Abstract
BARLEYmax, a barley variety, and cocoa polyphenols (CPPs) have been reported to affect bacterial metabolites in the colon. This study aimed to evaluate the combined effects of BARLEYmax and CPPs supplementation on fecal microbiota in vitro using pig feces for 48 h. The relative abundances of the family Clostridiaceae and the genus Clostridium and ammonia–nitrogen production were decreased by both BARLEYmax and CPP supplementation, and there was a positive correlation between their abundances and the ammonia–nitrogen concentration. Although acetate and n-butyrate production was decreased by CPP supplementation, their concentrations were maintained at a higher level in the BARLEYmax + CPP group than in the cellulose (control) and cellulose + CPP groups. Therefore, this study demonstrated that a combination of BARLEYmax and CPPs may be beneficial in maintaining higher short-chain fatty acid production and the elimination of potentially harmful factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-021-00959-z.
Keywords: BARLEYmax, n-butyrate, Cocoa polyphenol, Colonic microbiota, Short-chain fatty acid
Introduction
Barley (Hordeum vulgare L.) is one of the major cereals consumed worldwide and is widely used as a raw material in various food products. Barley contains β-glucan, a water-soluble dietary fiber consisting of β-(1-3)-(1-4) linkages of glucose residues. Supplementation with barley or barley β-glucan has been reported to alter the microbial composition and increase short-chain fatty acid (SCFA) production in the cecum of rats (Zhong et al., 2015) and in human feces in vitro (Hughes et al., 2008). In addition, the consumption of barley high in β-glucan has been reported to suppress elevated postprandial glucose levels in humans (Higa et al., 2019). Therefore, barley and barley β-glucan are known to have beneficial physiological properties.
BARLEYmax (Tantangara) is a barley variety developed with the non-genetic improvement of Himalaya 292 (Hordeum vulgare var. Himalaya 292) (Morell et al., 2003), which is rich in fructan, resistant starch (RS), and β-glucan (Aoe et al., 2019). Recently, the physiological properties of BARLEYmax has been investigated. It was reported that rats fed with BARLEYmax showed an altered microbial composition and increased SCFA production not only in the cecum but also in the distal colon due to the presence of fructan and RS in BARLEYmax (Aoe et al., 2019). Furthermore, an in vitro microbial fermentation study revealed that SCFA production was increased for an extended period due to the different compositions of indigestible carbohydrates in BARLEYmax compared with a conventional barley variety (Sato et al., 2020). Therefore, BARLEYmax was suggested to have greater beneficial effects on gut microbial fermentation compared with the effects of other conventional barley varieties.
Polyphenols are also regarded as materials that can alter the intestinal environment. Several polyphenols have bacteriostatic or bactericidal activities (Selma et al., 2009). It has been reported that polyphenol absorption in the small intestine is very low, and more than 90% of these compounds reach the colon (Tuohy et al., 2012). Several types of polyphenols and their metabolites have been reported to affect the gut microbial composition and fermentation characteristics (Kilua et al., 2019; Nagata et al., 2018; Zduńczyk et al., 2006). Cocoa (Theobroma cacao L.) has numerous health benefits owing to its high content of polyphenols, including condensed tannins (Hammerstone et al., 1999; Sánchez-Rabaneda et al., 2003). Cocoa-derived proanthocyanidins have been reported to be metabolized by the intestinal microbiota to products with lower molecular weights (Rios et al., 2003). In a previous study, the consumption of cocoa-derived procyanidins suppressed hyperglycemia and obesity in mice fed a high-fat diet (Yamashita et al., 2012), which demonstrated the potential biological effects of cocoa polyphenols (CPPs). However, in studies of CPPs, comprehensive microbial analyses using high-throughput 16S rRNA gene sequencing have rarely been reported. Overall, the evaluation of the effects of CPPs on the microbiota and their fermentation has been inadequate.
Several studies have reported the combined effects of fermentable carbohydrates and polyphenols on the intestinal microbiota (Kilua et al., 2019; Nagata et al., 2018; Zduńczyk et al., 2006). In a previous study, polyphenols derived from adzuki bean suppressed the production of ammonia–nitrogen, a toxic metabolite, both in vitro and in vivo (Nagata et al., 2018). In comparison with individual supplementation, a combination of adzuki bean polyphenols and inulin reduced the ammonia–nitrogen levels with a higher production of propionate in the rat cecum (Nagata et al., 2018). In another in vitro study, purple sweet potato polyphenols modulated the microbial composition depending on the fermentability of the added dietary fiber and suppressed the production of p-cresol (Kilua et al., 2019). Therefore, supplementation with a combination of fermentable carbohydrates and polyphenols instead of individual supplementation may have different beneficial physiological effects. However, the effects of BARLEYmax or CPPs in combination with other food-derived components on physiological properties are not well understand. Therefore, the aim of the current study was to assess the effects of a combination of BARLEYmax and CPPs on the intestinal microbiota and bacterial metabolites in vitro using pig fecal bacteria.
Although the microbial fermentation characteristics of several food materials in in vitro studies with pig feces have been reported (Han et al., 2014; Kilua et al., 2019; Nagata et al., 2018; Sato et al., 2020), it is not appropriate to generalize the results to humans. Nevertheless, it has been reported that the abundance of the predominant bacterial phyla Bacteroidetes and Firmicutes is similar between pig and human colons (Leser et al., 2002). Furthermore, in a previous study on adzuki bean polyphenols, similar microbial fermentation characteristics have been observed in the rat cecum and in vitro using pig feces (Nagata et al., 2018). Therefore, this study could serve as a reference for future investigations.
