICMR- National Institute for Research in Reproductive Health, Mumbai (Ethics no. 261/2014). Ethical guidelines laid down by Declaration of Helsinki, and other international committees were followed. Study participants were well-characterized women with PCOS and healthy controls undergoing controlled ovarian hyperstimulation (COH) for IVF and were enrolled after obtaining written consent. Women with PCOS (n = 50), were diagnosed as per Rotterdam criteria presented with at least any two of following three characteristics, i.e., oligomenorrhea and/or anovulation, biochemical/clinical hyperandrogenemia, and/or polycystic ovarian morphology on ultrasound. Women with other confounding factors, viz. non-classical adrenal hyperplasia, hyperprolactinemia, androgen secreting adrenal/ovarian tumor, and Cushing’s syndrome, were not included in study. Age and BMI-matched control group (n = 71) includes regularly menstruating women undergoing controlled ovarian hyperstimulation for IVF due to male factor infertility or as oocyte donors. Further it was ensured that none of the participants was taking medications for diabetes, thyroid medications, or medicines containing antioxidants.
All of the study participants had undergone same protocol of controlled ovarian hyperstimulation with GnRH agonist (0.5 mg buserlin acetate) which was administered from day 21 of previous menstrual cycle. Once the suppression was confirmed, ovarian stimulation was carried out by administrating hMG. After the maturation of follicles (16–18 mm diameter), human chorionic gonadotropin was given (rhCG, 10,000 IU), and oocyte retrieval was performed 34–36 h later.
FF was collected during ultrasound guided transvaginal retrieval of oocytes. Macroscopically clear FF without any blood contamination from dominant follicle was used. It was centrifuged at 10,000g at 4 °C for 20 min to remove residual debris and stored at − 80°C until further use in multiple aliquots to avoid freeze-thaw cycles, which is important given the dynamicity of redox parameters. GCs stripped off from oocyte were collected and washed with PBS, and cell lysis buffer was added to them to extract RNA. Number of follicles, total and MII oocytes retrieved, fertilized oocytes, embryo grade, pregnancy rate, pregnancy outcome, and other anthropometric parameters were obtained from clinical records. For embryo grading, “an Atlas of Human gametes and Conceptuses” by Lucinda (L. Veek, page no. 48) was followed (grading was done on day 3 or day 4). Fasting blood sample was collected from study participants on the day of oocyte pick-up (d-OPU) to obtain serum. Considering the link between PCOS, insulin resistance, and OS, we sub-grouped our study participants as insulin resistant (IR) and non-insulin resistant (NIR) groups based on 80th percentile value of homeostatic model assessment of insulin resistance (HOMA-IR) in control women (> 2.02). Further analysis was carried out segregating study subjects as lean (BMI < 23 kg/m2) or obese (BMIR > 23 kg/m2) [9]. In addition to this, women with PCOS were sub-grouped in four different PCOS phenotypes by using clinical features hyperandrogenemia (HA), oligo or anovulation (OA), and polycystic ovarian morphology (PCO). Phenotypic classification was done as follows [3]:
Phenotype A: HA + OA + PCO; Phenotype B: HA + OA; Phenotype C: HA + PCO and Phenotype D: OA + PCO.
Biochemical and hormonal assays
Sex hormone binding globulin (SHBG), estradiol (E2), progesterone (P4), testosterone (TT), and fasting insulin were estimated in serum and FF collected on d-OPU, by using immunoassay analyzer (Roche, Basel, Switzerland). Fasting blood glucose was analyzed by biochemical analyzer (Roche, Basel, Switzerland). Free and bioavailable testosterone was calculated using online calculator (http://www.issam.ch/freetesto.htm). Basal levels of follicular stimulating hormone (FSH), luteinizing hormone (LH), thyroid-stimulating hormone, anti-Mullerian hormone (AMH), and prolactin were obtained from clinical records. HOMA-IR was calculated as fasting insulin (microU/L) × fasting glucose (nmol/L)/22.5.
Assessment of redox parameters in follicular fluid
Commercially available kits were used for analyzing catalase activity and TAC (Cayman chemicals, USA) and SOD activity (Sigma Aldrich, USA). Total thiols were estimated by Ellman’s test [10]. Paraoxonase1 (PON1) arylesterase activity was analyzed using phenyl acetate substrate [11]. Glutathione reductase (GR) activity was estimated as previously described [12]. Total, reduced (GSH), and oxidized glutathione (GSSG) were measured by modified Tietz re-cycling assay of GSH, DTNB, and GR [13]. Glutathione peroxidase (GPX) activity was measured by rate of GSH oxidation by using cumene hydroperoxide as substrate [14]. Lipid peroxidation was measured by thiobarbituric acid reactive substances (TBARS) assay [15]. Advanced oxidized protein products (AOPP) were estimated by measuring oxidizing capacity of iodide [16]. Inter- and intra-assay CVs for all biochemical, hormonal, and redox parameters are enlisted in supplemental table 2.
Quantitative real-time PCR
Total RNA was extracted from GCs by using nucleo-spin triprep kit (Macherey-Nagel, Düren, Germany). cDNA was synthesized from 300 ng of RNA using first-strand cDNA synthesis kit (Takara Bio USA Inc.). Transcript levels of genes encoding antioxidant enzymes were assayed by SYBR green chemistry by using Dynamo color flash master mix (Thermo fisher scientific, MA, USA) and appropriate primers (Supplemental Table 1). 18s rRNA was used as housekeeping control. Fold change in gene expression levels in PCOS (n = 12) and controls (n = 11) was calculated by using 2-ΔΔCt method. Gene expression was normalized to 18s levels.
Statistical analysis
Mann-Whitney U test was used to compare univariate parameters, hormonal and baseline characteristics, FF redox parameters, and gene expression between control and PCOS group. Spearman correlation analysis was performed between redox parameters, gene expression, and oocyte/embryo quality parameters by using SPSS software (version 25.0). P < 0.05 was considered significant.
Results
Clinical characteristics of study participants
Baseline hormonal levels during early follicular phase, prior to COH, hormonal levels measured in FF and serum on d-OPU during IVF cycle, and oocyte quality parameters of PCOS women and controls are summarized in Table 1. In serum of d-OPU, we found SHBG levels significantly lower, while total, free, and bioavailable testosterone levels and free androgen index were significantly higher in PCOS women compared to controls. E2, P4 levels showed no significant differences between them. Fasting glucose and HOMA-IR were significantly increased in PCOS women compared to controls; however, fasting insulin showed increased trend in PCOS.
Table 1.
