Abstract
Seaweeds are macroalgae, which can be of many different morphologies, sizes, colors, and chemical profiles. They include brown, red, and green seaweeds. Brown seaweeds have been more investigated and exploited in comparison to other seaweed types for their use in animal feeding studies due to their large sizes and ease of harvesting. Recent in vitro and in vivo studies suggest that plant secondary compound-containing seaweeds (e.g., halogenated compounds, phlorotannins, etc.) have the potential to mitigate enteric methane (CH4) emissions from ruminants when added to the diets of beef and dairy cattle. Red seaweeds including Asparagopsis spp. are rich in crude protein and halogenated compounds compared to brown and green seaweeds. When halogenated-containing red seaweeds are used as the active ingredient in ruminant diets, bromoform concentration can be used as an indicator of anti-methanogenic properties. Phlorotannin-containing brown seaweed has also the potential to decrease CH4 production. However, numerous studies examined the possible anti-methanogenic effects of marine seaweeds with inconsistent results. This work reviews existing data associated with seaweeds and in vitro and in vivo rumen fermentation, animal performance, and enteric CH4 emissions in ruminants. Increased understanding of the seaweed supplementation related to rumen fermentation and its effect on animal performance and CH4 emissions in ruminants may lead to novel strategies aimed at reducing greenhouse gas emissions while improving animal productivity.
Keywords: Seaweed, Bromoform, Methane, Phlorotannins, Ruminant, Cattle
1. Introduction
The livestock industry contributes 14.5% to 19% of global greenhouse gas (GHG) emissions (Johnson and Johnson, 1995; Gerber et al., 2013) and accounts for approximately 11% of the GHG emissions in the US (Myhre et al., 2013; NASEM, 2018). Ruminal methane (CH4) emission is a consequence of anaerobic carbohydrate fermentation by ruminal microbiota that produce carbon dioxide (CO2) and hydrogen (H2) in a reduction pathway used by methanogens (Morgavi et al., 2010). It is estimated that sheep, goats, and cattle lose 2% to 12% of ingested gross energy to CH4 production depending on the diet (Johnson and Johnson, 1995). The ability of a CH4 inhibitor to increase metabolizable energy in the ruminant diet and effectively reduce enteric CH4 emissions is, therefore, an area of interest. Various dietary CH4 interventions including ionophores, chemical compounds, legumes, essential oils, fats, probiotics, and plant secondary metabolites (e.g., halogenated, phlorotannins, tannins, saponins, iodine) have been investigated as methanogenesis inhibitors (Patra, 2012; Min et al., 2020). However, in some cases, the desired antimethanogenic effect may coexist with adverse effects such as decreasing dry matter intake (DMI) and feed efficiency (average daily gain: feed intake ratio).
Seaweed, otherwise known as macroalgae, are primitive non-flowering photosynthetic macrophytes. There are three distinct seaweed groups: green (chlorophyta), brown (phaeophyta), and red (rhodophyta). Worldwide seaweed production through aquaculture was over 30 million tonnes (fresh) in 2016 (FAO, 2018a, FAO, 2018b; Rao et al., 2018). The capability of seaweed to promote well-being and health in livestock is facilitated to a great extent by bioactive secondary metabolites that are synthesized by some seaweed species (Abdul et al., 2016; Corona et al., 2016). Some of these secondary metabolites are responsible for antimethanogenic properties (Abecia et al., 2012; Roque et al., 2019a, b) but often health benefits come from various other nutrients (e.g., minerals, protein, and unsaturated fatty acids contents; Anderson et al., 2006; Cian et al., 2013). Recent in vitro and in vivo studies suggest that the halogenated compound-containing red seaweeds Asparagopsis taxiformis and Asparagopsis armata have the potential to reduce CH4 production when added to grass- and grain-based diets (Roque et al., 2019a, b, Kinley et al., 2020; Min et al., 2021). Red seaweed is effective in the short-term (Mitsumori et al., 2012). The long-term feeding efficacy of red seaweed is still unknown. When seaweed is added to cattle diets, the effects on diet palatability, animal health, and reproduction, as well as milk and meat quality are not consistent. Furthermore, seaweeds occasionally accumulate heavy metals, iodine and other minerals: feeding contaminant-laden seaweeds could have negative effects on animal and human health (Makkar et al., 2016).
Phlorotannins (polymers of phloroglucinol) are mainly found in brown seaweeds (Li et al., 2011), which can positively or negatively impact rumen function and CH4 production (Belanche et al., 2016; Huang et al., 2018). Supplementation of tannins at levels between 2% and 4% of dietary dry matter (DM) had positive effects in ruminants by increasing protein metabolism (Mueller-Harvey, 2006) and reducing bloat and enteric CH4 emissions (Rochfort et al., 2008; Min et al., 2020). Although the processes by which tannins act are somewhat unknown, among the most accepted are substrate depression (McMahon et al., 2000), enzyme inhibition (Jones et al., 1994), and direct inhibition of selected rumen microorganisms (Scalbert, 1991). Some tannins can directly inhibit CH4 production: an in vivo experiment in which ruminants (e.g., steers and lambs) were fed increasing doses of commercial brown seaweed (Ascophyllum nodosum meal; Tasco-14) had lower relative abundances of fecal Escherichia coli O157:H7 with no enhanced animal performance (Bach et al., 2008). Visser et al. (2017) identified that phlorotannins from Laminaria digitata decreased protein digestibility and CH4 production (40%) during a 24-h in vitro ruminal fermentation. However, dietary supplementation with brown seaweed does not always have a positive impact on digestion and metabolism, as these effects are dependent on the particular strain of seaweed used. Belanche et al. (2016) observed no changes on in vitro CH4 emissions when L. digitata or A. nodosum were included in the diet at 50 g/kg DM. Moneda et al. (2019) studied eight different seaweeds (Brown: Alaria esculenta, L. digitata, Pelvetia canaliculata, Saccharina latissima; Red: Mastocarpus stellatus, Palmaria palmata and Porphyra spp.; Green: Cladophora rupestris) that were included in an oat hay-based diet (1:1 oat hay:concentrate) at a rate of 50% and reported variable anti-methanogenic responses. Therefore, the use of brown seaweeds as CH4 mitigation options can be an alternative to conventional feedstuffs in ruminant diets, but it is necessary to assess their nutritive value and effectiveness prior to use in commercial feeding operations.
It has been reported that bromoform-containing seaweed or commercially available bromochloromethane (BCM; 5 to 10 μmol/L) supplementation are some of the most effective inhibitors of enteric CH4 emissions because they interfere with methanogenesis (Wood et al., 1968; McCrabb et al., 1997; Goel et al., 2009). Furthermore, studies demonstrated that commercially available BCM supplementation or bromoform-containing seaweed significantly reduced CH4 production (50% to 95%) and inhibited methanogenesis without negative effects on ruminal fermentation or animal growth performance (Tomkins and Hunter, 2004; Tomkins et al., 2009; Abecia et al., 2012; Kinley et al., 2016; Machado et al., 2018). However, most proposed mitigation strategies have shown inconsistent results among studies and may even lead to decreased DMI (McCrabb et al., 1997; Roque et al., 2019a), lower ruminal digestibility (Gojon-Baez et al., 1998; Machado et al., 2016; Tayyab et al., 2016), or altered rumen microbial community diversity including methanogen, bacteria, protozoa, and fungi populations (Goel et al., 2009, Mitsumori et al., 2012, Roque et al., 2019a, b). In addition, there is much variability in the anti-methanogenic potency between seasons and species of seaweed (Dubois et al., 2013; Sarojini et al., 2012; Pirian et al., 2017) and among animal species (McCrabb et al., 1997; Tomkins and Hunter, 2004; Li et al., 2018). Bromoform is the active ingredient in seaweed that causes the reduction in CH4 emissions, although other compounds such as dibromochloromethane and dibromoacetic acid have also been detected at lower concentrations (Marshall et al., 1999; Mata et al., 2011; Machado et al., 2016, 2018). This paper reviews progress utilizing naturally occurring plant secondary compounds from select seaweed varieties as an active ingredient for anti-methanogenesis thereby reducing CH4 emissions when ruminants are supplemented with seaweed.
2. Chemical composition and bioactive ingredients of seaweed
Nutritional and biochemical values of different seaweed have been studied by many researchers (Fleurence and Le Coeur, 1993; Ortega-Calvo et al., 1993; Rizk, 1997). The secondary compounds in seaweed contain various bioactive properties including anti-viral, anti-microbial, anti-tumor, anti-inflammatory, antioxidant, and many more (Table 1). Seaweeds are also the source of phytochemical compounds, including agar, carrageenan, and alginates (Cardozo et al., 2007; Rindi et al., 2011; Pal et al., 2014; Kolanjiathan et al., 2014; Neethu et al., 2017), these compounds are rich in valuable nutrients and have been used as a source of human food, various animal feeds, therapeutic agents, and fertilizer (Cardozo et al., 2007; Nunes et al., 2018). Seaweeds contain carbohydrates, proteins, minerals, vitamins, fats and oils, and amino acids (AA), and possess trace amounts of secondary compounds (e.g., phlorotannins, iodine, and halogenated compounds) in cell walls (McConnell and Fenical, 1977; El-Baroty et al., 2007; Pirian et al., 2017; Gaillard et al., 2018).
Table 1.
Nutraceutical and pharmacological potential of some seaweeds.
| Species | Compounds | Properties | References1 |
|---|---|---|---|
| Red seaweed (Rhodophyta) | |||
| Asparagopsis taxiformis | Alkaloides, flavonoids, anthraquinones, phenols, chlorophylls, halogenated compounds | Antioxidant, antiproliferative, free radical scavenging, antimethanogenesis | 1, 2, 34, 35 |
| Asparagopsis armata | Halogenated compounds | Antimicrobial, antitumor activity | 3, 35 |
| Chondria armata | Galactoglycerolipids | Antimicrobial | 4, 5 |
| Corallina pilulifera | Phlorotannins | Antioxidant and tyrosinase pathways | 6, 7 |
| Corallina tamariscifolia | Phlorotannins | Anti-inflammatory, antioxidant | 8 |
| Eucheuma cava | Phlorotannins, Lectins | Antioxidant, UV protection, Antibacterial, antiviral | 9, 10, 11 |
| Laurencia pacifica | Laurinterol, Bromophenols, Sesquiterpene | Antibacterial, antioxidant | 12, 13, 14 |
| Gracilaria spp. | Steroid, terpenoid, eiconoid | Antibacterial | 14 |
| Rhodomella spp. |
Bromophenols |
Antimicrobial activity |
14 |
| Green seaweed (Chlorophyta) | |||
| Cladophora glomerata | Chlorophylls | Antioxidant, antibacterial | 15, 16, 17 |
| Caulerpa sp. | Flavonoids, phenols, saponin | Tyrosinase inhibitor | 18 |
| Haematococcus lacustris | Carotenoids | Antioxidant, anti-inflammatory | 19, 20, 21 |
| Ulva lactuca | Chlorophylls | Antibacterial, antioxidant | 22, 23, 24 |
| Dunaliella tertiolecta |
Phenolics |
Anti-aging |
25 |
| Brown seaweed (Ochrophyta) | |||
| Ascophyllum nodosum | Phlorotannins | Anti-bacterial, inhibit rumen fermentation | 26, 27 |
| Cystoseira tamariscifolia | Phlorotannins | Anti-inflammatory | 28 |
| Ecklonia cava | Sulfated polysaccharide/Phlorotannins | Anti-viral, antioxidant, anti-inflammatory. Tyrosinase inhibition | 29, 30,31 |
| Ecklonia bicyclis | Sulfated polysaccharide | Antiviral, antioxidant, anti-inflammatory | 32, 33 |
| Himanthalia elongata | Volatile fatty acids | Antioxidant, antimicrobial | 34 |
| Laminaria digitata | Iodine | Control iodine deficiency disorders and animal weight gain | 35 |
Sources: 1 = Nunes et al. (2018), 2 = Neethu et al. (2017), 3 = Horta et al. (2019), 4 = Al-Fadhli et al. (2006), 5 = Fabrowska et al. (2015), 6 = Thomas and Kim (2013), 7 = Stengel et al. (2011), 8 = Ferreres et al. (2012), 9 = Heo et al. (2009), 10 = Ko et al. (2011), 11 = Samarakoon and Jeon (2012), 12 = Fenical (1976), 13 = Liu et al. (2011a), 14 = Kasanah et al. (2015), 15 = Spears (1988), 16 = Borowitzka (2013), 17 = Christaki et al. (2013), 18 = Demais et al. (2007), 19 = Goldberg (1943), 20 = Spears (1988), 21 = Lanfer-Marquez et al. (2005), 22 = Goldberg (1943), 23 = Spears (1988), 24 = Delaunay and Voile (2011), 25 = Norzagaray-Valenzuela et al. (2017), 26 = Wang et al. (2008, 2009a, b), 27 = Kannan et al. (2019), 28 = Ferreres et al. (2012), 29 = Robic et al. (2009), 30 = Samarakoon and Jeon (2012), 31 = Heo et al. (2009), 32 = Chizhov et al. (1999), 33 = Wijesinghea and Jeona (2012), 34 = Plaza et al. (2010), 35 = He et al. (2002).
In general, compared to green and brown seaweed, red seaweed contains a high amount of crude protein (CP; Table 2) reaching 38.1% CP (e.g., Porphyra spp.) of the DM content of the plant. These results are consistent with other data (Cian et al., 2015). In contrast, green seaweed contains moderate amounts (15.3% to 18.6% CP DM), while brown seaweed exhibit much lower CP contents (6.0% to 16.6% DM; Table 2). But some species of green seaweed, such as Ulva reticulate, Ulva lactuca, Ulva fasciata, and Enteromorpha, were reported to have higher CP content (12% to 23% DM) compared to other species collected from the Gulf of Mannar coast, India (Abirami and Kowsalva, 2012). In addition, Pirian et al. (2017) reported that CP contents were 12.3% and 9.0% in green algae (Caulerpa sertulariodes) and brown algae (Colpomenia) in Persian Gulf seaweed, respectively. In this regard, the CP content of red seaweed is comparable with that of high protein plant feeds such as soy and soybean meal (Kuiken and Lyman, 1949; Norziah and Ching, 2000). The CP content of seaweeds varies between species and also among seasonal periods (Mishra et al., 1993; Castro-Gonzalez et al., 1994; Fleurence, 1999; Guiry and Guiry 2014; Pirian et al., 2017). Therefore, seaweeds are an interesting potential source of food protein, and animal feed. However, research is needed to ascertain the appropriate seaweed type and feeding rate so that animal productivity is not negatively impacted.
Table 2.
