Abstract
Purpose
To describe the pregnancy and neonatal outcomes using fresh and vitrified/warmed blastocysts obtained from ovarian stimulation with follitropin delta in controlled trials versus follitropin alfa.
Methods
This investigation evaluated the outcome from 2719 fresh and frozen cycles performed in 1326 IVF/ICSI patients who could start up to three ovarian stimulations in the ESTHER-1 (NCT01956110) and ESTHER-2 (NCT01956123) trials, covering 1012 fresh cycles and 341 frozen cycles with follitropin delta and 1015 fresh cycles and 351 frozen cycles with follitropin alfa. Of the 1326 first cycle patients, 513 continued to cycle 2 and 188 to cycle 3, and 441 patients started frozen cycles after the fresh cycles. Pregnancy follow-up was continued until 4 weeks after birth.
Results
The overall cumulative take-home baby rate after up to three stimulation cycles was 60.3% with follitropin delta and 60.7% with follitropin alfa (−0.2% [95% CI: −5.4%; 5.0%]), of which the relative contribution was 72.8% from fresh cycles and 27.2% from frozen cycles in each treatment group. Across the fresh cycles, the ongoing implantation rate was 32.1% for follitropin delta and 32.1% for follitropin alfa, while it was 27.6% and 27.8%, respectively, for the frozen cycles. Major congenital anomalies among the live-born neonates up until 4 weeks were reported at an incidence of 1.6% with follitropin delta and 1.8% with follitropin alfa (−0.2% [95% CI: −1.9%; 1.5%]).
Conclusions
Based on comparative trials, the pregnancy and neonatal outcomes from fresh and frozen cycles provide reassuring data on the efficacy and safety of follitropin delta.
Trial registration
ClinicalTrials.gov Identifier: NCT01956110 registered on 8 October 2013; NCT01956123 registered on 8 October 2013.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02271-5.
Keywords: Follitropin delta, Fresh cycle, Frozen cycle, Neonatal outcome, Ovarian stimulation, Take-home baby rate
Introduction
Ovarian stimulation with recombinant follicle-stimulating hormone (FSH) preparations produced using Chinese Hamster Ovary (CHO) cells (i.e., follitropin alfa and follitropin beta) results in acceptable success rates in terms of pregnancy [1] and studies have provided reassuring information regarding the neonatal health after the use of fresh and vitrified/warmed embryos/blastocysts obtained from stimulation with these preparations [2, 3].
Today, recombinant FSH preparations are available from new types of mammalian cell lines. Follitropin delta is the most recently developed recombinant FSH preparation, and it is the first commercially available recombinant FSH expressed from a human cell line (PER.C6®). While follitropin alfa, follitropin beta, and follitropin delta have the same amino acid FSH sequence, follitropin delta resembles native human FSH with α2,6-linked sialic acid and bisecting N-acetylglucosamine, which are not present in follitropin alfa and follitropin beta [4–6]. These characteristics are reflected in a unique pharmacokinetic/pharmacodynamic profile of follitropin delta, resulting in a longer half-life, a slower clearance, and a greater pharmacodynamic response than follitropin alfa [7].
Comprehensive clinical trials have been performed in in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) patients undergoing ovarian stimulation to document the efficacy and safety of follitropin delta versus CHO-derived recombinant FSH preparations [8–13]. The trials have mainly focused on fresh cycles and have provided reassuring information on follitropin delta with respect to the clinical performance of fresh blastocysts leading to pregnancy and live birth as well as the safety of the patients. Nevertheless, two large trials have also included the use of cryopreserved blastocysts in frozen cycles in addition to up to three fresh cycles, facilitating a direct comparison of the cumulative live birth rate per cycle with follitropin delta versus a CHO-derived recombinant FSH preparation [9, 10, 13]. Moreover, neonatal health data have also been collected, including evaluation of congenital anomalies, gestational age, and birth weight. The present integrated analysis provides a comprehensive description of the pregnancy outcomes from women exposed in comparative controlled trials with follitropin delta as well as the neonatal outcomes.
Materials and methods
Trial designs
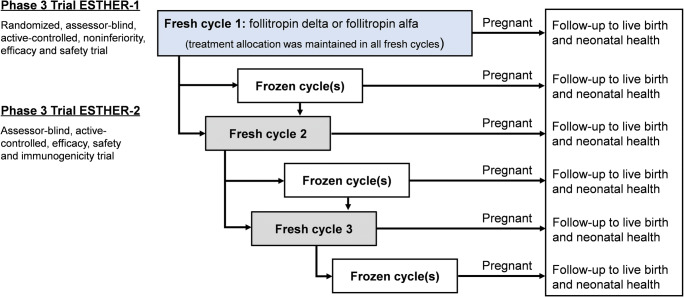
This integrated analysis was conducted with data from two controlled clinical trials in the development program for follitropin delta that were performed between October 2013 and January 2017 at 37 investigational sites in Europe and North and South America. The Evidence-based Stimulation Trial with Human rFSH in Europe and Rest of World 1 (ESTHER-1, NCT01956110) was a randomized, controlled, assessor-blind trial comparing individualized follitropin delta dosing versus conventional follitropin alfa dosing, following a gonadotropin-releasing hormone (GnRH) antagonist protocol (fresh cycle 1) [13]. ESTHER-1 included women who were within the age range 18-40 years, had regular menstrual cycles, and were diagnosed with tubal infertility, unexplained infertility, or endometriosis stage I/II [14], or had a partner diagnosed with male factor infertility. Patients who did not achieve an ongoing pregnancy in ESTHER-1 were offered to participate in the subsequent trial ESTHER-2 (NCT01956123). ESTHER-2 was a controlled, assessor-blind trial and covered up to two additional treatment cycles (fresh cycles 2 and 3) where patients maintained the same treatment allocation to either follitropin delta or follitropin alfa as in the first cycle [9]. Frozen cycles could be performed between or after the fresh cycles as per the patient’s preference (Fig. 1). A detailed patient flow after the first fresh cycle, detailing subsequent frozen cycles and new fresh cycles, has previously been presented [10].
