Abstract
The thermal effect of maesil on the content of ethyl carbamate and its precursors during one-year ripening of maesil liqueur was investigated. Fresh maesil (control), fruit blanched for 2 min (blanched), and fruit blanched and dried for 15 h at 50 °C (blanched/dried) were soaked in the liquor containing 25% alcohol at a ratio of 1:2 (w/w) for 100 days at 25 °C and the liquid was further ripened for 260 days. Ethyl carbamate ranged from 13.1 to 204.4 μg/kg with the highest value at 210 day. Thermally treated samples had higher ethyl carbamate concentration than the control, suggesting that thermal treatment increased the formation of ethyl carbamate. A positive correlation between ethyl carbamate content and β-glucosidase activity in all samples indicated that enzymatic hydrolysis of amygdalin by β-glucosidase determined ethyl carbamate concentration during the fermentation of maesil liqueur.
Keywords: Maesil liqueur, Ethyl carbamate, Blanching, Drying, β-glucosidase
Introduction
Maesil liqueur is produced from the fermentation of green maesil, an unripe fruit of Prunus mume, and soju which is a distilled liquor containing 20–25% alcohol (v/v) (Hwang et al., 2009; Kim et al., 2013; Lee et al., 2018). Green maesil is known to have a cyanogenic glycoside amygdalin that can form cyanide by enzymatic hydrolysis of β-glucosidase (Chung et al., 2013; Donald, 2009; Yu et al., 2015). Ethyl carbamate is naturally formed from the reaction of ethanol and cyanide during the fermentation of alcoholic beverages (Fang et al., 2018; Zimmerli and Schlatter, 1991). It is classified as a probable carcinogen to humans (Group 2A) by International Agency for Research on Cancer (IARC, 2020). Among 14 alcoholic beverages analyzed in Korean Total Diet Study, the highest value of 151 μg/kg was found in maesil liqueur (Ryu et al., 2015). The concentration of ethyl carbamate in maesil liqueur was reported to increase up to 216 μg/kg during fermentation (Kim et al., 2013).
The reduction of ethyl carbamate content in maesil liqueur has been achieved by either removing a kernel of maesil or inactivating β-glucosidase to produce cyanide. The removal of maesil seed resulted in a decrease of ethyl carbamate levels in maesil liqueur (Lachenmeier et al., 2005). Haisman and Knight (1967) demonstrated that β-glucosidase of plum kernels was inactivated up to 90% by thermal treatment at 60 °C. Oke (1994) reported that boiling reduced the cyanide content of cassava roots. Blanching and drying of the maesil are used in individual households to enhance the flavor of maesil liqueur. To date, the effect of thermal treatment on the content of ethyl carbamate and its precursors has never been reported.
Therefore, this study was aimed at elucidating the effects of thermal treatment of maesil on the contents of ethyl carbamate, its precursors such as cyanide and ethanol, amygdalin, and β-glucosidase to hydrolyze amygdalin for one-year fermentation of maesil liqueur.
Materials and methods
Chemicals
Sodium chloride, sodium hydroxide, sodium bicarbonate, ethyl carbamate, acetone, β-glucosidase, amygdalin, ρ-nitrophenyl-β-D-glucopyranoside (β-PNPGLU), 4-nitrophenol, amygdalin, potassium cyanide, potassium phosphate, and potassium diphosphate were purchased from Sigma-Aldrich (St Louis, MO, USA). An internal standard (d5-ethyl carbamate) was obtained from CDN isotopes (Pointe-Claire, QC, Canada). Dichloromethane, methanol, and ethanol were purchased from J. T. Baker (Center Valley, PA, USA). Picric acid was purchased from BDH Ltd (Poole, UK). Disposable diatomite ChemElut SPE column (50 mL) was obtained from Agilent Technology (Milwaukee, WI, USA).