Chemical composition of seaweed species (all values on DM basis)1.
| Type |
Red seaweed |
Green seaweed |
Brown seaweed |
|||||
|---|---|---|---|---|---|---|---|---|
| Species | Porphyra spp. | Aaparagopsis taxiformis | Asparagopsis armata | Ulva sp. | Ascophyllum nodosum | Macrocystis Sp. | Laminaria Sp. | Costaria Costata |
| Nutrients, % | ||||||||
| CP | 24.6-38.1 | 17.8 | 18.3 | 15.3-18.6 | 6.0-8.3 | 10.1 | 9.8-16.6 | 7.8 |
| NDF | 43.1 | 36.9 | 27.2 | 22.8-26.2 | 20.9-22.0 | 19.9 | 16.6 | – |
| ADF | 6.6 | 11.6 | 10.9 | 7.6-8.7 | 13.1 | 12.6 | Na | – |
| Either extract | 0.3-0.5 | 0.4 | 0.32 | 1.2 | 3.9 | 0.6 | 0.8 | – |
| Ash | 6.5-8.7 | – | 10.0 | 7.7-23.2 | 22.0-22.5 | 32.9 | 29.9-31.5 | – |
| Minerals, % | ||||||||
| Ca | 4.4 | 3.8 | 4.47 | 2.9 | 1.0-3.0 | 14.1 | 0.08 | 0.12 |
| P | 3.8 | 0.2 | 0.27 | 0.27 | 0.1-0.2 | 2.9 | – | – |
| Na | 4.1 | 6.6 | 9.36 | 2.0-3.3 | 2.4-4.0 | 36.5 | 25.3 | 4.16 |
| Mg | 4.9 | 0.8 | 1.38 | 1.7 | 0.5-1.09 | 39.2 | 5.5 | 0.96 |
| Minerals, mg/kg | ||||||||
| Fe | 2.2 | 6.2 | 1.188 | 1.24 | 134.0 | 117.0 | 233,2 | – |
| Mn | – | 0.1 | 0.63 | 0.10 | 10-50 | 11.0 | 6.2 | 1.48 |
| Zn | 0.15 | 0.24 | 0.07 | 0.05 | 35-100 | 12.0 | 111.7 | 10.8 |
| Cu | 0.51 | 0.87 | – | 7.07 | 4.0-15 | 2.0 | 14.9 | 6.4 |
| S | – | 4.5 | – | – | 2.0-2.3 | – | – | – |
| Iodine | 1.5 | 1.71-3.37 | 0.6-1.8 | 0.9 | 0.01-0.1 | ND | 0.9 | 0.03 |
| Bromoform2 | – | 1,723 | 1,320 | 1503 | 2.73 | 1503 | 49.7 | – |
| Phlorotannins | – | 5.0-6.04 | 5.05 | 1.0-2.05 | 20-14 | 21 | 2.0 | 2.0 |
DM = dry matter; OM = organic matter; CP = crude protein; NDF = neutral detergent fiber; ADF = acid detergent fiber; TDN = total digestible nutrient.
Sources: Abirami and Kowsalva (2012), Abudabos et al. (2013), Aminina et al. (2020), Anderson et al. (2006), Applegate and Gray (1995), Arasaki and Arasaki (1983), Baardseth (1970), Belanche et al. (2016), Cian et al. (2013), El-Baroty et al. (2007), Farley (2012), Hind et al. (2014), Holdt and Kraan (2011), Imbs et al. (2009), Leyton et al. (2016), Machado et al. (2016), Marino et al. (2016), Nunes et al. (2018), Roque et al. (2019a, b), Nunes et al. (2018), Ragan and Jensen (1978), Roque et al. (2019b).
Bromoform contents are μg/g DM, unless stated otherwise ng/g fresh weight.
Minor level of bromoform productions (ng/g of fresh weight): Manley et al. (1992), Carpenter and Liss (2000).
Minor levels of total phenolic compounds (Nunes et al., 2018).
Minor levels of condensed tannins (Kafhi et al., 2020; Mihaila, 2020).
Due to their high polysaccharide content, seaweeds have a high level of neutral detergent fiber (NDF) and acid detergent fiber (ADF) (Lahaye, 1991). Red seaweed generally contains higher levels of NDF (27.2% to 43.1% DM) than green (15.3% to 18.6% DM) and brown seaweed (16.6% to 22.0% DM; Table 2). Unlike land plants (which have cell walls made of mainly cellulose, hemicellulose, and lignin), the cell walls of seaweeds consist principally of alginates, with some cellulose, xylan, and xyloglucan (Rogers and Perkins, 1968). Regardless of this structural difference, the varied active polysaccharide components in seaweed polysaccharides are hydrolyzed and fermented by carbohydrate-active enzymes in the ruminant digestive system (Hehemann et al., 2010). The opportunity to reduce enteric CH4 with seaweed supplementation is a hot topic. There has been rising interest seaweed use for livestock feed, as the bioavailability of polysaccharides in some seaweed can result in CH4 reduction potential (Morais et al., 2020). Further animal nutrition studies are needed to evaluate both the nutritional benefit of seaweed supplementation and the efficacy of polysaccharide bioactivities at mitigating enteric CH4 emissions, as well as to determine any potential unfavorable effects on animal health, economics, or productivity.
2.1. Secondary metabolites
Seaweeds have an extended history of use as livestock feed. Seaweed has a greatly variable chemical composition, depending on the seaweed species, seasons, and environment (Makkar et al., 2016). Commonly, the most studied phytochemicals in seaweeds are phlorotannins and halogenated compounds. Studies of the effects of feed iodine and iodine adversaries on iodine status in animals could help to advance understanding of human iodine nutrition and physiology (Laurberg et al., 1998). The ocean is the primary source of iodine, containing between 50 and 60 μg/L (NRC, 2005). In both humans and livestock, iodine deficiency reduces the level of thyroid hormones resulting in hypothyroidism, goiter formation, depression of metabolism, growth, and a high rate of stillbirths (Schone and Rajendram, 2009). These intakes prevent iodine deficiency, facilitate a high performance (e.g., weight gain and low feed:gain ratio), maintain adequate iodine stores (>0.50 mg/g thyroid), and sustain thyroid function (Schone and Rajendram, 2009). Animal nutrition societies generally recommend iodine intakes in the range of 0.5–0.80 mg I/kg feed for growing calves and dairy cattle (Table 3). Lactating dairy cattle need more dietary iodine because over 10% of iodine intake may be excreted in milk, depending upon milk production rate (Miller et al., 1975). Based on published literature, however, maximum tolerable levels (mg I/kg diet) suggested for iodine are cattle, 50; sheep, 50; swine, 400; chicken and turkey, 300 (NRC, 1980). Additionally, iodine requirements may also be affected by animal genetic variances, temperature, and environment. Cattle, sheep, and goats display a significant reduction in thyroid hormone production during the summer (ARC, 1980). Iodine feed supplements are needed to produce thyroid hormones, maintain metabolism, and facilitate reproduction, growth, and development of the body (NRC, 2005). Iodates [Ca(IO3)2 × 6H2O; Ca(IO3)2], iodides (NaI; KI; EU, 2003, 2005), and seaweeds (Table 2) are recommended for feed supplements (NRC, 2005). However, the iodine content in some red and brown seaweeds is high reaching up to 3.7 mg/kg of DM (Table 2). In certain brown seaweeds, the concentration of iodine can reach very high levels, in particular, the genus Laminaria and Saccharina japonica (as Laminaria japonica) had the highest iodine content of 5.6 and 3.04 mg/kg DM, respectively (Misurcova, 2011; Holdt and Kraan, 2011). Therefore, the seaweed content of animal feeds may need to be limited to a maximum of 10% of the diet. There is a need to determine the mechanisms involved in iodine metabolism, particularly the interaction of iodine with other nutrients such as selenium, bromine, and iron (NRC, 2005). Required and recommended iodine supplementation of fed cattle, pigs, and poultry are presented in Table 3.
Table 3.
Required and recommended iodine supplementation of feed of cattle, pigs, and poultry in the US, UK, and Germany (mg/kg feed dry matter)1.
| Item | US (NRC, 1985, 1996, 1998, 2001, 2005) | UK (AFRC, 1981) | Germany (GfE, 1995, 1999, 2001, 2003, 2006) |
|---|---|---|---|
| Dairy cattle | 0.50 | 0.80 | 0.50 |
| Growing calves/bulls | 0.50 | 0.12 | 0.25 |
| Sows | 0.16 | 0.50 | 0.60 |
| Growing pigs | 0.16 | – | 0.15 |
| Laying hens | 0.32-0.49 | – | 0.50 |
| Broiler chickens | 0.35 | – | 0.50 |
US NRC = National Research Council (NRC) of the United States; GfE = Gesellschaft fur Ernahrungsphysiologie; AFRC = Agricultural Food Research Council.
Polyphenol compounds like phlorotannins were frequently reported in all genera of seaweeds but their presence tends to be highest in brown seaweed ranging from 20 to 140 g/kg DM (Table 2). Results of previous studies showed that when A. nodosum seaweed meal (brown seaweed; Tasco) was added (10 g/d; DM basis) to TMR rations in cannulated steers, the digestibility of that dietary ration was increased from 51.5% to 64.9% DM (Leupp et al., 2005). Results by Wang et al. (2008) proposed that not only can prebiotics alter the microbiota of the rumen but that phlorotannins found in A. nodosum may play a role in altering the fermentation patterns in the rumen of cattle. Williams et al. (2009a), reported that the rate of fiber (e.g., NDF) digestibility in situ by rumen-cannulated steers was increased by A. nodosum (Tasco) treatment. These effects are possibly related to changes in the rumen microbiome community diversity in cattle as reported by Ushakova et al. (2006). In addition, numerous studies have reported that phlorotannins containing seaweeds fed to beef cattle can decrease the shedding of foodborne pathogens such as E. coli O157:H7 (Wang et al., 2009a,b; Evans and Critchley, 2014; Huang et al., 2018). These results confirmed that feeding tannin-containing diets could be a useful method to decrease the presence of foodborne pathogens in the ruminant digestive tract thereby reduce the risk of carcass contamination and hence enhance food safety (Min et al., 2007; Huang et al., 2018).
The bromoform contents of red seaweed such as Asparagopsis spp. (Table 2) were lower than the polyphenol content, 1.32 to 1.72 mg/g DM. The seaweeds A. taxiformis and A. armata, are distributed across tropical and temperate marine ecosystems and contain high levels of halogen-containing (F, Cl, Br, and I) compounds including bromoform (CHBr3; 1.723 μg/g DM), followed by dibromochloromethane (CHBr2Cl; 0.158 μg/g DM), bromochloroacetate (C2H2BrClO2; 0.088 μg/g DM), and dibromoacetate (C2H2Br2O2; 0.009 μg/g DM; Table 2). The other seaweeds, including Macrocystis pyrifera, Ulva sp., Eisenia arborea, Laminaria farlowii, Egregia menziesii, and Cystoseira osmundacea, produce negligible amounts of halogenated compounds (Table 2) such as chloromethane (CH3Cl), bromomethane (CH3Br), methyl iodide (CH3I), bromomethane bromide (CH3Br2), and bromoform (Manley et al., 1992; Dembitsky and Tolstikov, 2003).
2.2. AA
Seaweed species and the season of the collection are the most common factors affecting both seaweed protein and AA composition (Fleurence, 1999). The protein content reported in brown seaweed is mostly low in comparison with green (10% to 26%) and red seaweed species (35% to 47%) with protein contents comparable to protein-rich foods such as soybean meal (Garcia-Vaquero and Hayes, 2016). The proteins from seaweeds have relatively high levels of the AA glycine (Gly), alanine (Ala), arginine (Arg), glutamic (Glu), and aspartic (Asp) acids whereas methionine (Met), cysteine (Cys), iso-leucine (Isol), and histidine (His) appear in a lower amount (Table 4). Glutamic (10.0-12.7 g/100g of protein) and aspartic acid (6.9-12.2 g/100 g of protein), which have acidic side chains at neutral pH, in seaweeds represent 10.0–12.78 g/100 g of protein. Commonly, all the six species of seaweeds (Table 4) are rich in essential and non-essential AA and showed a balanced sulfur-containing AA profile comparable to that of FAO, 2018a, FAO, 2018b and soybean meal, except A. nodosum. Red seaweed contained high levels of CP (17.8% to 38.1% DM) and sulfur-containing AA in that protein. Therefore, seaweeds might be important sources of proteins with a high level of essential AA. It was found that seaweeds could be a complementary source of food proteins for human and animal nutrition. However, Pirian et al. (2017) reported that essential AA concentrations such as leucine (7.6 to 8.8 g/100g of protein), alanine (3.4 to 4.9 g/100 g of protein), threonine (3.1 to 4.4 g/100 g of protein) are varied in the seaweed species, including Ulva linza, Sargassum vulgare and Gracilaria corticata collected from the Persian Gulf cost-line. Seasonal changes also affected the content of total AA profiles in Laminaria and Ulva seaweeds (Gaillard et al., 2018). However, seaweeds may contain non-protein-nitrogen (N; e.g., amine, amides, amino sugars, nitrates), resulting in a possible overestimation of their protein content (Misurcova, 2011, 2012). The accurate value of the N-to-protein conversion factor should be determined for each seaweed genera from the total N content based on AA composition and the distribution of N in protein and other nonprotein N compounds (Fujihara et al., 2001; Ezeagu et al., 2002; Lourenco et al., 1998; Salo-Vaananen and Koivistoinen, 1996). In different genera of green, brown, and red seaweed the assessments of N-to-protein conversion factors have been provided. The average rate of the N-to-protein conversion factor is 5.13 for green, 5.38 for brown, and 4.92 for red seaweeds (Lourenco et al., 2002). Data obtained from a previously published study (Lourenco et al., 2002) indicated that seaweed has the potential to become widely used as alternative feed ingredients for sustainable ruminant production.
Table 4.
