Abstract
Purpose
To determine the frequency of and factors associated with a patient being declined from pursuing a cycle of in vitro fertilization with autologous oocytes (IVF-AO).
Methods
A cross-sectional study using a nationwide cohort of female respondents aged 35 or over, who visited a US fertility clinic from 1/2015 to 3/2020, responded to the online FertilityIQ questionnaire (http://www.fertilityiq.com). All respondents were asked if they were previously declined from pursuing a cycle of IVF-AO. Examined demographic and clinical predictors included age, race/ethnicity, education, income, clinic type, care received in a mandated state, insurance coverage for fertility treatment, and self-reported infertility diagnosis. Logistic regression was used to calculate the adjusted odds ratios for factors associated with being declined from pursuing IVF-AO.
Results
Of 8660 women who met inclusion criteria, 418 (4.8%) reported previously being declined a cycle of IVF-AO. In the multivariate analysis, predictors of being declined from pursuing IVF-AO included increasing age, income of less than $50,000, and diagnoses of poor oocyte quality and diminished ovarian reserve. Predictors of being less likely to report decline included some college or college degree and diagnoses of male factor, unexplained or tubal infertility. Notably, diagnosis of PCOS or residence in a state with mandated fertility coverage was not predictive of patients being declined from pursuing IVF-AO.
Conclusion
Nearly 5% of patients who pursued IVF reported being declined from pursuing IVF-AO. Further studies are needed to confirm our findings and explore whether patients being declined treatment meet the criteria for futile or very poor prognosis.
Keywords: In vitro fertilization, Autologous oocytes, Ethics, Decline treatment
Introduction
Over the last several decades, the number of women seeking care with fertility specialists for assistance in building their families has continued to grow. In 2017, there were over 250,000 in vitro fertilization cycles performed, resulting in almost 70,000 live births [1]. While advances in reproductive medicine have been able to markedly improve outcomes for many couples and individuals, there remain a large number of women for which in vitro fertilization treatment, despite our best efforts, is unsuccessful in resulting in a live birth. Through thorough diagnostic testing, it is often possible to identify patients in which prognosis is poor prior to the initiation of treatment.
In 2019, the American Society of Reproductive Medicine’s Ethics committee published a committee opinion that guides clinicians in the care of patients with poor prognosis [2]. This document defines patients with a “very poor prognosis” as those with a 1–5% chance of success per cycle, and “futile” as those with < 1% chance of success per cycle. While decisions of whether to offer or refuse treatment to a couple or individual must always be individualized and patient-centered, it is ethical in some instances to decline care to those that meet the aforementioned criteria [2].
To date, there have been no published studies that have reported the percentage of patients who declined from pursuing in vitro fertilization with autologous oocytes (IVF-AO). Additionally, no studies to date have explored the factors associated with being declined IVF-AO, whether patient or clinic-centered. The objectives of this study are to determine the frequency of and factors associated with a patient being declined from IVF-AO.
Methods
Data source and sample
FertilityIQ is a website launched in 2015 with the intent of providing patients interested in or pursuing fertility treatment access to a nationwide educational resource where they could learn about treatment options and search nearby clinics. This cross-sectional population-based study includes data from the FertilityIQ database, which includes a voluntary questionnaire [3]. The complete survey can be found in Supplement 1. Through this online questionnaire, patients can disclose treatments they have received, review providers and clinics where they received care, and rate their overall experience and preferences. The data obtained is both qualitative and quantitative in nature. FertilityIQ partners with local fertility groups to recruit patients to complete reviews; patients are incentivized with the possibility of receiving grants for future treatments for their participation.
