Abstract
Owing to convenience, ease of preparation, and price, the consumption of commercial kimchi is gradually rising in South Korea. Here, we estimated the risk level posed by pathogenic Escherichia coli in commercial kimchi products using the quantitative microbial risk assessment (QMRA) approach to develop measures for preventing potential foodborne outbreaks from kimchi consumption. We collected 610 samples of commercial kimchi products produced in Korea, 267 kimchi samples from foreign countries imported to Korea, and 187 raw materials used in kimchi preparation, and analyzed them for contamination with pathogenic E. coli. A Predictive model was developed to observe the survival characteristics of pathogenic E. coli. A dose–response model was selected, and the risk level was estimated using @RISK software. Although a prior epidemiological study indicated the frequent occurrence of foodborne outbreaks arising from contaminated kimchi products consumed in food service facilities, we found a low probability of foodborne illness caused by pathogenic E. coli in commercial kimchi products.
Keywords: Kimchi, Pathogenic E. coli, Outbreak, Predictive model, Microbial risk assessment
Introduction
Kimchi is a Korean traditional food prepared using salted cabbage, radish, or other vegetables as the primary ingredient and combined with a spice mixture of powdered red pepper, green onion, ginger, salted seafood, and others, followed by fermentation (Park et al., 2003). Because of the wide variety and combinations of the types of major and minor ingredients, province of origin, and season of production, hundreds of kimchi varieties are available in South Korea (Chambers et al., 2012; Kim et al., 2012).
Recently, because of convenience and lower price, along with the trends in eating habits, commercial kimchi products are now widely manufactured and distributed by numerous food companies. As a result, commercial kimchi accounts for 37% of the total kimchi products (1,850,000 tons) in Korea (Wikim, 2020). Imported kimchi products were estimated to account for 13.7% of the total Korean kimchi consumption and 47.3% of kimchi consumption at food service facilities (Wikim, 2020). However, antiseptics, maggots, bacteria, viruses, and other contaminants have occasionally been detected in them. Therefore, it is necessary to develop measures to continuously monitor and assure the microbiological safety of commercial kimchi products.
However, foodborne illnesses arising from commercial kimchi provided by schools and food service facilities have persisted, and most cases were caused by contamination with pathogenic Escherichia coli (Choi et al., 2020; Kim et al., 2009). In general, raw materials used in the production of kimchi are manufactured without heating or sterilization. For this reason, contamination by microorganisms such as E. coli may occur during the manufacturing process when sufficient washing of raw materials is not performed. In Korea, pathogenic E. coli is major food poisoning bacteria (1784–2754 cases from 2010 to 2018) (Lee and Yoon, 2021), according to their molecular biological characteristics, mechanisms of pathogenesis, and toxin types, pathogenic E. coli is classified into five main groups: enterohaemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), and enteroaggregative E. coli (EAEC) (Nataro and Kaper, 1998).
In 2012, approximately 1200 students in seven schools across the Gyeonggi province developed food poisoning symptoms. An epidemiological investigation detected the same EAEC and ETEC genotypes in the radish and unfermented kimchi provided to the students and in the patients' stool samples (Cho et al., 2014). In 2013 and 2014, ETEC O6 was detected in samples from students who had experienced food poisoning symptoms after eating cabbage kimchi and young radish kimchi, provided as a part of the school lunch meal (Shin et al., 2016). Additionally, in 2017, pathogenic E. coli (ETEC and EAEC) in diced radish kimchi provided by food service facilities caused 37 food poisoning cases (KCDC, 2021a).
Quantitative microbial risk assessment (QMRA) supports scientific evidence in maintaining food safety (Nauta et al., 2009) and determines the probability of the risk of foodborne pathogens in food. MRA comprises four steps: hazard identification, exposure assessment, hazard characterization, and risk characterization (CAC, 2020). As each food has its own distribution chain and environment conditions, such as time and temperature, these aspects should also be reflected in the MRA scheme. For example, QMRA has been used to estimate the public health effects of E. coli O157:H7 in leafy green vegetables provided by salad bars and in fresh-cut lettuce distributed to consumers from farms as a guide for developing measures to prevent food poisoning. Accordingly, QMRA has been recognized as an effective tool to assure food safety (Franz et al., 2010; Pang et al., 2017).
The aims of this study were to develop a QMRA model for estimating the risk level posed by pathogenic E. coli in commercial kimchi, to compare the risk levels of EHEC, EPEC, ETEC, EIEC, and EAEC, and to propose measures to reinforce the safety of commercial kimchi against pathogenic E. coli. Overall, the developed QMRA model can provide a clear assessment of the risk of pathogenic E. coli-associated illness caused by the consumption of commercial kimchi in South Korea.
Materials and methods
Quantitative and qualitative determination of pathogenic E. coli
A total of 610 domestically produced kimchi samples were analyzed, including 240 cabbage kimchi, 85 diced radish kimchi, 55 radish roots kimchi, 120 pickled white radish, 90 kimchi without red pepper powder, and 20 unfermented kimchi. In addition, we sampled 267 imported cabbage kimchi products, along with 187 samples of raw materials such as cabbage (67), radish (50), ginger (18), garlic (21), powdered red pepper (15), and green onion (16), which were collected from Korean markets. The domestically produced kimchi samples were selected by the sales volume and market share while imported kimchi were collected from ‘Gwangju regional Korea food and drug administration/imported food inspection center’.