Demographic and clinical characteristics of the study participants
| Participant characteristics | PCOS (n = 50) Median (IQR) |
Control (n = 71) Median (IQR) |
P |
|---|---|---|---|
| Age, years | 31.0 (27.0–33.0) | 28.0 (26.5–31.0) | 0.098 |
| BMI (kg/m2) | 25.66 (22.68–29.87) | 24.7 (22.5–26.5) | 0.084 |
| Basal LH levels (μU/mL) | 7.68 (7.11–9.91) | 3.12 (1.61–6.90) | 0.040 |
| Basal FSH levels (μU/mL) | 5.25 (4.29–6.22) | 6.71 (3.54–8.03) | 0.730 |
| LH:FSH | 1.66 (1.04–1.97) | 0.57 (0.40–0.68) | 0.040 |
| Prolactin (ng/mL) | 12.30 (9.39–17.44) | 14.20 (6.97–17.63) | 0.461 |
| TSH (mIU/mL) | 2.05 (1.52–3.22) | 1.87 (1.72–2.92) | 0.244 |
| AMH (ng/mL) | 6.86 (3.58–9.74) | 4.76 (2.57–6.01) | 0.027 |
| hMG administered (IU) | 300 (225.0–300.0) | 300 (300.0–300.0) | 0.242 |
| Number of days of stimulation | 10.50 (9.00–12.25) | 10.50 (9.00–12.00) | 0.99 |
| Total follicles (n) | 19.00 (11.0–29.0) | 16.00 (12.0–24.25) | 0.648 |
| Total oocytes (n) | 15.00 (9.0–22.0) | 14.5 (11.0–23.0) | 0.635 |
| MII oocyte (n) | 11.0 (7.0–19.0) | 13.5 (8.0–17.75) | 0.857 |
| MII oocyte (%) | 83.33 (65.48–94.20) | 88.24 (75.18–100.0) | 0.104 |
| Fertilized MII oocyte (n) | 8.5 (6.0–14.0) | 10.0 (7.0–15.0) | 0.186 |
| ROF (%) | 78.89 (69.06–85.71) | 83.33 (76.39–95.58) | 0.039 |
| Grade I embryos (%) | 91.5 (50.0–100.0) | 100.0 (100.0–100.0) | 0.013 |
| aPregnancy rate per embryo transfer [% (proportion)] | 26.76 (19/71) | 42.85 (45/105) | 0.102 |
| $E2 (ng/mL) serum | 1.417 (0.880–2.561) | 1.09 (0.139–2.168) | 0.063 |
| $E2 (ng/mL) FF | 813.3 (336.4–1085) | 800.0 (505.5–1046) | 0.734 |
| $P4 (ng/mL) serum | 0.70 (0.292–3.25) | 1.35 (0.35–2.00) | 0.799 |
| $P4 (μg/mL) FF | 11.06 (5.83–14.42) | 17.06 (12.66–20.08) | 0.0005 |
| $TT (ng/dL) serum | 168.0 (123.5–226.9) | 123.3 (90.00–196.7) | 0.009 |
| $TT (ng/dL) FF | 457.8 (348.0–706.2) | 395.0 (304.1–497.4) | 0.014 |
| $SHBG (nmol/L) serum | 91.74 (54.33–152.1) | 177.8 (102.3–274.1) | 0.0003 |
| $SHBG (nmol/L) FF | 90.0 (80.33–144.8) | 150.0 (117.9–174.9) | 0.004 |
| $Free T (pmol/L) serum | 42.33 (31.47–78.08) | 27.52 (14.82–42.68) | 0.003 |
| $Free T (pmol/L) FF | 172.1 (117.6–300.0) | 92.13 (45.72–121.9) | < 0.0001 |
| $Bio T (nmol/L) serum | 0.992 (0.719–1.832) | 0.645 (0.348–0.999) | 0.003 |
| $Bio-T (nmol/L) FF | 4.03 (2.757–7.044) | 2.16 (1.07–2.86) | < 0.0001 |
| $FAI serum | 5.18 (3.43–11.29) | 2.96 (1.65–5.24) | 0.006 |
| $FAI FF | 22.56 (12.62–35.10) | 9.75 (4.96–13.19) | < 0.0001 |
| $Fasting glucose (mg/dL) serum | 91.00 (84.5–97. 5) | 87.00 (81.25–94.75) | 0.048 |
| $Fasting insulin (mIU/L) serum | 10.73 (7.940–13.98) | 8.85 (6.26–12.26) | 0.059 |
| HOMA-IR | 2.47 (1.54–3.43) | 1.55 (1.11–2.15) | 0.008 |
| $Fasting insulin (mIU/L) FF | 7.34 (4.6–10.63) | 8.64 (5.0–12.30) | 0.432 |
Data represented as median (interquartile-range) for clinical characteristics compared between women with PCOS (n = 50) and controls (n = 71). Parameters marked with “$” were measured in serum and follicular fluid obtained on the day of ovum pick up (d-OPU). Statistical comparison was performed using the Mann-Whitney U test. P < 0.05 considered significant. a: data available for 58 control and 35 PCOS women
IVF in vitro fertilization, BMI body mass index, LH luteinizing hormone, FSH follicle stimulating hormone, TSH thyroid stimulating hormone, hMG human menopausal gonadotrophin, ROF rate of fertilization, FF follicular fluid, E2 estradiol, P4 progesterone, TT total testosterone, SHBG sex hormone binding globulin, Free T free testosterone, Bio-T bioavailable testosterone, FAI free androgen index, HOMA-IR homeostasis model assessment for insulin resistance
In FF, P4 and SHBG levels were significantly lower, while total, free, and bioavailable testosterone levels and free androgen index were significantly higher in PCOS women compared to controls on the d-OPU, while E2 and fasting insulin levels were comparable between them.
Among IVF parameters, rate of fertilization and percent grade I embryos were significantly lower in PCOS than controls, and other parameters were comparable between both groups.
Redox parameters measured in follicular fluid
We evaluated the levels/activities of several pro/antioxidant molecules in FF of PCOS women in relation to controls (Fig. 1). SOD and PON1 arylesterase activities were significantly lower, whereas catalase activity showed decreased trend in FF of PCOS women. Glutathione status was found to be compromised in PCOS as evident from significantly lower amount of GSH, higher amount of GSSG, and lower values of GSH/GSSG ratio in them, though total glutathione was comparable. Furthermore, both GPX and GR showed lower activity in FF of PCOS women. Total thiol levels and TAC were significantly lower, while oxidative protein modification and lipid peroxidation were significantly higher in PCOS women. Insulin resistance was present in 16.9% of controls and 70% women with PCOS. Within control and PCOS group, IR and NIR women did not show significant difference in BMI. Redox parameters showed similar trends between controls and PCOS in total study population as well as in IR and NIR groups (Table 2). In PCOS group, we found increased TBARS levels and lower GR and GPX activity in IR women compared to NIR women. When classified on the basis of BMI, we found 36% of controls and 64% PCOS women were obese. When redox parameters were compared between control and PCOS women in obese (26 controls and 32 PCOS) and lean (45 controls and 18 PCOS) sub-groups, we observed that 9 out of 13 studied parameters showed similar trends between control and PCOS (Table 3) in both the sub-groups. Also, these trends were pretty much in line with total control vs total PCOS comparison. Within control group, lean and obese women showed no difference in redox parameters. However, in PCOS women, some of the markers were significantly different between lean and obese, viz. TBARS, total glutathione, reduced glutathione, GR activity, and GPX activities. AOPP values too were on higher side in obese PCOS women as compared to lean ones. The redox parameters were then compared between different PCOS phenotype sub-groups (phenotype A: n = 11/50; phenotype B: n = 9/50; phenotype C: n = 7/50; and phenotype D: n = 23/50), and between each of these phenotype sub-groups and controls and showed no significant difference except few (data not shown).
Fig. 1.
Comparative analysis redox parameters in follicular fluid. Activity/levels of pro/antioxidant molecules in follicular fluid of controls (n = 71) and women with PCOS (n = 50) are shown in the figure. Data are analyzed using the Mann-Whitney U test and represented as mean ± SEM, *P < 0.05
Table 2.