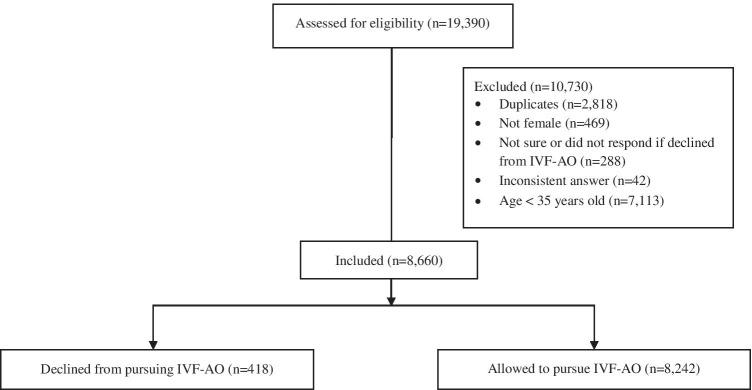
Responses from the de-identified FertilityIQ questionnaire completed between January 2015 and March 2020 were included in this analysis. Respondents who answered the question of whether they have previously been declined from IVF-AO (“Did any doctors decline to treat you for a cycle using your own eggs”), selected gender as “female” and self-reported age 35 or greater at the completion of the questionnaire were included in this cohort. Duplicate responses from the same IP address as well as inconsistent responses were excluded, as were those who were not sure if they were declined from IVF-AO (Fig. 1). Incomplete demographic variables were not inputted and instead left as incomplete. A total of 8660 respondents met inclusion criteria and were included in this analysis. This project was deemed to be exempt by the UCSF Institutional Review Board (IRB).
Fig. 1.
Consort flow diagram
Predictors
Certain characteristics were selected, a priori, as predictors of IVF-AO decline. Covariates included the following: self-reported age (35–37, 38–40, 41–42, 43+), self-reported race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic or Latino, non-Hispanic Asian, and other), highest education level attained (less than HS/HS/GED, some college, college degree, or master’s/professional degree), income (less than $49K, $50K–$99K, $100K–$199K, and over $200K), health insurance coverage for any aspect of fertility care (including diagnostic testing, office visits, medication, and/or procedures), whether the state of reported fertility treatment has insurance mandates for fertility care at the time the questionnaire was filled out, self-reported infertility diagnoses (including anovulation, diminished ovarian reserve (DOR), endometriosis, male factor, polycystic ovary syndrome, poor oocyte quality, tubal blockage, fibroids, immunologic, recurrent pregnancy loss, and unexplained infertility), and practice type (academic vs private).
Outcome
Participants were identified as being declined from using IVF with autologous oocytes if they answered “yes” to the question “Did any doctors declined to treat you for a cycle using your own eggs?” Women who responded “no” were classified as not being declined IVF-AO.
Data analysis
Descriptive statistics of participants’ demographic characteristics were reviewed. Chi-square analyses were performed to compare characteristics of women reporting a decline of IVF-AO compared to those who did not report a decline. Potential predictors were assessed through bivariate analyses and those deemed statistically associated with being declined IVF-AO to a significance level of 0.05 were included in the multivariable model. Using multivariate logistic regression, the adjusted odds were calculated for postulated predictors. All analyses were performed using the Statistical Analysis Software (SAS®) version 9.4.
Results
After exclusions, we identified 8660 participants who met inclusion criteria for analyses (Fig. 1). Among this cohort, 4.8% (n = 418) of respondents reported being declined a cycle of IVF-AO. Demographics of the study population are listed in Table 1. In the univariate analysis (Table 2), significant predictors of being declined IVF-AO included increasing age, household income of less than $50,000 per year, achievement of a master’s or professional degree, receiving care in a state with mandated fertility coverage, and self-reported diagnoses of DOR or poor oocyte quality. Significant predictors of not being declined included self-reported diagnoses of male factor infertility, PCOS, tubal blockage, and unexplained infertility. Self-reported race/ethnicity, diagnoses of endometriosis, fibroids, immunologic factors, and recurrent pregnancy loss, geographic location of residence, current practice type, presence of insurance coverage, and practice type were not associated with the report of IVF-AO decline in the univariate analysis.
Table 1.