Twenty-five grams of each test sample was aseptically and diluted with 225 mL of modified tryptone soy broth (TSB; Oxoid) and incubated at 35–37 °C for 24 h. The sample was then inoculated onto plates containing tellurite cefixime-sorbitol MacConkey agar (TC-SMAC) and 5-bromo-4-chloro-3indolyl-β-D-glucuronide (BCIG), respectively (both from Oxoid) and incubated at 35–37 °C for 18–24 h. More than five colorless colonies from the TC-SMAC (colonies that did not hydrolyze sorbitol) and more than five bluish-green colonies from BCIG were collected and transferred onto the nutrient agar (BD) and further incubated at 35–37 °C for 18–24 h. Next, suspect colonies were selected to detect the patho-types of E. coli using PowerCheck Diarrheal E. coli 4-plex Kit I and II (KogeneBiotech Co., Seoul, Korea). In addition, EHEC (VT1, VT2), EPEC (eaeA), EIEC (inV), EAEC (eggR), and ETEC (LT, ST) genes were detected by 7500 Fast Real-Time PCR (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
Prevalence and initial concentration of pathogenic E. coli in commercial kimchi products
The pathogenic E. coli prevalence data obtained in this study were converted to a beta distribution (α, β), where α is the number of positive samples plus one and β is the number of positive samples plus one, subtracted from the number of total samples (Sanaa et al., 2004). The beta distribution was the first input parameter to estimate the initial contamination level of pathogenic E. coli in commercial kimchi, and the qualitative detection level was converted into a quantitative value of pathogenic E. coli. The beta distribution (number of positive samples + 1, tested total samples–positive samples + 1) represents the probability distribution of an event when the number of success in total runs (n) is expressed as r, and the number of total and positive samples can be derived from the monitoring data (Lee et al., 2015). The equation (natural logarithm(1–prevalence data)/weight) was used to calculate the initial concentration of pathogenic E. coli in commercial kimchi products (Sanaa et al., 2004).
Development of a predictive model for the survival of pathogenic E. coli
The following pathogenic E. coli strains were obtained from the National Culture Collection for Pathogens (NCCP): EHEC (NCCP 11,076), EPEC (NCCP 13,715), ETEC (NCCP 15,732), EAEC (NCCP14039), and EIEC (NCCP 15,663). Single colonies of the individual strains were incubated in 10 mL of TSB (Oxoid) at 35 °C for 24 h. Centrifugation was conducted at 1,912 g and 4 °C for 15 min (VS-15000 N, Vision, Kyounggi, Korea) to obtain the cells, which were then washed with PBS (Oxoid) twice and used in development of the predictive model.
The commercial kimchi used in the development of the predictive model was obtained from a kimchi plant located at Cheongju-si, Korea. Cabbage kimchi purchased from this plant was transferred to the laboratory in a cooling container to maintain the temperature at 4 °C. The kimchi was dispensed into a 25 × 51-inch Whirl–pak (Nasco, WI, USA) (1 kg per pack), and a mixture of the pathogenic E. coli strains (i.e., EHEC O157:H7, EPEC, ETEC, EAEC, and EIEC) was inoculated to assure that the initial level was about 5–6 log colony-forming units (CFU)/g. The kimchi samples inoculated with pathogenic E. coli were stored at 4 °C, 10 °C, 25 °C, and 37 °C. Twenty-five grams of each sample was aseptically collected at each defined point and added to 225 mL of 0.85% saline (BNF, Korea), and the mixture was homogenized for 2 min using a homogenizer (BagMixer®, Interscience, St. Nom, France). Next, 10X dilutions were prepared with 0.85% peptone water containing saline (3 M, USA). The resultant microbial solution was spread over eosin methylene blue agar (Oxoid) and incubated at 37 °C for 24 h. Afterwards, colonies with metallic brilliance were counted to analyze the behavioral characteristics of pathogenic E. coli in cabbage kimchi.
To develop the primary predictive model, the Weibull equation was used as a survival model (GinaFiT V1.5) (Eq. (1)):
| 1 |
where N0 is the initial contamination level; t is time; δ is the time taken to reduce the initial contamination level by 1 log CFU; and ρ is the graph shape (Geeraerd et al., 2005).
Based on δ and ρ obtained from the first model, the secondary model for pathogenic E. coli in cabbage kimchi at different temperatures was developed using SigmaPlot13 (Systat Software, San Jose, CA, USA). An exponential equation (Eq. (2)) and a polynomial equation (Davey, 1991) (Eq. 3) were used to calculate delta (δ) and ρ, respectively:
[Exponential equation]
| 2 |
where Y is δ; a, b, c are constants; and T is temperature.
[Polynomial equation]
| 3 |
where Y is ρ; a, b, c are constants; and T is temperature.
Validation of the model's suitability
The root mean square error (RMSE) was calculated based on the difference in the maximum microbial growth rate between the predicted and actual values as shown below. When the value is close to 0, the model is considered to be more suitable (Baranyi et al., 1996) (Eq. (4)).
| 4 |
where obs is the observed value; pred is the predicted value; and n is the number of observations.