Materials and methods
Preparation of enzyme-resistant fraction (ERF) of BARLEYmax
Powdered BARLEYmax was supplied by Teijin Limited (Tokyo, Japan). For in vitro colonic fermentation, an ERF of BARLEYmax was obtained using amyloglucosidase (300 U/20 g BARLEYmax; Sigma-Aldrich, St. Louis, MO, USA) and pancreatin (0.75 g/20 g BARLEYmax; Sigma-Aldrich) as previously described (Han et al., 2014). The collected ERF was dried, weighed, and ground using a general mill (ACM-40; Shimomura, Niigata, Japan). The yield of the ERF was calculated based on the weight, which was 34.5%.
The proximate compositions of untreated BARLEYmax and the ERF of BARLEYmax were determined according to the analytical methods of the Association of Official Analytical Chemists (AOAC) as follows: moisture (AOAC 925. 10), protein (AOAC 920. 87, nitrogen-protein conversion factor: 5.83), lipids (AOAC 920. 85), ash (AOAC 923. 03), and total starch and RS (AOAC 2002. 02) (Table 1). Each measurement was repeated three times.
Table 1.
Proximate composition of BARLEYmax and ERF of BARLEYmax
| (g/100 g) | BARLEYmax | BARLEYmax-ERF |
|---|---|---|
| Moisture | 0.97 ± 0.11 | 1.07 ± 0.13 |
| Protein | 10.4 ± 0.1 | 12.8 ± 0.1 |
| Lipid | 5.94 ± 0.06 | 1.30 ± 0.06 |
| Ash | 1.90 ± 0.02 | 4.51 ± 0.04 |
| Carbohydrate | 80.8 ± 0.1 | 80.4 ± 0.1 |
| Total starch | 29.1 ± 0.4 | 11.9 ± 0.2 |
| RS | 2.70 ± 0.08 | NA |
Data are expressed as the mean ± SE (n = 3)
ERF, enzyme-resistant fraction; RS, resistant starch
Preparation of CPP extract
Cocoa powder was supplied by DAITOCACAO Co., Ltd. (Tokyo, Japan). Lipids in the cocoa powder were removed with a fourfold volume of hexane in an ultrasonic bath for 10 min and centrifuged at 2000 × g for 10 min at room temperature (20 °C). CPPs in the defatted cocoa powder were extracted and purified as previously described (Feliciano et al., 2014) with a slight modification. The defatted cocoa powder was suspended in 70% aqueous acetone (v/v) in an ultrasonic bath for 1 h. The suspension was centrifuged at 2000 × g for 10 min at room temperature (20 °C), and the supernatant was collected. Acetone was removed using a rotary evaporator, and the remaining extract was solubilized in distilled water. The extract was then centrifuged at 2000 × g for 20 min at room temperature (20 °C) to eliminate insoluble materials. The extract solution was applied directly to pretreated Sephadex LH-20 resin (GE Healthcare, Uppsala, Sweden). First, distilled water was added to the elution column to remove impurities such as free sugars, proteins, and low-molecular-weight polyphenol fractions. Subsequently, ethanol and 80% acetone (v/v) were added consecutively, and the extract was collected respectively. Acetone and ethanol were removed completely using a rotary evaporator. The remaining extract fraction was solubilized in distilled water, combined, and vacuum filtered to remove bacteria using a 0.22 µm sterile disposable filter (Nalgene Rapid-Flow Disposable Filter Units with PES Membrane; Thermo Fisher Scientific, Tokyo, Japan). The collected solution was used as a CPP source for in vitro fermentation.
The total polyphenol content of the solution containing CPPs was estimated using the Folin-Ciocalteu method as previously described (Kilua et al., 2019), which was 10.0 mg/mL catechin equivalents.
In vitro colonic fermentation
In vitro anaerobic fermentation was performed using a mixture of fresh pig feces as previously described (Kilua et al., 2019). This experimental design was approved by the Animal Experiment Committee of Obihiro University of Agriculture and Veterinary Medicine (Obihiro, Japan) (approval number, 18–32). In brief, fresh feces were collected directly from the anus of three live pigs (Mangalitsa, approximately 6 months old), which were reared outside without any antibiotic administration, from the Tokachi Royal Mangalica Farm of Marukatsu Co., Ltd. (Obihiro, Japan). Fecal slurry (10 × dilution) was prepared by homogenizing equal amounts of samples from the three pigs with 0.85% NaCl solution and used as inocula. The pH-controlled small-scale fermenters (220 mL working volume; Able & Biott, Tokyo, Japan) were filled with fecal slurry, nutrient broth (NB; Difco, Sparks, MD, USA), and one of the following groups of samples: cellulose (CEL; Sigma-Aldrich), ERF of BARLEYmax (BM), CEL + CPP, and BM + CPP. The final concentrations of the carbohydrate source (cellulose or carbohydrates in BARLEYmax), nitrogen source (NB or NB + proteins in BARLEYmax), CPP extract, and pig feces in the fermenters were 3%, 0.8%, 0.16%, and 2% (w/v), respectively. The fermenters were maintained anaerobically using CO2 gas at 37 °C with a lower pH limit of 5.50. The composition of the fermenters and experimental conditions have been established in our previous studies (Han et al., 2014; Kilua et al., 2019; Nagata et al., 2018). Sampling (6 mL) and pH measurements were performed at 0, 6, 12, 24, and 48 h. After 48 h of incubation, aliquots (1 mL) were collected and stored at −80 °C until DNA extraction, and the remaining samples were stored at −30 °C until further analysis. In vitro fermentation was performed five times for each experimental group.