Comparison of redox parameters in PCOS and control women after subgrouping as insulin resistant (IR) and non-insulin resistant (NIR)
| IR | NIR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Redox parameters | PCOS (n = 35) | Controls (n = 12) | Pa | PCOS (n = 15) | Controls (n = 59) | Pb | Pc | Pd | Pe |
| Catalase activity (U/mL) | 4.44 (0.99, 6.62) | 6.53 (3.02, 9.45) | 0.070 | 3.94 (2.07, 6.72) | 4.49 (3.02, 8.85) | 0.50 | 0.219 | 0.470 | 0.465 |
| TBARS (MDA μM) | 7.72 (4.12, 12.79) | 4.58 (2.55, 7.05) | 0.044 | 4.91 (1.41, 9.76) | 2.88 (1.55, 4.27) | 0.048 | 0.009 | 0.460 | 0.041 |
| GR activity (U/mL) | 0.006 (0.005, 0.008) | 0.008 (0.005, 0.012) | 0.046 | 0.004 (0.003, 0.006) | 0.008 (0.006, 0.01) | 0.001 | 0.002 | 0.804 | 0.028 |
| PON1 arylesterase activity (KU/L) | 23.90 (17.19, 34.39) | 30.95 (27.68, 51.84) | 0.038 | 20.98 (9.46, 41.37) | 32.84 (19.34, 46.89) | 0.046 | 0.028 | 0.830 | 0.715 |
| SOD activity (% inhibition) | 62.41 (54.64, 68.53) | 69.66 (60.82, 72.5) | 0.049 | 49.59 (44.56, 72.08) | 66.73 (61.26, 72.97) | 0.025 | 0.041 | 0.824 | 0.989 |
| GPX activity (U/ml) | 60.32 (32.17, 88.47) | 82.44 (46.58, 141.1) | 0.049 | 80.43 (69.03, 95.84) | 91.62 (79.87, 118.0) | 0.055 | 0.045 | 0.174 | 0.022 |
| Total thiols (μM) | 168.10 (100.00, 198.60) | 198.60 (130.40, 239.10) | 0.045 | 192.8 (123.2, 218.1) | 208.7 (175.4, 253.6) | 0.049 | 0.010 | 0.715 | 0.166 |
| TAC (mM of trolox eqval) | 0.62 (0.44, 0.85) | 0.97 (0.58, 1.41) | 0.031 | 0.69 (0.43, 1.00) | 0.92 (0.61, 1.32) | 0.046 | 0.042 | 0.558 | 0.162 |
| Total glutathione | 12.97 (10.43, 16.61) | 14.63 (10.78, 19.67) | 0.450 | 15.38 (9.62, 20.14) | 15.68 (12.27, 19.47) | 0.490 | 0.755 | 0.416 | 0.990 |
| GSSG (μM) | 5.93 (3.00, 7.13) | 3.99 (2.53, 4.80) | 0.040 | 3.57 (2.24, 5.84) | 2.07 (0.86, 4.04) | 0.027 | 0.035 | 0.855 | 0.08 |
| GSH (μM) | 7.63 (4.07, 9.50) | 10.02 (8.29, 11.88) | 0.048 | 8.35 (4.66, 9.64) | 9.40 (7.82, 11.32) | 0.029 | 0.033 | 0.978 | 0.085 |
| GSH/GSSG | 2.07 (1.13, 2.96) | 4.95 (1.61, 9.92) | 0.023 | 2.39 (1.65, 3.46) | 3.79 (2.24, 7.7) | 0.040 | 0.008 | 0.805 | 0.198 |
| AOPP (μM) | 29.22 (25.93, 31.72 | 27.84 (18.27, 29.02) | 0.047 | 28.41 (26.23, 30.30) | 24.72 (20.41, 28.06) | 0.044 | 0.007 | 0.337 | 0.585 |
Data represented as median (interquartile range) for redox parameters measured in follicular fluid, compared between different subgroups. Statistical comparison was performed using Mann-Whitney U test. P < 0.05 considered significant. TBARS thiobarbituric acid reactive substances, GR glutathione reductase, SOD superoxide dismutase, GPX glutathione peroxidase, TAC total antioxidant capacity, GSSG oxidized glutathione, GSH reduced glutathione, AOPP advanced oxidized protein products, IR insulin resistant, NIR non-insulin resistant
P values obtained for different comparisons:
Pa = IR PCOS (n = 35) vs. IR controls (n = 12)
Pb = NIR PCOS (n = 15) vs. NIR controls (n = 59)
Pc = All PCOS (n = 50) vs. All controls (n = 71)
Pd = IR controls (n = 12) vs. NIR controls (n = 59)
Pe = IR PCOS (n = 35) vs. NIR PCOS (n = 15)
Table 3.
Comparison of redox parameters in PCOS and control women after subgrouping as lean and obese
| Lean | Obese | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Redox parameters | PCOS (n = 18) | Controls (n = 45) | Pf | PCOS (n = 31) | Controls (n = 26) | Pg | Pc | Ph | Pi |
| Catalase activity (U/ml) | 4.207 (2.255–7.568) | 4.662 (0.785–8.511) | 0.893 | 4.370 (1.32–6.70) | 1.321 (1.32–9.60) | 0.624 | 0.219 | 0.885 | 0.772 |
| TBARS (MDA μM) | 4.862 (1.429–9.864) | 3.879 (2.270–7.704) | 0.323 | 7.83 (4.63–12.84) | 3.97 (2.00–5.54) | 0.002 | 0.009 | 0.530 | 0.045 |
| GR activity (U/mL) | 0.004 (0.003–0.006) | 0.008 (0.006–0.011) | 0.0002 | 0.006 (0.005–0.009) | 0.009 (0.007–0.011) | 0.036 | 0.002 | 0.870 | 0.004 |
| PON1 arylesterase activity (KU/L) | 22.01 (9.28–39.37) | 32.67 (19.99–46.69) | 0.025 | 22.61 (15.77–33.61) | 32.32 (19.26–47.01) | 0.036 | 0.028 | 0.565 | 0.558 |
| SOD activity (% inhibition) | 61.18 (50.11–71.59) | 68.34 (62.36–73.41) | 0.037 | 62.22 (51.03–69.28) | 68.05 (61.12–72.55) | 0.042 | 0.041 | 0.849 | 0.476 |
| GPX activity (U/mL) | 84.45 (50.94–94.67) | 88.14 (62.00–118.0) | 0.221 | 44.24 (29.16–77.41) | 72.39 (40.21–103.2) | 0.044 | 0.045 | 0.266 | 0.0004 |
| Total thiols (μM) | 185.5 (134.1–223.9) | 215.9 (176.1–256.5) | 0.042 | 185.5 (106.5–210.1) | 198.6 (162.3–238.0) | 0.048 | 0.010 | 0.955 | 0.569 |
| TAC (mM of trolox eqval) | 0.6411 (0.435–0.946) | 0.92 (0.526–1.385) | 0.049 | 0.559 (0.458–0.936) | 0.776 (0.383–1.232) | 0.464 | 0.042 | 0.245 | 0.281 |
| Total glutathione | 18.59 (11.46–21.16) | 16.50 (14.33–21.16) | 0.877 | 12.97 (9.911–16.75) | 14.02 (10.23–16.40) | 0.961 | 0.755 | 0.529 | 0.023 |
| GSSG (μM) | 4.293 (3.385– 5.520) | 2.550 (1.782–4.607) | 0.043 | 3.649 (2.290–4.796) | 1.924 (1.045–3.765) | 0.028 | 0.035 | 0.313 | 0.710 |
| GSH (μM) | 9.065 (3.993–11.72) | 9.741 (8.497–11.78) | 0.371 | 6.785 (4.448–9.496) | 9.151 (7.238–11.53) | 0.040 | 0.033 | 0.845 | 0.009 |
| GSH/GSSG | 2.644 (1.667–3.614) | 4.096 (2.326–8.153) | 0.036 | 1.792 (1.203–2.946) | 4.772 (2.205–8.693) | 0.004 | 0.008 | 0.220 | 0.131 |
| AOPP (μM) | 27.29 (25.79–29.50) | 24.32 (20.13–28.16) | 0.040 | 29.26 (25.28–31.55) | 26.91 (20.05–29.30) | 0.038 | 0.007 | 0.264 | 0.057 |
Data represented as median (interquartile range) for redox parameters measured in follicular fluid, compared between different subgroups. Statistical comparison was performed using Mann-Whitney U test. P < 0.05 considered significant. TBARS Thiobarbituric acid reactive substances, GR glutathione reductase, SOD superoxide dismutase, GPX glutathione peroxidase, TAC total antioxidant capacity, GSSG oxidized glutathione, GSH reduced glutathione, AOPP advanced oxidized protein products
P values obtained for different comparisons:
Pf = lean PCOS (n = 18) vs. lean controls (n = 45)
Pg = obese PCOS (n = 32) vs. obese controls (n = 26)