Consumption of kimchi
To reflect the recent trends in kimchi consumption, we used the 2016 data from the Korea National Health and Nutrition Examination Survey of the Korea Centers for Disease Control and Prevention, which is a database containing essential food consumption patterns (KCDC, 2021c). It contains information from 7,040 individuals surveyed to identify kimchi consumption patterns for five kinds of kimchi products on the basis of food consumption data.
Dose–response model
For healthy adults, the minimum infectious dose of E. coli was reported to be about 10 organisms for EHEC O157:H7, 108 organisms for ETEC, 106 organisms for EPEC, and 106–1010 organisms for EIEC (PHAC, 2021). We used the Beta-Poisson models for EHEC O157:H7 (α = 0.49, β = 1.90 × 105), ETEC (α = 0.16, β = 9.98 × 107), and EPEC (α = 0.1008, β = 1.78 × 106) (Huertas et al., 2008; Loge et al., 2002; Teunis et al., 2004). Although microbial risk assessment has not been conducted on EIEC data, the minimum infection level for EIEC is approximately 106 cells, and the usual infection level is 106–108 cells. Accordingly, the exponential model (k = 9.70 × 10–9) (DuPont et al., 1971) was selected as a dose–response model for EIEC. Because there is no dose–response model developed for EAEC, it was excluded from the risk assessment.
Distribution environment
There are various wholesale and retail channels for the distribution of commercial kimchi products distributed by local manufacturers and importers (Wikim, 2020). These distribution channels can be divided into channels for the distribution of kimchi products to general consumers (for households) and to restaurants and food service facilities. The retail distribution channels, such as grocery stores, convenience stores, department stores, internet shopping sites, and traditional markets, where consumers can directly purchase kimchi products, account for approximately 22%, whereas 78% of the kimchi is distributed to restaurants and food service facilities. We also included factors associated with the distribution environments in the model, such as temperature and time. Various product types (small-volume to large-volume packs) are available in the consumer channels, and most products are handled and provided at controlled temperatures. For example, majority are stored in a cold warehouse and marketed at display stands maintained at or below 7 °C with the actual temperature of the products ≤ 10.6 °C.
Restaurants and food service facilities serve commercial kimchi and/or in-house kimchi. Most products are stored at cold temperature (approximately 4 °C) and exposed to room temperature for 1–2 h before they are served to the consumers. The collected temperature and time data for each step were also analyzed with @RISK (Palisade Corporation, Ithaca, NY, USA) to obtain appropriate probabilistic distributions.
Risk characterization using @RISK
A microbial risk assessment scheme was established using @RISK, as shown in Table 1. As mentioned above, the simulation model included the initial contamination level data for pathogenic E. coli, predictive models, probabilistic distributions for temperature and time, probabilistic distribution for consumption, and the dose–response model, which were compiled in Microsoft Excel (version 2010, Microsoft Corporation, Seattle, WA, USA). That is, the contamination levels of pathogenic E. coli in commercial kimchi (log CFU/g) were estimated in consideration of (1) storage at the sales stand, (2) shipping, and (3) storage before consumption. In addition, the level of pathogenic E. coli ultimately consumed was determined in consideration of the cabbage kimchi consumption pattern by simulation with @RISK for 10,000 iterations to calculate the risk of pathogenic E. coli foodborne illness from the intake of commercial kimchi products (one person per day).
Table 1.
Simulation model and formulae used to estimate the risk of pathogenic E. coli in commercial kimchi using @RISK 7.0
| Symbol | Unit | Definition | Formulae | Reference |
|---|---|---|---|---|
| Contamination level of pathogens | ||||
| DP | Detection prevalence of pathogenic E. coli in cabbage kimchi | = RiskBeta(2, 1,064) | Sanaa et al., (2004) | |
| CL | CFU/g | Contamination level of pathogenic E. coli in cabbage kimchi | = − LN(1 − DP)/25 | |
| IC | log CFU/g | Initial contamination level | = LOG(CL) | |
| Market storage | ||||
| Mark-time | h | Storage time in market | = RiskUniform(0,1440) | This study |
| Mark-temp | °C | Storage temperature in market | = RiskPert(4,7,10) | |
| Transportation | ||||
| Trans-time | h | Time (market to home) | = RiskPert(0.33,0.813,1.171) | Nam et al., (2018) |
| Trans-temp | °C | Storage temperature during transportation | = RiskNormal(6.6,2.6) | Carrasco et al., (2007) |
| Home storage | ||||
| Home-time | h | Storage time until consumption | = RiskPert(0,123.2,720) | Nam et al., (2018) |