Sample preparation
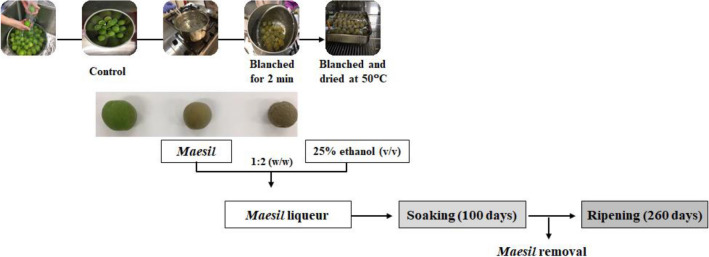
Maesil liqueur was prepared using the methods of Park et al. (2007) and Lee et al. (2017) with a slight modification. Green fruits of maesil were purchased from a local farm (Hadong, Korea) in 2017. As shown in Fig. 1, whole fruits were washed with pure water, wiped with a soft cotton cloth, and then placed on the table to volatilize the water on the fruit surface. The treatment conditions of the fruit were as follows: fresh maesil (control), 500 g of maesil fruits was heated in 5 L of boiling water for 2 min and then cooled to room temperature (blanched), and maesil fruits (500 g) were blanched and dried for 15 h in a drying oven at 50 °C (blanched/dried). Each sample (each 1 kg) was immersed in 2 kg of soju containing 25% ethanol (v/v) at a ratio of 1:2 (w/w) in a transparent jar. Each pretreatment of maesil fruit was performed three times. The jars were put in a thermostatic incubator (IL3-25A, Jeio Tech, Daejeon, Korea) at 25 °C under a 12:12 h light–dark cycle to simulate a ripening condition at individual houses. After being soaked for 100 days, the fruit was taken out from the jar, and the remaining liquid was further ripened for 260 days. The fruits were freeze-dried for 5 days (EYELA freeze-drier FDU-1200, Tokyo, Japan) and ground (Hibell Super grinder, Hwaseong, Korea), The fruit powder (0.4 g) was mixed with 10 mL of distilled water for 100 min at 37 °C at 120 rpm (JSSB-30 T shaker, JS Research Inc., Gongju, Korea),centrifuged at 16,582 × g for 10 min at 4 °C and supernatant and maesil liqueur were analyzed for ethyl carbamate, amygdalin, total cyanide, and ethanol, and β-glucosidase activity. All experiments were performed in triplicate.
Fig. 1.
Preparation procedure for maesil liqueur in different maesil pretreatment. Control, fresh maesil; Blanched, heated for 2 min in boiling water; Blanched/dried, heated for 2 min in boiling water and dried for 15 h at 50 °C
Ethyl carbamate analysis
Ethyl carbamate was analyzed using the method of Choi and Koh (2016). Maesil liqueur was neutralized using 1 N sodium hydroxide solution. Ten grams of the neutralized sample was mixed with d5-ethyl carbamate (100 ng), distilled water (30 mL), and sodium chloride (5 g). After being loaded into ChemElut cartridge (50 mL), ethyl carbamate was eluted with 160 mL of dichloromethane, concentrated to about 2–3 mL using a rotary evaporator at 18 °C, transferred into a v-vial, and then concentrated to 1 mL at 37 °C under a nitrogen stream. The concentrated sample was injected into a 7820A gas chromatograph coupled with a 5977E mass selective detector (Agilent, Santa Clara, CA, USA) equipped with a DB-WAX column (30 m × 0.25 mm, film thickness 0.25 μm, J&W, Folsom, CA). The oven temperature was programmed as follows: 60 °C at 0 min, 10 °C/min to 90 °C, 2 °C/min to 130 °C held for 5 min, 20 °C/min to 220 °C, and then held for 3 min. The mass selective detector was operated in the selected ion monitoring (SIM) with an electron impact energy of 70 eV. The MS transfer line and ion source were 240 °C and 230 °C, respectively. Mass to charge (m/z) of 62 and 64 were major fragment ions of ethyl carbamate and d5-ethyl carbamate. The peak of ethyl carbamate in the sample was confirmed by comparing the area ratios of m/z 62 vs m/z 74 that were the major fragment ions of ethyl carbamate.
Amygdalin determination
Amygdalin was analyzed according to the method of Kim et al. (2010) and Bolarinwa et al. (2015) with some modifications. Ethanol existing in the sample (2 mL) was removed using a rotary evaporator. After being filtered with a polytetrafluoroethylene (PTFE) membrane (Millipore, Milford, MA, USA), amygdalin was determined using a high-performance liquid chromatograph (HPLC) coupled with a diode array detector (1260 Infinity, Agilent Technologies, Waldbronn, Germany). A Kinetex C18 column (150 mm × 4.6 mm, 5 μm) was used for amygdalin separation (Torrance, CA, USA). Amygdalin was monitored at 214 nm using 25% methanol at a rate of 1 mL/min. The linear range of amygdalin quantification was 10–1000 μg/mL.