Fig. 1.
Schematic overview of ESTHER-1 and ESTHER-2 trial designs. The flow of patients is provided for fresh and frozen cycles using day 5 blastocysts obtained after ovarian stimulation with follitropin delta and follitropin alfa. Frozen cycles (optional) were started within 1 year after start of stimulation of the last fresh cycle in either of the trials. ESTHER Evidence-based Stimulation Trial with Human rFSH in Europe and Rest of World.
In fresh cycle 1, the follitropin delta (Rekovelle; Ferring Pharmaceuticals, Saint-Prex, Switzerland) dosing regimen was individualized based on patient’s serum anti-Müllerian hormone (AMH, Elecsys® AMH immunoassay; Roche Diagnostics, Rotkreuz, Switzerland) and body weight, and no dose adjustments were made during stimulation. The follitropin alfa (Gonal-f; Merck Serono, Geneva, Switzerland) dose was 150 IU/day for the first 5 days and could thereafter be adjusted. In subsequent cycles, the starting doses were maintained or adjusted according to the ovarian response in the previous cycle. The maximum allowed daily dose of follitropin delta was gradually increased from 12 μg in fresh cycle 1 to 18 μg in fresh cycle 2 and to 24 μg in fresh cycle 3. The maximum allowed daily starting dose of follitropin alfa was 150 IU, 225 IU, and 300 IU in fresh cycles 1, 2, and 3, respectively, with a maximum allowed daily dose of 450 IU in all three cycles. Patients with surplus of day 5 blastocysts as well as patients who underwent triggering of final follicular maturation with GnRH agonist could undergo frozen cycles. Blastocysts were cryopreserved in individual straws using the vitrification method. The transfer policy in the fresh cycles was determined by age and blastocyst quality, with guidance to transfer either one or two blastocysts. In the frozen cycles, one or two blastocysts could be transferred from one or several of the fresh cycles at the discretion of the investigator and patient, and in accordance with local practice. Both natural cycle and programmed regimens were allowed. Pregnancy follow-up was done until 4 weeks after birth for all pregnancies resulting from fresh cycles or frozen cycles started within 1 year after start of stimulation of the last fresh cycle in either of the trials. Additional details on trial design, population and baseline characteristics, procedures, and results, including CONSORT flow diagrams, are available in previous publications [9, 13].
Outcomes and definitions
The main outcomes were the overall cumulative take-home baby rate for women exposed to follitropin delta and follitropin alfa in the ESTHER trials as well as the neonatal health data up until 4 weeks after birth.
Live birth was defined as the birth of at least one live baby after ≥24 weeks of gestational age. The cumulative take-home baby rate (i.e., live rate at 4 weeks after birth) from fresh and frozen cycles for each stimulation cycle in which the women participated was calculated. A started frozen cycle was defined as a cycle with warming of at least one blastocyst with the intention of transfer. The ongoing implantation rate (defined as the number of intrauterine viable fetuses 10-11 weeks after transfer divided by number of blastocysts transferred) was determined across all fresh cycles as well as all frozen cycles.
Neonatal outcomes included neonatal characteristics such as gestational age, gender, birth weight, and length at birth as well as safety variables like congenital anomalies, stillbirth (defined as death of a fetus after ≥24 weeks of gestation), neonatal death (defined as death of a live-born neonate within 4 weeks after birth), admission to neonatal intensive care unit (NICU) or neonatal care unit (NCU) within 24 h after birth, and also hospitalization occurring between 24 h and 4 weeks after birth.
Singleton and multiple status were based on the number of intrauterine viable fetuses at the ongoing pregnancy visit performed 10-11 weeks after transfer. Gestational age was calculated as the days between blastocyst transfer and birth plus 19 days. A birth weight <2500 g was defined as low birth weight, and <1500 g was defined as very low birth weight [15]. Preterm birth was defined as a live birth at <37 weeks gestation and very preterm birth was defined as <32 weeks gestation [16].
Congenital anomalies detected in fetuses, neonates within 24 h after birth (referred to as “at birth”), and neonates between 24 h and 4 weeks after birth (referred to as “at 4 weeks after birth”) were reported after assessment and diagnosis by the neonate’s physician and coded using the Medical Dictionary for Regulatory Activities (MedDRA) versions 18.1 and 19.1. As per regulatory guidelines, major congenital anomalies were defined as a life-threatening structural anomaly or one likely to cause significant impairment of health or functional capacity and which needs medical or surgical treatment, and minor congenital anomalies were defined as a relatively frequent structural anomaly not likely to cause any medical or cosmetic problems [17]. The categorization of minor and major congenital anomalies was made at the time of database lock.
Statistical analyses
Descriptive statistics are presented without accounting for multiple records for a patient. Cumulative take-home baby rates were compared between treatments by constructing a two-sided 95% confidence interval (CI) for the estimated mean difference in rates (follitropin delta - follitropin alfa) using the Mantel-Haenszel method to combine risk differences across age strata (<35, 35–37, and 38–40 years). Ongoing implantation rates per started cycle with blastocyst transfer were compared between treatments using a mixed effect logistic regression model with treatment, age stratum, and single/double transfer as fixed factors and patient as random effect, assuming normally distributed log-odds. The estimated mean difference and 95% CI were derived using the delta method. Similarly, the take-home baby rates per started cycle were compared between treatments using a mixed effect logistic regression model with treatment and age stratum as fixed factors and patient as random effect. The 95% CIs were calculated to estimate the mean difference in incidence of major and minor congenital anomalies using the method of Wald. All statistical analyses were performed using the SAS software (SAS Institute Inc., version 9.4, Cary, NC, USA).