Bacterial DNA extraction, next-generation sequencing, and analysis of 16S rRNA gene sequences
Bacterial DNA in the aliquots was extracted as described by Yu and Morrison (2004) and purified using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The variable regions V3 to V4 of the 16S rRNA gene were amplified from the purified DNA, and paired-end sequencing of the amplicons was performed using the Illumina MiSeq System (Illumina, San Diego, CA, USA) as previously described (Nagata et al., 2020).
Sequence data of the 16S rRNA gene were analyzed using the Quantitative Insight Into Microbial Ecology (QIIME) software (version 1.9.1) (Warren et al., 2018), and the generated biome table was normalized using an equal subsampling size of 8484 sequences, which is the minimum number among the samples. The number of sequences before normalization and Good’s coverage after normalization for each sample are shown in Supplementary Table S1. The remaining high-quality reads were clustered into operational taxonomic units (OTUs) with a 97% sequence identity threshold. The distances between bacterial communities in different samples were calculated using the weighted UniFrac distance metric, and principal coordinate analysis (PCoA) plots were generated in QIIME (Lozupone and Knight, 2005). Calypso version 8.84 was used for the quantitative visualization of the microbial community composition at the phylum, family, and genus levels (Zakrzewski et al., 2017).
SCFA analysis
SCFA concentrations in the aliquots were determined by high-performance liquid chromatography (HPLC; LC-10AD; Shimadzu, Kyoto, Japan) according to a previously described procedure (Nagata et al., 2020). Samples for HPLC were prepared as previously described (Han et al., 2016).
Ammonia–nitrogen analysis
Ammonia–nitrogen concentrations in the aliquots were determined using a commercially available kit (Wako Pure Chemical Industry, Ltd., Tokyo, Japan) according to the manufacturer’s instructions. The absorbance was read at 620 nm using a microplate reader (Multiscan FC; Thermo Fisher Scientific).
Statistical analysis
Data are presented as the mean ± standard error (SE) (n = 5). Two-way ANOVA (SPSS version 17; IBM Inc., Chicago, IL, USA) was performed for all data to assess the effect of the carbohydrate source (cellulose and BARLEYmax), CPP extract, and their interaction. When significant interactions were found, individual means were analyzed by one-way ANOVA with Tukey’s test (SPSS version 17). Correlations between parameters were assessed by Pearson’s correlation analysis. A p value less than 0.05 was considered as statistically significant.
Results and discussion
Microbial diversity and abundance in fermenters
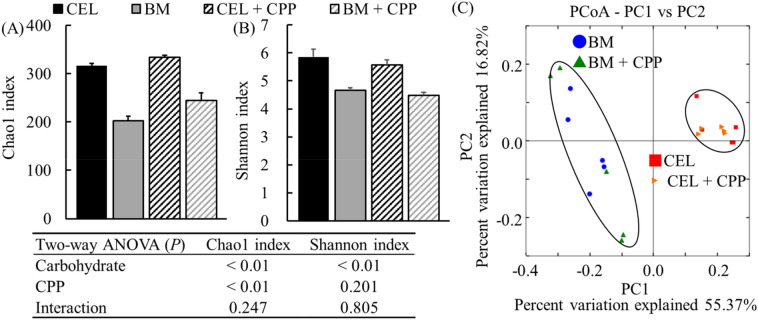
Microbial 16S rRNA gene sequences from samples obtained at 48 h of incubation were analyzed. The α-diversity at the OTU level was measured using the Chao1 and Shannon indices. These indices were significantly decreased by BARLEYmax (p < 0.01; Fig. 1A, B). In addition, the Chao1 index was significantly increased in the presence of CPPs (p < 0.01; Fig. 1A). The β-diversity of the microbial community data in the PCoA plot showed distinct clustering due to different carbohydrate sources (Fig. 1C). A lower α-diversity is often reported to be associated with higher SCFA production (Genda et al., 2018; Nagata et al., 2020). Furthermore, in a previous in vitro study, BARLEYmax decreased the α-diversity with increased SCFA production and altered the microbial composition (Sato et al., 2020). In this study, BARLEYmax increased SCFA production (discussed in detail later); thus, the results are in agreement with previous findings.
Fig. 1.