β-Glucosidase activity
β-Glucosidase activity was determined by the method of Carrao-Panizzi and Bordingnon (2000) with a slight modification. β-Glucosidase concentrations were 0, 40, 60, 80, and 100 μg/mL. The maesil liqueur (0.5 mL) was mixed with 2 mL of β-PNPGLU and then reacted for 30 min at 37 °C. Then, 0.5 M sodium carbonate solution (1.5 mL) was added and placed in the water bath at 37 °C for 30 min. The absorbance was measured at 400 nm using a UV–Vis spectrophotometer (Biochrom Libra S22, Santa Barbara, CA, USA). The blank was prepared with a substrate (β-PNPGLU) instead of the sample extract.
Cyanide determination
Cyanide content was measured using the enzyme-picrate acid method of Egan et al. (1998), Bradbury et al. (1999), and Kim et al. (2010). Cyanide solutions for calibration were prepared in six concentrations of 0, 2, 10, 20, 50, and 100 μg/mL in 0.1 M phosphate buffer. Picric paper was prepared by immersing a Whatman paper (Whatman 3MM Chr, Kent, UK) in 40 mL of distilled water that contained 5% sodium bicarbonate and 0.5% picric acid. The paper was dried for 10 min at 50 °C and then put in a test tube containing maesil liqueur (500 μL) and β-glucosidase (50 μL, 3.43 unit). The paper was placed in a water bath at 45 °C for 3 h and then put in 3 mL of distilled water for 30 min. Absorbance of the resulting solution was measured at 510 nm.
Ethanol determination
Ethanol was determined using an alcolyzer Wine M/ME (Anton Parr GmbH, Graz, Austria) based on the Near Infrared (NIR) spectroscopy method. The alcolyzer was conditioned with distilled water for zero point and 20% ethanol solution. The sample was sonicated to remove residual carbon dioxide and then injected into the alcolyzer.
Statistical analysis
All experiments were performed in triplicates, and the results were presented as the mean ± standard deviations. An analysis of variance (ANOVA) and Duncan’s multiple range tests were conducted to express the difference among three pretreated samples. Pearson’s correlation analysis was used to evaluate the significance of the correlations. All statistical analyses were performed using SPSS IBM version 21.0 (SPSS Inc., Chicago, IL, USA).
Results and discussion
Ethyl carbamate precursors in maesil fruit
Ethyl carbamate is mostly formed from the chemical reaction between ethanol and cyanate produced enzymatically from cyanogenic glycoside amygdalin present in maesil (Donald, 2009; Chung et al., 2013). This study was performed to elucidate thermal condition that affects the activity of β-glucosidase that involves in the formation of cyanide from amygdalin. The contents of amygdalin, β-glucosidase, and cyanide in the control and thermally pretreated maesil fruits are presented in Table 1. Compared with the fresh maesil (88.5%), moisture content was 80.6% in the blanched maesil and 70.0% in the blanched/dried maesil, respectively. The contents of amygdalin were 187.2 mg/100 g dry weight in the blanched maesil and 237.7 mg/100 g dry weight in the blanched/dried maesil, respectively. The maesil pretreatments resulted in a significant decrease of amygdalin content (55.1% in blanched maesil and 43.1% in blanched/dried maesil) compared with control. While the blanched maesil showed no significant difference in the β-glucosidase activity compared with control, the blanched/dried maesil reduced its activity up to 31.7%. It is lower than the result of Haisman and Knight (1967), who reported that 90% of β-glucosidase in the plum kernel was inactivated by heating for 0.6 min at 60 °C. The content of total cyanide in the blanched/dried maesil was 86.7% of the control, which can be attributed to the vaporization of cyanide during thermal treatment. Similarly, Nambisan and Sundaresan (1985) reported that cyanide content in cassava roots was decreased into 75% by boiling for 30 min. These indicate that the content of cyanide in maesil can be controlled either by reducing β-glucosidase activity or by vaporizing cyanide produced from the enzymatic hydrolysis of amygdalin.
Table 1.