Results
Pregnancy outcomes
In this integrated analysis of data from comparative controlled trials with fresh and frozen cycles, 1326 women underwent a total of 2719 cycles: 1353 cycles in the follitropin delta group and 1366 cycles in the follitropin alfa group (Table 1). These cycles were distributed as 2027 (74.5%) fresh cycles (1012 cycles in the follitropin delta group and 1015 cycles in the follitropin alfa group) and 692 (25.5%) frozen cycles (341 cycles in the follitropin delta group and 351 cycles in the follitropin alfa group). A total of 1326 IVF/ICSI patients (665 for follitropin delta and 661 for follitropin alfa) were randomized and exposed in fresh cycle 1, of whom 513 patients (252 for follitropin delta and 261 for follitropin alfa) continued to fresh cycle 2 and of these, 188 patients (95 for follitropin delta and 93 for follitropin alfa) also started fresh cycle 3. In total, 441 of the 1326 patients also started frozen cycles (222 for follitropin delta and 219 for follitropin alfa). The average number of started fresh and frozen cycles was 2.0 per patient in the follitropin delta group and 2.1 per patient in the follitropin alfa group, and the average number of cycles with transfer was 1.8 per patient for both treatment groups. Fresh and frozen cycles with transfer of one and two blastocysts are presented in Table S1. After fresh cycle 1, 12.5% (14.1% for follitropin delta and 10.9% for follitropin alfa) of the patients had neither achieved an ongoing pregnancy nor proceeded to further fresh or frozen cycles, while this was the case for 19.7% (17.5% for follitropin delta and 21.8% for follitropin alfa) of the patients after fresh cycle 2.
Table 1.
Number of started cycles and ongoing implantation rate in fresh and frozen cycles
| Outcome | Fresh cyclesa | Frozen cyclesb | ||
|---|---|---|---|---|
| Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | |
| N | 665 | 661 | 222 | 219 |
| Started cycles | 1012 | 1015 | 341 | 351 |
| Ongoing implantation ratec | 312/971 (32.1) | 313/976 (32.1) | 126/457 (27.6) | 132/475 (27.8) |
aThe women underwent a maximum of three fresh cycles
bThe women underwent a maximum of eight frozen cycles
cThe number of intrauterine viable fetuses 10–11 weeks after transfer divided by number of blastocysts transferred (%)
N total number of women
Across the fresh cycles, the overall ongoing implantation rate per started cycle with transfer was 32.1% for follitropin delta and 32.1% for follitropin alfa (estimated mean difference 0.3% [95% CI: −4.2%; 4.7%]) (Table 1). For the frozen cycles, the overall ongoing implantation rate per started cycle with transfer was 27.6% in the follitropin delta group and 27.8% in the follitropin alfa group (estimated mean difference 0.7% [95% CI: −6.1%; 7.6%]). Thus, comparable ongoing implantation rates were achieved between the two treatment groups after fresh or frozen cycles.
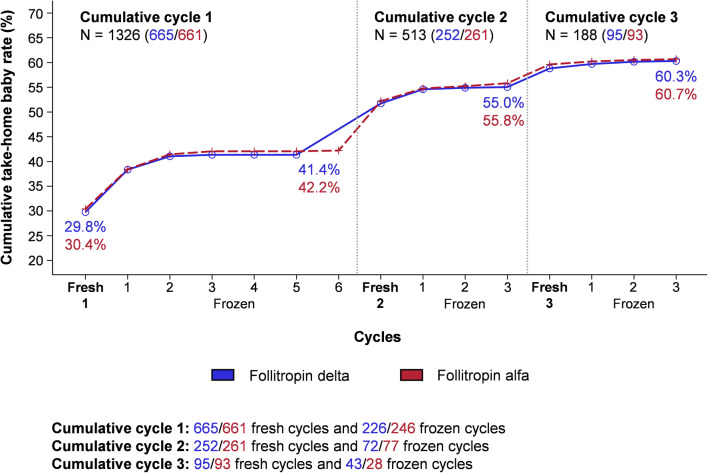
As displayed in Figure 2, the cumulative take-home baby rate after the first ovarian stimulation cycle was 41.4% in the follitropin delta group and 42.2% in the follitropin alfa group. Following the second ovarian stimulation cycle, the cumulative take-home baby rate across the fresh and frozen cycles increased to 55.0% in the follitropin delta group and 55.8% in the follitropin alfa group. The overall take-home baby rate for women who participated in the ESTHER trials was 60.3% with follitropin delta and 60.7% with follitropin alfa (estimated mean difference −0.2% [95% CI: −5.4%; 5.0%]). For the 802 women (401 in each treatment group) with at least one live neonate at 4 weeks after birth, the relative contribution to the take-home baby rate was 72.8% (292/401) from fresh cycles and 27.2% (109/401) from frozen cycles in the follitropin delta group and 72.8% (292/401) from fresh cycles and 27.2% (109/401) from frozen cycles in the follitropin alfa group. The cumulative take-home baby rate is presented by age (<35 and ≥35 years) in Table S2.
Fig. 2.
Cumulative take-home baby rate in fresh and frozen cycles. The cumulative take-home baby rate is shown by fresh and frozen cycles with follitropin delta and follitropin alfa. N total number of women.
In relation to the data obtained in frozen cycles, the availability of cryopreserved blastocysts was similar in the two treatment groups, with 69.5% of the women in the follitropin delta group and 68.8% of the women in the follitropin alfa group having at least one blastocyst cryopreserved (Table S3). The overall survival rate for warmed blastocysts proceeding to transfer was 87.4% in the follitropin delta group and 88.8% in the follitropin alfa group. In each treatment group, 48.1% of the women with frozen blastocysts underwent at least one frozen cycle with transfer within 1 year after start of stimulation of the last fresh cycle. When evaluating the outcome from the frozen cycles, the overall take-home baby rate per started cycle was 32.0% (109/341) in the follitropin delta group and 31.1% (109/351) in the follitropin alfa group (estimated mean difference 1.5% [95% CI: −6.0 %; 8.9 %]).