Characteristics of fertility IQ questionnaire respondents, age 35 years or greater, who reported whether they were allowed to pursue IVF-AO between 2015 and 2020
| Characteristic | Participant cohorta (%) |
|---|---|
| Highest education level achieved | N = 8637 |
| GED or less | 217 (2.5) |
| Some college | 1187 (13.7) |
| College | 3170 (36.7) |
| Master’s or professional degree | 4063 (47.0) |
| Income | N = 7948 |
| $0–$49K | 505 (6.4) |
| $50K–$99K | 2395 (30.1) |
| $100K–$199K | 3286 (41.3) |
| $200K+ | 1762 (22.2) |
| Race/ethnicity | N = 7768 |
| White | 4306 (55.4) |
| Black or African American | 525 (6.8) |
| Latino | 652 (8.4) |
| Asian | 783 (10.1) |
| Other | 1502 (19.3) |
| Age | N = 8610 |
| 35–37 | 3275 (38.0) |
| 38–40 | 2727 (31.7) |
| 41–42 | 1219 (14.2) |
| 42+ | 1389 (16.1) |
| Self-reported diagnoses | N = 8660 |
| Anovulation | 326 (3.8) |
| DOR | 2084 (24.1) |
| Endometriosis | 1043 (12.0) |
| Male | 1790 (20.7) |
| PCOS | 1033 (11.9) |
| Poor oocyte quality | 1701 (19.6) |
| Tubal blockage | 803 (9.3) |
| Fibroid | 777 (9.0) |
| Immunologic | 211 (2.4) |
| RPL | 790 (9.1) |
| Unexplained | 2832 (32.7) |
| State with mandated coverage | N = 8507 |
| Yes | 5096 (59.9) |
| No | 3411 (40.1) |
| Location | N = 8660 |
| Northeast | 2776 (33.0) |
| Midwest | 1291 (15.3) |
| South | 2187 (26.0) |
| West | 2166 (25.7) |
| Current practice type | N = 8652 |
| Academic | 1524 (17.6) |
| Private | 7128 (82.4) |
| Insurance coverage | N = 7116 |
| None | 2150 (30.2) |
| Some fertility coverage (office, medication, testing, and/or procedures) | 4966 (69.8) |
| Declined IVF prior to current care | N = 8660 |
| Yes | 418 (4.8) |
| No | 8242 (95.2) |
Table 2.
Univariate analysis of predictors of reporting IVF-AO decline among respondents aged 35 and greater from 2015 through 2020
| Respondents declined: n (%) | Respondents not declined: n (%) | OR (95% CI) | |
|---|---|---|---|
| Total: 418 (4.8) | Total: 8242 (95.2) | ||
| Highest education level achieved* | |||
| GED or less | 8 (2.0) | 209 (2.5) | 0.64 (0.31–1.32) |
| Some college | 40 (9.6) | 1147 (13.9) | 0.57 (0.40–0.81) |
| College | 130 (32.1) | 3035 (36.9) | 0.72 (0.58–0.90) |
| Master’s or professional degree | 228 (56.3) | 3829 (46.6) | Reference |
| Income* | |||
| $0–$49K | 35 (9.6) | 468 (6.2) | 1.53 (1.02–2.30) |
| $50K–$99K | 102 (27.9) | 2288 (30.2) | 0.91 (0.68–1.23) |
| $100K–$199K | 147 (40.2) | 3137 (41.4) | 0.96 (0.73–1.26) |
| $200K+ | 82 (22.4) | 1678 (22.2) | Reference |
| Race/ethnicity | |||
| White | 199 (55.6) | 4100 (55.4) | Reference |
| Black or African American | 31 (8.7) | 493 (6.7) | 1.30 (0.88–1.90) |