Amino acid (AA) composition (g/100g of protein) of various seaweed species1.
| Type |
Red seaweed |
Green seaweed |
Brown seaweed |
Soybean meal |
|||
|---|---|---|---|---|---|---|---|
| Species | Porphyra columbina | Asparagopsis taxiformis | Ulva Spp. | Ascophyllum nodosum | Macrocystis pyrifera | Laminaria digitata | N × 6.25 |
| Essential AA | |||||||
| Methionine | 1.68 | 2.32 | 1.6-6.7 | 0.7 | 2.05 | 1.49 | 1.4 |
| Cysteine | 1.89 | 4.32 | 2.01-5.9 | trace | 3.5 | 1.96 | 1.38 |
| Valine | 5.85 | 6.19 | 4.4-6.7 | 3.7 | 4.45 | 6.01 | 5.34 |
| Iso-leucine | 2.71 | 5.09 | 2.6-3.7 | 2.8 | 3.20 | 2.61 | 5.31 |
| Leucine | 7.38 | 8.25 | 5.2-6.7 | 4.6 | 5.76 | 4.45 | 7.58 |
| Phenylalanine | 3.7 | 5.86 | 3.5-4.68 | 2.3 | 3.27 | 2.82 | 5.08 |
| Tyrosine | 2.55 | 3.67 | 1.4-3.0 | 0.9 | 2.68 | 1.74 | 3.35 |
| Histidine | 1.26 | 1.48 | 2.0-3.01 | 1.3 | 1.30 | 2.38 | 2.33 |
| Lysine | 6.01 | 4.32 | 3.8-4.4 | 4.9 | 5.05 | 4.77 | 6.65 |
| Threonine |
5.91 |
5.86 |
3.8-9.4 |
2.8 |
4.78 |
3.41 |
3.90 |
| Non-essential AA | |||||||
| Serine | 6.16 | 5.93 | 4.2-6.4 | 3.0 | 4.44 | 2.45 | 5.18 |
| Arginine | 6.19 | 7.15 | 4.5-5.0 | 8.0 | 3.83 | 2.96 | 7.72 |
| Glutamic acid | 10.5 | 10.89 | 13.5-12.7 | 10.0 | 13.83 | 3.86 | 18.4 |
| Aspartic acid | 12.2 | 12.24 | 7.9-12.4 | 6.9 | 10.04 | 4.69 | 14.14 |
| Proline | 3.96 | 5.15 | 0.0-2.8 | 2.6 | 3.73 | 1.91 | 5.99 |
| Glycine | 8.87 | 5.15 | 5.4-7.7 | 5.0 | 4.83 | 3.31 | 5.54 |
| Alanine | 12.54 | 7.35 | 5.98.7 | 5.3 | 11.43 | 4.51 | 4.54 |
N = nitrogen.
Sources: Anderson et al. (2006), Angell et al. (2012), Castro-Gonzalez et al. (1994), Cian et al. (2013), Imbs et al. (2009). Arieli et al. (1993), Dawczynski et al. (2007), Kolb et al. (2004), Makkar et al. (2016), Kuiken and Lyman (1949), Ortiz et al. (2006). Phorphyra columbina was collected from Punta Maqueda, Argentina (Cian et al., 2013); Asparagopsis taxiformis and Ulva spp. were collected from shallow reefs at Nelly Bay, Magnetic Island; Anderson et al., 2006); Ascophyllum nodosum was harvested off the coast of Nova (Angell et al., 2012); The samples of Macrocystis pyrifera was collected in summer in Bahía Tortu-gas, Baja California Sur (Castro-Gonzalez et al., 1994); Laminaria digitata was collected in Troitsa Bay of the Peter the Great Bay of the Sea of Japan (Kolb et al., 2004; Imbs et al. (2009). Soybean meals (mean value of 20 strains of soybean meals) were obtained through the cooperation of the United States Regional Soybean Laboratory at Urbana (Kuiken and Lyman, 1949). .
2.3. Lipids and fatty acids
Brown seaweed normally has the greatest total lipid content, followed by green and red seaweeds (Gosch et al., 2012). In recent years, lipid composition in seaweeds has raised considerable interest due to their high content of unsaturated fatty acids (USFA). Seaweed lipids generally comprise long-chain fatty acids, especially polyunsaturated fatty acids (PUFA) with 18- and 22- carbon (C) atoms, depending on species. The average contribution of saturated and unsaturated fatty acids contents is presented in Table 5. Saturated fatty acids (SFA) and USFA varied among seaweed species and the yielding of SFA/USFA ratio was accounted to 0.35, 0.83, 0.93, 0.90, and 0.33 for A. taxiformis, Porphyra dioica, Ulva rigida, Codium tomentosum, and A. nodosum, respectively (Table 5). Among the selected seaweed species, both A. taxiformis and A. nodosum were the most abundant in USFA. It has been reported that high levels of monounsaturated fatty acids (MUFA) and PUFA were also found in S. vulgare (35.1% and 21.5%) and U. linza (30% and 21%), respectively (Pirian et al., 2017). Furthermore, average USFA contents varied from 26% of the total fatty acid content in U. rigida to 75.0% in A. nodosum (Table 5). Certain seaweeds (red and brown) also contain high levels of omega-3, omega-6, and other PUFA (Table 5; Holdt and Kraan, 2011; van Ginneken et al., 2011) which could aid meat and milk qualities, immune systems, and reproduction rates through improved conception rates and reduced pregnancy losses (Moallem, 2018). Therefore, PUFA is believed to be an essential nutritional component in humans and animals, playing an important role in improved animal health.
Table 5.
Fatty acids (FA, % DM) profile of red, green, and brown seaweed species1.
| Item | Red |
Green |
Brown |
||
|---|---|---|---|---|---|
| Asparagopsis taxiformis | Porphyra dioica | Ulva rigida | Codium tomentosum | Ascophyllum nodosum | |
| C14:00 | 3.77 | 23.3 | 20.2 | 22.3 | 9.4 |
| C16:00 | 3.73 | 18.3 | 2.1 | 4.9 | 13.4 |
| C18:00 | 1.18 | 4.9 | 2.9 | 2.6 | 0.76 |
| C18:1 | 3.52 | 3.3 | 9.5 | 11.1 | 27.8 |
| C18:2n-6 | 7.75 | 1.7 | 1.5 | – | 7.47 |
| C20:1 | – | 0.6 | 1.2 | – | 0.22 |
| C20:2n-6 | 1.38 (C20:3) | 0.6 | 1.2 | – | 5.05 |
| C20:4n-6 | 1.19 (C20:4) | 2.7 | – | 4.5 | 17.24 |
| C20:5n-3 | 1.6 (C20: 5) | 20.5 | 1.4 | 7.9 | 7.24 |
| C22:6 |
32.77 |
– |
– |
– |
– |
| SFA | 23.17 | 37.5 | 24.1 | 30.2 | 25.1 |
| MUFA | 19.52 | 22.5 | 13.0 | 16.8 | 31.5 |
| PUFA | 46.97 | 22.6 | 13.0 | 16.8 | 43.5 |
| Ave. USFA | 66.49 | 45.1 | 26.0 | 33.6 | 75.0 |
| SFA:USFA | 0.35 | 0.83 | 0.93 | 0.90 | 0.33 |
SFA = saturated fatty acids; USFA = unsaturated fatty acids; MUFA = mono-unsaturated fatty acids; PUFA = poly-unsaturated fatty acids.
Sources: Cian et al. (2013), Lorenzo et al. (2017), Lopes et al. (2020), Mellouk et al. (2017). Major fatty acids were presented in this Table.
3. The effect of seaweed on methanogenesis
Anti-methanogenic, halogenated compounds (e.g., BCM) in seaweed, have been reported to inhibit enteric CH4 emissions when fed to ruminants (Table 6), but limited studies have assessed how seaweed supplementation might impact the ruminal microbiota and methanogenesis. The addition of red seaweed and BCM has been reported to depress CH4 production both in vivo and in vitro (Table 6, Table 7). In steers and dairy cattle fed forage- and grain-based diets, the addition of BCM (<0.6 g/100 kg BW) or red seaweed of Asparagopsis spp. (<1.0% OM basis) decreased CH4 production by 50.0% to 99.5%, while feeding seaweed also decreased DMI (38.0%) in dairy cattle (Table 6). These results are consistent with other data. Dairy cattle fed the red seaweed, A. armata, supplemented diet (0.5% and 1.0% inclusions; OM basis) had reduced DMI and milk yield (kg/d) by up to 38.0% and 13.5%, respectively (Roque et al., 2019a, Table 6). Recently, however, Kinley et al. (2020) reported that the low levels of red seaweed (A. taxiformis; 0.05% to 0.2% OM) in a beef total mixed ration (TMR)-based diet reduced enteric CH4 emissions by up to 98% without any reduction of DMI in beef cattle (Table 6). These results were consistent with the previous study reported in Angus-Hereford beef steers fed a high-forage-based TMR diet compared to low-forage-based TMR diets (Roque et al., 2020). McCrabb et al. (1997) reported a reduction in DMI (7.4%) of forage-based diet contained BCM (1.2% of DM BCM) for steers fed low and medium-quality alfalfa hay diets over 10 to 12 weeks. These results indicate that increasing BCM supplementation or BCM-containing seaweed (ranging from 0.5% to 1.0% of DM) progressively decreased DMI in beef and dairy cattle. However, DMI was not different between treatment groups in sheep, lactating dairy goats (Table 6), and steers fed concentrate-based diet (Tomkins et al., 2009). Li et al. (2018) reported the effects of five dietary inclusion levels of A. taxiformis (0%, 0.5%, 1%, 2%, and 3% OM) on CH4 emissions when fed to sheep consuming a high fiber diet for 72 h. It appears that dairy or beef cattle seem to have palatability issues when Asparagopsis seaweed supplementation was included up to 1.0% of DM in a diet, or more than 0.3 g bromoform/100 kg BW (Tomkins and Hunter, 2004; Tomkins et al., 2009), respectively, compared to sheep (Orpin et al., 1985; Hansen et al., 2003).
Table 6.
In vivo studies of methane (CH4) emissions from seaweed and commercial bromochloromethane (BCM) supplementation.
| Animal | Basal diet | Treatment | DMI, kg/d | CH4 production | Reference1 |
|---|---|---|---|---|---|
| Beef steers |
Feedlot TMR (total mixed ration) | BCM, g/100 kg of BW | CH4, g/kg DMI | 1 | |
| 0 (control) | 6.2b | 8.7a | |||
| 0.15 | 7.4a | 3.8ab | |||
| 0.30 | 5.6b | 1.4b | |||
| 0.60 | 5.5b | 0.8b | |||
| Rate of change, % |
−11.3 |
−95.2 |
|||
| Beef steers |
Alfalfa hay | BCM, g/100 kg of BW | CH4, mL/min | 2 | |
| 0 (control) | 8.1a | 205.5a | |||
| 1.2 | 7.5b | 0.24b | |||
| Rate of change, % |
−7.4 |
−90.6 |
|||
| Beef steers |
Feedlot TMR | BCM, g/100 kg of BW | CH4, g/kg DMI | 3 | |
| 0 (control) | 10.4 | 20.0a | |||
| 0.98 | 10.3 | 0.1b | |||
| Rate of change, % |
−0.96 |
−99.5 |
|||
| Dairy cows |
Dairy TMR | A. armata, %, OM basis | CH4, g/kg DMI | 4 | |
| 0 (control) | 27.9a | 15.0a | |||
| 0.5 | 24.9b | 12.0b | |||
| 1.0 | 17.3c | 7.5b | |||
| Rate of change, % |
−38.0 |
−50.0 |
|||
| Beef steers |
Feedlot TMR |
A. taxiformis, %, OM basis | CH4, g/kg DMI | 5 | |
| 0 (control) | 8.4 | 10.4 | |||
| 0.05% | 8.0 | 10.0 | |||
| 0.10% | 10.3 | 6.2 | |||
| 0.2% | 8.8 | 0.2 | |||
| Rate of change, % |
0.4 |
−98.0 |
|||
| Sheep |
High-fiber pellet | A. taxiformis, %, OM basis | CH4, g/kg DMI | 6 | |
| 0 (control) | 1.0 | 15.0a | |||
| 0.5 | 1.1 | 12.7ab | |||
| 1.0 | 1.0 | 7.0b | |||
| 2.0 | 1.1 | 5.6c | |||
| 3.0 | 1.0 | 2.9c | |||
| Rate of change, % |
0.0 |
−80.7 |
|||
| Sheep |
Feedlot TMR | BCM, g/100 kg of BW | CH4, % of GE intake | 7 | |
| 0 (control) | 1.0 | 6.1a | |||
| 0.15 | 1.0 | 1.0b | |||
| 0.3 | 1.0 | 0.9b | |||
| 0.45 | 1.0 | 0.8b | |||
| Rate of change, % |
0.0 |
−86.9 |
|||
| Dairy goats | Alfalfa + concentrate | BCM, g/100 kg of BW | CH4, g/kg DMI | 8 | |
| 0 (control) | 0.99 | 29.95 | |||
| 0.3 | 1.04 | 19.9 | |||
| Rate of change, % | 0.5 | −33.6 | |||
DMI = dry matter intake; TMR = total mixed ration; A. armata = Asparagopsis armata; A. taxiformis = Asparagopsis taxiformis; GE = gross energy.
a, b, c Values in a column with different superscript letters were significantly different (P < 0.05).
Sources: 1 = Tomkins and Hunter (2004); 2 = McCrabb et al. (1997), 3 = Johnson et al. (1972), 4 = Roque et al. (2019a), 5 = Kinley et al. (2020), 6 = Li et al. (2018), 7 = Sawyer et al. (1974), 8 = Abecia et al. (2012).
Table 7.
In vitro studies of methane (CH4) emissions from red seaweed or bromochloromethane (BCM) supplementation.
| System | Basal diet | Treatment | CH4 production | Reference1 |
|---|---|---|---|---|
| Ankom |
Rhodes grass (Chloris gayana) | A. taxiformis, % DM | CH4, mL/g OM | 1 |
| Control (no seaweed) | 22.2a | |||
| 0.5 | 19.6b | |||
| 1.0 | 3.4c | |||
| 5.0 | <0.05c | |||
| 10.0 | <0.05c | |||
| Rate of change, % |
−99.8 |
|||
| Rhodes grass | Oedogonium sp., % DM | CH4, mL/g OM | 2 | |
| Control (no seaweed) | 22.2a | |||
| 10.0 | 20.9a | |||
| 50.0 | 18.4b | |||
| 100 | 6.1c | |||
| Rate of change, % |
−72.5 |
|||
| Batch |
Grass-hay | BCM, μmol/L | CH4, mL/100 mL | 2 |
| Control (no BCM) | 15.8a | |||
| 5.0 | 3.5b | |||
| 10.0 | 1.1b | |||
| Rate of change, % |
−93.0 |
|||
| Batch |
Meadow hay/corn silage | Seaweeds, 25% DM | CH4, mL/g DM | 3 |
| Control (no seaweed) | 1.75a | |||
| Ulva sp. (green) | 1.30b | |||
| L. ochroleua (brown) | 1.98a | |||
| S. latissima (brown) | 1.81a | |||
| Gigartina sp. (red) | 1.17b | |||
| G. vermiculopphylla (red) | 1.07b | |||
| Rate of change, % |
−38.9 |
|||
| CC | Dairy TMR | A. taxiformis, 5% OM | CH4, mL/g OM | 4 |
| Control (no seaweed) | 12.08a | |||
| 5.0 | 0.59b | |||
| Rate of change, % | −95.1 |
A. taxiformis = Asparagopsis taxiformis; DM = dry matter; OM = organic matter; L. ochroleua = Laminaria ochroleua, S. latissima = Saccharina latissimi;G. vermiculopphylla = Gracilaria vermiculopphylla; CC = continuous system; TMR = total mixed ration.
a, b, c Values in a column with different superscript letters were significantly different (P < 0.05).