Neonatal outcomes
The total number of live-born neonates was 873; distributed as 308 from fresh cycles with follitropin delta, 125 from frozen cycles with follitropin delta, 310 from fresh cycles with follitropin alfa, and 130 from frozen cycles with follitropin alfa (Table S4). In fresh cycles, stillbirth was reported for 2 fetuses in the follitropin delta group and 3 fetuses in the follitropin alfa group. There were no stillbirths in the frozen cycles. Neonatal characteristics of live-born neonates are presented in Table 2 for fresh and frozen cycles. Overall, the mean gestational age for all live-born neonates was 38.7 weeks in both treatment groups, and the birth weight, length at birth, and gender distribution were also similar between treatment groups. The number of singletons and multiples was comparable between the follitropin delta and follitropin alfa groups after fresh and frozen cycles, and there were no higher-order pregnancies than twins. As a result of more double blastocyst transfers in the last fresh cycle and the frozen cycles (Table S1), the number of twins born in these cycles was higher than in the initial fresh cycles; this was observed for both treatment groups. Among singletons and multiples, there was also no difference between treatment groups in neonatal characteristics.
Table 2.
Characteristics of live-born neonates at birth in fresh and frozen cycles
| Characteristic | Fresh cycles | Frozen cycles | Total | |||
|---|---|---|---|---|---|---|
| Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | |
| N | 308 | 310 | 125 | 130 | 433 | 440 |
| Gestational age (days) | ||||||
| All | 271.0 ± 16.4 | 271.5 ± 16.1 | 271.2 ± 14.7 | 268.6 ± 15.2 | 271.1 ± 15.9 | 270.7 ± 15.9 |
| Singletons | 273.5 ± 14.3 | 274.5 ± 11.7 | 276.5 ± 11.4 | 274.7 ± 12.1 | 274.2 ± 13.7 | 274.6 ± 11.7 |
| Multiples | 249.9 ± 18.4 | 247.5 ± 25.3 | 256.4 ± 12.5 | 254.9 ± 12.3 | 253.2 ± 15.9 | 251.5 ± 19.6 |
| Gender | ||||||
| All | ||||||
| Boy | 166 (53.9) | 168 (54.2) | 58 (46.4) | 62 (47.7) | 224 (51.7) | 230 (52.3) |
| Girl | 142 (46.1) | 142 (45.8) | 67 (53.6) | 68 (52.3) | 209 (48.3) | 210 (47.7) |
| Singletons | ||||||
| Boy | 153 (55.4) | 149 (54.0) | 39 (42.4) | 42 (46.7) | 192 (52.2) | 191 (52.2) |
| Girl | 123 (44.6) | 127 (46.0) | 53 (57.6) | 48 (53.3) | 176 (47.8) | 175 (47.8) |
| Multiples | ||||||
| Boy | 13 (40.6) | 19 (55.9) | 19 (57.6) | 20 (50.0) | 32 (49.2) | 39 (52.7) |
| Girl | 19 (59.4) | 15 (44.1) | 14 (42.4) | 20 (50.0) | 33 (50.8) | 35 (47.3) |
| Birth weight (g) | ||||||
| All | 3133 ± 647 | 3127 ± 610 | 3160 ± 607 | 3034 ± 654 | 3141 ± 635 | 3100 ± 624 |
| Singletons | 3223 ± 586 | 3229 ± 518 | 3365 ± 510 | 3325 ± 491 | 3258 ± 571 | 3252 ± 512 |
| Multiples | 2359 ± 635 | 2306 ± 682 | 2590 ± 482 | 2377 ± 476 | 2476 ± 570 | 2344 ± 577 |
| Length at birth (cm) | ||||||
| All | 49.7 ± 3.6 | 49.6 ± 3.6 | 49.9 ± 3.8 | 49.5 ± 3.9 | 49.8 ± 3.7 | 49.6 ± 3.7 |
| Singletons | 50.1 ± 3.3 | 50.0 ± 3.0 | 50.7 ± 3.6 | 51.0 ± 3.3 | 50.3 ± 3.4 | 50.3 ± 3.1 |
| Multiples | 46.3 ± 4.8 | 46.1 ± 5.8 | 47.1 ± 2.9 | 46.0 ± 3.0 | 46.7 ± 4.0 | 46.0 ± 4.5 |
Values are mean ± SD or n (%), unless otherwise stated. Singleton and multiple status were based on the number of intrauterine viable fetuses at the ongoing pregnancy visit
N total number of live-born neonates, n number of live-born neonates with observations, SD standard deviation
As summarized for fresh and frozen cycles in Table 3, the incidence of preterm births was 15.5% in the follitropin delta group and 15.9% in the follitropin alfa group, with a slightly higher incidence in the frozen cycles than in the fresh cycles due to more twin pregnancies. Admission to NICU/NCU immediately after birth, with the most frequent cause being prematurity, and new hospitalization occurring within the initial 4 weeks were reported at a similar incidence in the two treatment groups after fresh and frozen cycles. No neonatal deaths within 4 weeks after birth were reported in the follitropin delta group, while neonatal deaths were reported for 4 neonates in the follitropin alfa group, of which 3 deaths were associated with prematurity and 1 death was caused by a congenital anomaly.
Table 3.