| Latino | 33 (9.2) | 618 (8.4) | 1.10 (0.75–1.61) |
| Asian | 40 (11.2) | 743 (10.0) | 1.10 (0.78–1.57) |
| Other | 55 (15.4) | 1446 (19.5) | 0.78 (0.58–1.06) |
| Age (years)* | |||
| 35–37 | 74 (18.4) | 3199 (39.0) | Reference |
| 38–40 | 105 (26.0) | 2620 (32.0) | 1.73 (1.28–2.34) |
| 41–42 | 55 (13.7) | 1163 (14.2) | 2.04 (1.43–2.92) |
| 42+ | 169 (41.9) | 1213 (14.8) | 6.02 (4.55–8.00) |
| Self-reported diagnoses* | |||
| Anovulation | 216 (10.4) | 1868 (89.6) | 1.09 (0.66–1.79) |
| DOR | 46 (4.4) | 997 (95.6) | 3.65 (2.99–4.45)* |
| Endometriosis | 56 (3.1) | 1734 (96.9) | 0.90 (0.66–1.23) |
| Male | 28 (2.7) | 1005 (97.3) | 0.58 (0.44–0.77)* |
| PCOS | 156 (9.2) | 1545 (90.8) | 0.52 (0.35–0.76)* |
| Poor oocyte quality | 18 (2.2) | 785 (97.8) | 2.58 (2.10–3.17)* |
| Tubal blockage | 39 (5.0) | 738 (95.0) | 0.43 (0.27–0.69)* |
| Fibroid | 16 (7.6) | 195 (92.4) | 1.05 (0.75–1.47) |
| Immunologic | 40 (5.1) | 750 (94.9) | 1.64 (0.98–2.76) |
| RPL | 92 (3.3) | 2740 (96.7) | 1.06 (0.76–1.48) |
| Unexplained | 42 (6.0) | 653 (94.0) | 0.57 (0.45–0.72)* |
| State with mandated fertility coverage* | |||
| Yes | 267 (66.6) | 4819 (59.5) | Reference |
| No | 134 (33.4) | 3275 (40.5) | 0.74 (0.60–0.91) |
| Location | |||
| Northeast | 146 (37.0) | 2622 (32.7) | 1.21 (0.93–1.58) |
| Midwest | 57 (14.4) | 1233 (15.4) | 1.01 (0.72–1.41) |
| South | 96 (24.3) | 2089 (26.1) | Reference |
| West | 96 (24.3) | 2069 (25.8) | 1.01 (0.76–1.35) |
| Current practice type | |||
| Academic | 66 (16.4) | 1455 (17.7) | Reference |
| Private | 337 (83.4) | 6782 (82.3) | 1.10 (0.84–1.44) |
| Insurance coverage | |||
| None | 109 (31.8) | 2039 (30.2) | 1.08 (0.86–1.36) |
| Some fertility coverage (office, medication, testing, and/or procedures) | 234 (68.2) | 4723 (69.9) | Reference |
| Type of practice declining | |||
| Academic | 56 (19.5) | 1455 (17.7) | Reference0.89 (0.66–1.19) |
| Private | 231 (80.5) | 6782 (82.3) | |
| Both | 12 | N/A | |
| Missing | 85 | N/A | |
OR odds ratio modeled for reported decline of IVF-AO, CI confidence interval
*Significance with chi-square p value ≤ 0.05 level
In the multivariate analysis (Table 3), the predictors of being declined from pursuing IVF-AO which remained significant in the multivariate model included increasing age (p < 0.0001), income of less than $50,000 (p = 0.0035), and diagnoses of poor oocyte quality (p = 0.0034) and DOR (p < 0.0001). Predictors of being less likely to report decline from pursuing IVF-AO included some college or college degree (p = 0.0084) and self-reported diagnoses of male factor infertility (p = 0.0316), unexplained infertility (p = 0.0073), or tubal blockage (p = 0.0019).
Table 3.