The content of amygdalin, β-glucosidase activity, and total cyanide of control and pretreated maesil
| Amygdalin (mg/100 g dry weight) |
β-glucosidase activity (μg/mL) |
Total cyanide (mg/100 g dry weight) |
|
|---|---|---|---|
| Control | 417.3 ± 4.6a | 42.4 ± 0.3a | 6.9 ± 0.4a |
| Blanched | 187.2 ± 9.4c | 39.1 ± 0.5a | 6.5 ± 0.3a |
| Blanched/dried | 237.7 ± 14.7b | 29.0 ± 1.2b | 5.9 ± 0.4b |
Each value is expressed as mean ± standard deviation (n = 3)
Different letters (a–c) in the same column indicate a significant difference among three samples at p < 0.05 using tDuncan’s multiple test
Ethyl carbamate
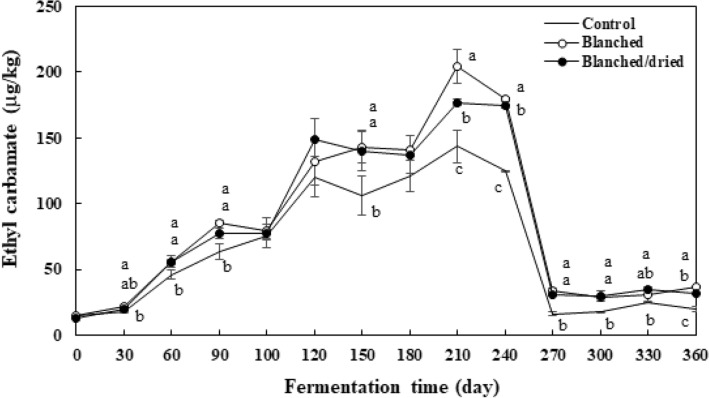
Figure 2 shows ethyl carbamate concentrations in three maesil liqueur samples for one-year fermentation. The content of ethyl carbamate in the control ranged from 14.6 to 143.3 μg/kg. The contents of ethyl carbamate in the blanched and blanched/dried maesil liqueur ranged from 15.0 to 204.4 μg/kg and from 13.1 to 176.8 μg/kg, respectively. The pretreated samples contained higher ethyl carbamate content than the control. The ethyl carbamate contents of the blanched maesil liqueur were either similar or higher than those of the blanched/dried maesil liqueur. This suggests that the formation of ethyl carbamate was favorable in the blanched maesil liqueur compared to the blanched/dried maesil liqueur. Interestingly, the contents of ethyl carbamate in all samples increased until day 210 and then drastically decreased on day 270. This indicates that both ethyl carbamate formation and its degradation occurred during fermentation. Perez-Martin et al. (2013) found that lactic acid bacteria isolated from red wines had an esterase activity, which hydrolyzes ethyl carbamate to form ammonia and carbon dioxide. Wu et al. (2013) demonstrated that urethanase of Rhodotorula mucilaginosa from Chinese rice wine decreased ethyl carbamate level by 80%. This stain is resistant in acidic conditions (pH 3.5–4.5) and 18–20% ethanol during fermentation. Given that maesil liqueur was acidic (pH 3–4) and contained about 20% ethanol, esterase or urethanase can be active in maesil liqueur. Further studies are needed to find the presence of the enzyme to hydrolyze ethyl carbamate.
Fig. 2.
Changes in the ethyl carbamate content of maesil liqueur during one-year fermentation. Each value is expressed as mean ± standard deviation (n = 3). Different letters (a–c) indicate a significant difference among three samples at each analyzing point (p < 0.05)
Amygdalin
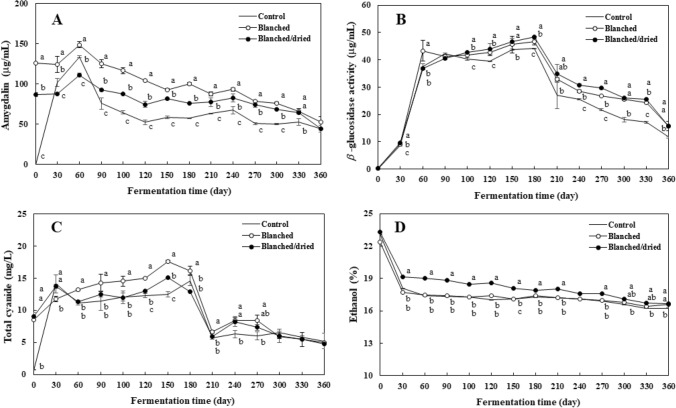
Amygdalin is a cyanogenic glycoside found in maesil (Bolarinwa et al., 2015; Chung et al., 2013; Donald, 2009; Yu et al., 2015). The content of amygdalin ranged from not detected (ND) to 134.0 μg/mL in the control, from 52.8 to 148.9 μg/mL in the blanched liqueur, and from 44.4 to 114.3 μg/mL in the blanched/dried sample, respectively (Fig. 3A). Amygdalin content in all maesil liqueurs reached a maximum value at 60 day and then gradually decreased until 120 day. This indicates that amygdalin present in maesil fruit was gradually released into 25% ethanol phase during early soaking stages and then was enzymatically hydrolyzed into cyanide. In the early stages of maesil soaking, amygdalin content of the blanched/dried sample in the liquid phase was lower than fresh and blanched samples. This can be explained that the drying step caused a loss of water molecule from the fruit and led to a crumpled surface of the fruit (Fig. 1), which resulted in a slow rate of amygdalin release from the maesil. The amygdalin content after 60 day was steadily decreased (Fig. 3A), indicating that β-glucosidase was active to hydrolyze amygdalin during fermentation.