Prematurity, low birth weight, admission to NICU/NCU, and hospitalization in fresh and frozen cycles
| Outcome | Fresh cycles | Frozen cycles | Total | |||
|---|---|---|---|---|---|---|
| Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | |
| N | 308 | 310 | 125 | 130 | 433 | 440 |
| Preterm birth | ||||||
| All | ||||||
| <32 weeks | 8 (2.6) | 6 (1.9) | 1 (0.8) | 1 (0.8) | 9 (2.1) | 7 (1.6) |
| 32–36 weeks | 37 (12.0) | 35 (11.3) | 21 (16.8) | 28 (21.5) | 58 (13.4) | 63 (14.3) |
| Singletons | ||||||
| <32 weeks | 4 (1.4) | 1 (0.4) | 1 (1.1) | 1 (1.1) | 5 (1.4) | 2 (0.5) |
| 32–36 weeks | 21 (7.6) | 18 (6.5) | 3 (3.3) | 6 (6.7) | 24 (6.5) | 24 (6.6) |
| Multiples | ||||||
| <32 weeks | 4 (12.5) | 5 (14.7) | 0 | 0 | 4 (6.2) | 5 (6.8) |
| 32–36 weeks | 16 (50.0) | 17 (50.0) | 18 (54.5) | 22 (55.0) | 34 (52.3) | 39 (52.7) |
| Low birth weight | ||||||
| All | ||||||
| <1500 g | 8 (2.6) | 3 (1.0) | 0 | 1 (0.8) | 8 (1.8) | 4 (0.9) |
| 1500–2499 g | 35 (11.4) | 35 (11.3) | 14 (11.2) | 28 (21.5) | 49 (11.3) | 63 (14.3) |
| Singletons | ||||||
| <1500 g | 4 (1.4) | 0 | 0 | 1 (1.1) | 4 (1.1) | 1 (0.3) |
| 1500–2499 g | 19 (6.9) | 19 (6.9) | 1 (1.1) | 3 (3.3) | 20 (5.4) | 22 (6.0) |
| Multiples | ||||||
| <1500 g | 4 (12.5) | 3 (8.8) | 0 | 0 | 4 (6.2) | 3 (4.1) |
| 1500–2499 g | 16 (50.0) | 16 (47.1) | 13 (39.4) | 25 (62.5) | 29 (44.6) | 41 (55.4) |
| Admission to NICU/NCUa | ||||||
| All | 29 (9.4) | 30 (9.7) | 17 (13.6) | 22 (16.9) | 46 (10.6) | 52 (11.8) |
| Hospitalizationb | ||||||
| All | 7 (2.3) | 12 (3.9) | 3 (2.4) | 3 (2.3) | 10 (2.3) | 15 (3.4) |
Values are n (%), unless otherwise stated. Singleton and multiple status were based on the number of intrauterine viable fetuses at the ongoing pregnancy visit
aWithin 24 h after birth
bBetween 24 h and 4 weeks after birth
N total number of live-born neonates, n number of live-born neonates with observations, NCU neonatal care unit, NICU neonatal intensive care unit
Congenital anomalies
Major congenital anomalies detected among live-born neonates up until 4 weeks after birth are presented in Table 4, with the baseline characteristics for the mothers displayed in Table S5. The incidence of live-born neonates with major congenital anomalies was 1.6% (5/308) in fresh cycles with follitropin delta and 2.3% (7/310) in fresh cycles with follitropin alfa (estimated mean difference −0.6% [95% CI: −2.8%; 1.5%]), while it was 1.6% (2/125) in frozen cycles with follitropin delta and 0.8% (1/130) in frozen cycles with follitropin alfa (estimated mean difference 0.8% [95% CI: −1.8%; 3.5%]). In total, the incidence of live-born neonates with major congenital anomalies was comparable between the two treatment groups, with 1.6% (7/433) for follitropin delta and 1.8% (8/440) for follitropin alfa (estimated mean difference −0.2% [95% CI: −1.9%; 1.5%]). Of these 15 live-born neonates, 10 live-born neonates had only 1 major congenital anomaly, whereas 5 live-born neonates had 2 or 3 major anomalies. The most commonly reported major congenital anomalies were cardiac and vascular disorders as well as renal and urinary tract disorders. In the follitropin delta group, all major congenital anomalies were detected at birth with no new events observed at 4 weeks after birth. Of the 8 live-born neonates with major congenital anomalies in the follitropin alfa group, 5 neonates had major congenital anomalies only at birth, 1 neonate had events both at birth and 4 weeks, and 2 neonates had events only at 4 weeks. The incidence of live-born neonates with major congenital anomalies is presented by patient age (<35 and ≥35 years) in Table S6.
Table 4.
Major congenital anomalies among live-born neonates in fresh and frozen cycles
| MedDRA high-level group term Preferred term |
Fresh cycles | Frozen cycles | Total | |||
|---|---|---|---|---|---|---|
| Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | Follitropin delta | Follitropin alfa | |
| N | 308 | 310 | 125 | 130 | 433 | 440 |
| Number of neonates with any event (%) | 5 (1.6) | 7 (2.3) | 2 (1.6) | 1 (0.8) | 7 (1.6) | 8 (1.8) |
| Total number of events | 6 | 11 | 3 | 1 | 9 | 12 |
| Cardiac and vascular disorders congenital | ||||||
| Atrial septal defect | 1 (0.3)a | 1 (0.2)a | ||||
| Bicuspid aortic valve | 1 (0.3)a | 1 (0.2)a | ||||
| Coarctation of the aorta | 1 (0.3)a | 1 (0.2)a | ||||
| Double outlet right ventricle | 1 (0.8) | 1 (0.2) | ||||
| Patent ductus arteriosus | 1 (0.3) | 1 (0.8) | 2 (0.5) | |||
| Transposition of the great vessels | 1 (0.3) | 1 (0.2) | ||||
| Ventricular septal defect | 1 (0.3) | 2 (0.6)a | 1 (0.2) | 2 (0.5)a | ||
| Chromosomal abnormalities and abnormal gene carriers | ||||||
| Beckwith-Wiedemann syndrome | 1 (0.3)a | 1 (0.8) | 1 (0.2) | 1 (0.2)a | ||
| Gastrointestinal tract disorders congenital | ||||||
| Cleft palate | 1 (0.3) | 1 (0.3) | 1 (0.2) | 1 (0.2) | ||
| Musculoskeletal and connective tissue disorders congenital | ||||||
| Adactyly | 1 (0.3) | 1 (0.2) | ||||
| Polydactyly | 1 (0.8) | 1 (0.2) | ||||
| Renal and urinary tract disorders congenital | ||||||
| Congenital pyelocaliectasis | 1 (0.3) | 1 (0.2) | ||||
| Pelvic kidney | 1 (0.3) | 1 (0.2) | ||||
| Urethral valves | 1 (0.3) | 1 (0.3) | 1 (0.2) | 1 (0.2) | ||
| Reproductive tract and breast disorders congenital | ||||||
| Cryptorchism | 1 (0.3) | 1 (0.2) | ||||
Values are n (%), unless otherwise stated
aDetected between 24 h and 4 weeks after birth
MedDRA Medical Dictionary for Regulatory Activities, N total number of live-born neonates, n number of live-born neonates with events
In addition to the major congenital anomalies in live-born neonates, major congenital anomalies leading to elective termination of the pregnancy were reported for 1.1% (5/474) of the clinical pregnancies detected at 5–6 weeks after transfer in the follitropin delta group and 1.0% (5/486) of the clinical pregnancies in the follitropin alfa group after fresh and frozen cycles (estimated mean difference 0% [95% CI: −1.3%; 1.3%]), including 6 cases of trisomy 13, 18, and 21 (4 for follitropin delta and 2 for follitropin alfa). Finally, minor congenital anomalies, defined as relatively frequent structural anomalies not likely to cause any medical or cosmetic problems, were more common than major congenital anomalies. The incidence of live-born neonates with minor congenital anomalies after fresh and frozen cycles was 4.8% (21/433) in the follitropin delta group and 3.0% (13/440) in the follitropin alfa group (estimated mean difference 1.9% [95% CI: −0.7%; 4.5%]).