Pc = all PCOS (n = 50) vs. all controls (n = 71)
Ph = lean controls (n = 45) vs. obese controls (n = 26)
Pi = lean PCOS (n = 18) vs. obese PCOS (n = 32)
Expression of antioxidant genes in granulosa cells
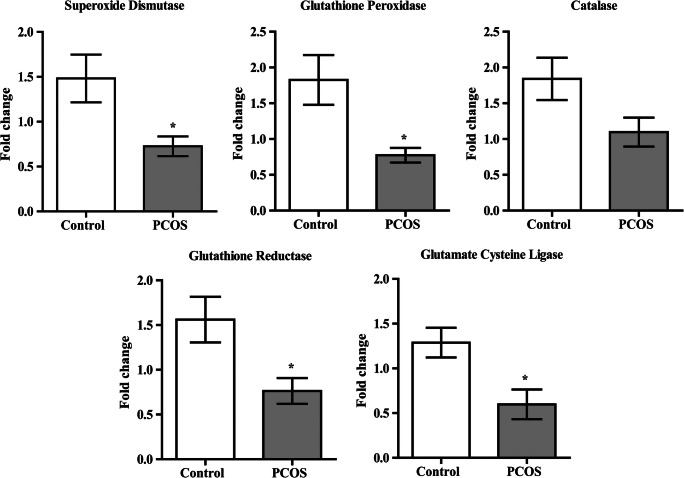
Transcript levels of SOD, GPX, GR, and glutamate cysteine ligase (GCL) in GCs (Fig. 2) were significantly lower in PCOS women compared to controls, and catalase transcript showed lower trends. It is interesting to note that all mRNA expression trends were accordant with their respective activity in FF.
Fig. 2.
Relative expression of antioxidant enzymes in granulosa cells. Transcript expression of antioxidant enzymes in granulosa cells was studied by real time PCR. Bar graphs representing transcript expression profiles of antioxidant genes between controls (n = 11) and women with PCOS (n = 12) are shown in the figure. Analysis was carried out by Mann-Whitney U test and represented as mean ± SEM. *P < 0.05
All parameters showing significant differences between controls and PCOS women were adequately powered at sample size used (calculated from https://www.sphanalytics.com/statistical-power-calculator-using-average-values/, last accessed 09/09/2020).
Relationship between oxidative stress parameters and oocyte quality
To investigate the influence of redox status of FF on oocyte/embryo quality parameters, we first performed correlation analysis in all of our total study participants (controls + PCOS) (Table 4). We found positive correlation between glutathione reductase activity and rate of fertilization and PON1 arylesterase activity with number of follicles retrieved, MII oocytes, and number of fertilized oocytes. When we looked at control and PCOS groups separately, we found positive correlations of PON1 activity with pregnancy outcome, and total glutathione with percent grade I embryos and pregnancy outcome in control group. In PCOS group, PON1 activity showed positive correlation with number of fertilized oocytes and percent grade I embryos. Further, we investigated any influence of insulin resistance on relationship of redox parameters with oocyte quality parameters. In NIR individuals, PON1 arylesterase activity positively correlated with number of follicles retrieved. In IR individuals, glutathione reductase activity positively correlated with rate of fertilization. Also, PON1 arylesterase activity showed positive correlation, and AOPP showed negative correlation with number of oocytes retrieved and oocytes fertilized. Additionally, AOPP showed negative correlation with number of MII oocytes. In lean individuals, catalase activity positively correlated with rate of fertilization, whereas GR activity positively correlated with percentage of grade I embryos and pregnancy outcome. In obese individuals, PON1 showed positive relationship with number of follicles retrieved, oocytes retrieved, and fertilized and MII oocytes. AOPP negatively correlated with number of MII, fertilized oocytes, and percentage of grade I embryos. However, transcript levels of antioxidant enzymes in GCs did not show any significant correlation with oocyte/embryo quality parameters.
Table 4.
Correlation analysis between follicular fluid redox markers and oocyte/embryo quality parameters
| FF redox marker | Oocyte/embryo quality parameter | Coefficient of correlation | P |
|---|---|---|---|
| All study participants (n = 121) | |||
| Glutathione reductase activity | Rate of fertilization | 0.31 | 0.008 |
| PON1 arylesterase activity | Number of follicles retrieved | 0.267 | 0.025 |
| PON1 arylesterase activity | Number of MII oocytes | 0.218 | 0.043 |
| PON1 arylesterase activity | Number of oocytes fertilized | 0.289 | 0.014 |
| PCOS (n = 50) | |||
| PON1 arylesterase activity | Percent grade I embryos | 0.399 | 0.048 |
| PON1 arylesterase activity | Number of oocytes fertilized | 0.355 | 0.043 |
| Controls (n = 71) | |||
| Catalase activity | Number of fertilized oocytes | 0.343 | 0.035 |
| TBARS | Number of fertilized oocytes | 0.495 | 0.002 |
| TBARS | Percent grade I embryos | − 0.531 | 0.034 |
| PON1 arylesterase activity | Pregnancy outcome | 0.513 | 0.035 |
| Total thiols | Number of follicles retrieved | 0.36 | 0.031 |
| Total glutathione | Percent grade I embryos | 0.661 | 0.027 |
| Total glutathione | Pregnancy outcome | 0.592 | 0.033 |
| Non-IR (PCOS n = 15, controls n = 59) | |||
| PON1 arylesterase activity | Number of follicles retrieved | 0.3 | 0.040 |
| IR (PCOS n = 35, controls n = 12) | |||
| Glutathione reductase activity | Rate of fertilization | 0.319 | 0.045 |
| PON1 arylesterase activity | Number of oocytes retrieved | 0.302 | 0.041 |
| PON1 arylesterase activity | Number of oocytes fertilized | 0.377 | 0.015 |
| AOPP | Number of oocytes retrieved | − 0.39 | 0.011 |
| AOPP | Number of MII oocytes | − 0.363 | 0.018 |
| AOPP | Number of oocytes fertilized | − 0.339 | 0.037 |
| Lean (PCOS n = 18, controls n = 45) | |||
| Catalase activity | Rate of fertilization | 0.305 | 0.039 |
| Glutathione reductase activity | Percent grade I embryos | 0.467 | 0.025 |
| Glutathione reductase activity | Pregnancy outcome | 0.406 | 0.029 |
| Obese (PCOS n = 32, controls n = 26) | |||
| PON1 arylesterase activity | Number of follicles retrieved | 0.432 | 0.002 |
| PON1 arylesterase activity | Number of oocytes retrieved | 0.429 | 0.001 |
| PON1 arylesterase activity | Number of MII oocytes | 0.378 | 0.005 |
| PON1 arylesterase activity | Number of oocytes fertilized | 0.505 | < 0.001 |
| AOPP | Number of MII oocytes | − 0.317 | 0.03 |
| AOPP | Number of oocytes fertilized | − 0.328 | 0.039 |
| AOPP | Percent grade I embryos | − 0.477 | 0.012 |
List of significant correlations obtained between follicular fluid redox markers and oocyte/embryo quality parameters (spearman’s correlation analysis). TBARS thiobarbituric acid reactive substances, AOPP advanced oxidized protein products, IR insulin resistant, NIR non-insulin resistant. P < 0.05 considered significant.