| Home-temp | °C | Storage temperature until consumption | = RiskUiform(4,10) | This study |
| Predictive inactivation model applied during transportation, followed by home storage, followed by consumption | ||||
| δ | h | Treatment time for the first decimal reduction | = 2368.9026*exp(− 0.2825*Mark and Trans and Home-temp) | This study |
| ρ | Shape of the Weibull model | = 3.124209 − 0.17709* Mark and Trans and Home-temp + 0.003736* Mark and Trans and Home-temp2 | This study | |
| C | log CFU/g | Contamination level of pathogenic E. coli at the home | = IC − (Mark and Trans and Home-time/δ3)ρ | Geeraerd et al., (2005) |
| Consumption | ||||
| Consump | g | Daily consumption average amount | = RiskInvGauss(59.819,7.3315,RiskShift(− 2.1173)) | KCDC, (2017) |
| ConFre | % | Daily consumption frequency | = 77.44 | |
| CF(0) | Daily non-consumption frequency (rate) | = 1 − ConFre/100 | ||
| CF(1) | Daily consumption frequency (rate) | = ConFre/100 | ||
| CF | Distribution for consumption frequency | = RiskDiscrete({0,1},CF(0):CF(1)) | ||
| Amount | Daily consumption average amount considered frequency | = IF(ConFre = 0,0,Consump) | ||
| Dose–Response | ||||
| D | Amount of pathogenic E. coli | = 10C × Amount | ||
| EHEC | αEHEC, βEHEC | Beta-Poison | = 0.49, = 1.90*105 | Huertas et al., (2008) |
| ETEC | αETEC, βETEC | = 0.16, = 9.98*107 | Loge et al., (2002) | |
| EPEC | αEPEC, βEPEC | = 0.1008, = 1.78*106 | Strachan et al., (2005) | |
| EIEC | kEIEC | Exponential | = 9.7*10–9 | DuPont et al., (1971) |
Results and discussion
Table 2 presents the prevalence of E. coli contamination in the commercial kimchi samples. The E. coli detected was further analyzed by TC-SMAC and BCIG cultures and by real-time PCR. EPEC with the eaeA gene was detected in one out of the 877 kimchi products, which was an imported cabbage kimchi. In addition, although majority of the raw materials did not contain generic and pathogenic E. coli, generic E. coli contamination (4.03 log CFU/g) was found in cabbage, the main ingredient of kimchi. Lee et al. (2018) did not detect any pathogenic E. coli in 200 raw materials used for kimchi production and in 100 commercial kimchi products distributed in the Korean market. Moreover, from hygiene index microorganism, generic E. coli was not detected in most spices used for commercial kimchi. However, different products, such as commercial kimchi paste, showed a contamination level of 1.39 or 1.65 log CFU/g (Cheon et al., 2017). In the present study, we found that generic E. coli was more frequently detected in imported commercial cabbage kimchi than in domestic commercial cabbage kimchi, implying that Korean kimchi manufacturers are producing hygienic products and adequately conforming to safety measures according to the HACCP system.
Table 2.
Analysis of E. coli contamination in raw materials, domestic, and imported commercial kimchi in S. Korea
| Category | No. of sample | E. coli | Pathogenic E. coli (Qualitative) | ||
|---|---|---|---|---|---|
| No. of positive sample | Log CFU/g (Quantitative) | ||||
| Domestic commercial kimchi | Cabbage kimchi | 240 | 17 | 1.78 ± 0.56 | (1/877) |
| Diced radish kimchi | 85 | 5 | 2.15 ± 0.03 | ||
| Radish roots kimchi | 55 | 5 | 1.85 ± 0.15 | ||
| Kimchi without red pepper powder | 90 | 3 | 0.80 ± 0.17 | ||
| Pickled white radish | 120 | 3 | 0.80 ± 0.17 | ||
| Unfermented kimchi | 20 | 1 | 0.70 ± 0.00 | ||
| Imported commercial kimchi | Cabbage kimchi | 267 | 88 | 1.50 ± 0.64 | |
| Raw materials | Cabbage | 67 | 1 | 4.03 ± 0.00 | (0/187) |
| Radish | 50 | ND† | |||
| Garlic | 21 | ND | |||
| Ginger | 18 | ND | |||
| Red pepper powder | 15 | ND | |||
| Green onion | 16 | ND | |||
†ND: Not detected
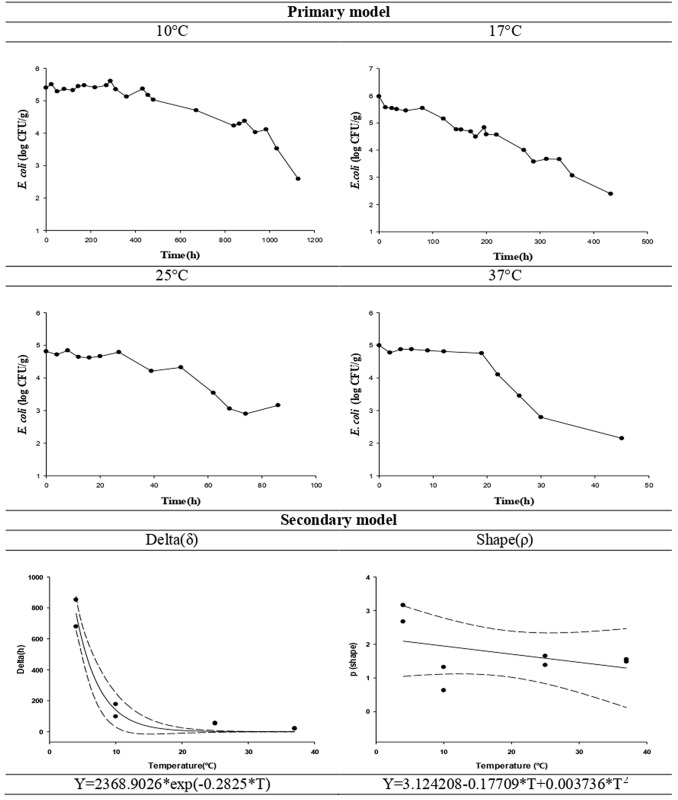
The survival prediction parameters for pathogenic E. coli are provided in Fig. 1 and Table 3. In our previous study (data not shown), we observed the survival of 5 different pathotypes separately. They all had almost identical growth curves and the differences are statistically insignificant. Thus, based on our research data and literature, we used a single (mixture of the pathogenic E. coli strains) survival model. The δ value (1 log reduction time) for pathogenic E. coli in cabbage kimchi proportionally decreased at higher storage temperatures. Pathogenic E. coli in commercial kimchi products (cabbage kimchi) was found to be affected by temperature. The ρ value of the Weibull survival model indicates the shape of the graph, i.e., when ρ > 1, the graph takes on a convex curve, indicating the gradual death of contaminated microorganisms, whereas when ρ < 1, the curve is concave, indicating more abrupt death (Albert and Mafart, 2005). In this study, the ρ value obtained was greater than 1 at 4 °C, indicating a gradual decrease in the number of pathogens.