Multivariate analysis of predictors of reporting IVF-AO decline among respondents aged 35 and greater from 2015 through 2020
| Adjusted OR(95% CI) | Chi-squarep-value | |
|---|---|---|
| Highest education level achieved* | 0.0084 | |
| GED or less | 0.582 (0.261–1.297) | |
| Some college | 0.550 (0.368–0.821) | |
| College | 0.754 (0.592–0.961) | |
| Master’s or professional degree | Reference | |
| Income* | 0.0035 | |
| $0–$49K | 2.276 (1.434–3.611) | |
| $50K–$99K | 1.211 (0.884–1.660) | |
| $100K–$199K | 1.062 (0.796–1.416) | |
| $200K+ | Reference | |
| Age (years)* | < 0.0001 | |
| 35–37 | Reference | |
| 38–40 | 1.500 (1.087–2.070) | |
| 41–42 | 1.442 (0.975–2.133) | |
| 42+ | 4.434 (3.267–6.018) | |
| Self-reported diagnoses | ||
| DOR* | 2.893 (2.287–3.660) | < 0.0001 |
| Male* | 0.706 (0.514–0.970) | 0.0316 |
| PCOS | 0.975 (0.643–1.479) | 0.9068 |
| Poor oocyte quality* | 1.437 (1.128–1.832) | 0.0034 |
| Tubal blockage* | 0.441 (0.263–0.740) | 0.0019 |
| Unexplained* | 0.697 (0.535–0.907) | 0.0073 |
| State with mandated fertility coverage | 0.1004 | |
| Yes | Reference | |
| No | 0.822 (0.650–1.039) | |
OR adjusted odds ratio, adjusted for all other variables in table, CI confidence interval
*Significance with chi-square p value ≤ 0.05 level
Notably, none of the following variables was predictive of patients being declined from pursuing IVF-AO in the multivariate model: self-reported diagnosis of PCOS or residence in a state with mandated fertility coverage.
Discussion
Our study is the first to report that approximately 5% of patients who seek in vitro fertilization with autologous oocyte are declined by their providers from pursuing treatment. Respondents reporting a decline in IVF-AO were more likely to be older, of lower income (< $50,000/year), and have self-reported diagnoses of DOR or poor oocyte quality. Conversely, respondents with some college or college degree as compared to a professional degree and infertility diagnoses of male factor, tubal blockage, or unexplained infertility were found to be less likely to report being declined.
Our results are consistent with prior literature, as it is well described that age and DOR are features associated with poor prognosis and it is reasonable for these patients to be declined with higher frequency [4, 5]. Furthermore, patients with self-reported diagnoses of male factor, tubal factor, and unexplained infertility are declined at lower rates, which can be expected as these would not be typical indications for proceeding directly to gamete donation. The association of patients being declined with both lower income and higher education is surprising, as higher education tends to correlate with higher income [6]. However, when considering the univariate analysis, these associations are prominent at the extremes suggesting separate yet correlated reasons one would be declined. Those who report an annual household income of less than $50,000 may be declined if the likelihood of success is low in the setting of undue financial implications of IVF with autologous oocytes. As the high cost of treatment is often cited as a barrier to pursuing ART, patients with higher income for whom the high cost of treatment is not a deterrent may be more likely to pursue treatments with a low chance of success compared to those with limited resources [7]. Conversely, patients who have attained a master’s or professional degree have delayed childbearing and are more likely to be older, thus making their chances of success lower. These patients often have causes of infertility like DOR and poor oocyte quality that have been described as reasons to move to gamete donation sooner. Additionally, these individuals may have higher health literacy and be better able to understand poor prognosis or futile treatment.
While age and education were included in the model, it is possible based on multicollinearity that the effects of age and education could not be completely disentangled. Interestingly, when limiting the analysis by age (results not shown), the only variable that remained universally as a significant predictor of decline was a diagnosis of DOR, which was shown to be associated with increased report of decline irrespective of age. This underscores our findings that while the presence of an infertility diagnosis which portends a poor prognosis is the most predictive of IVF decline with autologous oocytes, this relationship is likely moderated by socioeconomic factors,
While this is explicitly deemed unethical by the ASRM guidance, some have anecdotally described the protection of a fertility center’s success rates as a reason why some centers may choose to decline poor prognosis patients. Following this line of reasoning, one might expect private practices, facing competitive pressures, may be more likely to decline patients than academic centers. Nonetheless, our study did not find a difference in the type of clinic that declined patients.