Fig. 3.
Changes in amygdalin (A), β-glucosidase activity (B), total cyanide (C), and ethanol (D) of maesil liqueur during one-year fermentation. Each value is expressed as mean ± standard deviation (n = 3). Different letters (a–c) indicate a significant difference among three samples at each analyzing point (p < 0.05)
β-Glucosidase activity
β-Glucosidase hydrolyzes amygdalin to yield cyanide that reacts with ethanol, thus resulting in the formation of ethyl carbamate (Ahmed et al., 2017; Cueto et al., 2018). The activity of β-glucosidase was observed in Lactobillus species isolated from maesil fruit and juice (Lee and Paik, 2017). This suggests that β-glucosidase may contributed to the degradation of amygdalin in the maesil liqueur during fermentation. As shown in Fig. 3B, the activities of β-glucosidase in the control ranged from ND to 44.2 μg/mL, those of β-glucosidase in the blanched and blanched/dried maesil liqueur ranged from ND to 46.6 μg/mL and from ND to 48.4 μg/mL, respectively. The activities of β-glucosidase were highest in 60–180 day. This corresponded to the time when amygdalin content rapidly decreased during ripening. Vasconcelo et al. (1990) reported that the hydrolysis of cyanogenic glycosides in cassava roots occurred in acid environment (pH 3.8) during lactic fermentation. Tuncel et al. (1995) reported that β-glucosidase activity caused a degradation of amygdalin in ground apricot kernels soaked at 20 °C. On the contrary, Bolarinwa et al. (2015) reported that β-glucosidase from apple seeds has highly stable at 70 °C. Haisman and Knight (1967) showed that β-glucosidase in canned plum kernels was inactivated by heat treatment for 12 min at 100 °C. These indicate that the β-glucosidase of maesil liqueur was not completely inactivated during thermal treatment employed in this study.
Total cyanide
Cyanide is an important precursor of ethyl carbamate in stone fruit spirit (EFSA, 2007). The contents of total cyanide are presented in Fig. 3C. In the maesil fruit, total cyanide contents in the blanched and blanched/dried maesil liqueur samples were 8.5 mg/mL and 9.0 mg/mL, while total cyanide in the control was not detected. During the soaking and ripening, the content of amygdalin and the β-glucosidase activities of the blanched and blanched/dried samples were higher than those of the control (Fig. 3A and B). The blanched and blanched/dried samples also showed a higher trend in the content of total cyanide than the control. Ethyl carbamate contents of thermally treated samples were higher than those of the control (Fig. 2). This confirms that β-glucosidase of maesil liqueur was not inactivated by the thermal treatment. Similarly, Montagnac et al. (2009) reported that boiling and drying were not effective in removing cyanide in cassava roots. In addition to thermal treatment, microorganisms involved in the fermentation of maesil liqueur may also affect the content of cyanide. Kobawila et al. (2005) reported that the decrease of cyanide content reduced more than 70% by bacterial enzymes during fermentation of cassava roots and leaves. Knowles (1976) found that certain strains of Bacillus pumilus had the capacity to use cyanide for their nutrition.
Ethanol
Cyanate generated from the oxidation of cyanide reacts with ethanol to form ethyl carbamate (Zimmerli and Schlatter, 1991). The change of ethanol contents during fermentation is shown in Fig. 3D. The contents of ethanol decreased from 23.1 to 16.2% in the control, while both the blanched and blanched/dried samples decreased from 22.4 to 16.4% and from 23.3 to 16.6%, respectively. This suggests that ethanol content was not critical in determining ethyl carbamate content in maesil liqueur. Considering that ethyl carbamate had a maximum level on 210 day, the rapid decrease of cyanide content at 180 day can be explained by the reaction with ethanol to form ethyl carbamate.