Measurement of α-diversity using the Chao1 (A) and Shannon indices (B) and PCoA plot of β-diversity (C). Samples were obtained from the fermenters at 48 h during in vitro colonic fermentation. The data on α-diversity measured at the OTU level are expressed as mean ± SE (n = 5). Two-way ANOVA was performed to assess the effects of the carbohydrate source, CPP extract, and their interaction. Differences at p < 0.05 were considered as statistically significant. The weighted UniFrac distance metric in QIIME at the OTU level was used to determine the β-diversity. CEL, cellulose; BM, BARLEYmax; CPP, cocoa polyphenol; PCoA, principal coordinate analysis
The relative abundances of the phyla Bacteroidetes and Actinobacteria were significantly increased by BARLEYmax (p < 0.01); however, the abundance of the phylum Firmicutes was not affected by either BARLEYmax or CPP supplementation (Table 2). The relative abundances of the genera Prevotella and Bifidobacterium, belonging to the phyla Bacteroidetes and Actinobacteria, respectively, were significantly increased by BARLEYmax (p < 0.01; Table 2), which are consistent with the findings of a previous study using BARLEYmax (Sato et al., 2020). The genus Prevotella has been reported to readily ferment barley β-glucan and increase its abundance (Hughes et al., 2008), and Prevotella spp. are known to produce acetate and propionate (Koh et al., 2016; Payne et al., 2011). The relative abundance of the genus Lactobacillus was also significantly increased by BARLEYmax (p < 0.01; Table 2). In previous studies, the genera Bifidobacterium and Lactobacillus were increased by supplementation with indigestible carbohydrates, including β-glucan, fructan, and RS (Bird et al., 2007; Ito et al., 2011; Snart et al., 2006).
Table 2.
Relative abundance of microbial taxa at the phylum, family, and genus levels in fermenters at 48 h of in vitro colonic fermentation
| Two-way ANOVA (P) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CEL | BM | CEL + CPP | BM + CPP | Carbohydrate | CPP | Interaction | |||
| Phylum | Family | Genus | Relative abundance (%) | ||||||
| Firmicutes | 54.1 ± 3.7 | 57.9 ± 3.6 | 57.9 ± 3.3 | 51.7 ± 1.2 | 0.705 | 0.705 | 0.127 | ||
| Lactobacillus | 1.68 ± 0.19b | 13.1 ± 3.3a | 6.95 ± 0.64ab | 9.36 ± 1.5a | < 0.01 | 0.672 | < 0.05 | ||
| Clostridiaceae | 3.23 ± 0.49 | 0.84 ± 0.31 | 1.87 ± 0.18 | 0.29 ± 0.03 | < 0.01 | < 0.01 | 0.201 | ||
| Clostridium | 2.79 ± 0.58 | 0.26 ± 0.09 | 1.54 ± 0.22 | 0.23 ± 0.03 | < 0.01 | 0.080 | 0.071 | ||
| Bacteroidetes | 31.7 ± 3.1b | 35.9 ± 3.6ab | 21.7 ± 0.9c | 41.7 ± 1.1a | < 0.01 | 0.414 | < 0.01 | ||
| Prevotella | 16.2 ± 1.3 | 35.1 ± 3.7 | 14.8 ± 0.9 | 40.7 ± 1.4 | < 0.01 | 0.322 | 0.117 | ||
| Actinobacteria | 2.02 ± 0.30 | 5.68 ± 1.15 | 3.61 ± 0.70 | 5.63 ± 1.08 | < 0.01 | 0.394 | 0.365 | ||
| Bifidobacterium | 0.11 ± 0.02 | 4.53 ± 1.17 | 0.15 ± 0.02 | 4.05 ± 1.02 | < 0.01 | 0.775 | 0.741 | ||
Data are expressed as the mean ± SE (n = 5). Two-way ANOVA was performed to assess the effects of the carbohydrate source, CPP extract, and their interaction. When significant interactions were observed (p < 0.05), the means among the groups were analyzed by ANOVA paired with Tukey’s test.
CEL, cellulose; BM, BARLEYmax; CPP, cocoa polyphenol
a−cMean values within a row with different letters are significantly different (p < 0.05).
The relative abundance of the family Clostridiaceae was significantly decreased by both BARLEYmax and CPP supplementation (p < 0.01; Table 2). In addition, the abundance of the genus Clostridium was significantly decreased by BARLEYmax (p < 0.01); however, its decrease in the presence of CPPs was not statistically significant (p = 0.080; Table 2). Furthermore, the abundances of Clostridiaceae and Clostridium were the lowest in the BM + CPP group among the groups; however, there was no statistical interaction. In previous in vitro and in vivo studies, the relative abundance of the genus Clostridium was decreased by supplementation with the BARLEYmax (Aoe et al., 2019; Sato et al., 2020). Among the Clostridium spp., C. perfringens is well known as a causative agent of several forms of enteric diseases due to the production of exotoxins (Shindo et al., 2015), and it has been reported to decrease with higher SCFA production and lower pH (Pelpolage et al., 2019). Furthermore, green tea extract containing flavanols, including catechins, has been reported to inhibit the growth of Clostridium spp. in vitro (Ahn et al., 1991) and in human feces (Okubo et al., 1992). Therefore, in this study, BARLEYmax and CPPs might inhibit the growth of Clostridium spp. independently, and their combination might have the maximum inhibitory effect.
The relative abundances of the phylum Bacteroidetes and the genus Lactobacillus were significantly affected by the interaction between the carbohydrate source and CPP extract (p < 0.05). The abundances of the phylum Bacteroidetes and the genus Lactobacillus were not significantly different between the BM and BM + CPP groups, and their abundances were lower and higher, respectively, in the CEL + CPP group than in the CEL group. Purple sweet potato polyphenols have been reported to affect the microbial composition in vitro depending on the fermentability of the added dietary fiber (Kilua et al., 2019). Therefore, a some of the bacterial taxa might be affected by the CPP extract when supplemented with non-fermentable dietary fibers such as cellulose.
pH and SCFA concentrations in fermenters
The pH of the fermenters was significantly reduced by BARLEYmax after 6 h of incubation (p < 0.01; Fig. 2).