As described previously, the 2019 ASRM guidance on fertility treatment when the prognosis is very poor or futile deems it ethical to refuse treatment [2]. Through shared decision-making and open communication, physicians can counsel patients about their treatment prognosis with the goal of reaching mutually agreed upon decisions. To support physicians in this process, ASRM recommends that practices develop policies to guide evidenced-based decisions regarding care of this patient population. The current study was not designed to evaluate how many clinics have these policies in place, but a critical assessment of clinic policies is both pertinent and necessary to determine they’re evidence-based and standardized.
This study has several strengths including its large national multi-state sample increasing the generalizability of the findings. Additionally, substantial demographic, medical, and other variables were available for review to complete a robust evaluation and support findings seen in this analysis. Lastly, this is the first study in the USA to our knowledge to investigate the prevalence of and factors associated with a decline in IVF-AO use.
As a self-report survey, this sample is at risk for recall bias due to its retrospective nature. The FertilityIQ database does not include clinical data such as ovarian reserve markers or number of prior cycles, limiting our ability to understand other factors that may have driven the decision to decline the patient. Furthermore, there is the potential for sampling bias as some patients with infertility do not seek care and of those who do seek care and may be at risk for decline, only a subset will complete a FertilityIQ questionnaire. Given this limitation, the respondents may not be representative of all patients who receive fertility care or those who were declined using IVF-AO. Lastly, this questionnaire does not elucidate the reasons why someone may have been declined and while this study was limited to an older population, there could be reasons a patient was declined irrespective of prognosis that was not accounted for.
Conclusion
Nearly 5% of patients who pursued IVF reported being declined from pursuing reproductive care with autologous oocytes, with patients who are older, of low household income, who have attained advanced degrees, or with self-reported diagnoses of DOR or poor oocyte quality being declined at a greater rate. Further studies that include clinical data are needed to confirm these findings, explore whether patients that are being declined treatment meet criteria for futile or very poor prognosis, and if opportunities to proceed with IVF-AO are further moderated by socioeconomic factors that would enhance the access and utilization of these family building services.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by EH, JM, and AP. The first draft of the manuscript was written by EH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Data availability
All data and materials as well as software applications comply with field standards.
Declarations
Conflict of interest
Jake Anderson-Bialis and Deborah Anderson-Bialis are owners and cofounders of FertilityIQ. All other authors report no conflicts of interest/competing interests.
Ethical approval
This study was considered exempt from IRB approval.
Consent to participate
No identifiable data was used in this study so consent for specific individuals was not obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eduardo Hariton and Jerrine R. Morris are co-first authors.
References
- 1.Assisted Reproductive Technology (ART): ART Success Rates. Centers for Disease Control and Prevention. https://www.cdc.gov/art/artdata/index.html. Accessed 29 Oct 2020.
- 2.Ethics Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org; Ethics Committee of the American Society for Reproductive Medicine. Fertility treatment when the prognosis is very poor or futile: an Ethics Committee opinion. Fertil Steril. 2019;111(4):659–63. [DOI] [PubMed]
- 3.FertilityIQ: About Us. FertilityIQ. https://www.fertilityiq.com/about-us. Accessed 29 Oct 2020.
- 4.Ferraretti AP, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (Oxford, England) 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 5.Van Voorhis BJ. Clinical practice. In vitro fertilization. N Engl J Med. 2007;356:379–386. doi: 10.1056/NEJMcp065743. [DOI] [PubMed] [Google Scholar]
- 6.Boshara R, Emmons WR, Noeth B. The demographics of wealth: how age, education and race separate thrivers from strugglers in today’s economy. Essay No. 2: Education and Wealth, May 2015
- 7.Mosalanejad L, et al. Barriers to infertility treatment: an integrated study. Glob J Health Sci. 2013;6(1):181–191. doi: 10.5539/gjhs.v6n1p181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials as well as software applications comply with field standards.