Correlation between ethyl carbamate and its precursors
Pearson's correlation coefficients among ethyl carbamate, amygdalin, β-glucosidase activity, total cyanide, and ethanol are presented in Table 2. The content of amygdalin and ethanol had no correlation between ethyl carbamate content. On the other hand, a positive correlation between ethyl carbamate content and β-glucosidase activity was observed in all examined samples (p < 0.01), suggesting that β-glucosidase played an important role in determining ethyl carbamate content in maesil liqueur. In addition, a significant relation between ethyl carbamate content and total cyanide content was found in the control and blanched samples (p < 0.05). A similar result was observed in the previous studies demonstrating that positive correlation was found between ethyl carbamate and cyanide in Brazilian sugar cane spirits and Prunus mume liqueur (Aresta et al., 2001; Hashiguchi et al., 2010). A positive relation between total cyanide content and β-glucosidase activity confirms the assumption that cyanide is produced from the enzymatic hydrolysis of amygdalin via β-glucosidase, which resulted in the formation of ethyl carbamate during fermentation. These findings indicate that β-glucosidase played a key role of ethyl carbamate formation during fermentation of maesil liqueur. Meanwhile, total cyanide had no relation with ethyl carbamate in the blanched/dried sample (Table 2). The cyanide must be oxidized into cyanate and then form ethyl carbamate by a nucleophilic attack of ethanol (Aresta et al., 2001). It is noteworthy that ethanol content of the blanched/dried maesil liqueur was significantly higher than the other samples throughout the fermentation (Fig. 3D), indicating that ethanol remained as an intact form in the liqueur. These results suggest that the chemical conversion of cyanide to cyanate as well as enzymatic formation of cyanide by β-glucosidase should be considered together to find the influencing factor of ethyl carbamate content in the maesil liqueur.
Table 2.
Pearson correlation coefficients between ethyl carbamate, amygdalin, β-glucosidase activity, total cyanide, and ethanol in maesil liqueur
| Amygdalin | β-glucosidase activity | Total cyanide | Ethanol | ||
|---|---|---|---|---|---|
| Control | Ethyl carbamate | 0.158 | 0.658** | 0.345* | − 0.241 |
| Amygdalin | 0.377* | 0.557** | − 0.440** | ||
| β-glucosidase activity | 0.693** | − 0.468** | |||
| Total cyanide | − 0.395** | ||||
| Blanched | Ethyl carbamate | 0.004 | 0.579** | 0.316* | − 0.274 |
| Amygdalin | − 0.014 | 0.303 | 0.469** | ||
| β-glucosidase activity | 0.660** | − 0.532** | |||
| Total cyanide | − 0.017 | ||||
| Blanched/dried | Ethyl carbamate | 0.196 | 0.684** | 0.262 | − 0.249 |
| Amygdalin | 0.153 | 0.401** | 0.401** | ||
| β-glucosidase activity | 0.432** | − 0.447** | |||
| Total cyanide | 0.286 |
*A significant difference at p < 0.05
**A significant difference at p < 0.01
In conclusion, the content of ethyl carbamate was higher in the liqueurs fermented with thermally treated maesil compared with the control. Thermal treatment might make the flesh of maesil soften to release amygdalin from the maesil fruit. However, β-glucosidase remained active to produce cyanate, thus formed ethyl carbamate through the reaction with ethanol. Thermal condition to inactivate β-glucosidase and identification of microorganisms to hydrolyze ethyl carbamate should be further investigated to minimize ethyl carbamate content in the maesil liqueur.
Acknowledgements
This work was supported by a research grant from Seoul Women’s University (2021-0153).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bogyoung Choi, Email: bogyoungchoi@kist.re.kr.
Eunmi Koh, Email: kohem7@swu.ac.kr.