Discussion
The present comprehensive data set from comparative controlled trials provided reassurance on all outcomes, including ongoing implantation rate, take-home baby rate, and neonatal health, in fresh and frozen cycles following ovarian stimulation with follitropin delta, a recombinant FSH preparation expressed from a human cell line.
Following follitropin delta treatment, the ongoing implantation rate in fresh cycles, the availability of cryopreserved blastocysts, the survival rate for warmed blastocysts proceeding to transfer, and the ongoing implantation rate in frozen cycles were comparable to follitropin alfa. Moreover, the risk of stillbirth was low, which is in line with previous reports [18]. Across all fresh and frozen cycles, an overall cumulative take-home baby rate of about 60% was achieved in the follitropin delta group. The major relative contribution to the cumulative take-home baby rate was from fresh cycles (approximately three-quarters), and a similar contribution of fresh and frozen cycles was observed within each of the cycles in this analysis. The take-home baby rate reached a plateau after two frozen cycles in the first cumulative cycle and after one frozen cycle in the two next cumulative cycles. In conclusion, these pregnancy outcome findings provide data on the efficacy of follitropin delta in terms of take-home baby rates in fresh and frozen cycles and add reassuring information to the clinical performance of fresh and cryopreserved blastocysts derived from ovarian stimulation with follitropin delta.
In terms of neonatal outcomes, the vast majority of births in these trials resulted in the delivery of healthy neonates. The neonates born after ovarian stimulation with follitropin delta and follitropin alfa demonstrated comparable neonatal outcomes, with no difference regarding gestational age and birth weight. The incidence of congenital anomalies after the use of follitropin delta was within the range of what previous studies on congenital anomalies in neonates born after ovarian stimulation have found [2, 19–25], with the numbers reported in the literature highly dependent on the definitions used and the population studied. The relevant observation, which is made possible by the comparative design of the present data set, is that the overall incidence and distribution of congenital anomalies were similar for the two recombinant FSH preparations in the present analysis. Both major and minor congenital heart anomalies were found after treatment with follitropin delta and follitropin alfa, consistent with this being the most frequently reported anomaly in neonates born after ovarian stimulation [26]. Most importantly, no pattern or clustering of specific types of congenital anomalies was identified.
There are several studies in the literature reporting differences in neonatal outcomes between fresh and frozen cycles [3, 27–33], including higher birth weight in frozen cycles, which was also observed in the present analysis. Although the present data material was too limited to warrant such an analysis, there was no indication of additional concerns regarding neonatal outcomes in either fresh or frozen cycles following ovarian stimulation with follitropin delta compared to follitropin alfa.
The present analysis focused on the take-home baby rate using the data available at 4 weeks after birth. This endpoint is clinically relevant, as it accounts for the losses occurring in the immediate period after birth that are not reflected in the standard reporting of live birth rate. Furthermore, the 4-week follow-up time frame allowed for reporting of those congenital anomalies that are difficult to detect at birth, and comprised 6 of the 21 major congenital anomalies in the present analysis, and therefore also ensured a more complete analysis of neonatal health. The time period of the analyzed frozen cycles covered 1 year after start of the last ovarian stimulation cycle, which seems adequate considering that the cumulative take-home baby rate plateaued after one or two frozen cycles. Nevertheless, this integrated analysis of data was influenced by the individual trial designs, including that a limited proportion of patients proceeded to a new ovarian stimulation cycle before using all cryopreserved blastocysts.
This report compiles the most comprehensive data set of pregnancy and neonatal outcomes in fresh and frozen cycles following ovarian stimulation with a recombinant FSH preparation expressed from a human cell line. The reported take-home baby rate and observed neonatal outcomes with follitropin delta across fresh and frozen cycles in controlled trials with follitropin alfa as a reference add reassuring information on the clinical performance of follitropin delta in terms of efficacy and safety.
Supplementary information
(DOC 215 kb)
Acknowledgements
The authors acknowledge the patients, investigators, and staff at participating sites in the two trials, and the clinical teams at Ferring Pharmaceuticals. The authors also thank Lisbeth Helmgaard and Maria Gullberg (Global Medical Writing, Ferring Pharmaceuticals, Denmark) for assistance in writing the manuscript, Marie Goethberg (Global Biometrics, Ferring Pharmaceuticals, Denmark) for statistical analysis support, and Ankur Chakraborty (Global Biometrics, Ferring Pharmaceuticals, Denmark) for statistical programming.
Appendix: The ESTHER-1 and ESTHER-2 Trial Groups’ participating sites and principal investigators are presented in Table 5
Table 5.