Discussion
Currently, morphological features of oocyte and embryo are mostly used for their selection in ARTs. Redox dynamics in oocyte microenvironment can be one of the crucial factors influencing oocyte/embryo quality, especially in PCOS considering its cryptic pathophysiology and link with OS. On the quest of delving into non-invasive approaches, redox markers have been investigated by several groups. Our study showed that redox homeostasis in follicular microenvironment of women with PCOS is compromised, and few markers are correlated with oocyte/embryo quality parameters. In addition, our data indicates that GCs may have fair role in maintaining redox balance in follicle, as transcript expression of antioxidant enzymes in GCs was in line with their activity in FF.
We found decreased activity of SOD, involved in dismutation of superoxide anion to hydrogen peroxide in FF of PCOS women on similar lines with other reports [17–19]. Complementing this, we found that transcript expression of SOD was low in GCs which is in line with a previous study [20]. In contrast, another study reported higher SOD activity in PCOS than controls [21]. Hydrogen peroxide produced after SOD activity is neutralized by catalase and GPX. We observed decreased trend in catalase activity in FF of PCOS women in line with an Iranian study [18]. A German study found lower FF catalase activity in obese women irrespective of PCOS status [22]. Further, catalase mRNA expression in GCs also tended to be on lower side in women with PCOS. Glutathione is ubiquitous non-protein thiol involved in antioxidant defense, detoxification, maintenance of thiol status, cell proliferation, differentiation, apoptosis, etc. Both in vitro and in vivo studies have demonstrated that GSH content in oocytes and GCs can be modulated by gonadotropins. Moreover, Ggt1 and Nrf2 (involved in GSH synthesis and regulation) knockout mice have shown deleterious effect on fertility [23]. We observed a lower trend in total glutathione levels in women with PCOS, whereas Japanese study reported significantly lower total glutathione levels in FF from women with PCOS [24]. GPX and GR in concert with glutathione form a very crucial antioxidant system. GPX quenches hydrogen peroxide to water and molecular oxygen by utilizing GSH. Then, GSSG formed is recycled back to its active form (GSH) by GR. We observed significantly higher levels of GSSG, along with lower levels of GSH in PCOS than controls. The lower GPX activity we found in FF of PCOS women corroborates with an earlier report [18]. The lower expression of GPX transcript in GCs from PCOS women is in similar lines with other studies; additionally, it has been suggested to be a potential marker for embryo quality in PCOS [18, 25]. We observed reduced GR activity in FF as well as lower GR transcript expression in GCs of PCOS women; however, a study involving German women reported higher GR activity in FF in obese women as compared to lean ones irrespective PCOS status [22]. Another notable observation was significantly low transcript expression of GCL (the rate limiting enzyme in glutathione synthesis), in women with PCOS compared to controls which may contribute to lower level of reduced glutathione in women with PCOS. These results indicate that components of glutathione system are majorly compromised in follicular niche of PCOS women and may have consequences on oocyte/embryo quality. Further, transcript levels of antioxidant enzymes being in accordance with their respective activities in FF imply that possibly GCs are also involved in maintaining redox homeostasis in the oocyte microenvironment. Apart from glutathione, large pool of sulfhydryl group containing organic compounds is instrumental in tackling oxidative insults and maneuvering thiol reactive signaling. We found significantly lower levels of thiols in PCOS women compared to controls which matched with an Iranian study [26], but was in contrast to a Turkish study which observed unchanged levels between control and PCOS group [27]. TAC which represents sum of all exogenous and endogenous antioxidants was significantly lower in FF from PCOS women in our study which corroborates with other studies [18, 21, 24, 26, 28]. In contrast, others did not find any difference in TAC levels [29, 30].
We also measured activity of PON1 in FF, a multifunctional antioxidant enzyme which protects the cells from OS by preventing lipid peroxidation and protein homocysteinylation [31]. A previous study had reported lower PON1 activity in serum of women with PCOS [11]. PON1 is associated with HDL which is the sole lipoprotein present in FF; hence, PON1 has vital contribution in the antioxidative property of HDL in ovarian follicle [32]. An Italian study reported lower PON1 activity along with lower TAC, CoQ10, and higher HDL oxidation in FF in obese as compared to non-obese women. Further, PON1 activity negatively correlated with lipid peroxidation and positively correlated with number of oocytes retrieved and number of good quality oocytes [33]. A Spanish group found that PON1 activity was lower in small compared to large sized follicles in sub-fertile and fertile women [34]. To the best of our knowledge, we report for the first time significantly reduced PON1 activity in FF of women with PCOS compared to controls.
All this data on PON1 activity in FF supports the notion that it may be an important regulator in follicular niche by directly neutralizing pro-oxidants and by protecting HDL via catabolism of oxidized cholesteryl esters and phospholipids.
Among pro-oxidant markers which are indicative of oxidative damage to macromolecules, we studied lipid peroxidation and protein oxidation. TBARS levels indicate that the extent of oxidative insults to cell membrane and intracellular lipids, was increased in FF of women with PCOS in our study similar to other reports [21, 26]. AOPP is a marker for oxidative protein modification and is involved in formation of structures like di-tyrosine many of which are irreversible tempering of protein residues. To the best of our knowledge, we demonstrate significantly higher level of AOPP for the first time in FF of PCOS women.