Fig. 1.
Predictive models of pathogenic E. coli on commercial kimchi (cabbage kimchi) as a function of temperature. δ (delta) and ρ (shape) are the best-fit values from the Weibull model fit. T, Temperature (•)
Table 3.
Survival kinetic parameters of pathogenic E. coli in commercial kimchi (cabbage kimchi)
| Bacteria | Temperature (°C) | Cabbage kimchi | |||
|---|---|---|---|---|---|
| δ† (h) | ρ†† (shape) | Initial level (log CFU/g) | R2 | ||
| Pathogenic E. coli | 10 | 765.54 ± 122.19 | 2.92 ± 0.35 | 4.98 ± 0.58 | 0.944 ± 0.016 |
| 17 | 133.76 ± 56.46 | 0.98 ± 0.49 | 5.47 ± 0.33 | 0.944 ± 0.037 | |
| 25 | 54.34 ± 2.49 | 1.52 ± 0.19 | 4.83 ± 0.04 | 0.902 ± 0.004 | |
| 37 | 20.59 ± 1.67 | 1.52 ± 0.05 | 5.04 ± 0.04 | 0.926 ± 0.019 | |
†δ(delta): Treatment time for the first decimal reduction †† p: Graph shape
The secondary model equations for δ and ρ calculated from the primary model were developed using the exponential equation and the square root model, respectively, as functions of temperature. To validate the suitability of the predictive model, the same experiment was conducted at 15 °C (which was not used for the development of the predictive model). The RMSE between the actual and predicted values was close to 0 (0.512), confirming the suitability of the predictive model for pathogenic E. coli in cabbage kimchi.
In line with several previous studies on the various kinds of kimchi and fermented vegetables, we identified a clear effect of fermentation on the reduction of pathogenic microorganisms. The decrease in pathogenic microorganisms in kimchi is attributed to the production of organic acids, especially lactic acid, and the consequent change to a more acidic pH in the course of fermentation (Cho et al., 2011; Niksic et al., 2005). Various kinds of lactic acid-producing bacteria appear during kimchi fermentation, such as Leuconostoc, Lactobacillus, and Pediococcus species, and significantly affect the taste, flavor, and quality of the product. Although the acidity of kimchi is generally effective to suppress the growth of pathogenic microorganisms during storage, E. coli O157:H7 could survive in radish kimchi at ≤ pH 4.0 and in typical fresh apple cider at pH 3.7 (Iu et al., 2001; Lee et al., 2020). This could be related to the gradual rate of pH decrease during fermentation, allowing for E. coli to adapt to the acidic environment by developing acid resistance, thereby facilitating its survival (Cho et al., 2011).
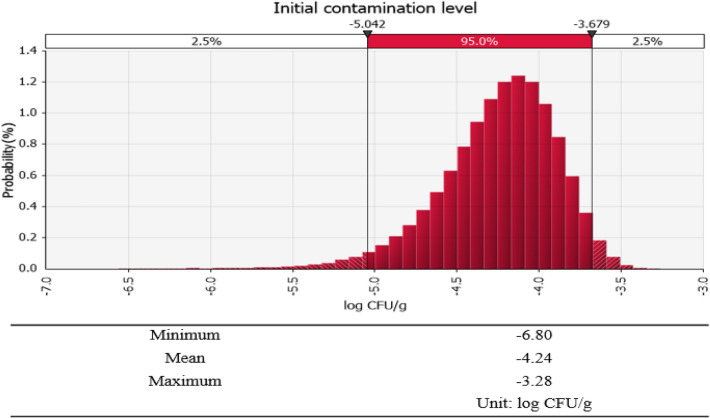
QMRA of pathogenic E. coli in cabbage kimchi was conducted using the @RISK program by considering the one positive sample for EPEC (eaeA), among the 1,064 samples tested, according to the equation for assessing the initial contamination level proposed by Sanaa et al. (2004). The initial contamination level was estimated at approximately –4.24 log CFU/g (Fig. 2). The daily mean consumption amounts were 57.50 g (77.44%) for cabbage kimchi, 8.47 g (14.64%) for diced radish kimchi, 10.07 g (10.07%) for young radish kimchi, 3.55 g (3.55%) for pickled white radish, and 1.25 g (1.26%) for kimchi without red pepper powder. Based on the @RISK simulation, upon consumption of commercial kimchi products in the Korean market, the probability of developing food poisoning caused by pathogenic E. coli in a given day was determined to be the highest for EHEC (6.60E-09), followed by EPEC (1.36E-10), EIEC (2.33E-11), and ETEC (4.68E-12). Overall, the risk of food poisoning arising from pathogenic E. coli in commercial kimchi was found to be relatively low, and EHEC showed a relatively higher risk than the other pathogenic E. coli strains (Table 4).