ESTHER-1 and ESTHER-2 Trial Groups’ participating sites and principal investigators
| Country | Participating sites and principal investigatorsa |
|---|---|
| Belgium | Herman Tournaye, UZ Brussel; Petra De Sutter, UZ Gent; Wim Decleer, AZ Jan Palfijn AV, Gent. |
| Brazil | Alvaro Petracco, Fertilitat – Centro de Medicina Reproductiva, Porto Alegre; Edson Borges, Fertility – Centro de Fertilizacao Assistida, São Paulo; Caio Parente Barbosa, Instituto Ideia Fértil de Saúde Reproductiva, São Paulo. |
| Canada | Jon Havelock, Pacific Centre for Reproductive Medicine, Burnaby, British Columbia; Paul Claman, Ottawa Fertility Centre, Ottawa, Ontario; Albert Yuzpe, Olive Fertility Centre, Vancouver, British Columbia. |
| Czech Republic | Hana Visnova, IVF CUBE SE, Prague; Pavel Ventruba, Centre of Assisted Reproduction, Brno; Petr Uher, Institute of Reproductive Medicine and Genetics, Karlovy Vary; Milan Mrazek, GYNEM, Prague. |
| Denmark | Anders Nyboe Andersen, The Fertility Clinic, Copenhagen University Hospital, Copenhagen; Ulla Breth Knudsen, The Fertility Clinic, Aarhus University Hospital, Skejby. |
| France | Didier Dewailly, Department of Endocrine Gynaecology and Reproductive Medicine, Hôpital Jeanne de Flandre; Anne Guivarc'h Leveque, Clinique Mutualiste La Sagesse. |
| Italy | Antonio La Marca, University of Modena and Reggio Emilia, Modena; Enrico Papaleo, Centro Natalità San Raffaele, Milan. |
| Poland | Waldemar Kuczynski, Kriobank, Bialystok; Katarzyna Kozioł, nOvum Fertility Clinic, Warsaw. |
| Russia | Margarita Anshina, Centre of Reproduction & Genetics – LLC, Moscow; Irina Zazerskaya, Federal State Budgetary Institution “Federal Center of Heart, Blood & Endocrinology named after V.I. Almazov” of Ministry of Health of the Russian Federation, Saint Petersburg; Alexander Gzgzyan, Institute of Russian Academy of Medical Science Scientific Research Institute of Gynaecology and Obstetrics named after D.O. Ott of North-West Department of RAMS, Saint Petersburg; Elena Bulychova, State Budgetary Health Institution of Moscow Region “Moscow Regional Scientific Research Institute of Obstetrics & Gynaecology.” |
| Spain | Victoria Verdú, Genefiv, Madrid; Pedro Barri, Hospital Universitario Quirón Dexeus, Barcelona; Juan Antonio García-Velasco, IVI Madrid, Madrid; Manuel Fernández-Sánchez, IVI Sevilla, Seville; Fernando Sánchez Martin, Ginemed, Seville; Ernesto Bosch, IVI Valencia, Valencia; José Serna, IVI Zaragoza, Zaragoza; Gemma Castillon; IVI Barcelona, Barcelona; Rafael Bernabeu, Instituto Bernabeu, Alicante; Marcos Ferrando, IVI Bilbao, Bilbao. |
| UK | Stuart Lavery, Boston Place Clinic, London, England; Marco Gaudoin, Glasgow Centre for Reproductive Medicine, Glasgow, Scotland. |
aIn total, 37 sites from 11 countries participated in ESTHER-1 and 32 of the 37 sites participated in ESTHER-2
ESTHER Evidence-based Stimulation Trial with Human rFSH in Europe and Rest of World
Author contribution
JH participated in project conception and design, assisted with data collection and manuscript revision, and contributed to interpretation of the data. AKAH and BM contributed to interpretation of the data and assisted with manuscript revision. J-CA led project conception and design, and was responsible for interpretation of the data and preparation of the manuscript. All authors have read and approved the final manuscript to be published.
Funding
The trials were funded by Ferring Pharmaceuticals.
Data availability
Not applicable.
Declarations
Ethics approval
The trials were approved by the applicable ethics committees (i.e., independent ethics committees in Europe, ethics committees in Brazil and Russia, and research ethics boards in Canada) and performed in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonisation Guidelines for Good Clinical Practice, and local regulatory requirements.
Consent to participate
Informed consent was obtained from all participants included in the trials. Informed consent for collection of follow-up data of neonatal outcomes was obtained from each woman and her partner, as applicable per local regulations.
Consent for publication
Consent for publication was obtained from all participants included in the trials as part of the trial informed consent. Trial data were de-identified.
Competing interests
AKAH has received a consulting fee from Ferring Pharmaceuticals for review of all cases of congenital anomalies in the ESTHER-1 and ESTHER-2 trials. BM and J-CA are employees of Ferring Pharmaceuticals. J-CA has patent applications on follitropin delta granted and pending. The authors report no other relevant financial or non-financial conflicts of interests related to the trials.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Niederberger C, Pellicer A, Cohen J, Gardner DK, Palermo GD, O’Neill CL, et al. Forty years of IVF. Fertil Steril. 2018;110(2):185–324.e5. doi: 10.1016/j.fertnstert.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Bonduelle M, Mannaerts B, Leader A, Bergh C, Passier D, Devroey P. Prospective follow-up of 838 fetuses conceived after ovarian stimulation with corifollitropin alfa: comparative and overall neonatal outcome. Hum Reprod. 2012;27(7):2177–2185. doi: 10.1093/humrep/des156. [DOI] [PubMed] [Google Scholar]
- 3.Vuong LN, Ly TT, Nguyen NA, Nguyen LMT, Le XTH, Le TK, et al. Development of children born from freeze-only versus fresh embryo transfer: follow-up of a randomized controlled trial. Fertil Steril. 2020;114(3):558–566. doi: 10.1016/j.fertnstert.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Ulloa-Aguirre A, Timossi C. Biochemical and functional aspects of gonadotrophin-releasing hormone and gonadotrophins. Reprod BioMed Online. 2000;1(2):48–62. doi: 10.1016/S1472-6483(10)61901-3. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Chen X, Zhang X, Zhang W, Li Y, Yin H, Shao H, Chen G. Comparative assessment of glycosylation of a recombinant human FSH and a highly purified FSH extracted from human urine. J Proteome Res. 2016;15(3):923–932. doi: 10.1021/acs.jproteome.5b00921. [DOI] [PubMed] [Google Scholar]
- 6.World Intellectual Property Organization (WO 2009/127826 Al). Recombinant FSH including alpha 2,3- and alpha 2,6-sialylation. Available from: https://patentscope.wipo.int/search/en/WO2009127826. Accessed 20 April 2021.