Fig. 2.
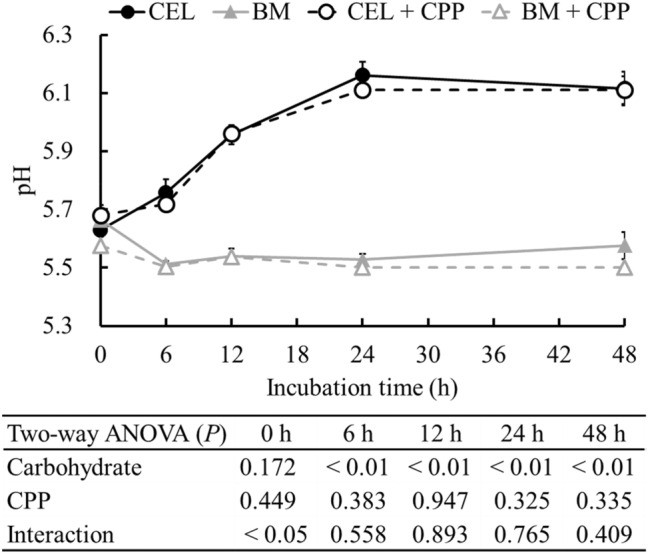
pH in fermenters during in vitro colonic fermentation at 0, 6, 12, 24, and 48 h of incubation. Data are expressed as the mean ± SE (n = 5). Two-way ANOVA was performed to assess the effects of carbohydrate source, CPP, and their interaction. Differences at p < 0.05 were considered as statistically significant. CEL, cellulose; BM, BARLEYmax; CPP, cocoa polyphenol
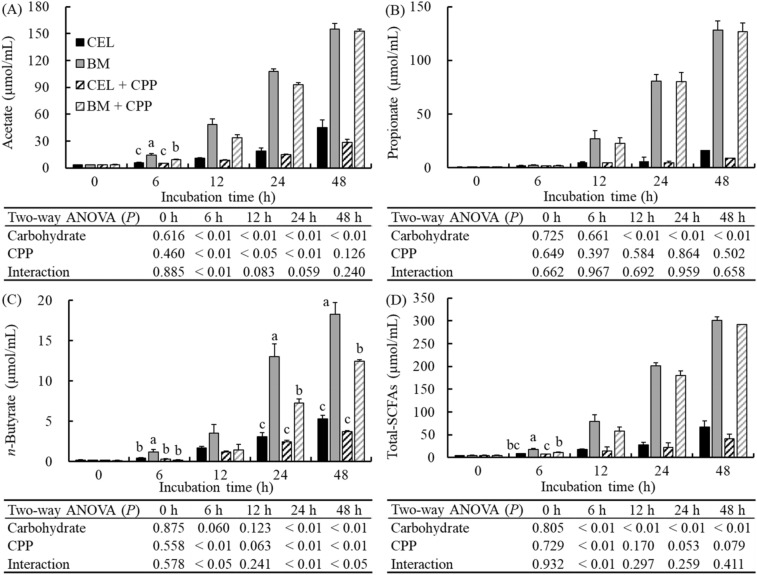
Supplementation with BARLEYmax significantly increased the acetate and total SCFA concentrations in the fermenters after 6 h of incubation (p < 0.01; Fig. 3A, D), and the propionate and n-butyrate concentrations were significantly increased after 12 and 24 h of incubation respectively (p < 0.01; Fig. 3B, C), which are consistent with previous findings (Aoe et al., 2019; Sato et al., 2020).
Fig. 3.
Concentration of acetate (A), propionate (B), n-butyrate (C), and total-SCFAs (D) in fermenters during in vitro colonic fermentation at 0, 6, 12, 24, and 48 h of incubation. Data are expressed as the mean ± SE (n = 5). Two-way ANOVA was performed to assess the effects of carbohydrate source, CPP, and their interaction. If significant interactions were observed (p < 0.05), means among the groups were analyzed by ANOVA paired with Tukey’s test. a−cMean values at the same time point designated by different letters are significantly different (p < 0.05). CEL, cellulose; BM, BARLEYmax; CPP, cocoa polyphenol
In addition, the acetate and total SCFA concentrations were significantly decreased by CPP supplementation during the 6–24 h of incubation and at 6 h of incubation, respectively (p < 0.05; Fig. 3A, D). After 24 h of incubation, the total SCFA concentration tended to be decreased by CPP supplementation; however, this was not statistically significant (24 h, p = 0.053; 48 h, p = 0.079; Fig. 3D). Furthermore, the acetate and total SCFA concentrations at 6 h of incubation were significantly affected by the interaction between the carbohydrate source and CPP extract (p < 0.01); their concentrations were significantly lower in the BM + CPP group than in the BM group (p < 0.05; Fig. 3A, D). In addition, the acetate concentration was significantly higher in the BM + CPP group than in the CEL and CEL + CPP groups, and the total SCFA concentration was significantly higher in the BM + CPP group than in the CEL + CPP group (p < 0.05; Fig. 3A, D). The n-butyrate concentration was significantly decreased by CPP supplementation (p < 0.01) and was significantly affected by the interaction of the carbohydrate source and CPP extract at 6, 24, and 48 h of incubation (p < 0.05). In addition, its concentration tended to be decreased by CPP supplementation after 12 h of incubation; however, this was not statistically significant (p = 0.063; Fig. 3C). The concentration of n-butyrate in the BM group was the highest among the groups (p < 0.05), and it was significantly higher in the BM + CPP group than in the CEL and CEL + CPP groups at 24 and 48 h of incubation (p < 0.05; Fig. 3C). The propionate concentration was not affected by CPP supplementation.