Sources:1 = Machado et al. (2015b), 2 = Goel et al. (2009), 3 = Maia et al. (2016), 4 = Roque et al. (2019b).
With high potency and wide-spectrum efficacy against rumen methanogens, a red seaweed (Asparagopsis spp.) represents a promising natural intervention strategy for reducing enteric CH4 emissions from ruminants if animal production levels can be maintained. Such a conclusion is supported by in vitro data (Table 7). Recent studies suggest that the red seaweed A. taxiformis has the potential to reduce CH4 emission from beef cattle by up to 95% (Table 7). Inclusion of 5% A. taxiformis (OM basis) in a dairy ration resulted in a 95% reduction in CH4 emissions with no negative impacts on rumen fermentation (Roque et al., 2019a, Roque et al., 2019b). This in vitro experiment was similar to in vivo results reported from Kinley et al. (2016) and Machado et al. (2016), in which a strong anti-methanogenic activity of A. taxiformis was observed when included in the diet at 22.7 g/kg DM. Both A. taxiformis and A. armata supplementation, used at dietary inclusion levels at 0%, 2%, and 4% as-fed basis in an anaerobic in vitro study, increased total gas, butyrate, and valerate production (P < 0.01), while production of CH4 (mg/g DM), acetate, propionate, acetate/propionate ratios and in vitro dry matter digestibility (% DM) were reduced (P < 0.01) as both red seaweed supplementation increased (Min et al., 2021). Therefore, it may be possible to suppress methanogenesis both directly and indirectly by the addition of red seaweeds.
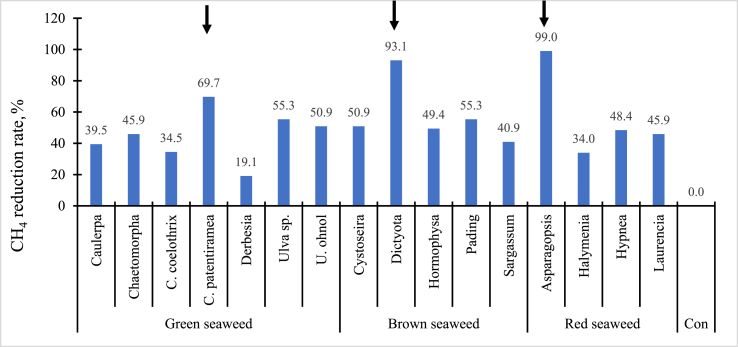
Among the 17 seaweed species tested in vitro (Fig. 1), Cladophora patentiramea (green seaweed), Dictyota (brown seaweed), and Asparagopsis (red seaweed) had the strongest effects, inhibiting CH4 production by 69.7%, 93.1%, and 99.0%, respectively (Machado et al., 2014). Molina-Alcaide et al. (2017) and Moneda et al. (2019) observed similar effects of anti-methanogenic activities for brown (P. canaliculata) and red (M. stellatus) seaweeds in ruminant diets at 200 g/kg DM. However, Belanche et al. (2016) reported no changes in vitro CH4 production when brown seaweeds (L. digitata or A. nodosum) were included in the diet at 50 g/kg DM. The data suggest that inhibition of methanogenesis varies among seaweed species and their secondary metabolites (Lanigan, 1972; Ungerfeld et al., 2004).
Fig. 1.
Various seaweed species (0.2 g OM/seaweed species; green-, brown-, and red-seaweed) and in vitro methane (CH4) production (mL/g OM) (Adapted from Machado et al., 2014). Con = Control (1 g of Flinders grass + 0.2 g of decorticated cottonseed meal as a positive control, OM basis). Samples of 1 g of Flinders grass + 0.2 g of all other seaweed (OM basis) were used in this study). C. coelothrix = Cladophora coelothrix; C. patentiramea = Cladophora patentiramea; U. ohnoi = Ulva ohnoi. Pooled rumen fluid as an in vitro inoculum was collected from three fistulated steers (Bos indicus) fed Flinders grass (Iseilema membranacea) hay. A arrow indicates a lower CH4 production from seaweed species.
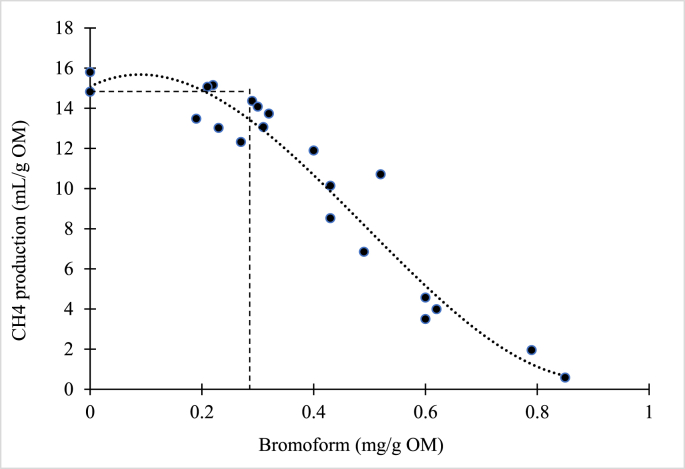
In addition, bromoform or BCM concentration could be used as an indicator of anti-methanogenic properties, when red seaweeds are used as the active ingredient in ruminant diets (Fig. 2). A polynomial correlation between the concentration of bromoform and in vitro CH4 emissions shows enteric CH4 production decreases curvilinearly with increasing bromoform concentration (Fig. 2). Independent of the fit, BCM does not reduce CH4 production until somewhere around 0.25 mg/g OM and then linearly decreases until CH4 production is 0 when BCM concentration ranges from 0.8 to 0.9 mg/g OM. It has been found that BCM in red seaweed inhibits methanogen populations in both batch- and continuous-culture systems (Goel et al., 2009). However, most of the research was conducted with freeze-dried seaweed without considering other post-harvest processing methods. Vucko et al. (2017) assessed in vitro influences of different processing methods of A. taxiformis in a factorial design based on rinsing (unrinsed vs. dip rinsed/submerged), freezing (frozen vs. not frozen), and drying (freeze-dried vs. kiln-dried/dehydrated) on CH4 production and the concentration of bromoform. A. taxiformis that had been frozen and freeze-dried, irrespective of rinsing, was the most effective at inhibiting CH4 emissions. Of these, the unrinsed treatment had the highest bromoform concentration (4.4 mg/g DM) followed by either oven-dried or dehydrated without freezing.
Fig. 2.
In vitro correlation between the concentration of halogenated compounds (bromoform or bromochloromethane (BCM; mg/g OM) and the methane (CH4) emissions (mL/g OM) in the Asparagopsis taxiformis (cut off: < 1.0% of bromoform or BCM (mg/g OM). Adapted from Goel et al. (2009) and Vucko et al. (2017). OM = organic matter.
Seaweeds are particularly abundant in their production of haloperoxidase enzymes, and these particular molecules play influential roles in shaping biotic interactions and in marine chemical ecology (Thapa et al., 2020). However, the bromoform is somewhat soluble in water and readily evaporates into the air during handling (e.g., rinsing, freezing, or drying), possibly allowing more to volatilize (EPIC, 2020). The supplementation of green seaweed, Oedogonium (0.2 g OM) to different basal diets (1 g OM) decreased CH4 emission at different rates, by approximately 40% (Dubois et al., 2013), 30% (Machado et al., 2014), and 15% (Machado et al. 2016), when Rhodes grass, Finders grass (Iseilema spp.), and Rhodes grass hay were used as basal ingredients, respectively. Therefore, interrelationships between seaweed species and different dietary ingredients are unclear and need to be further studied.
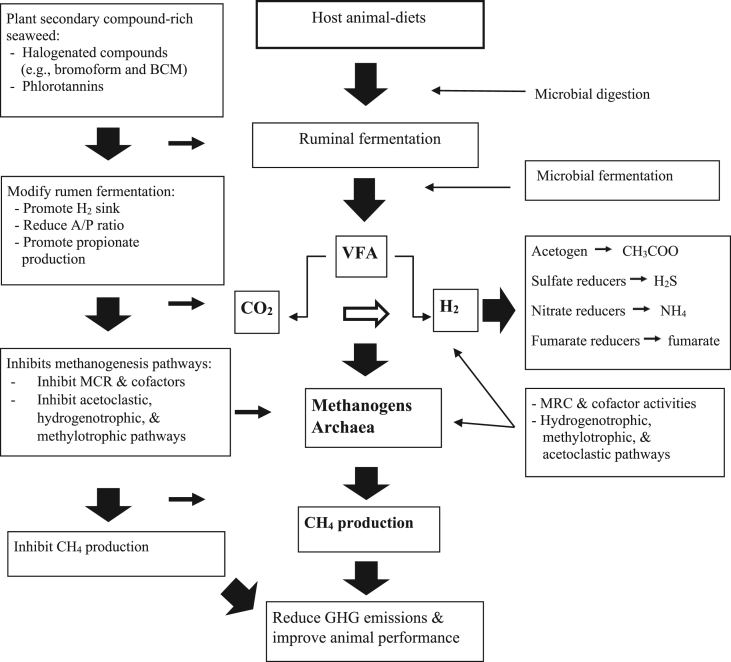
The plant secondary metabolites and the interactions between anti-methanogenic compounds and bioactive ingredients in seaweed are proposed in Fig. 3. The dietary carbohydrates that have been digested by a group of rumen microbiota in the rumen with the production of volatile fatty acids (VFA), carbon dioxide (CO2), and hydrogen (H2) (Fig. 3). During rumen fermentation, H2 is emitted into the rumen through the re-oxidation of the various cofactors (NADH, NADPH, and FADH). The produced CO2 and H2 are the key substrates utilized by methanogenic archaea, which is widely accepted as the major methanogenesis pathway in the rumen (Ellis et al., 2008). This is an active process, in which methanogens effectively affect the metabolism of rumen fermentative and acetogenic bacteria via interspecies H2 transfer (Stams and Plugge, 2009). In addition, other groups of methanogens also use formate, acetate, methanol, methylamines, and alcohol (Ellis et al., 2008). Methanogenic archaea use one of three pathways for methanogenesis: (1) hydrogen-dependent and CO2-reducing, or hydrogenotrophic (most common); (2) methylotrophic; and, (3) acetoclastic (Berghuis et al., 2019). All three pathways require the gene cluster for CH4 production known as methyl-coenzyme M reductase (MCR; Ferry and Kastead, 2007; Conrad, 2009).
Fig. 3.
Proposed schematic microbial fermentation of plant secondary compound (e.g., bromoform, BCM)-rich seaweed and methane (CH4) reduction pathways in the rumen. Three major pathways of methanogenesis are known: hydrogenotrophic, methylotrophic, and acetoclastic pathways. A/P ratio = acetate-to-propionate ratio; BCM = bromochloromethane, MCR = methyl CoM reductase; VFA = volatile fatty acids; GHG = global greenhouse gas. Sources: Wood et al. (1968), Bapteste et al. (2005), Denman et al., 2007a, Denman et al., 2007b, Attwood and McSweeney (2008), Frey (2010), Allen et al. (2014), Patra (2012, 2016), Machado et al. (2016), Danielsson et al. (2017), Machado et al. (2018), and Roque et al. (2020).
The schematic diagram of dietary manipulation with seaweed addition, which alters the pathway of fermentation to reduce CH4, is summarized in Fig. 3. One of the halogenated compounds such as bromoform or chloroform (CHCl3) is identified to block the function of corrinoid enzymes and to inhibit MCR (Oremland and Capone, 1998). The CHCl3 (10 μmol/L) can inhibit the production of CH4 from both H2/CO2 and acetate, which means the acetoclastic and hydrogenotrophic methanogens can all be inhibited (Scholten et al., 2000; Liu et al., 2011b). In addition, bromoform-rich Asparagopsis spp. is known as an inhibitor of methanogenesis by serving as competitive inhibitors (or analogs) of the MCR, preventing the final catalysis step (Goel et al., 2009). The mode of action is cross-reacting with reduced vitamin B12 and inhibiting the cobamide-dependent methyl-transferase step of methanogenesis (Wood et al., 1968; Chalupa, 1977). However, fluoroacetate (FCH2COO−) only inhibits acetoclastic methanogenesis (Chidthaisong and Conrad, 2000). Therefore, these halogenated compounds block the function of specific enzymes and inhibit MCR together with methyl group transfer in methanogenesis (Fig. 3; Wood et al., 1968; Ellermann et al., 1988; Liu and Whitman, 2008; Yu and Smith, 2000; Ungerfeld et al., 2004; Attwood and McSweeney, 2008; Frey, 2010; Hedderich and Whitman, 2013; Allen et al., 2014; Costa and Leigh, 2014). In addition, bromoform compounds found in Gracilaria sp. (red seaweed) are the effect of the reduction in the methanogen population (Prayitno et al., 2018). The same authors reported that bromoform-rich red seaweed inhibited the work of methanogens specifically. Denman et al. (2007b) reported BCM would reduce the activity of coenzyme cobalamin (vitamin B12) and coenzyme MCR. Therefore, halogen-rich seaweed could be a useful tool for the mitigation of enteric GHG emissions as a potential anti-methanogenic agent.
4. The effect of seaweed on ruminal fermentation
The in vitro DM digestibility of various seaweeds (brown, red, and green) has been evaluated (Tayyab et al., 2016; Gojon-Baez et al., 1998; Gaillard et al., 2018). After 72 and 96 h in vitro rumen incubation, the DM or OM digestibility of seaweed species (e.g., M. pyrifera and Sargassum spp.) varied between 27.9% and 94.6% DM (Tayyab et al., 2016; Gojon-Baez et al., 1998). Inclusion greater than 10% (DM basis) of A. taxiformis or Oedogonium reduced in vitro OM digestibility of Rhodes grass hay (Machado et al., 2016). Inclusion of A. armata in lactating dairy cows fed a dairy TMR diet reduced DMI (Roque et al., 2019a, b) and protein digestibility (Tayyab et al., 2016). However, the in vivo trials of Castro et al. (2009) and Marín et al. (2009), with the inclusion of up to 30% of different seaweed species, M. pyrifera and Sargassum sp., did not negatively impact digestibility. In addition, North Ronaldsay sheep demonstrated a dietary preference among seaweed species, preferring brown seaweed (Laminaria digitate and Laminaria hyperborean) (Hansen et al., 2003; Orpin et al., 1985). However, a direct comparison of results between studies is limited due to the use of different methodologies to determine digestibility, different levels of red seaweed inclusion, various plant secondary compounds, and the nature of the basal substrates.