Fig. 2.
Probability distribution of the initial contamination level of pathogenic E. coli in commercial kimchi using @RISK
Table 4.
Probability of illness per day per person caused by pathogenic E. coli in commercial kimchi with @RISK scenario
| 25% | 50% | Mean | 95% | Maximum | |||
|---|---|---|---|---|---|---|---|
| Pathogenic E. coli | 1 | EHEC | 0 | 3.81 × 10–10 | 6.60 × 10–09 | 2.73 × 10–08 | 7.99 × 10–07 |
| 2 | EPEC | 0 | 7.92 × 10–12 | 1.36 × 10–10 | 5.60 × 10–10 | 1.99 × 10–08 | |
| 3 | EIEC | 0 | 1.32 × 10–12 | 2.33 × 10–11 | 9.47 × 10–11 | 3.82 × 10–09 | |
| 4 | ETEC | 0 | 2.98 × 10–13 | 4.68 × 10–12 | 2.90 × 10–11 | 5.24 × 10–10 |
The goal of the present study was to propose safety measures to reduce the incidence of foodborne outbreaks caused by pathogenic E. coli based on the QMRA results. Several factors in the production and manufacturing chain need to be considered for a complete risk assessment. First, it is necessary to consider the potential risk from the cultivation of cabbage used as the main ingredient in the production of kimchi. Pathogenic E. coli in the environment are derived from strains in the large intestine of animals. When groundwater, river water, or livestock wastewater containing soil or animal feces is used as agricultural water to cultivate vegetables, there is a high likelihood of contamination of the crops with pathogenic E. coli, norovirus, and other pathogenic bacteria (Graciaa et al., 2018). Indeed, a high E. coli contamination level of agricultural water used in the cultivation of fresh-cut lettuce was found to increase the probability of food poisoning and other diseases caused by E. coli O157:H7 (Pang et al., 2017). Similarly, Yun et al. (2017) isolated 164 E. coli strains from surface water and underground water used as agricultural water for the cultivation of cabbage in four areas in Korea, including 10 strains (6.1%) harboring the eaeA gene indicative of EPEC contamination. The presence of E. coli indicates potential contamination of agricultural water with other ecologically related pathogenic microorganisms.
Second, commercially salted cabbage has been increasingly used in kimchi production for convenience as it eliminates the time-consuming salting step. Except for washing, there is no additional processing, including heat treatment and the microbial control measure in the manufacturing of salted cabbage (Kim and Yoon, 2008; Song et al., 2019). Therefore, the probability of E. coli contamination will be increased if washing is not sufficiently conducted in the production of salted cabbage, contaminated agricultural water is used, or agricultural water is repeatedly used.
Third, the microbiological control of commercial kimchi in Korea has thus far focused only on EHEC (negative for n = 5, c = 0, m = 0/25 g). However, epidemiological investigations revealed that the major causative microorganisms of foodborne illness from kimchi were EPEC (44.7%), ETEC (34.2%), EAEC (10.5%), and EHEC (9.2%) (KCDC, 2021b; Lee et al., 2012). Our results and previous case reports did not show any food poisoning case caused by EHEC in the last decade. Therefore, in addition to the continued control of EHEC to prevent food poisoning, careful attention also needs to be paid to other pathogenic E. coli (EPEC, ETEC, and EAEC) to assure the appropriate control of commercial kimchi products.
In this study, we selected the commercial cabbage kimchi products sold in Korea for the QMRA, which demonstrated a low probability of foodborne illness (one person per day). To decrease the risk of foodborne illness of pathogenic E. coli in kimchi, it is highly recommended to minimize the initial contamination level and maintain appropriate refrigeration distribution system.
These risk assessment results were obtained upon establishing certain assumptions using limited data; thus, re-evaluation may be warranted if additional data become available. It is also necessary to continuously perform risk assessment to establish reasonable scientific standards and specifications by considering the risk, exposure frequency, and other factors.
Acknowledgements
The authors would like to thank all of the reviewers of this study and their great contributions
Abbreviations
- BCIG
5-Bromo-4-chloro-3indolyl-β-D-glucuronide
- EAEC
Enteroaggregative E. coli
- EHEC
Enterohaemorrhagic E. coli
- EIEC
Enteroinvasive E. coli
- EPEC
Enteropathogenic E. coli
- ETEC
Enterotoxigenic E. coli
- PR
Prevalence data
- QMRA
Quantitative microbial risk assessment
- RMSE
Root mean square error
- TC-SMAC
Tellurite cefixime-sorbitol MacConkey agar
Funding
This research was supported by the Korea Ministry of Food and Drug Safety (Grant number: 17161MFDS031).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gun Woo Nam, Email: jpgv9@korea.kr.
Myeongkyo Jeong, Email: img2025@korea.kr.
Eun Jeong Heo, Email: dvmheo@korea.kr.
Oun Ki Chang, Email: okchang@korea.kr.