- 7.Olsson H, Sandström R, Grundemar L. Different pharmacokinetic and pharmacodynamic properties of recombinant follicle-stimulating hormone (rFSH) derived from a human cell line compared with rFSH from a non-human cell line. J Clin Pharmacol. 2014;54(11):1299–1307. doi: 10.1002/jcph.328. [DOI] [PubMed] [Google Scholar]
- 8.Arce J-C, Nyboe Andersen A, Fernández-Sánchez M, Visnova H, Bosch E, García-Velasco JA, et al. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimüllerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102(6):1633–40.e5. doi: 10.1016/j.fertnstert.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Bosch E, Havelock J, Martin FS, Rasmussen BB, Klein BM, Mannaerts B. et al; ESTHER-2 Study Group. Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind Phase 3 safety trial. Reprod BioMed Online. 2019;38(2):195–205. doi: 10.1016/j.rbmo.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Sánchez M, Visnova H, Yuzpe A, Klein BM, Mannaerts B. Arce J-C; ESTHER-1 and ESTHER-2 Study Group. Individualization of the starting dose of follitropin delta reduces the overall OHSS risk and/or the need for additional preventive interventions: cumulative data over three stimulation cycles. Reprod BioMed Online. 2019;38(4):528–537. doi: 10.1016/j.rbmo.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara O, Klein BM, Arce J-C, Japanese Follitropin Delta Phase 2 Trial Group. Randomized, assessor-blind, antimüllerian hormone–stratified, dose-response trial in Japanese in vitro fertilization/intracytoplasmic sperm injection patients undergoing controlled ovarian stimulation with follitropin delta. Fertil Steril. 2021;115(6):1478-86. 10.1016/j.fertnstert.2020.10.059. [DOI] [PubMed]
- 12.Ishihara O, Arce J-C, Japanese Follitropin Delta Phase 3 Trial (STORK) Group. Individualized follitropin delta dosing reduces OHSS risk in Japanese IVF/ICSI patients: a randomized controlled trial. Reprod BioMed Online. 2021;42(5):909-18. 10.1016/j.rbmo.2021.01.023. [DOI] [PubMed]
- 13.Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce J-C, ESTHER-1 Study Group Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107(2):387–96.e4. doi: 10.1016/j.fertnstert.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 14.American Society for Reproductive Medicine Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 15.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, van der Poel S, International Committee for Monitoring Assisted Reproductive Technology. World Health Organization The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum Reprod. 2009;24(11):2683–2687. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency guideline EMEA/CHMP/313666/2005. Guideline on the exposure to medicinal products during pregnancy: need for post-authorisation data. May 2006. Available from: https://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/11/WC500011303.pdf. Accessed 20 April 2021.
- 18.Henningsen AA, Wennerholm UB, Gissler M, Romundstad LB, Nygren KG, Tiitinen A, Skjaerven R, Nyboe Andersen A, Lidegaard Ø, Forman JL, Pinborg A. Risk of stillbirth and infant deaths after assisted reproductive technology: a Nordic study from the CoNARTaS. Hum Reprod. 2014;29(5):1090–1096. doi: 10.1093/humrep/deu031. [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 20.Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(4):330–353. doi: 10.1093/humupd/dmt006. [DOI] [PubMed] [Google Scholar]
- 21.Henningsen AKA, Bergh C, Skjaerven R, Tiitinen A, Wennerholm UB, Romundstad LB, Gissler M, Opdahl S, Nyboe Andersen A, Lidegaard Ø, Forman JL, Pinborg A. Trends over time in congenital malformations in live-born children conceived after assisted reproductive technology. Acta Obstet Gynecol Scand. 2018;97(7):816–823. doi: 10.1111/aogs.13347. [DOI] [PubMed] [Google Scholar]
- 22.Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, Otterblad PO. Congenital malformations in infants born after in vitro fertilization in Sweden. Birth Defects Res A Clin Mol Teratol. 2010;88(3):137–143. doi: 10.1002/bdra.20645. [DOI] [PubMed] [Google Scholar]
- 23.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(5):485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 24.Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437–443. doi: 10.1007/s10815-004-8760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen J, Jiang J, Ding C, Dai J, Liu Y, Xia Y, et al. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97(6):1331–7.e4. doi: 10.1016/j.fertnstert.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 26.Giorgione V, Parazzini F, Fesslova V, Cipriani S, Candiani M, Inversetti A, Sigismondi C, Tiberio F, Cavoretto P. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):33–42. doi: 10.1002/uog.18932. [DOI] [PubMed] [Google Scholar]
- 27.Belva F, Bonduelle M, Roelants M, Verheyen G, Van Landuyt L. Neonatal health including congenital malformation risk of 1072 children born after vitrified embryo transfer. Hum Reprod. 2016;31(7):1610–1620. doi: 10.1093/humrep/dew103. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101(1):128–133. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil Steril. 2016;106(7):1703–1708. doi: 10.1016/j.fertnstert.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 30.Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. 2018;24(1):35–58. doi: 10.1093/humupd/dmx031. [DOI] [PubMed] [Google Scholar]
- 31.Pinborg A, Henningsen AA, Loft A, Malchau SS, Forman J, Nyboe AA. Large baby syndrome in singletons born after frozen embryo transfer (FET): is it due to maternal factors or the cryotechnique? Hum Reprod. 2014;29(3):618–627. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- 32.Sha T, Yin X, Cheng W, Massey IY. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril. 2018;109(2):330–42.e9. doi: 10.1016/j.fertnstert.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Stormlund S, Sopa N, Zedeler A, Bogstad J, Prætorius L, Nielsen HS, Kitlinski ML, Skouby SO, Mikkelsen AL, Spangmose AL, Jeppesen JV, Khatibi A, la Cour Freiesleben N, Ziebe S, Polyzos NP, Bergh C, Humaidan P, Andersen AN, Løssl K, Pinborg A. Freeze-all versus fresh blastocyst transfer strategy during in vitro fertilisation in women with regular menstrual cycles: multicentre randomised controlled trial. BMJ. 2020;370:m2519. doi: 10.1136/bmj.m2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 215 kb)
Data Availability Statement
Not applicable.