References
- Ahmed A, Nasim F, Batool K, Bibi A. Microbial β-glucosidase: sources, productions and applications. Journal of Applied and Environmental Microbiology. 2017;5:31–375. doi: 10.12691/jaem-5-2-2. [DOI] [Google Scholar]
- Aresta M, Boscolo M, Franco DW. Copper (II) catalysis in cyanate conversion into ethyl carbamate in spirits, and relevant reactions. Journal of Agricultural and Food Chemistry. 2001;6:2819–2824. doi: 10.1021/jf001346w. [DOI] [PubMed] [Google Scholar]
- Bolarinwa LF, Orfila C, Morgan MRA. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chemistry. 2015;152:133–139. doi: 10.1016/j.foodchem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Bradbury MG, Egan SV, Bradbury JH. Picrate paper kits for determination of total cyanogens in cassava roots and all forms of cyanogens in cassava products. Journal of the Science of Food and Agriculture. 1999;79:593–601. doi: 10.1002/(SICI)1097-0010(19990315)79:4<593::AID-JSFA222>3.0.CO;2-2. [DOI] [Google Scholar]
- Carrao-Panizzi MC, Bordingnon JR. Activity of beta-glucosidase and levels of isoflavone glucosides in soybean cultivars affected by the environment. Pesquisa Agropecuaria Brasileira. 2000;35:873–878. doi: 10.1590/S0100-204X2000000500002. [DOI] [Google Scholar]
- Choi B, Koh E. Changes of ethyl carbamate and its precursors in maesil (Prunusmume) during one-year fermentation. Food Chemistry. 2016;209:318–322. doi: 10.1016/j.foodchem.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Chung HS, Kim DS, Kim HS, Lee YG, Seong JH. Ethyl carbamate of freezing pretreatment on the quality of juice extracted from Prunusmume fruit by osmosis with sucrose. Food Science and Technology. 2013;54:30–34. [Google Scholar]
- Cueto JD, Moller BL, Dicenta F, Sanchez-Perez R. β-glucosidase activity in almond seeds. Plant Physiology and Biochemistry. 2018;126:163–172. doi: 10.1016/j.plaphy.2017.12.028. [DOI] [PubMed] [Google Scholar]
- Donald GB. Cyanogenic foods (cassava, fruit kernels, and cycad seeds) Medical Toxicology of Natural Substances. 2009;55:336–352. doi: 10.1016/j.disamonth.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Egan SV, Yeah HH, Bradbury JH. Simple picrate paper kit for determination of the cyanogenic potential of cassava flour. Journal of the Science of Food and Agriculture. 1998;76:39–48. doi: 10.1002/(SICI)1097-0010(199801)76:1<39::AID-JSFA947>3.0.CO;2-M. [DOI] [Google Scholar]
- European Food Safety Authority (EFSA) Ethyl carbamate and hydrocyanic acid in food and beverages. EFSA Journal. 2007;551:1–44. [Google Scholar]
- Fang F, Qiu Y, Du G, Chen J. Evaluation of ethyl carbamate formation in Luzhou-flavor spirit during distillation and storage processes. Food Bioscience. 2018;23:137–141. doi: 10.1016/j.fbio.2018.02.007. [DOI] [Google Scholar]
- Haisman DR, Knight DJ. β-glucosidase activity in canned plums. International Journal of Food Science and Technology. 1967;2:241–248. doi: 10.1111/j.1365-2621.1967.tb01348.x. [DOI] [Google Scholar]
- Hashiguchi T, Horii S, Izu SH, Sudo S. The concentration of ethyl carbamate in commercial ume (Prunusmume) liqueur products and a method of reducing it. Bioscience Biotechnology Biochemistry. 2010;74:2060–2066. doi: 10.1271/bbb.100364. [DOI] [PubMed] [Google Scholar]
- Hwang LH, Kim AK, Park KA, Kim JY, Hwang IS, Chae YZ. The ethyl carbamate of raw material, alcohol content, and trans-resveratrol on the formation of ethyl carbamate in plum wine. Journal of Food Hygiene and Safety. 2009;24:194–199. [Google Scholar]
- International Agency for Research on Cancer (IARC). Alcoholic beverage consumption and ethyl carbamate (urethane). Available from: http://monographs.iarc.fr/ENG/Meetings/vol96-summanry.pdf. Accessed Mar. 29, 2020.