Women with PCOS are often insulin resistant which may influence their redox status. However, no reports are available on redox markers in FF of PCOS women according to insulin resistance status. Towards this, we grouped our study population according to insulin resistance and found that most of the studied redox parameters were not influenced by insulin resistance, indicating that the higher OS observed in PCOS women is due to PCOS status irrespective of insulin resistance. It should be noted that there was no significance difference in BMI between control and PCOS women and insulin resistance was also moderate in PCOS group. Few groups studied redox markers (TBARS, catalase, SOD, GPX, and glutathione) in serum of control and PCOS women, according to insulin resistance status [35–37]. Majority of their results accorded with our observations; however, some of their trends differed in PCOS group between IR and NIR women. Higher TBARS levels in insulin-resistant women in our study can be attributed to increased free fatty acid levels and their accumulation in the vicinity of mitochondria, a prominent site for ROS generation leading to higher lipid peroxidation [38]. The lower GPX and GR activity observed in IR group than NIR PCOS group may be due to lower levels of reduced glutathione and competition by other metabolic enzymes for NADPH, respectively, which are known to be associated with insulin resistance [39]. Obesity is usually seen in many of the women with PCOS, and it may be related to other features in PCOS, namely, metabolic dysfunction, cardiovascular risk, oxidative stress, and ovulatory dysfunction [40, 41]. Higher activity of SOD and catalase was reported in FF of obese women than in lean women in both PCOS and control groups, which is in contrast to our results. Higher GPX activity was also reported in FF of obese control women than that of lean controls; however, it was similar in PCOS group between lean and obese women [22]. In an Iraian study, Nasiri and his group reported that TAC was lower in obese women than lean women in PCOS group which differed from our results, but in control group, no difference was found similar to our findings. Lipid peroxidation levels were unchanged in lean and obese women in PCOS group, whereas they were higher in obese women than lean women in control group which partly matches our results [40]. Similar to our observations, Bacchetti et al. reported lower TAC and higher lipid peroxidation levels in FF of obese women than in lean women [33]. The increased lipolysis in obesity leads to accumulation of fats which in turn can activate pro-inflammatory cytokines and macrophage infiltration that may result in higher lipid peroxidation [42]. Lower activities of GPX and GR enzymes in obese PCOS women as compared to lean PCOS women may be due to lower levels of total glutathione in obese PCOS women as observed by us. We have compiled the redox parameters studied in FF by our group and other investigators in Table 5. The variations in results between studies may be due to several reasons, like sample size, diagnostic criteria, selection of controls, and ethnicity.
Table 5.
The changes in pro-/antioxidant markers measured follicular fluid samples in women with PCOS in comparison with control subjects
| Pro-/antioxidant marker | Trends reported by us in women with PCOS as compared to controls | Trends reported in other studies in women with PCOS as compared to controls |
|---|---|---|
| Superoxide dismutase | Activity in FF ↓ | Seleem et al 2014 ↓, Masjedi et al, 2019 ↓, Sabatini et al 2000 ↓, Pekel et al 2015 ↑, Bausenwein et al 2009 ↑ (in obese women than in lean women in both control and PCOS groups) |
| Transcript in GCs ↓ | Avila et al 2016 ↓ | |
| Catalase | Activity in FF (unchanged) | Masjedi et al 2019 ↓, Bausenwein et al 2009 ↑ (in obese women than in lean women in both control and PCOS groups) |
| Transcript in GCs (unchanged) | - | |
| Total glutathione | FF levels (unchanged) | Nishihara et al 2018 ↓ |
| GSSG | FF levels ↑ | - |
| GSH | FF levels ↓ | |
| Transcript in GCs ↓ (GCL, rate limiting enzyme, catalyses first step) | ||
| GSH/GSSG | FF levels ↓ | |
| Glutathione Peroxidase | Activity in FF ↓ | Masjedi et al 2019 ↓, Bausenwein et al ↑ (in obese women than in lean women in control group, unchaged in PCOS group) |
| Transcript in GCs ↓ | Masjedi et al 2019 ↓, Huang et al 2014 ↓ | |
| Glutathione reductase | Activity in FF ↓ | Bausenwein et al ↑ (in obese women than in lean women in PCOS group) |
| Total thiols | FF levels ↓ | Artimani et al 2018 ↓, Tola et al 2018 (unchanged) |
| Total antioxidant capacity | FF levels ↓ |
Becatti et al 2018 ↓, Masjedi et al 2019 ↓, Pekel et al 2015 ↓, Nishihara et al 2018 ↓, Artimani et al 2018 ↓, Nasiri et al 2015 ↓, Yilmaz et al 2016 (unchanged), Appasamy et al 2008 (unchanged) |
| PON1 arylesterase | Activity in FF ↓ |
Bacchetti et al 2019 ↓ (in obese women than in lean women), Meijide et al 2017 ↓ (in smaller follicles than in larger ones), Dadachanji et al 2018 (serum activity) ↓ |
| TBARS | FF levels ↑ |
Pekel et al 2015 ↑, Artimani et al 2018 ↑, Nasiri et al 2015 ↑ |
↑ higher, ↓ lower, FF follicular fluid, GSSG oxidized glutathione, GSH reduced glutathione, PON1 paraoxonase1, TBARS thiobarbituric acid reactive substances, AOPP advanced oxidized protein products
Studying relationship between FF redox markers and oocyte/embryo quality parameters may provide insights about potential factors which may have greater influence on oocyte quality. Interestingly, in our study, PON1 activity consistently showed positive correlation with various oocyte/embryo quality parameters in all of our sub-groups Additionally, components of glutathione system too were found to be associated with IVF parameters in all study participants. Further, in IR individuals, AOPP negatively correlated with number of oocytes retrieved, MII oocytes, and fertilized oocytes which is a noteworthy observation, as AOPP is known to be associated with insulin resistance, T2DM, and advanced glycation end products [43]. Also, AOPP circulate in blood for longer periods of time and may further trigger OS by inducing activation of neutrophil and monocyte oxidative metabolism. Higher levels of AOPP can negatively affect the oocyte quality by impeding the appearance of first polar body and cytoplasmic maturation and hence can arrest maturation of oocytes and in turn embryo development [44]. Grossly, our results draw attention to the negative influence of OS and plausible positive impact of PON1 enzyme and the glutathione system on oocyte development. Several other groups have also attempted to delineate relationship between FF redox markers and IVF parameters. Studies in Turkish women showed positive correlations of clinical pregnancy rates with TAC levels of FF in both PCOS and controls [29], and of better fertilization rates with native and total thiols levels in PCOS [28]. We did not find any correlation between TAC and IVF parameters; however, total thiols showed positive correlation with number of follicles retrieved in control group. Also, positive correlation of catalase activity with number of fertilized oocytes in control women was observed. In another study, a good correlation was found between ROS levels and spindle imaging which can predict oocyte quality [45]. On the contrary, Seleem et al. [17] found no correlation between SOD activity in FF and fertilization rate/oocyte quality in controls or PCOS which is similar to our results. Becatti et al. [28] reported negative correlation of percent MII oocytes and fertilization rate with TBARS levels, redox index, but not with TAC levels in both fertile and infertile women. We found that TBARS levels negatively correlated with percent grade I embryos and positively correlated with number of fertilized oocytes in control group. Other studies observed no association between oocyte quality parameters and redox markers [25, 46] in women undergoing IVF. In our study, neither GPX activity nor oxidized or reduced glutathione or their ratio showed correlation with any IVF parameter. As far as insulin resistance aspect is concerned, very few and contrasting reports are available wherein insulin resistance negatively influenced oocyte quality in Iranian women with PCOS, while no difference was reported in IVF parameters in Turkish PCOS women between IR and NIR groups [47, 48].