Mi-Gyeong Kim, Email: angelmg@korea.kr.
Hyo-Sun Kwak, Email: sunkwak@korea.kr.
Soo Hwan Suh, Email: soohsuh@korea.kr, Email: qmw0@cdc.gov.
References
- Albert I, Mafart P. A modified Weibull model for bacterial inactivation. International Journal of Food Microbiology. 100: 197-211 (2005) [DOI] [PubMed]
- Baranyi J, Ross T, McMeekin T, Roberts T. Effects of parameterization on the performance of empirical models used in ‘predictive microbiology'. Food Microbiology. 13: 83-91 (1996)
- Carrasco E, Perez-Rodriguez F, Valero A, Garcia-Gimeno R, Zurera G. Survey of temperature and consumption patterns of fresh-cut leafy green salads: risk factors for listeriosis. Journal of Food Protection. 70: 2407-2412 (2007) [DOI] [PubMed]
- Chambers IV E, Lee J, Chun S, Miller AE. Development of a lexicon for commercially available cabbage (baechu) kimchi. Journal of Sensory Studies. 27: 511-518 (2012)
- Cheon SH, Lee SI, Hwnag IM, Seo HY. Quality characteristics of commercial kimchi paste. Korean Journal of Food and Cookery Science. Sci. 33: 9-19 (2017)
- Cho G, Lee MH, Choi C. Survival of Escherichia coli O157: H7 and Listeria monocytogenes during kimchi fermentation supplemented with raw pork meat. Food Control. 22: 1253-1260 (2011)
- Cho J, Joo I, Park K, Han M, Son N, Jeong S, Heo J, Kim Y, Oh M, Kim S. Characterization of pathogenic Escherichia coli strains linked to an outbreak associated with kimchi consumption in South Korea, 2012. Food Science and Technology. 23: 209-214 (2014)
- Choi YK, Kang JH, Lee YW, Seo YG, Lee HY, Kim SJ, Lee JY, Ha JM, Oh HM, Kim YJ, Byun KH, Ha SD, Yoon YH. Quantitative microbial risk assessment for Clostridium perfringens foodborne illness following consumption of kimchi in South Korea. Food Science and Biotechnology. 29: 1131-1139 (2020) [DOI] [PMC free article] [PubMed]
- Codex Alimentarius Commission (CAC). Principles and guidelines for the conduct of microbiological risk assessment CAC/GL 30-1999. Available from: http://www.fao.org/3/y1579e/y1579e05.htm. Accessed 2 July 2020
- Davey K. Applicability of the Davey (linear Arrhenius) predictive model to the lag phase of microbial growth. Journal of Applied Microbiology. 70: 253-257 (1991)
- DuPont HL, Formal SB, Hornick RB, Snyder MJ, Libonati JP, Sheahan DG, LaBrec EH, Kalas JP. Pathogenesis of Escherichia coli diarrhea. The New England Journal of Medicine. 285: 1-9 (1971) [DOI] [PubMed]
- Franz E, Tromp S, Rijgersberg H, Van Der Fels-Klerx H. Quantitative microbial risk assessment for Escherichia coli O157: H7, Salmonella, and Listeria monocytogenes in leafy green vegetables consumed at salad bars. Journal of Food Protection. 73: 274-285 (2010) [DOI] [PubMed]
- Geeraerd A, Valdramidis V, Van Impe J. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. International Journal of Food Microbiology. 102: 95-105 (2005) [DOI] [PubMed]
- Graciaa DS, Cope JR, Roberts VA, Cikesh BL, Kahler AM, Vigar M, Hilborn ED, Wade TJ, Backer LC, Montgomery SP. Outbreaks associated with untreated recreational water-United States, 2000-2014. American Journal of Transplantation. 18: 2083-2087 (2018) [DOI] [PMC free article] [PubMed]
- Huertas E, Salgot M, Hollender J, Weber S, Dott W, Khan S, Schafer A, Messalem R, Bis B, Aharoni A. Key objectives for water reuse concepts. Desalination. 218: 120-131 (2008)
- Iu J, Mittal GS, Griffiths MW. Reduction in levels of Escherichia coli O157:H7 in apple cider by pulsed electric fields. Journal of Food Protection. 64: 964-969 (2001) [DOI] [PubMed]
- Kim JG, Yoon JS. Changes of index microorganisms and lactic acid bacteria of Korean fermented vegetables (kimchi) during the ripening and fermentation-part 2. Journal of Environmental Health Sciences. 34: 70-75 (2008)
- Kim J, Bang J, Beuchat LR, Kim H, Ryu J. Controlled fermentation of kimchi using naturally occurring antimicrobial agents. Food Microbiology. 32: 20-31 (2012) [DOI] [PubMed]
- Kim MJ, Kim SH, Kim TS, Kee H, Seo J, Kim E, Park J, Chung JK, Lee J. Identification of Shiga toxin-producing E. coli isolated from diarrhea patients and cattle in Gwangju area, Korea. Journal of Bacteriology and Virology. 39: 29-39 (2009)
- KCDC. Korea Centers for Disease Control and Prevention. Epidemiological Investigation of Infectious Diseases in Korea Annual Report 2017. Available from: http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=5&pblctDtaSn=1010. Accessed Octorber. 1, 2021a)
- KCDC. Korea Centers for Disease Control and Prevention. Infectious Disease Portal (2011–2017). South Korean Ministry of Welfare and Health. Available from: http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaMain.do (Accessed Octorber. 1, 2021b)