The results of this study are in agreement with those of previous studies on grapefruit flavonoids in the rat cecum (Zduńczyk et al., 2006) and adzuki bean-derived polyphenols in vitro (Nagata et al. 2018), which showed the suppression of acetate and n-butyrate production. Several n-butyrate-producing bacteria have been identified in recent years (Koh et al., 2016). However, the effect of CPPs on n-butyrate-producing bacteria was not observed in this study (Supplementary Table S2). In addition, n-butyrate-producing bacteria were not detected in previous polyphenol studies (Nagata et al., 2018; Zduńczyk et al., 2006). The relationship between the abundance of each microbial taxon and decreased n-butyrate production due to polyphenols was unclear in this study; thus, further studies should be carried out to clarify their relationship.
Ammonia–nitrogen concentration in fermenters
Ammonia is a toxic metabolite produced by intestinal bacteria, which promotes tumorigenesis in the colon (Ichikawa and Sakata, 1998). In this study, ammonia–nitrogen production in the fermenters was significantly suppressed by BARLEYmax during the 6–48 h of incubation (p < 0.01; Fig. 4), which was accompanied by higher SCFA production and lower pH, as observed in previous studies using BARLEYmax (Sato et al., 2020) and other indigestible carbohydrates such as RS and dietary fiber (Han et al., 2014). The production of ammonia–nitrogen was also significantly suppressed by CPP supplementation during the 6–24 h of incubation (p < 0.01; Fig. 4). Moreover, the ammonia–nitrogen concentration was affected by the interaction of the carbohydrate source and CPP extract at 6 and 24 h of incubation, and the concentration was significantly lower in the CEL + CPP group than in the CEL group (p < 0.05; Fig. 4).
Fig. 4.
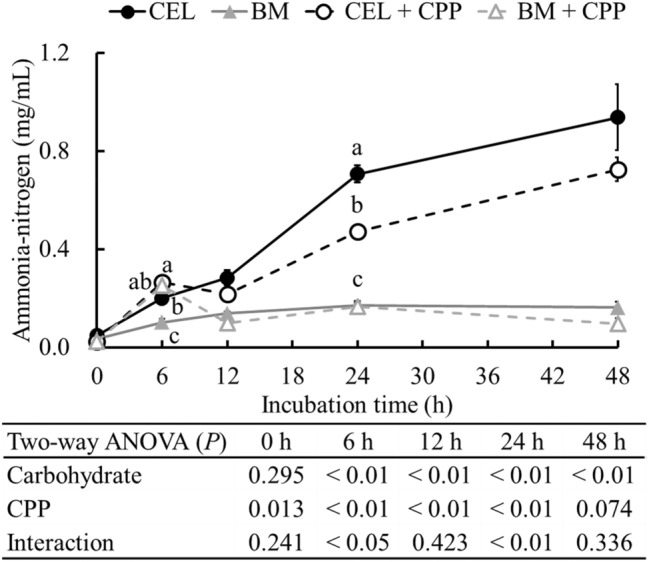
Ammonia–nitrogen concentration in fermenters during in vitro colonic fermentation at 0, 6, 12, 24, and 48 h of incubation. Data are expressed as the mean ± SE (n = 5). Two-way ANOVA was performed to assess the effects of the carbohydrate source, CPP extract, and their interaction. If significant interactions were observed (p < 0.05), means among the groups were analyzed by ANOVA paired with Tukey’s test. a−cMean values at the same time point designated by different letters are significantly different (p < 0.05). CEL, cellulose; BM, BARLEYmax; CPP, cocoa polyphenol
In previous studies, adzuki bean-derived polyphenols (Nagata et al., 2018) and grapefruit flavonoids (Zduńczyk et al., 2006) have been reported to suppress ammonia–nitrogen production. In this study, the ammonia–nitrogen concentration at 48 h of incubation was positively correlated with the relative abundances of the Clostridiaceae family (r = 0.640; p < 0.01) and the genus Clostridium (r = 0.607; p < 0.01) (Supplementary Fig. S1). Clostridium spp., especially C. perfringens, are known as ammonia-producing bacteria, and their growth can be inhibited by higher levels of SCFAs and polyphenols. In addition, a decrease in both ammonia–nitrogen production and the abundance of the genus Clostridium has been observed in previous in vitro studies (Kilua et al., 2019; Sato et al., 2020). Therefore, the growth inhibition of Clostridium spp. by BARLEYmax with higher SCFA production and CPP levels could reduce ammonia–nitrogen production. However, the ammonia–nitrogen concentration was not significantly different between the BM and BM + CPP groups; thus, further analysis of other toxic metabolites, such as p-cresol, should be carried out. A combination of fermentable carbohydrates and polyphenols might decrease p-cresol production (Kilua et al., 2019).