Decreasing methanogenesis could free molecular H2 for use in pathways that yield rumen fermentation end products (e.g., VFA) that provide an additional energy supply to the host animal, thus increasing the efficiency of feed utilization for growth and milk production. Effects of BCM or seaweed supplementation on rumen fermentation and animal performance are presented in Table 8. In the present study (Table 8), BCM supplementation (0.29 to 0.30 g/100 kg BW) in steers fed various diets decreased acetate (2.0% to 29.4%) and the acetate-to-propionate (A/P) ratio (4.3% to 14.2%) and increased the propionate concentration (3.4% to 11.1%). Furthermore, Bromoform in red seaweed or BCM supplementation markedly reduced A/P ratio in both in vitro and in vivo and resulted in improved average daily gain (ADG; < 21%) or feed efficiency (gain-to-feed [G/F] ratio; 7.6% to 15.4%) in beef steers or dairy cattle (Table 8). These results are consistent with others (Machado et al., 2015b). Their research indicated that total VFA was not affected by A. taxiformis treatment, compared with the control. In the presence of A. taxiformis (2% OM), however, the concentration of acetate and A:P ratio was decreased by 20%: whereas, propionate concentration was increased by 50%. If ruminal VFA production promotes less acetate production relative to propionate (i.e., lower A/P ratio), the net balance of H2 in the rumen decreases, resulting in reduced CH4 formation (van Nevel and Demeyer, 1996), which confirms similar responses in fermentation patterns of ruminants where CH4 production was inhibited with various halogenated compounds of CH4 (Trei et al., 1971, 1972; Cole and McCroskey 1975), and other antimethanogenic agents such as ionophores (Goodrich et al., 1984) and plant tannins (Min et al., 2019). Previous results suggested that moderate levels (<1% DM) of A. taxiformis supplementation directly affects propionate production in the rumen (Mitsumori et al., 2012; Roque et al., 2020). When BCM (0.3g/100 kg body weight) was fed to cannulated Brahman-crossbred (Bos indicus) beef steers, the total enteric CH4 emission was reduced by 30% with a subsequent increase in propionate, iso-butyrate, valerate, and iso-valerate concentrations (Denman et al., 2007a, b). These results were consistent with an average decrease of 34% in the number of methanogens enumerated by mcrA-targeted real time-PCR. These same authors reported that alternative methanogens (e.g., Methanomicrobium, Methanosarcina, and Methanococcus) are established following the suppression of major methanogens such as Methanobacterium by BCM (Denman et al., 2007a, b; Kobayashi, 2010). However, constraining one biochemical response in a complex system such as that in the rumen may result in many other interconnected effects, one of which is the inhibition of fiber digestion because of changes in the microbial digestion (van Nevel and Demeyer 1995). Despite this research, mechanisms of associative effects of seaweeds and methanogenesis are not well understood.
Table 8.
Effect of bromochloromethane (BCM) and seaweed supplementation upon the in vitro and in vivo ruminal fermentation profiles, average daily gain (ADG), and feed efficiency (gain:feed [G:F] ratio) in ruminants.
| Item | Acetate, % | Propionate,% | A/P ratio | ADG, kg/d | G:F ratio | Reference1 |
|---|---|---|---|---|---|---|
| In vivo | ||||||
| Steers (Brahman-crossbred) | ||||||
| Control (no BCM) | 79.6 | 17.0 | 4.7 | 0.5 | 0.08 | 1 |
| BCM (0.3 g BCM/100 kg BW) | 78.0 | 17.6 | 4.5 | 0.5 | 0.09 | |
| Rate of change, % | −2.0 | 3.4 | −4.3 | 0.0 | 11.1 | |
| Steers (Brahman) | 2 | |||||
| Exp. 1 (n = 11): Angleton grass-based diet | ||||||
| Control (no BCM) | 61.6a | 21.7b | 2.9a | 0.23 | 0.012 | |
| 0.29 g BCM/100 kg BW | 59.4b | 24.4a | 2.5b | 0.22 | 0.013 | |
| Rate of change, % | −3.6 | 11.1 | −13.8 | −4.34 | 7.6 | |
| Exp. 2 (n = 8): Rhodes gras-based diet | ||||||
| Control | 64.8 | 18.6 | 3.5 | 0.59 | 0.033 | |
| 0.29 g BCM/100 kg BW | 45.7 | 20.8 | 3.0 | 0.62 | 0.039 | |
| Rate of change, % | −29.4 | 10.6 | −14.2 | 4.8 | 15.4 | |
| Steers (Brahman-crossbred) | 3 | |||||
| Control (no BCM) | – | – | – | 1.4 | 0.18 | |
| 0.3 g BCM/100 kg BW | – | – | – | 1.5 | 0.19 | |
| Rate of change, % | – | – | – | 6.7 | 5.3 | |
| Holstein steers, g/100 kg BW | 4 | |||||
| Control (no BCM) | – | – | 4.75a | 0.56b | 0.05 | |
| 0.18 g BCM/100 kg BW | – | – | 2.27b | 0.71a | 0.07 | |
| Rate of change, % | – | – | −52.2 | 21.1 | 28.6 | |
| Sheep (A. taxiformis), % DM | 5 | |||||
| 0 (control) | 65.0 | 20.8 | 3.19 | – | – | |
| 0.5 | 56.3 | 27.7 | 2.10 | – | – | |
| 1.0 | 54.4 | 31.5 | 1.76 | – | – | |
| 2.0 | 55.0 | 30.8 | 1.86 | – | – | |
| 3.0 | 54.5 | 32.0 | 1.77 | – | – | |
| Wether | 6 | |||||
| Control (no BCM) | 51.9 | 26.8 | 1.94 | – | – | |
| 2.5, mg BCM/kg BW | 53.6 | 24.6 | 2.18 | – | – | |
| 3.0, mg BCM/kg BW | 49.3 | 28.1 | 1.75 | – | – | |
| Dairy goats | ||||||
| Control (no BCM) | 61.4 | 11.1b | 5.71a | −6.1 | – | 7 |
| 3.0 mg BCM/kg BW |
60.3 |
15.5a |
3.92b |
−6.6 |
– |
|
| In vitro | ||||||
| A. taxiformis, % OM | 8 | |||||
| 0 (control) | 66.4a | 22.5c | 3.0a | – | – | |
| 0.5 | 57.2a | 27.9b | 2.1b | – | – | |
| 1.0 | 47.4b | 33.2b | 1.4b | – | – | |
| 5.0 | 31.5b | 46.8a | 0.7c | – | – | |
| 10.0 | 29.1b | 46.7a | 0.6c | – | – | |
| BCM, % OM | 8 | |||||
| 0 (control) | 74.0a | 19.4b | 3.8 | – | – | |
| 1.0 | 69.5b | 20.8b | 3.4 | – | – | |
| 5.0 | 61.9b | 26.8b | 2.4 | – | – | |
| 10.0 | 57.4b | 29.6a | 2.0 | – | – | |
| 25.0 | 57.4b | 29.3a | 2.0 | – | – | |
| A. taxiformis, % OM | ||||||
| 0 (control) | 75.0a | 19.2b | 3.9a | – | – | 9 |
| 2 | 60.4b | 28.7a | 2.1b | – | – | |
| Macroalgae2, 0.2 g OM/g of grass | 10 | |||||
| Freshwater algae | 65.5 | 24.7 | 2.7 | – | – | |
| Green seaweed | 64.6 | 25.9 | 2.5 | – | – | |
| Brown seaweed | 63.3 | 29.3 | 2.7 | – | – | |
| Red seaweed | 59.3 | 28.4 | 2.1 | – | – | |
BW = body weight; DM = dry matter; A. taxiformis = Asparagopsis taxiformis; A/P ratio = acetate-to-propionate ratio.
a, b, c Values in a column with different superscript letters were significantly different (P < 0.05).
Sources: 1 = Denman et al. (2007b), 2 = McCrabb et al. (1997), 3 = Tomkins and Hunter (2004), 4 = Johnson et al. (1972), 5 = Li et al. (2018), 6 = Sawyer et al. (1974), 7 = Abecia et al. (2012), 8 = Machado et al. (2015a), 9 = Machado et al. (2016), 10 = Machado et al. (2014).
Data was presented as an average mean value from the fresh-water algae (3 species), green seaweed (7 species), brown seaweed (6 species), and red seaweed (4 species) species.
5. The effect of seaweed on animal performance and carcass traits
Brown seaweed (A. nodosum) is one of the most used and studied seaweed species in livestock industries (Allen et al., 2001a, b; Makkar et al., 2016). A. nodosum is a plentiful source of bioactive ingredients such as iodine, minerals, PUFA, vitamins, and phlorotannins (Ragan and Glombitza, 1986; Cvetkovic et al., 2004; Antaya et al., 2015; Makkar et al., 2016). Phlorotannins have the potential benefits of inhibiting ruminal proteolysis (Wang et al., 2008; Zhou et al., 2018) and foodborne pathogens (Connan et al., 2004; Belanche et al., 2016; Zhou et al., 2018; Huang et al., 2018). Although previous studies evaluated the effects of brown seaweed on milk production, heat stress, and animal health in dairy cows (Pompeu et al., 2011; Antaya et al., 2015), the effects of long-term seaweed supplementation on nutrient utilization and plant secondary metabolism are not clear in dairy and beef cattle. Although previous studies evaluated the effects of BCM supplementation on ADG or feed efficiency (G:F ratio) in beef steers (Table 8), the effects of long-term seaweed supplementation on nutrient utilization and animal performance (e.g., ADG and milk production) are not clear in dairy and beef cattle. It has been reported that no differences or variable responses were found in animal performance (ADG) and carcass quality in Angus-Hereford beef steers along with no significant differences in milk yield and milk components (e.g., fat, protein, lactose, and solid not fat) in Jersey cows fed TMR diets with low levels of A. taxiformis (0.25% to 0.5% DM) and A. nodosum (113 g/d), respectively (Antaya et al., 2019; Roque et al., 2020). Likewise, no significant effects of 10% seaweed meal (A. nodosum and Laminaria cloustoni) as a percentage of DM in the diet were observed in Ayrshire dairy cows on milk yield or fat percentage (Burt et al., 1954). In addition, blood concentrations of cortisol, glucose, fatty acids, and thyroxine did not change with feeding control diet or brown seaweed (A. nodosum) supplementation (Antaya et al., 2019).
In contrast, the ability of bromoform-containing red seaweed (e.g., A. taxiformis and A. armata) to reduce CH4 emissions while improving animal production in ruminants was reported. In multiparous Holstein dairy cow fed a grain-based diet, the addition of a high level of bromoform-containing A. armata (1% OM; 1.32 mg/g DM of bromoform) supplementation decreased CH4 emission (67.2%), DMI (38%), and milk production (11.6%), but no significant changes in body weight and milk composition were noted (e.g., fat, protein, lactose, solid-not fat and bromoform concentration [0.11 vs. 0.15 μg/L]) between cows in the control group compared with those that received the low level of A. armata (0.5% OM) inclusion (Roque et al., 2019a). Abecia et al. (2012) reported that milk production was greater (36%) for dairy goats in the BCM-containing diet due to higher proportions of short-chain fatty acid (e.g., propionate; Park et al., 2007), although the compositions of milk components (fat, protein, lactose, casein, and total solids) were not affected by BCM treatment. Moreover, seaweed supplemented (Sargassum wightii) Sahiwal cows had significantly higher milk yield and 4% fat corrected milk (Singh et al., 2015) indicating a potential for optimizing the level of seaweed supplementation to dairy cattle.
According to Anderson et al. (2006), the addition of 2% A. nodosum (% DM of seaweed) to a grain-based diet increased carcass marbling scores and increased the percent grading choice by 39.6% of English crossbred steers (n = 32) and heifers (n = 32). This could explain the improved ADG (1.52 vs. 1.45 kg/d; P = 0.06) that was observed in steers fed a corn-based diet with 2% A. nodosum supplementation during two 14-d (28-d) feedlot feeding trials, compared to the control diet (Anderson et al., 2006). It has been reported that beef steers grazing tall fescue (Festuca arundinacea) grass that had been sprayed with a seaweed extract solution (Tasco-EX; extracted from A. nodosum, Nova Scotia, Canada) had more carcass marbling at harvest in retail cuts than control steers (Allen et al., 2001b). Additionally, Tasco-14, a proprietary brown seaweed meal (Acadian Seaplants Ltd., Dartmouth, Nova Scotia, Canada) has been found to increase marbling score and USDA quality grade in feedlot cattle when supplemented in two 14 d periods (28-d) before slaughter (Braden et al., 2007). Brown seaweed (A. nodosum) or supplementation with its extract has had a positive effect on animal health, heat stress tolerance, immune function, increased antioxidant levels, and enhances meat shelf-life, color, and marbling score in beef cattle (Zaki et al., 1994; Behrends et al., 2000; Allen et al., 2001a, b; Montgomery et al., 2001; Saker et al., 2001). Although previous studies evaluated the effects of brown seaweed supplementation to improve animal health, food safety, and carcass characteristics (Fike et al., 2001; Montgomery et al., 2001; Braden, 2003; Braden et al., 2007), the mechanisms involved in seaweed supplementation are currently not well understood for beef cattle diets.
Even though brown seaweed supplementation has the potential to mitigate iodine deficiency in humans via milk consumption (Brito, 2017), there are concerns of excess iodine intake particularly for children (IOM, 2001; Zimmermann et al., 2005). Currently, no conclusive standards exist for iodine levels in milk, but a maximum of 500 μg/L has been advised (EFSA, 2012). A linear increase in milk iodine, which averaged 177, 602, 1,015, and 1,370 μg/L in multiparous Jersey cows fed, respectively, 0, 57, 113, and 170 g/d of brown seaweed (A. nodosum) was observed in cows during the winter season (Antaya et al., 2015). Additional research is needed to determine if seaweed type, inclusion rate, and feeding duration impact milk production, milk composition profiles, and animal performance (ADG and feed efficiency).
6. Rumen microbiome adaptation to seaweed
The North Ronaldsay sheep consume a variety of seaweed species (P. palmata, A. esculenta, A. nodosum, Fucus sp., and Laminaria spp.), but due to animal dietary preference and availability, Laminaria spp. accounts for approximately 90% of their total diet (Hansen et al., 2003). North Ronaldsay sheep fed a diet containing L. digitate seaweed had rumen microbial communities that differed greatly in ciliate protozoa (e.g., Dasytricha ruminantium species) and bacterial populations (Streptococcus bovis, Selenomonas ruminantium, Butyrivibrio fibrisolvens, and lactate-utilizing bacterial species) compared to those on a pasture-based diet (Greenwood et al., 1983; Orpin et al., 1985). This is similar to findings of Eadie (1957) and Mitsumori et al. (2012) who reported decreased relative abundance of methanogen, protozoa, and fungi populations when sheep and goats were fed diets containing brown seaweed (Laminaria sp.) or BCM (0, 0.5, 2.0, and 5 g/100 kg BW) supplementation. These results, however, are inconsistent with other data (Belache et al., 2016). Their research indicates that tannin-rich (phlorotannins) brown seaweed (A. nodosum and Laminaria digita) had no substantial effect on rumen fermentation (VFA, ammonia), feed digestibility, or CH4 emissions. These same authors reported that the richness of total bacteria, anaerobic fungi, biodiversity indices, and abundances of the main bacterial and methanogen genera were also unaffected by brown seaweed supplementation (Belanche et al., 2016). Likewise, both A. taxiformis and A. armata have strong activity against ruminal gram-negative and gram-positive bacteria (Paul et al., 2006; Salvador et al., 2007). Besides, A. taxiformis has confirmed antimethanogenic activity in in vitro ruminal fermentation studies (Machado et al., 2015b, 2016). Recently, 16S ribosomal RNA (rRNA) gene amplicon sequencing showed that the relative abundance of methanogens in the fermentation bottles incubated with A. taxiformis (1,723 μg bromoform/g DM; Machado et al., 2016) decreased significantly compared to control diets, but this reduction in methanogen richness along with CH4 production was significant when averaged throughout the experiment (Roque et al., 2019b). This suggests that A. taxiformis has a direct effect on the metabolic functionality of rumen methanogens whereas its impact on microbiome congregation, specifically methanogen abundance, is hindered. It strongly inhibits the production of CH4 when added at a dose of 2% of the OM incubated (Roque et al., 2019b), demonstrating that these red seaweeds are active against archaea for the microbial production of CH4. These results demonstrated that the impact of seaweed on the rumen microbial community differs according to seaweed species.