- KCDC. Korea Centers for Disease Control and Prevention. Korea Health Statistic 2016: Korea national health and nutrition examination survey. South Korean Ministry of Welfare and Health. Available from: https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do (Accessed Octorber. 2, 2021c)
- Lee D, Gwack J, Youn S. Enteropathogenic Escherichia coli outbreak and its incubation period: is it short or long?. Osong Public Health and Research Perspectives. 3: 43-47 (2012) [DOI] [PMC free article] [PubMed]
- Lee H, Kim K, Choi K, Yoon Y. Quantitative microbial risk assessment for Staphylococcus aureus in natural and processed cheese in Korea. Journal of Dairy Science. 98: 5931-5945 (2015) [DOI] [PubMed]
- Lee H, Yoon Y. Etiological agents implicated in foodborne illness world wide. Food Science of Animal Resources. 41: 1-7 (2021) [DOI] [PMC free article] [PubMed]
- Lee HS, Choi HE, Choi UK, Yuk HG. Evaluation of standard enrichment broths for recovery of healthy and chlorine-injured Escherichia coli O157:H7 cells in kimchi. Food Science and Biotechnology. 29: 1439-1445 (2020) [DOI] [PMC free article] [PubMed]
- Lee J, Ha J, Lee H, Lee JY, Hwang Y, Lee HM, Kim SH, Kim S. Analysis of microbiological contamination in kimchi and its ingredients. Journal of Food Hygiene and Safety. 33: 94-101 (2018)
- Loge FG, Thompson DE, Call D. PCR detection of specific pathogens in water: a risk-based analysis. Environmental Science & Technology. 36: 2754-2759 (2002) [DOI] [PubMed]
- Nam GW. Quantitative microbial risk assessment of Clostridium perfringens in beef jerky. Korean Journal of Food Science and Technology. 2018;50(621-628):621. [Google Scholar]
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clinical Microbiology Reviews. 11: 142-201 (1998) [DOI] [PMC free article] [PubMed]
- Nauta M, Hill A, Rosenquist H, Brynestad S, Fetsch A, van der Logt P, Fazil A, Christensen B, Katsma E, Borck B, Havelaar A. A comparison of risk assessments on Campylobacter in broiler meat. International Journal of Food Microbiology. 129: 107-123 (2009) [DOI] [PubMed]
- Niksic M, Niebuhr SE, Dickson JS, Mendonca AF, Koziczkowski JJ, Ellingson JL. Survival of Listeria monocytogenes and Escherichia coli O157: H7 during sauerkraut fermentation. Journal of Food Protection. 68: 1367-1374 (2005) [DOI] [PubMed]
- Pang H, Lambertini E, Buchanan RL, Schaffner DW, Pradhan AK. Quantitative microbial risk assessment for Escherichia coli O157: H7 in fresh-cut lettuce. Journal of Food Protection. 80: 302-311 (2017) [DOI] [PubMed]
- Park K, Cho E, Rhee S, Jung K, Yi S, Jhun BH. Kimchi and an active component, β-sitosterol, reduce oncogenic H-Rasv12-induced DNA synthesis. Journal of Medicinal Food. 6: 151-156 (2003) [DOI] [PubMed]
- PHAC. Public Health Agency of Canada. Pathogen Safety Data Sheets. Available from: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/escherichia-coli-enterotoxigenic.html (Accessed September. 30, 2021)
- Sanaa M, Coroller L, Cerf O. Risk assessment of listeriosis linked to the consumption of two soft cheeses made from raw milk: Camembert of Normandy and Brie of Meaux. Risk Analysis. 24: 389-399 (2004) [DOI] [PubMed]
- Shin J, Yoon K, Jeon D, Oh S, Oh K, Chung GT, Kim SW, Cho S. Consecutive outbreaks of enterotoxigenic Escherichia coli O6 in schools in South Korea caused by contamination of fermented vegetable kimchi. Foodborne Pathogens and Disease. 13: 535-543 (2016) [DOI] [PubMed]
- Song W, Chung H, Kang D, Ha J. Microbial quality of reduced-sodium napa kimchi and its processing. Food Science and Nutrition. 7: 628-635 (2019) [DOI] [PMC free article] [PubMed]
- Strachan NJ, Doyle MP, Kasuga F, Rotariu O, Ogden I D. Dose response modelling of Escherichia coli O157 incorporating data from foodborne and environmental outbreaks. International Journal of Food Microbiology. 103: 35-47 (2005) [DOI] [PubMed]
- Teunis P, Takumi K, Shinagawa K. Dose response for infection by Escherichia coli O157: H7 from outbreak data. Risk Analysis. 24: 401-407 (2004) [DOI] [PubMed]
- Wikim. World Institute of Kimchi. Humanistic Understanding of Kimchi and Kimjang Culture. Available from: https://www.wikim.re.kr/upload/board/0012/14588990163930.pdf (Accessed September. 29, 2020)
- Yun B, Team MS, Nas R, Kim M, Ryu J, Park B. Investigation of microbiological and physiochemical quality for irrigation water used in napa cabbage cultivation. Journal of Food Hygiene and Safety. 32: 396-403 (2017)