In conclusion, both BARLEYmax and CPP supplementation inhibited the growth of Clostridiaceae and Clostridium in an additive manner. Consequently, ammonia–nitrogen production might be suppressed. Although the beneficial effects of cocoa-derived procyanidins on hyperglycemia and obesity in mice have been previously reported (Yamashita et al., 2012), CPP supplementation was found to disrupt the production of SCFA, especially n-butyrate, in the current study. However, SCFA concentrations were maintained at a higher level in the BARLEYmax + CPP group compared with the basal level in the control group. Therefore, BARLEYmax may alleviate the reduction in SCFA production in the presence of CPPs, and simultaneous supplementation with BARLEYmax and CPPs may eliminate harmful factors, such as bacterial toxic metabolites, in the host. However, the effects of their combination in the human body remain unclear as pig fecal bacteria were used in this study. Therefore, further studies with animals and humans or in vitro studies with human colonic bacteria should be conducted. In addition, the polyphenol composition of CPP extracts should be determined to clarify which polyphenols are responsible for these effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Marukatsu Co., Ltd. (Obihiro, Hokkaido, Japan) for providing fresh pig feces for the study and Editage (www.editage.com) for English language editing.
Funding
This research was funded by Teijin Limited.
Declarations
Conflict of interest
Michihiro Fukushima received financial research support from Teijin Limited (Tokyo, Japan).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn YJ, Kawamura T, Kim M, Yamamoto T, Mitsuoka T. Tea polyphenols: selective growth inhibitors of Clostridium spp. Agricultural and Biological Chemistry. 1991;55:1425–1426. doi: 10.1080/00021369.1991.10870770. [DOI] [Google Scholar]
- Aoe S, Yamanaka C, Fuwa M, Tamiya T, Nakayama Y, Miyoshi T, Kitazono E. Effects of BARLEYmax and high-β-glucan barley line on short-chain fatty acids production and microbiota from the cecum to the distal colon in rats. PLoS ONE. 2019;14:e0218118. doi: 10.1371/journal.pone.0218118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AR, Vuaran M, Brown I, Topping DL. Two high-amylose maize starches with different amounts of resistant starch vary in their effects on fermentation, tissue and digesta mass accretion, and bacterial populations in the large bowel of pigs. British Journal of Nutrition. 2007;97:134–144. doi: 10.1017/S0007114507250433. [DOI] [PubMed] [Google Scholar]
- Feliciano RP, Meudt JJ, Shanmuganayagam D, Krueger CG, Reed JD. Ratio of “A-type” to “B-type” proanthocyanidin interflavan bonds affects extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells. Journal of Agricultural and Food Chemistry. 2014;62:3919–3925. doi: 10.1021/jf403839a. [DOI] [PubMed] [Google Scholar]
- Genda T, Kondo T, Sugiura S, Hino S, Shimamoto S, Nakamura T, Ukita S, Morita T. Bacterial fermentation of water-soluble cellulose acetate raises large-bowel acetate and propionate and decreases plasma cholesterol concentrations in rats. Journal of Agricultural and Food Chemistry. 2018;66:11909–11916. doi: 10.1021/acs.jafc.8b04093. [DOI] [PubMed] [Google Scholar]
- Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. Journal of Agricultural and Food Chemistry. 1999;47:490–496. doi: 10.1021/jf980760h. [DOI] [PubMed] [Google Scholar]
- Han KH, Azuma S, Fukushima M. In vitro fermentation of spent turmeric powder with a mixed culture of pig faecal bacteria. Food & Function. 2014;5:2446–2452. doi: 10.1039/C4FO00142G. [DOI] [PubMed] [Google Scholar]
- Han KH, Lee CH, Kinoshita M, Oh CH, Shimada K, Fukushima M. Spent turmeric reduces fat mass in rats fed a high-fat diet. Food & Function. 2016;7:1814–1824. doi: 10.1039/C5FO00764J. [DOI] [PubMed] [Google Scholar]
- Higa M, Fuse Y, Miyashita N, Fujitani A, Yamashita K, Ichijo T, Aoe S, Hirose T. Effect of high β-glucan barley on postprandial blood glucose levels in subjects with normal glucose tolerance: assessment by meal tolerance test and continuous glucose monitoring system. Clinical Nutrition Research. 2019;8:55–63. doi: 10.7762/cnr.2019.8.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SA, Shewry PR, Gibson GR, McCleary BV, Rastall RA. In vitro fermentation of oat and barley derived β-glucans by human faecal microbiota. FEMS Microbiology Ecology. 2008;64:482–493. doi: 10.1111/j.1574-6941.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sakata T. Stimulation of epithelial cell proliferation of isolated distal colon of rats by continuous colonic infusion of ammonia or short-chain fatty acids is nonadditive. Journal of Nutrition. 1998;128:843–847. doi: 10.1093/jn/128.5.843. [DOI] [PubMed] [Google Scholar]
- Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, Conlon MA, Morita T. Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune functions, and immunoglobulin a secretion in the rat cecum. Journal of Agricultural and Food Chemistry. 2011;59:5771–5778. doi: 10.1021/jf200859z. [DOI] [PubMed] [Google Scholar]