Goel et al. (2009) reported that the populations of total bacteria and protozoa were not affected when BCM was added to in vitro batch cultures, but methanogenesis and growth of methanogens were reduced. There was a concomitant decrease in the relative abundance of major methanogens (Methanobacterials, Methanomassiliicoccales, and Methanomicrobiales) although bacterial communities were similar (Machado et al., 2018).) The relative abundance of methanogen that received supplementation with A. taxiformis (5% OM) were significantly decreased compared to the control diet in the continuous culture system (Roque et al., 2019b). These results, along with recent studies, are in close agreement with the microbial community changes in vitro and in vivo studies (Goel et al., 2009; Mitsumori et al., 2012). In contrast, BCM (3.0 mg/100 kg BW) supplementation did not inhibit the population of bacterial, protozoa, and methanogenic archaea in lactating dairy goats over 57-d although CH4 emissions were reduced by 33% (Abecia et al., 2012). The disparity in results between Abecia et al. (2012) and Mitsumori et al. (2012) might be explained by the duration of the trial (57-d vs. 8-d feeding trials) and the final concentration of BCM (up to 3 mg BCM vs. 5.0 mg BCM/100 kg body weight) in the diets. The increased duration of the Abecia et al. (2012) study may have provided time for the microbial ecosystem to adapt to the dietary treatment. Williams et al. (2009b) reported that methanogens take longer than 4-weeks to adapt to dietary changes, compared with approximately 15-d for the rumen bacterial community as a whole. Additional research is needed to determine if the duration of feeding BCM impacts the ruminal microbiota population and if methanogenic adaptation occurs.
7. Benefits and challenges of seaweed
Promoting seaweed as a dietary supplement for adaption-based climate change animal production strategies requires a value-added outcome for cattle producers. In recent years, seaweed has been studied as a promising and sustainable feedstock for the livestock industry for the following reasons:
-
1)
Seaweed can be used to provide an alternative source of nutrients. Seaweeds are a source of various nutritious compounds including proteins, lipids, vitamins, fatty acids, AA, carbohydrates, minerals, and other nutraceuticals. Seaweed also contains bioactive compounds such as anti-methanogenic, antioxidant, anti-inflammatory, anti-bacterial, or anti-viral agents (Pal et al., 2014; Pirian et al., 2017; Gaillard et al., 2018; Nunes et al., 2018; Roque et al., 2020).
-
2)
Dietary supplementation with seaweed biomass would allow for the delivery of phlorotannins or halogenated CH4 analogs as a holistic approach for the mitigation of enteric CH4 emissions and animal health compared to the use of extracts or metabolites (Tomkins et al., 2009, Machado et al., 2018; Wang et al., 2009a, b; Kinley et al., 2020). Therefore, seaweed could be a useful tool for mitigation of enteric GHG emissions without detrimental effects on ruminal fermentation. Although, additional research is needed to determine the seaweed inclusion rate that has positive impacts on animal performance, intake, efficiency, carcass traits, fatty acids profiles in milk and meat, and ruminal health (e.g. anti-inflammatory).
8. Summary of findings
Current research findings support the hypothesis that certain seaweeds decreased CH4 emissions. However, the available supply of this seaweed (specially bromoform-rich red seaweed) is a dilemma and there are some concerns over its sustainable production and potential negative impacts on the rumen digestibility and health impacts of bromoform. Seaweeds may be alternative feed ingredients for sustainable ruminant production. Although there have been several recent advances in our knowledge of anti-methanogenesis using seaweeds, there are still significant gaps in the in vitro and in vivo experiments to date (e.g., dairy and feedlot cattle performance). Moreover, reducing enteric CH4 emissions is challenging, and any improved mitigation strategy needs to be sustainable, practical, and economically feasible, thus ensuring the functional capacity of the rumen microbiome for ruminal fermentation and improved animal productivity. Future research will need to address the unsolved issues in existing animal performance, rumen microbiome changes, reproduction performance, immune-related animal health, and milk composition and milk quality. Additionally, to attain a comprehensive considerate of the methanogenesis responsible for the significant reduction of CH4, and its probable long-term influence on ruminants, rumen fluid metabolomic profiles associated with feed efficiency and the host animal are warranted.
Author contributions
B.R. Min and D. Brauer designed the model, the computational framework, analyzed the data, and wrote the manuscript. D. Parker and H. Waldrip assisted with data analysis and helped the overall directions. C. Lockard, K. Hales, A. Akbay, and S. Augyte provided critical feedback and helped shape the analysis and manuscript. All authors discussed the results and commented on the manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Footnotes
The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA/ARS.
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdul K.H.P.S., Tye Y.Y., Chow S.T., Saurabh C.K., Pariday M.T., Syakir M.I. Cellulosic pulp fiber as reinforcement materials in seaweed-based film. BioResources. 2016;12:29–42. [Google Scholar]
- Abecia L., Toral P.G., Martin-Garcia A.I., Martinez G., Tomkins N.W., Molina-Alcaide E., Newbold C., Yanez-Rui A. Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats. J Dairy Sci. 2012;95:2027–2036. doi: 10.3168/jds.2011-4831. [DOI] [PubMed] [Google Scholar]
- Abirami R.G., Kowsalva S. Phytochemical screening, microbial load and antimicrobial activity of underexploited seaweeds. Int Res J Microbiol. 2012;3(10):328–332. [Google Scholar]
- Abudabos A.M., Okab A.B., Aljumaah R.S., Samara E.M., Abdoun K.A., Al-Haidary A.A. Nutritional value of green seaweed (Ulva lactuca) for broiler chickens. Ital J Anim Sci. 2013;12:177–181. [Google Scholar]
- AFRC (Agricultural Food Research Council) Commonwealth Agricultural Bureaux; London: 1981. The nutrient requirement of pigs. [Google Scholar]
- Al-Fadhli A., Wahidulla S., D'Souza L. Glycolipids from the red alga Chondria armata. Glycobiology. 2006;16:902–915. doi: 10.1093/glycob/cwl018. [DOI] [PubMed] [Google Scholar]
- Allen V.G., Pond K.R., Saker K.E., Fontenot J.P., Bagley C.P., Ivy R.L., Evans R.R., Schmidt R.E., Fike J.H., Zhang X., Ayad J.Y., Brown C.P., Miller M.F., Montgomery J.L., Mahon J., Wester D.B., Melton C. Tasco: influence of brown seaweed on antioxidants in forages and livestock A review. J Anim Sci. 2001;79(E. Suppl):E.21–E.31. [Google Scholar]
- Allen V.G., Pond K.R., Saker K.E., Fontenot J.P., Bagley C.P., Ivy R.L., Evans R.R., Brown C.P., Miller M.F., Montgomery J.L., Dettle T.M., Wester D.M. Tasco Forage: III. Influence of a seaweed extract on performance, monocyte immune cell response, and carcass characteristics in feedlot-finished steers. J Anim Sci. 2001;79:1032–1040. doi: 10.2527/2001.7941032x. [DOI] [PubMed] [Google Scholar]
- Allen K.D., Wegener G., White R.H. Discovery of multiple modified F430 coenzymes in methanogens and anaerobic methanotropic archaea suggest possible new roles in nature. Appl Environ Microbiol. 2014;80:6403–6412. doi: 10.1128/AEM.02202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminina N.M., Karaulova E.P., Vishnevskaya T.I., Yakush E.V., Kim Y.K., Name K.H., Son K.T. Characteristics of polyphenolic content in Brown algae of the pacific coast of Russia. Molecules. 2020;25:1–13. doi: 10.3390/molecules25173909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.J., Blanton J.R., Jr., Gleghorn J., Kim S.W., Johnson J.W. Ascophyllum nodosum supplementation strategies that improve overall carcass merit of implanted English crossbred cattle. Asian-Aust J Anim Sci. 2006;19:1514–1518. [Google Scholar]
- Angell A.R., Pirozzi I., de Nys R., Paul N.A. Feeding preferences and the nutritional value of tropical algae for the Abalone Haliotis asinina. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0038857. e38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antaya N.T., Soder K.J., Kraft J., Whitehouse N.L., Guindon N.E., Erickson P.S., Conroy A.B., Brito A.F. Incremental amounts of Ascophyllum nodosum meal do not improve animal performance but do increase milk iodine output in early lactation dairy cows fed high-forage diets. J Dairy Sci. 2015;98:1991–2004. doi: 10.3168/jds.2014-8851. [DOI] [PubMed] [Google Scholar]
- Antaya N., Ghelichkhan M., Perira A.B.D., Soder K.J., Brito A.F. Production, milk iodine, and nutrient utilization in Jersey cows supplemented with brown seaweed (Ascophyllum nodosum) during the grazing season. J Dairy Sci. 2019;102:8040–8058. doi: 10.3168/jds.2019-16478. [DOI] [PubMed] [Google Scholar]
- Applegate R.D., Gray P.B. Nutritional value of seaweed to ruminants. Rangifer. 1995;15:15–18. 2012. [Google Scholar]
- Arasaki S., Arasaki T. Distributors, Kodansha International/USA through Harper and Row, Japan Publications; Tokyo: New York: 1983. Vegetables from the sea: low calorie, high nutrition to help you look and feel better. [Google Scholar]
- ARC (Agriculture Research Council) Commonwealth Agriculture Bureaux; Slough, UK: 1980. The nutrient requirements of ruminant livestock. [Google Scholar]
- Arieli A., Sklan D., Kissil G. A note on the nutritive value of Ulva lactuca for ruminants. Anim Prod. 1993;57:329–331. [Google Scholar]
- Attwood G., McSweeney C. Methanogen genomics to discover targets for methane mitigation technologies and option for alternative H2 utilization in the rumen. Aust J Exp Agric. 2008;48:28–37. [Google Scholar]
- Baardseth E. Synopsis of biological data on knobbed wrack Ascophyllum nodosum (L.) Le Jolis Fish. Synop FAO. 1970;38:44. https://www.fao.org/3/b0672e/b0672e.pdf [Google Scholar]
- Bach S.J., Wang Y., McAllister T.A. Effect of feeding sun-dried seaweed (Ascophyllum nodosum) on fecal shedding of Escherichia coli O157:H7 by feedlot cattle and on growth performance of lambs. Anim Feed Sci Technol. 2008;142:17–32. doi: 10.1016/j.anifeedsci.2007.05.033. [DOI] [Google Scholar]
- Bapteste E., Brochier C., Boucher Y. Higher-level classification of the Archaea: evolution pf methanogenesis and methanogens. Archaea. 2005;1:353–363. doi: 10.1155/2005/859728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends L.L., Blanton J.R., Jr., Miller M.F., Pond K.R., Allen V.G. Tasco supplementation in feedlot cattle: effects on pathogen loads. J Anim Sci. 2000;78(Suppl. 1):106. [Abstract] [Google Scholar]
- Belanche A., Jones E., Parveen I., Newbold C. A metagenomics approach to evaluate the impact of dietary supplementation with Ascophyllum nodosum or Laminaria digitata on rumen function in Rusitec fermenters. Front Microbiol. 2016;10:1–14. doi: 10.3389/fmicb.2016.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis B.A., Yu F.B., Schulz F., Blainey P.C., Woyke T., Quake S.R. Hydrogenotrophic methanogenesis in archaeal phylum Verstraetearchaeota reveals the shared ancestry of all ethanogens. PNAS. 2019;116:5037–5044. doi: 10.1073/pnas.1815631116. www.pnas.org/cgi/doi/10.1073/pnas.1815631116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka M.A. High-value products from microalgae-their development and commercialization. J Appl Phycol. 2013;25:743–756. [Google Scholar]
- Braden K.W. Texas Tech University; Lubbock: 2003. Effects of 2% Ascophyllum nodosum on carcass characteristics, retail display, and microbial loads of feedlot steers. M. S. Thesis. [Google Scholar]
- Braden K.W., Blanton J.R., Montgomery J.L., Van Santen E., Allen V.G., Miller M.F. Tasco supplementation: effects on carcass characteristics, sensory attributes, and retail display shelf-life. J Anim Sci. 2007;85:754–768. doi: 10.2527/jas.2006-294. [DOI] [PubMed] [Google Scholar]
- Brito A.F. Invited commentary in response to the paper entitled ‘Iodine concentration of milk-alternative drinks available in the UK in comparison with cows’ milk’ by Sarah Bath and colleagues. Br J Nutr. 2017;118:879–880. doi: 10.1017/S0007114517003117. [DOI] [PubMed] [Google Scholar]
- Burt A.W.A., Bartlett S., Rowland S.J. The use of seaweed meals in concentrate mixtures for dairy cows. J Dairy Res. 1954;21:299–304. [Google Scholar]
- Cardozo K.H.M., Guaratini T., Barros M.P., Falcao V.R., Tonon A.P., Lopes N.P., Campos S., Torres M.A., Souza A.O., Colepicolo P., Pinto E. Review: metabolites from algae with economical impact. Comp Biochem Physiol Toxicol Pharmacol. 2007;146:60–78. doi: 10.1016/j.cbpc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Carpenter L.J., Liss P.S. On temperate sources of bromoform and other reactive organic bromine gases. J Geophys Res. 2000;105:20539–20547. [Google Scholar]
- Castro M.N., Casas Valdez M., Marin Alvarez A., Aguila Ramirez R.N., Sanche, Rodriguez I., Hernandez Contreras H., Sangines Garcia L. The kelp Macrocystis pyrifera as nutritional supplement for goats. Revista Científica de Veterinaria. 2009;19:63–70. [Google Scholar]