- Kim EJ, Lee HJ, Jang JW, Kim IY, Kim DH, Kim HA, Lee SM, Jang HW, Kim SY, Jang YM, Im DK, Lee SH. Analytical of determination of cyanide in maesil (Prunusmume) extracts. Korean Journal of Food Science and Technology. 2010;2:130–135. [Google Scholar]
- Kim NY, Eom MN, Do YS, Kim JB, Kang SH, Yoon MH, Lee JB. Determination of ethyl carbamate in maesil wine by alcohol content and ratio of maesil (Prunusmume) during ripening period. Korean Journal of Food Preservation. 2013;20:429–434. doi: 10.11002/kjfp.2013.20.3.429. [DOI] [Google Scholar]
- Knowles CJ. Microorganisms and cyanide. Bacteriology Reviews. 1976;40:652–680. doi: 10.1128/br.40.3.652-680.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobawila SC, Louembe D, Keleke S, Hounhouigan J, Gamba C. Reduction of the cyanide content during fermentation of cassava roots and leaves to produce Bikedi and Ntoba Mbodi, two food products from Congo. African Journal of Biotechnology. 2005;4:689–696. doi: 10.5897/AJB2005.000-3128. [DOI] [Google Scholar]
- Lachenmeier DW, Schehl B, Kuballa T, Frank W, Senn T. Retrospective trends and current status of ethyl carbamate in German stone-fruit spirits. Food Additives and Contaminants. 2005;22:397–405. doi: 10.1080/02652030500073360. [DOI] [PubMed] [Google Scholar]
- Lee NK, Paik HD. Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. Journal of Microbiology and Biotechnology Research. 2017;27:869–877. doi: 10.4014/jmb.1612.12005. [DOI] [PubMed] [Google Scholar]
- Lee S, Koh K, Yang J, Oh S, Kim J. Balhyo Sikpumhak (Fermented food science) Korea: Hyoil press Seoul; 2017. pp. 201–202. [Google Scholar]
- Lee JB, Kim MK, Kim BK, Chung YH, Lee KG. Analysis of ethyl carbamate in plum wines produced in Korea. Food Science and Biotechnology. 2018;27:277–282. doi: 10.1007/s10068-017-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnac JA, Davis CR, Tanumihardjo SA. Processing techniques to reduce toxicity and antiutrients of cassava for use as a stable food. Comprehensive Reviews in Food Science and Food Safety. 2009;8:17–26. doi: 10.1111/j.1541-4337.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- Nambisan B, Sundaresan S. Effect of processing on the cyanoglucoside content of cassava. Journal of the Science of Food and Agriculture. 1985;36:1197–1203. doi: 10.1002/jsfa.2740361126. [DOI] [Google Scholar]
- Oke OL. Eliminating cyanogens from cassava through processing: technology and tradition. Acta Horticulturae. 1994;375:163–174. doi: 10.17660/ActaHortic.1994.375.14. [DOI] [Google Scholar]
- Park LY, Chae MH, Lee SH. Effect of ratio of maesil (Prunusmume) and alcohol on quality changes of maesil liqueur during leaching and ripening. Korea Journal of Food Preservation. 2007;14:645–649. [Google Scholar]
- Perez-Martin F, Sesena S, Izquierdo PM, Palop ML. Esterase activity of lactic acid bacteria isolated from malolactic fermentation of red wines. International Journal of Food Microbiology. 2013;163:153–158. doi: 10.1016/j.ijfoodmicro.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Ryu D, Choi B, Kim E, Park S, Paeng H, Kim C, Lee J, Yoon HJ, Koh E. Determination of ethyl carbamate in alcoholic beverages and fermented foods sold in Korea. Toxicology Research. 2015;31:289. doi: 10.5487/TR.2015.31.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncel G, Nout MJR, Brimer L. Degradation of cyanogenic glycosides of bitter apricot seeds (Prunusarmeniaca) by endogeneous and added enzymes as affected by heat treatments and particle size. Food Chemistry. 1995;63:65–69. doi: 10.1016/S0308-8146(97)00217-3. [DOI] [Google Scholar]
- Vasconcelos AT, Twiddy DR, Wesstby A, Reilly PJA. Detoxification of cassava during gari preparation. International Journal of Food Science and Technology. 1990;25:198–203. doi: 10.1111/j.1365-2621.1990.tb01074.x. [DOI] [Google Scholar]
- Wu Q, Zhao Y, Xu Y. Immobilized Rhodotorulamucilaginosa: a novel urethanase producing strain for degrading ethyl carbamate. Applied Biochemistry and Biotechnology. 2013;171:2220–2232. doi: 10.1007/s12010-013-0493-7. [DOI] [PubMed] [Google Scholar]
- Yu Y, Xiao G, Xu Y, Wu J, Zhang Y, Chen W. Changes of quality in the fruits of Prunusmume during deacidification by fermentation with Lactobacillusfermentium. Journal of Food Science. 2015;80:405–410. doi: 10.1111/1750-3841.12769. [DOI] [PubMed] [Google Scholar]
- Zimmerli B, Schlatter J. Ethyl carbamate: analytical methodology, occurrence, formation, biological activity and risk assessment. Mutation Research. 1991;259:325–350. doi: 10.1016/0165-1218(91)90126-7. [DOI] [PubMed] [Google Scholar]