- Kilua A, Nomata R, Nagata R, Fukuma N, Shimada K, Han KH, Fukushima M. Purple sweet potato polyphenols differentially influence the microbial composition depending on the fermentability of dietary fiber in a mixed culture of swine fecal bacteria. Nutrients. 2019;11:1495. doi: 10.3390/nu11071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Moøller K. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Applied and Environmental Microbiology. 2002;68:673–690. doi: 10.1128/AEM.68.2.673-690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell MK, Kosar-Hashemi B, Cmiel M, Samuel MS, Chandler P, Rahman S, Buleon A, Batey IL, Li Z. Barley sex6 mutants lack starch synthase lla activity and contain a starch with novel properties. Plant Journal. 2003;34:173–185. doi: 10.1046/j.1365-313X.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- Nagata R, Echizen M, Yamaguchi Y, Han KH, Shimada K, Ohba K, Kitano-Okada T, Nagura T, Uchino H, Fukushima M. Effect of a combination of inulin and polyphenol-containing adzuki bean extract on intestinal fermentation in vitro and in vivo. Bioscience, Biotechnology, and Biochemistry. 2018;82:489–496. doi: 10.1080/09168451.2018.1429886. [DOI] [PubMed] [Google Scholar]
- Nagata R, Kamibayashi R, Bochimoto H, Fukuma N, Shimada K, Tachibe M, Takaishi Y, Han KH, Fukushima M. Chemical modification of cornstarch by hydroxypropylation enhances cecal fermentation-mediated lipid metabolism in rats. Starch - Stärke. 2020;72:1900050. doi: 10.1002/star.201900050. [DOI] [Google Scholar]
- Okubo T, Ishihara N, Oura A, Serit M, Kim M, Yamamoto T, Mitsuoka T. In vivo effects of tea polyphenol intake on human intestinal microflora and metabolism. Bioscience, Biotechnology, and Biochemistry. 1992;56:588–591. doi: 10.1271/bbb.56.588. [DOI] [PubMed] [Google Scholar]
- Payne AN, Chassard C, Zimmermann M, Müller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutrition and Diabetes. 2011;1:e12. doi: 10.1038/nutd.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelpolage SW, Goto Y, Nagata R, Fukuma N, Furuta T, Mizu M, Han KH, Fukushima M. Colonic fermentation of water soluble fiber fraction extracted from sugarcane (Sacchurum officinarum L.) bagasse in murine models. Food Chemistry. 2019;292:336–345. doi: 10.1016/j.foodchem.2019.04.063. [DOI] [PubMed] [Google Scholar]
- Rios LY, Gonthier MP, Rémésy C, Mila I, Lapierre C, Lazarus SA, Williamson G, Scalbert A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. American Journal of Clinical Nutrition. 2003;77:912–918. doi: 10.1093/ajcn/77.4.912. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rabaneda F, Jáuregui O, Casals I, Andrés-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventós RM. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao) Journal of Mass Spectrometry. 2003;38:35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- Sato S, Nagata R, Fukuma N, Shimada K, Tamiya T, Nakayama Y, Han KH, Fukushima M. Abundant indigestible carbohydrate fraction in BARLEYmax influences colonic fermentation properties in vitro. Journal of Japan Society of Nutrition and Food Sciences. 2020;73:81–91. doi: 10.4327/jsnfs.73.81. [DOI] [Google Scholar]
- Selma MV, Espín JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. Journal of Agricultural and Food Chemistry. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- Shindo Y, Dobashi Y, Sakai T, Monma C, Miyatani H, Yoshida Y. Epidemiological and pathobiological profiles of Clostridium perfringens infections: review of consecutive series of 33 cases over a 13-year period. International Journal of Clinical and Experimental Pathology. 2015;8:569–577. [PMC free article] [PubMed] [Google Scholar]
- Snart J, Bibiloni R, Grayson T, Lay C, Zhang H, Allison GE, Laverdiere JK, Temelli F, Vasanthan T, Bell R, Tannock GW. Supplementation of the diet with high-viscosity beta-glucan results in enrichment for lactobacilli in the rat cecum. Applied and Environmental Microbiology. 2006;72:1925–1931. doi: 10.1128/AEM.72.3.1925-1931.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy KM, Conterno L, Gasperotti M, Viola R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. Journal of Agricultural and Food Chemistry. 2012;60:8776–8782. doi: 10.1021/jf2053959. [DOI] [PubMed] [Google Scholar]
- Warren FJ, Fukuma NM, Mikkelsen D, Flanagan BM, Williams BA, Lisle AT, Cuív PÓ, Morrison M, Gidley MJ. Food starch structure impacts gut microbiome composition. Msphere. 2018;3:e00086–18. doi: 10.1128/mSphere.00086-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Archives of Biochemistry and Biophysics. 2012;527:95–104. doi: 10.1016/j.abb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques. 2004;36:808–812. doi: 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]
- Zakrzewski M, Proietti C, Ellis JJ, Hasan S, Brion MJ, Berger B, Krause L. Calypso: a user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics. 2017;33:782–783. doi: 10.1093/bioinformatics/btw725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zduńczyk Z, Juśkiewicz J, Estrella I. Cecal parameters of rats fed diets containing grapefruit polyphenols and inulin as single supplements or in a combination. Nutrition. 2006;22:898–904. doi: 10.1016/j.nut.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Marungruang N, Fåk F, Nyman M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. British Journal of Nutrition. 2015;113:1558–1570. doi: 10.1017/S0007114515000793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.