- Castro-Gonzalez M.I., Carrillo-Dominguez S., Pérez-Gil F. Chemical composition of Macrocystis pyrifera (giant sargazo) collected in summer and winter and its possible use in animal feeding. Cienc Mar. 1994;20:33–40. [Google Scholar]
- Chalupa W. Manipulating rumen fermentation. J Anim Sci. 1977;45:585–599. [Google Scholar]
- Chidthaisong A., Conrad R. Specificity of chloroform, 2-bromoethanesulfonate and fluoroacetate to inhibit methanogenesis and other anaerobic processes in anoxic rice field soil. Soil Biol Biochem. 2000;32:977–988. [Google Scholar]
- Chizhov A.O., Dell A., Morris H.R., Haslam S.M., McDowell R.A., Shashkov A.S., Nifantev N.E., Khatuntseva E.A., Usov A.I. A study of fucoidan from the brown seaweed Chorda filum. Car Res. 1999;320:108–119. doi: 10.1016/s0008-6215(99)00148-2. [DOI] [PubMed] [Google Scholar]
- Christaki E., Bonos E., Giannenas I., Florou-Paner P. Functional properties of carotenoids originating from algae. J Sci Food Agric. 2013;93:5–11. doi: 10.1002/jsfa.5902. [DOI] [PubMed] [Google Scholar]
- Cian R.E., Fajardo M.A., Alaiz M., Vioque J., Gonza´lez R.J., Drago S.R. Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int J Food Sci Nutr. 2013;1:1–7. doi: 10.3109/09637486.2013.854746. Informa UK Ltd. [DOI] [PubMed] [Google Scholar]
- Cian R.E., Drago S.R., De Medina F.S., Martínez-Augustin O. Proteins and carbohydrates from red seaweeds: evidence for beneficial effects on gut function and microbiota. Mar Drugs. 2015;13:5358–5383. doi: 10.3390/md13085358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole N.A., McCroskey J.E. Effects of hemiacetyl of chloral and starch on the performance of beef steers. J Anim Sci. 1975;41:1735–1741. [Google Scholar]
- Connan S., Goulard F., Stiger V., Deslandes E., Gall E.A. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot Mar. 2004;47:410–416. doi: 10.1515/BOT.2004.057. [DOI] [Google Scholar]
- Conrad R. The global methane cycle: recent advances in understanding the microbial processes involved. Environ Microbiol Rep. 2009;1:285–292. doi: 10.1111/j.1758-2229.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- Corona G., Ji Y., Anegboonlap P., Hotchkiss S., Gill C., Yaqoob Y., Spencer J.P.E., Rowland I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br J Nutr. 2016;115:1240–1253. doi: 10.1017/S0007114516000210. [DOI] [PubMed] [Google Scholar]
- Costa K.C., Leigh J.A. Metabolic versatility in methanogens. Curr Opin Biotechnol. 2014;29:70–75. doi: 10.1016/j.copbio.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Cvetkovic B., Brouk M.J., Shirley J.E. Dairy day (report of progress 941). Kansas state university agricultural experiment station and cooperative extension service. 2004. Impact of dried seaweed meal on heat-stressed lactating dairy cattle; pp. 59–61.https://www.ksre.kstate.edu/historicpublications/pubs/SRP941.pdf [Google Scholar]
- Danielsson R., Dicksved J., Sun L., Gonda H., Muller B., Schnurer A., Bertilsson J. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front Microbiol. 2017;8(266):1–15. doi: 10.3389/fmicb.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawczynski C.H., Sch¨afer U., Leiterer M., Jahreis G. Nutritional and toxicological importance of macro, trace, and ultra-trace elements in algae food products. J Agric Food Chem. 2007;55:10470–10475. doi: 10.1021/jf0721500. [DOI] [PubMed] [Google Scholar]
- Delaunay D., Voile I. 2011. Composition Dermatologique et/ou Cosmétique Utilisée Pour la Régénération de la Peau, European Patent EP2488149 B1.https://data.epo.org/publicationserver/rest/v1.0/publicationates/20131002/patents/EP2488149NWB1/document.html Available online: [Google Scholar]
- Demais H., Brendle J., Le Deit H., Laza Anca L., Lurton L., Brault D. 2007. Argiles intercalés. European patent EP1786862 A1.https://patents.google.com/patent/EP1786862A1 Available online: [Google Scholar]
- Dembitsky M.V., Tolstikov G.A. Natural halogenated alkanes, cycloalkanes and their derivatives. Chem Sustain Dev. 2003;11:803–810. [Google Scholar]
- Denman K.L., Brasseur G., Chidthaisong A., Ciais P., Cox P.M., Dickinson R.E., Hauglustaine D., Heinze C., Holland E., Jacob D., et al. In: Climate change: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on climate change. Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K.V., Tignor M., Miller H.L., editors. Cambridge University Press; Cambridge, UK and New York, NY, USA: 2007. Couplings between changes in the climate system and biogeochemistry; pp. 499–587. [Google Scholar]
- Denman S.E., Tomkins N.W., McSweeney C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol Ecol. 2007;62:313–322. doi: 10.1111/j.1574-6941.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- Dubois B., Tomkins N.W., Kinley R.D., Bai M., Seymour S., Paul N.A., de Nys R. Effect of tropical algae as additives on rumen in vitro gas production and fermentation characteristics. Am J Plant Sci. 2013;4:34–43. [Google Scholar]
- Eadie J.M. Proceedings of the Royal Society of Edinburgh Section B; 1957. The mid-winter rumen microfauna of the seaweed eaten sheep of North Ronaldsay; pp. 661276–661287. [Google Scholar]
- EFSA. European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for protein. EFSA J. 2012;10:1–66. doi: 10.2903/j.efsa.2012.2557. 2557. [DOI] [Google Scholar]
- El-Baroty G.S., Moussa M.A., Shallan M.A., Ali A., Sabh Z., Shalaby E.A. Contribution to the aroma, biological activities, minerals, protein, pigments and lipid contents of the red alga: Asparagopsis taxiformis. J Appl Sci Res. 2007;3:1825–1834. [Google Scholar]
- Ellermann J., Hedderich R., Bocher R., Thauer R.K. The final step in methane formation. Eur J Biochem. 1988;172:669–677. doi: 10.1111/j.1432-1033.1988.tb13941.x. [DOI] [PubMed] [Google Scholar]
- Ellis J.L., Dijkstra J., Kebreab E., Bannink A., Odongo N.E., McBride B.W., France J. Aspects of rumen microbiology central to mechanistic modeling of methane production in cattle. J Agric Sci. 2008;146:213–233. [Google Scholar]
- EPIC . 2020. Bromodichloromethane.https://www.epicwaterfilters.ca/pages/bromodichloromethane-water-filter [Google Scholar]
- EU European Union. OJEU. 2003;46:L268/29–L268/43. [Google Scholar]
- EU European Union. OJEU. 2005;48:L233/8–L233/10. [Google Scholar]
- Evans F.D., Critchley A.T. Seaweeds for animal production use. J Appl Phycol. 2014;26:891–899. [Google Scholar]
- Ezeagu I.E., Petzke J.K., Metges C., Akinsoyinu A.O., Ologhobo A.D. Seed protein contents and nitrogen-to-protein conversion factors for some uncultivated tropical plant seeds. Food Chem. 2002;78:105–109. [Google Scholar]
- Fabrowska J., Eska L., Schroeder B., Messyasz G.B., Pikosz M. In: Marine algae extracts. Kim S.K., Chojnacka K., editors. Wiley-VCH, Verlag GmbH and Co. KGaA; Weinheim, Germany: 2015. Biomass and extracts of algae as material for cosmetics; pp. 681–706. ISBN 9783527337088. [Google Scholar]
- FAO . 2018. Food and Agriculture Organizations. Amino acid scoring patterns (FAO). FAO/WHO/UNUEPR/81/31.http://www.fao.org/3/M3013E/M3013E00.htm Rome. [Google Scholar]
- FAO . 2018. Food and agriculture organization of the United Nations (FAO). The state of the world fisheries and aquaculture.http://www.fao.org/state-of-fisheries-aquaculture/en Accessed 2018. [Google Scholar]
- Farley R.H. 2012. Chemistry and the aquarium: iodine in marine aquaria.https://www.austinreefclub.com/FAQ/reef-chemistry/water-chemistry-101/iodine-r31/ [Google Scholar]
- Fenical W. Chemical variation in a new bromochamigrene derivative from the red seaweed Laurencia pacifica. Phytochemistry. 1976;15:511–512. [Google Scholar]
- Ferreres F., Lopes G., Gil-Izquierdo A., Andrade P.B., Sousa C., Mouga T., Valentão P. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MSn: approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar Drugs. 2012;10:2766–2781. doi: 10.3390/md10122766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry J.G., Kastead K.A. In: Archaea: molecular and cellular biology. Cavicchioli R., editor. ASM Press; Washington, DC: 2007. Methanogenesis; pp. 288–314. 9. [Google Scholar]
- Fike J.H., Allen V.G., Schmidt R.E., Zhang X., Fontenot J.P., Bagley C.P., Ivy R.L., Evans R.R., Coelho R.W., Wester D.B. Tasco-Forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and ruminants. J Anim Sci. 2001;79:1011–1021. doi: 10.2527/2001.7941011x. [DOI] [PubMed] [Google Scholar]
- Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol. 1999;10(1):25–28. doi: 10.1016/S0924-2244(99)00015-1. [DOI] [Google Scholar]
- Fleurence J., Le Coeur C. Influence of mineralization methods on the determination of the mineral content of brown seaweed Undaria pinnatifida by atomic absorption spectrophotometry. Hydrobiologia. 1993;260/261:531–534. [Google Scholar]
- Frey P.A. In: Comprehensive natural products II. 1st ed. Mander L., Liu H.W., editors. Elsevier; Oxford, United Kingdom: 2010. Cobalamin coenzymes in enzymology; pp. 501–546. [Google Scholar]
- Fujihara S., Kasuga A., Aoyagi Y. Nitrogen-to-protein conversion factors for common vegetables in Japan. J Food Sci. 2001;66:412–415. doi: 10.1111/j.1750-3841.2008.00665.x. [DOI] [PubMed] [Google Scholar]
- Gaillard C., Bhatti H.S., Garrido M.N., Lind V., Roleda M.Y., Weisbjerg M.R. Amino acid profiles of nine seaweed species and their in situ degradability in dairy cows. Anim Feed Sci Technol. 2018;241:210–222. [Google Scholar]
- Garcia-Vaquero M., Hayes M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev Int. 2016;32(1):15–45. [Google Scholar]
- Gerber P.J., Steinfeld H., Henderson B., Mottet A., Opio C., Dijkman J., Falcucci A., Tempio G. FAO; Rome: 2013. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. [Google Scholar]
- GfE (Gesellschaft fur Ernahrungsphysiologie) Mastrinder, DLG-Verlag; Frankfurt (Main): 1995. Energieund nahrstoffbedarf landwirt-schaftlicher nutztiere, nr. 6; p. 85. [Google Scholar]
- GfE (Gesellschaft fur Ernahrungsphysiologie) DLG-Verlag; Frankfurt: 1999. Empfehlungen zur Energie- und Nahrstoffversorgung der Legehennen und Masthuhner (Broiler). Nr. 7”; p. 185. [Google Scholar]
- GfE (Gesellschaft fur Ernahrungsphysiologie der Haustiere) DLG-Verlags GmbH; Frankfurt am Main: 2001. Empfehlungen zur Energie- und Nahrstoffversorgung der Milchkuhe und Aufzuchtrinder; p. 136. [Google Scholar]
- GfE (Gesellschaft fur Ernahrungsphysiologie) No. 9. DLG-Verlag; Frankfurt: 2003. p. 121. (Recommendations for supply of energy and nutrients to goats). [Google Scholar]
- GfE (Gesellschaft fur Ernahrungsphysiologie) Nr. 4. Schweine. DLG-Verlag; Frankfurt GfE: 2006. p. 247. (Society of Nutritional Physiology (of domestic animals. Energie- und Nahrstoffbedarf landwirt-schaftlicher Nutztiere). [Google Scholar]
- Goel G., Makkar H.P.S., Becker K. Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations. Br J Nutr. 2009;101:1484–1492. doi: 10.1017/S0007114508076198. 2009. [DOI] [PubMed] [Google Scholar]
- Gojon-Baez H.H., Siqueiros-Beltrones D.A., Hernandez-Contreras H. In situ ruminal digestibility and degradability of Macrocystis pyrifera and Sargassum spp. in bovine livestock. Cienc Mar. 1998;24(4):463–481. [Google Scholar]
- Goldberg S.L. The use of water-soluble chlorophyll in oral sepsis: an experimental study of 300 cases. Am J Sur. 1943;62:117–123. [Google Scholar]
- Goodrich R.D., Garnett J.E., Gast D.R., Kirick M.A., Larson D.A., Meiske J.C. Influence of monensin on the performance of cattle. J Anim Sci. 1984;58:1484–1498. doi: 10.2527/jas1984.5861484x. [DOI] [PubMed] [Google Scholar]
- Gosch B.J., Magnusson M., Paul N.A., Nys R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. Gcb Bioenergy. 2012;4(6):919–930. [Google Scholar]
- Greenwood Y., Hall F.J., Orpin C.G., Paterson I.W. Microbiology of seaweed digestion in Orkney sheep. J Appl Bacteriol. 1983;58:585–596. doi: 10.1111/j.1365-2672.1985.tb01715.x. [DOI] [PubMed] [Google Scholar]
- Guiry M., Guiry G. National University of Ireland; Galway: 2014. AlgaeBase. World-wide electronic publication.http://www.algaebase.org [Google Scholar]
- Hansen H.R., Hector B.L., Feldmann J. A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim Feed Sci Technol. 2003;105:21–28. [Google Scholar]
- He M.L., Hollwich W., Rambeck W.A. Supplementation of algae to the diet of pigs: a new possibility to improve the iodine content in the meat. J Anim Physiol Anim Nutr. 2002;86:97–104. doi: 10.1046/j.1439-0396.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Hedderich R., Whitman W.B. In: The prokaryotes. Rosenberg E., DeLong E.F., Lory S., Stackebrandt E., Thompson F., editors. Springer; Berlin Heidelberg: 2013. Physiology and biochemistry of the methane-producing archaea; pp. 635–662. [Google Scholar]
- Hehemann J.H., Correc G., Barbeyron T., Helbert W., Czjze M., Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- Heo S.J., Ko S.C., Cha S.H., Kang D.H., Park H.S., Choi Y.U., Kim D., Jung W.K., Jeon Y. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol Vitro. 2009;23:1123–1130. doi: 10.1016/j.tiv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Hind Z., Rabah A., Christelle B., Hacène B., Yves B. Chemical and biological evaluation of the nutritive value of Algerian green seaweed Ulva lactuca using in vitro gas production technique for ruminant animals. Int J Adv Res. 2014;2:916–925. [Google Scholar]