As a limitation of this study, we acknowledge that embryo quality and pregnancy outcome can be influenced by quality of sperms and other exogenous factors like storage, freezing, handling of embryos, and also embryo culture medium; hence, composition of follicular niche may not be the only contributing element towards these two factors.
In our study, we have tried to cover a broad range of redox markers in follicular fluid and transcript expression in GCs of women with PCOS. Collectively, our results show higher levels of OS and lower levels/activity of antioxidants in PCOS. Further, we demonstrated correlation of few redox markers with oocyte/embryo quality parameters. Our data strongly indicates that glutathione system which forms the cornerstone of redox homeostasis and multifactorial paraoxonase1 enzyme could be explored further as surrogates for IVF prognosis/outcome in PCOS. Additionally, we speculate that GCs may endow considerable contribution in maintaining redox homeostasis in follicular environment. In order to reach a consensus on the impact of FF redox markers on oocyte/embryo quality in PCOS, considerably more work needs to be done to shed light on these relationships. Furthermore, the insulin resistance aspect with regard to oocyte/embryo quality needs further exploration. Considering that functional studies may not be feasible with human oocyte/embryo, this correlation data may serve as an alternative to comprehend how the dynamicity of molecules involved in redox metabolism influence oocyte/embryo quality and which of them merit further inspection. Moreover, treatment directed towards correcting disrupted pro and antioxidant balance may possess potential to improve oocyte/embryo quality in ART settings.
Supplementary Information
Supplementary data to this article can be found online at https://doi.org/.
(DOCX 14 kb)
Acknowledgements
We thank all study participants and our collaborator late Dr. Kusum Zhaveri. Authors extend their gratitude to Dr. Veena Bangera, Ms. Leena Mukadam (P.D. Hinduja National Hospital and Medical Research Centre) who collected clinical samples, and Ms. Pushpa Dharwadkar who provided IVF data records. We thank Department of Science and Technology, India, for providing fellowship to AN. We thank Mrs. Sushma Khavale, Mrs. Gayatri Shinde, and Mrs. Nanda Joshi from NIRRH for technical support. We acknowledge necessary support from NIRRH and Indian Council of Medical Research (NIRRH/RA/934/07–2020), India.
Authors’ contributions
Aalaap Naigaonkar: Acquisition of data, analysis and interpretation of data, drafting the article, or revising it critically for important intellectual content
Roshan Ddachanji: Acquisition of data, drafting the article, or revising it critically for important intellectual content
Indira Hinduja: Provided patient samples
Srabani Mukherjee: The conception and design of the study, drafting the article, or revising it critically for important intellectual content, final approval of the version to be submitted
Funding
This work was supported by Board of Research in Nuclear Sciences (BRNS) (37(1)/14/57/2014-BRNS), Department of Atomic Energy (DAE), Government of India; NIRRH (NIRRH/RA/934/07–2020) and Indian Council of Medical Research (ICMR), New Delhi, India; and Department of Science and Technology (DST), New Delhi, Government of India provided fellowship to AN.
Declarations
Ethics approval
This study was approved by both Ethics committees of ICMR-National Institute for Research in Reproductive Health (ICMR-NIRRH) and P. D. Hinduja National Hospital, Mumbai, India.
Consent to participate
Informed written consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aalaap Naigaonkar, Email: aalaap.na@gmail.com.
Roshan Dadachanji, Email: r_dadachanji@yahoo.co.in.
Indira Hinduja, Email: indirahinduja@gmail.com.
Srabani Mukherjee, Email: srabanimuk@yahoo.com, Email: mukherjees@nirrh.res.in.
References
- 1.Sagvekar P, Dadachanji R, Patil K, Mukherjee S. Pathomechanisms of polycystic ovary syndrome: multidimensional approaches. Front Biosci - Elit. 2018;10:384–422. doi: 10.2741/e829. [DOI] [PubMed] [Google Scholar]
- 2.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. Elsevier Inc.; 2015;103:303–16. Available from: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed]
- 3.Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. Elsevier Ltd; 2017;28:186–98. Available from: 10.1016/j.tem.2016.11.008 [DOI] [PubMed]
- 4.Ambekar AS, Kelkar DS, Pinto SM, Sharma R, Hinduja I, Zaveri K, Pandey A, Prasad TSK, Gowda H, Mukherjee S. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab. 2015;100:744–753. doi: 10.1210/jc.2014-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16:1–18. doi: 10.1186/s12958-017-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108:1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, He G, Chen M, Zuo T, Xu W, Liu X. The role of antioxidant enzymes in the ovaries. Franco R, editor. Oxid Med Cell Longev [Internet]. Hindawi; 2017;2017:4371714. Available from: 10.1155/2017/4371714 [DOI] [PMC free article] [PubMed]
- 8.Zuo T, Zhu M, Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev. 2016;2016. [DOI] [PMC free article] [PubMed]
- 9.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes Care. United States; 2003;26:1380–4. [DOI] [PubMed]
- 10.Bulaj G, Kortemme T, Goldenberg DP. Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry. 1998;37:8965–8972. doi: 10.1021/bi973101r. [DOI] [PubMed] [Google Scholar]
- 11.Dadachanji R, Shaikh N, Patil A, Shah N, Mukherjee S. PON1 promoter polymorphisms contribute to PCOS susceptibility and phenotypic outcomes in Indian women. Gene. Elsevier B.V; 2018;661:34–44. Available from: 10.1016/j.gene.2018.03.083. [DOI] [PubMed]
- 12.Mavis RD, Stellwagen E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J Biol Chem. 1968;243:809–814. doi: 10.1016/S0021-9258(19)81737-4. [DOI] [PubMed] [Google Scholar]
- 13.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 14.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 15.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 16.Taylor EL, Armstrong KR, Perrett D, Hattersley AT, Winyard PG. Optimisation of an advanced oxidation protein products assay: its application to studies of oxidative stress in diabetes mellitus. Oxidative Med Cell Longev. 2015;2015:1–10. doi: 10.1155/2015/496271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seleem AK, El Refaeey AA, Shaalan D, Sherbiny Y, Badawy A. Superoxide dismutase in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection. J Assist Reprod Genet. 2014;31:499–504. doi: 10.1007/s10815-014-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masjedi F, Keshtgar S, Agah F, Karbalaei N. Association between sex steroids and oxidative status with vitamin D levels in follicular fluid of non-obese PCOS and healthy women. J Reprod Infertil. 2019;20:132–142. [PMC free article] [PubMed] [Google Scholar]
- 19.Sabatini L, Al Shawaf T, Wilson C, Lower A, Grudzinskas J. Follicular fluid superoxide dismutase (SOD) activity in women with polycystic ovarian syndrome (PCOS) Fertil Steril. 2000;74:S253–S254. doi: 10.1016/S0015-0282(00)01473-4. [DOI] [Google Scholar]
- 20.Ávila J, González-Fernández R, Rotoli D, Hernández J, Palumbo A. Oxidative stress in granulosa-lutein cells from in vitro fertilization patients. Reprod Sci. 2016;23:1656–1661. doi: 10.1177/1933719116674077. [DOI] [PubMed] [Google Scholar]
- 21.Pekel A, Gönenç A, Turhan NÖ, Kafalı H. Changes of sFas and sFasL, oxidative stress markers in serum and follicular fluid of patients undergoing IVF. J Assist Reprod Genet. 2015;32:233–241. doi: 10.1007/s10815-014-0396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bausenwein J, Serke H, Eberle K, Hirrlinger J, Jogschies P, Hmeidan FA, et al. Elevated levels of oxidized low-density lipoprotein and of catalase activity in follicular fluid of obese women. Mol Hum Reprod. 2009;16:117–124. doi: 10.1093/molehr/gap078. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura BN, Fielder TJ, Hoang YD, Lim J, McConnachie LA, Kavanagh TJ, et al. Lack of maternal glutamate cysteine ligase modifier subunit (Gclm) decreases oocyte glutathione concentrations and disrupts preimplantation development in mice. Endocrinology. 2011;152:2806–2815. doi: 10.1210/en.2011-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishihara T, Matsumoto K, Hosoi Y, Morimoto Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod Med Biol. 2018;17:481–486. doi: 10.1002/rmb2.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang B, Li Z, Ai J, Zhu L, Li Y, Jin L, Zhang H. Antioxidant capacity of follicular fluid from patients undergoing in vitro fertilization. Int J Clin Exp Pathol. 2014;7:2273–2282. [PMC free article] [PubMed] [Google Scholar]
- 26.Artimani T, Karimi J, Mehdizadeh M, Yavangi M, Khanlarzadeh E, Ghorbani M, et al. Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS). Gynecol Endocrinol. Informa UK Ltd.; 2018;34:148–52. Available from: 10.1080/09513590.2017.1371691. [DOI] [PubMed]
- 27.Tola EN, Köroğlu N, Ergin M, Oral HB, Turgut A, Erel Ö. The role of follicular fluid thiol/disulphide homeostasis in polycystic ovary syndrome. Balkan Med J. 2018;35:306–310. doi: 10.4274/balkanmedj.2017.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becatti M, Fucci R, Mannucci A, Barygina V, Mugnaini M, Criscuoli L, et al. A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed]
- 29.Yilmaz N, Inal HA, Gorkem U, Sargin Oruc A, Yilmaz S, Turkkani A. Follicular fluid total antioxidant capacity levels in PCOS. J Obstet Gynaecol (Lahore) 2016;36:654–657. doi: 10.3109/01443615.2016.1148683. [DOI] [PubMed] [Google Scholar]
- 30.Appasamy M, Jauniaux E, Serhal P, Al-Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil Steril. 2008;89:912–921. doi: 10.1016/j.fertnstert.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Manolescu BN, Busu C, Badita D, Stanculescu R, Berteanu M. Paraoxonase 1 - an update of the antioxidant properties of high- density lipoproteins. Maedica (Buchar) 2015;10:173–177. [PMC free article] [PubMed] [Google Scholar]
- 32.Browne RW, Shelly WB, Bloom MS, Ocque AJ, Sandler JR, Huddleston HG, Fujimoto VY. Distributions of high-density lipoprotein particle components in human follicular fluid and sera and their associations with embryo morphology parameters during IVF. Hum Reprod. 2008;23:1884–1894. doi: 10.1093/humrep/den183. [DOI] [PubMed] [Google Scholar]
- 33.Bacchetti T, Morresi C, Vignini A, Tiano L, Orlando P, Montik N, Ciavattini A, Ferretti G. HDL functionality in follicular fluid in normal-weight and obese women undergoing assisted reproductive treatment. J Assist Reprod Genet. 2019;36:1657–1664. doi: 10.1007/s10815-019-01523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meijide S, Pérez-Ruiz I, Hernández ML, Navarro R, Ferrando M, Larreategui Z, et al. Paraoxonase activities in human follicular fluid: role in follicular maturation. Reprod Biomed Online. Elsevier Ltd; 2017;35:351–62. Available from: 10.1016/j.rbmo.2017.06.008. [DOI] [PubMed]
- 35.Turan V, Sezer ED, Zeybek B, Sendag F. Infertility and the presence of insulin resistance are associated with increased oxidative stress in young, non-obese Turkish women with polycystic ovary syndrome. J Pediatr Adolesc Gynecol. Elsevier Ltd; 2015;28:119–23. Available from: 10.1016/j.jpag.2014.05.003. [DOI] [PubMed]
- 36.Kurdoglu Z, Ozkol H, Tuluce Y, Koyuncu I. Oxidative status and its relation with insulin resistance in young non-obese women with polycystic ovary syndrome. J Endocrinol Investig. 2012;35:317–321. doi: 10.3275/7682. [DOI] [PubMed] [Google Scholar]
- 37.Schrauwen P, Hoeks J, Hesselink MK. Lipid-induced cell stress and insulin resistance. Scand J Food Nutr. 2006;50:62–67. doi: 10.1080/17482970601066132. [DOI] [Google Scholar]
- 38.Özer A, Bakacak M, Kiran H, Ercan Ö, Köstü B, Kanat-Pektaş M, et al. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol Pol. 2016;87:733–738. doi: 10.5603/GP.2016.0079. [DOI] [PubMed] [Google Scholar]
- 39.Lutchmansingh FK, Hsu JW, Bennett FI, Badaloo AV, Norma MA, Georgiana MGS, et al. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS One. 2018;13:1–12. doi: 10.1371/journal.pone.0198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Zolfaghari Z, et al. Abdominal obesity can induce both systemic and follicular fluid oxidative stress independent from polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. Elsevier Ireland Ltd; 2015;184:112–6. Available from: 10.1016/j.ejogrb.2014.11.008. [DOI] [PubMed]
- 41.Bannigida DM, Nayak BS, Vijayaraghavan R. Insulin resistance and oxidative marker in women with PCOS. Arch Physiol Biochem. Taylor & Francis; 2020;126:183–6. Available from: 10.1080/13813455.2018.1499120. [DOI] [PubMed]
- 42.Pillon N, Soulage C. Lipid peroxidation by-products and the metabolic syndrome. In: Catala A, editor. Lipid Peroxidation. IntechOpen. 2012. 10.5772/46019.
- 43.Koçak H, Öner-Iyidoǧan Y, Gürdöl F, Öner P, Süzme R, Esin D, et al. Advanced oxidation protein products in obese women: Its relation to insulin resistance and resistin. Clin Exp Med. 2007;7:173–178. doi: 10.1007/s10238-007-0143-x. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, Liu J, Qiu Z, Chen D, Luo C, Liu X, Hua R, Zhu X, Lin Y, Li L, Liu W, Quan S. Advanced oxidation protein products from the follicular microenvironment and their role in infertile women with endometriosis. Exp Ther Med. 2018;15:479–486. doi: 10.3892/etm.2017.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chakravarty B, Chattopadhyay R, Ghosh S, Goswami SK, Rajani S, Sharma S. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J Hum Reprod Sci. 2012;5:187–193. doi: 10.4103/0974-1208.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Hassani F, Oryan S, Eftekhari-Yazdi P, Bazrgar M, Moini A, Nasiri N, Ghaheri A. Association between the number of retrieved mature oocytes and insulin resistance or sensitivity in infertile women with polycystic ovary syndrome. Int J Fertil Steril. 2019;12:310–315. doi: 10.22074/ijfs.2019.5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cakiroglu Y. The impact of body mass index and insulin resistance on IVF outcomes of polycystic ovary syndrome. North Clin Istanbul. 2017;4:218–224. doi: 10.14744/nci.2017.79663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)