- Holdt S.L., Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- Horta A., Alves C., Pinteus S., Lopes C., Fino N., et al. Identification of Asparagopsis armata- associated bacteria and characterization of their bioactive potential. Microbiol. 2019;8:1–9. doi: 10.1002/mbo3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Liu X., Zhao G., Hu T., Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbs T.I., Krasovskaya N.P., Ermakova S.P., Makarieva T.N., Shevchenko N.M., Zvyagintseva T.N. Comparative study of chemical composition and antitumor activity of aqueous–ethanol extracts of brown algae Laminaria cichorioides, Costaria costata, and Fucus evanescens. Russ J Mar Biol. 2009;35:164–170. [Google Scholar]
- IOM (United States Institute of Medicine) Dietary reference intakes report of the panel on micronutrients. 2001. Food and Nutrition Board. Natl. Acad. Press; Washington, DC: 2001. Iodine; pp. 258–289. [DOI] [Google Scholar]
- Johnson K.A., Johnson D.E. Methane emissions from cattle. J Anim Sci. 1995;73:2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- Johnson E.D., Wood A.S., Stone J.B., Moran E.T., Jr. Some effects of methane inhibition in ruminants (steers) Can J Anim Sci. 1972;52:703–712. doi: 10.4141/cjas72-083. [DOI] [Google Scholar]
- Jones G.A., McAllister T.A., Muir A.D., Cheng K.J. Effects of sainfoin (Onobrychis viciifolia scop) condensed tannins on growth and proteolysis by 4 strains of ruminal bacteria. Appl Environ Microbiol. 1994;60:1374–1378. doi: 10.1128/aem.60.4.1374-1378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafhi S.E., Hsaine L., Samri N., Etahiri S., Khlifi S. Phenolic compounds and antioxidant activity of nine seaweeds on the coast of El Jadida-Morocco. Int J Pharmaceut Sci Rev Res. 2020;63:18–24. [Google Scholar]
- Kannan G., Lee J.H., Kouakou B., Terrill T.H. Reduction of microbial contamination of goat meat using dietary brown seaweed (Ascophyllum nodosum) supplementation and chlorinated wash. Can J Anim Sci. 2019;99:570–577. [Google Scholar]
- Kasanah N., Triyanto D.S., Seto W., Amelia B., Isnansetyo A. Antibacterial compounds from red seaweeds. Indo J Chem. 2015;15:201–209. [Google Scholar]
- Kinley R.D., Nys R.D., Vucko M.J., Machado L., Tomkins N.W. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci. 2016;56:282–289. [Google Scholar]
- Kinley R.D., Martinez-Fernandez G., Mathews M.K., Nys R.D., Magnusson M., Tomkins N.W. Mitigating the carbon footprint and improving the productivity of ruminant livestock agriculture using red seaweed. J Clean Prod. 2020;259(6):1–10. [Google Scholar]
- Ko S.C., Cha S.H., Heo S.J., Lee S.H., Kang S.M., Jeon Y.J. Protective effect of Ecklonia cava on UVB- induced oxidative stress: in vitro and in vivo zebrafish model. J Appl Phycol. 2011;23:697–708. [Google Scholar]
- Kobayashi Y. Abatement of methane production from ruminants: trends in the manipulation of rumen fermentation. Asian-Aust. J Anim Sci. 2010;23:410–416. [Google Scholar]
- Kolanjiathan K., Ganesh P., Saranrai P. Pharmacological importance of seaweeds: a review. World J Fish Mar Sci. 2014;6:1–15. [Google Scholar]
- Kolb N., Vallorani L., Milanovic N., Stocchi V. Evaluation of marine algae Wakame (Undaria pinnatifida) and Kombu (Laminaria digitata japonica) as food supplements. Food Technol Biotechnol. 2004;42:57–61. [Google Scholar]
- Kuiken K., Lyman C.M. Essential amino acid composition for soybean meals prepared from twenty strains of soybeans. J Biol Chem. 1949;177:29–36. [PubMed] [Google Scholar]
- Lahaye M. Marine algae as sources of fibers: determination of soluble and insoluble dietary fiber contents in some sea vegetables. J Sci Food Agric. 1991;54:587–594. [Google Scholar]
- Lanfer-Marquez U.M., Barros R.M.C., Sinnecker P. Antioxidant activity of chlorophylls and their derivatives. Food Res Int. 2005;38:885–891. [Google Scholar]
- Lanigan G. Metabolism of pyrrolizidine alkaloids in the ovine rumen. IV. Effects of chloral hydrate and halogenated methane on rumen methanogenesis and alkaloid metabolism in fistulated sheep. Crop Pasture Sci. 1972;23:1085–1091. [Google Scholar]
- Laurberg P., Pedersen K.M., Hreidarsson A., Sigfusson N., Iversen E., Knudsen P.R. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and Jutland, Denmark. J Clin Endocrinol. 1998;83:765–769. doi: 10.1210/jcem.83.3.4624. [DOI] [PubMed] [Google Scholar]
- Leupp J.L., Caton J.S., Soto-Navarro S.A., Lardy G.P. Effects of cooked molasses blocks and fermentation extract or brown seaweed meal inclusion on intake, digestion and microbial efficiency in steers fed low-quality hay. J Anim Sci. 2005;83:2938–2945. doi: 10.2527/2005.83122938x. [DOI] [PubMed] [Google Scholar]
- Leyton A., Conte P., Barriga A.B., Buschmann A.H., Arvela P.M., Mikkola J.P., Lienqueo M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016;16:201–208. doi: 10.1016/j.algal.2016.03.019. [DOI] [Google Scholar]
- Li Y.X., Wijesekara I., Li Y., Kim S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011;46:2219–2224. doi: 10.1016/j.procbio.2011.09.015. [DOI] [Google Scholar]
- Li X., Norman H.C., Kinley R.D., Laurence M., Wilmot M., Bender H., Tomkins N. Asparagopsis taxiformis decreases enteric methane production from sheep. Anim Prod Sci. 2018;58:681–688. [Google Scholar]
- Liu Y., Whitman W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N Y Acad Sci. 2008;1125:171–189. doi: 10.1196/annals.1419.019. [DOI] [PubMed] [Google Scholar]
- Liu M., Hansen P.L., Lin X. Bromophenols in marine algae and their bioactivities. Mar Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang J., Wang A., Chen J. Chemical inhibitors of methanogenesis and putative applications. Appl Microbial Biotech. 2011;89:1333–1340. doi: 10.1007/s00253-010-3066-5. [DOI] [PubMed] [Google Scholar]
- Lopes D., Melo T., Rey T., Meneses J., Monteiro F.L., Helguero L.A., Abreu M.H., Lillebø A.I., Calado R., Domingues M.R. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules. 2020;25(17):1–18. doi: 10.3390/molecules25173883. 3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.M., Agregán I.D.R., Munekata P.E.S., Franco D., Carballo J., Sahin S.S., Lacomba R., Barba F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcate. Mar Drugs. 2017;15:1–11. doi: 10.3390/md1511036. 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco S.O., Barbarino E., Marquez U.M.L., Aidar E. Distribution of intracellular nitrogen in marine microalgae: basis for the calculation of specific nitrogen-to-protein conversion factors. J Phycol. 1998;34:798–811. [Google Scholar]
- Lourenco S.O., Barbarino E., De-Paula J.C., Ot´avioda Pereira L.S., Marquez U.M.L. Amino acid composition, protein content and calculation nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycol Res. 2002;50:233–241. [Google Scholar]
- Machado L., Magnusson M., Paul N.A., Nys R.D., Tomkins N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One. 2014;e85289(9):1–11. doi: 10.1371/journal.pone.0085289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado L., Kinley R.D., Magnusson M., de Nys R., Tomkins N.W. The potential of macroalgae for beef production systems in Northern Australia. J Appl Phycol. 2015;27:2001–2005. doi: 10.1007/s10811-014-0439-7. [DOI] [Google Scholar]
- Machado L., Magnusson M., Paul N.A., Kinley R.D., de Nys R., Tomkins N.W. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J Appl Phycol. 2015;28:1443–1452. doi: 10.1007/s10811-015-0639-9. [DOI] [Google Scholar]
- Machado L., Magnusson M., Paul N.A., Kinley R., de Nys R., Tomkins N. Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J Appl Phycol. 2016;28:3117–3126. [Google Scholar]
- Machado L., Tomkins N., Magnusson M., Midgley D.J., de Nys R., Rosewarne C.P. In vitro response of rumen microbiota to the antimethanogenic red macroalga Asparagopsis taxiformis. Microb Ecol. 2018;75:811–818. doi: 10.1007/s00248-017-1086-8. [DOI] [PubMed] [Google Scholar]
- Maia M.R.G., Fonscea A.J.M., Oliveira H.M., Mendonca C., Cabrita A.R.J. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci Rep. 2016;6:1–9. doi: 10.1038/srep32321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar H.P.S., Tran G., Heuze V., Giger-Reverdin S., Lessire M., Lebas F., Ankers P. Seaweeds for livestock diets: a review. Anim Feed Sci Technol. 2016;212:1–17. [Google Scholar]
- Manley L., Goodwin K., North W.J. Laboratory production of bromoform, methylene bromide, and methyl iodide by macroalgae and distribution in nearshore southern California waters. Limnol Oceanogr. 1992;37:1652–1659. [Google Scholar]
- Marín A., Casas-Valdez M., Carrillo S., Hernandez H., Monroy A., Sangines L., Perez-Gil F. The marine algae Sargassum spp. (Sargassaceae) as feed for sheep in tropical and subtropical regions. Rev Biol Trop. 2009;57:1271–1281. doi: 10.15517/rbt.v57i4.5464. [DOI] [PubMed] [Google Scholar]
- Marino F., Caro G.D., Gugliandolo C., Spano A., Faggio C., et al. Preliminary study on the in vitro and in vivo effects of Asparagopsis taxiformis bioactive phyco derivatives on teleosts. Front Physiol. 2016;7(459):1–11. doi: 10.3389/fphys.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.A., Harper D.B., McRoberts W.C., Dring M.J. Volatile bromocarbons produced by falkenbergia states of Asparagopsis spp. (Rhodophyta) Limnol Oceanogr. 1999;44:1348–1352. [Google Scholar]
- Mata L., Gaspar H., Justino F., Santos R. Effects of hydrogen peroxide on the content of major volatile halogenated compounds in the red alga Asparagopsis taxiformis (Bonnemaisoniaceae) J Appl Phycol. 2011;23:827–832. [Google Scholar]
- McConnell O., Fenical W. Halogen chemistry of the red alga Asparagopsis. Phytochemistry. 1977;16:367–374. [Google Scholar]
- McCrabb G.J., Berger K.T., Magner T., May C., Hunter R.A. Inhibiting methane production in Brahman cattle by dietary supplementation with a novel compound and the effects on growth. Aust J Agric Res. 1997;48:323–329. [Google Scholar]
- McMahon L.R., McAllister T.A., Berg B.P., Majak W., Acharya S.N., Popp J.D., et al. A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can J Plant Sci. 2000;80:469–485. doi: 10.4141/P99-050. [DOI] [Google Scholar]
- Mellouk Z., Benammar I., Krouf D., Goudjil M., Okbi M., Malaisse W. Antioxidant properties of the red alga Asparagopsis taxiformis collected on the North West Algerian coast. Exp Ther Med. 2017;13:3281–3290. doi: 10.3892/etm.2017.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaila A. The University of Waikato; New Zealand: 2020. Investigating the anti-methanogenic properties of select species of seaweed in New Zealand. MS Thesis. file:///C:/Published%20data/seaweed%20and%20methane-thesis.pdf. [Google Scholar]
- Miller J.K., Swanson E.W., Spalding G.E. Iodine absorption, excretion, recycling and tissue distribution in the dairy cow. J Dairy Sci. 1975;58:1578–1593. doi: 10.3168/jds.S0022-0302(75)84753-9. 1975. [DOI] [PubMed] [Google Scholar]
- Min B.R., Pinchak W., Anderson R.C., Callaway T.R. Effect of tannins on the in vitro growth of Escherichia coli O157: H7 and in vivo growth of generic Escherichia coli excreted from steers. J Food Protect. 2007;70:543–550. doi: 10.4315/0362-028x-70.3.543. [DOI] [PubMed] [Google Scholar]
- Min B.R., Castleberry L., Allen H., Parker D., Waldrip H., Brauer D., Willis W. Associative effects of wet distiller's grains plus solubles and tannin-rich peanut skin supplementation on in vitro rumen fermentation, greenhouse gas emissions, and microbial changes. J Anim Sci. 2019;97:4668–4681. doi: 10.1093/jas/skz317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.R., Solaiman S., Waldrip H.M., Parker D., Todd R.W., Brauer D. Dietary mitigation of enteric methane emissions from ruminants: a review of plant tannins mitigation options. Anim Nutr. 2020;6:231–246. doi: 10.1016/j.aninu.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.R., Genovese G., Castleberry L., Lockard C., Waldrip H.M., Parker D.B., Miller D.N., Akbay A., Morabito M., Manghisi A., Spagnuolo D., Brauer D. The potential role of two red seaweeds that promote anti-methanogenic activity and rumen fermentation profiles under laboratory conditions. J Anim Sci. 2021;(Suppl 2):328. [Google Scholar]
- Mishra V.K., Temelli F., Ooraikul B., Shacklock P.F., Craigie J.S. Lipids of the red alga, palmaria-palmata. Bot Mar. 1993;36:169–174. doi: 10.1515/botm.1993.36.2.169. 1993. [DOI] [Google Scholar]
- Misurcova L. Isolation and chemical properties of molecules derived from seaweeds chemical composition of seaweeds. Handbook Mar Macroalgae. 2011:171–192. [Google Scholar]
- Misurcova L. In: Handbook of marine macroalgae: biotechnology and applied phycology. Kim S.K., editor. John Wiley & Sons; 2012. Chemical composition of seaweeds; p. 567. 2012. [Google Scholar]
- Mitsumori M., Shinkai T., Takenaka A., Enishi O., Higuchi K., Kobayashi Y., Nonaka I., Asanuma N., Denman S.E., McSweeney C.S. Responses in digestion, rumen fermentation and microbial populations to inhibition of methane formation by a halogenated methane analogue. Br J Nutr. 2012;108:482–491. doi: 10.1017/S0007114511005794. [DOI] [PubMed] [Google Scholar]
- Moallem U. Invited review: roles of dietary n-3 fatty acids in performance, milk fat composition, and reproductive and immune systems in dairy cattle. J Dairy Sci. 2018;101:8641–8661. doi: 10.3168/jds.2018-14772. [DOI] [PubMed] [Google Scholar]
- Molina-Alcaide E.M.D., Carro M.Y., Roleda M.R., Weisbjerg V., Novoa-Garrido M. In vitro ruminal fermentation and methane production of different seaweed species. Anim Feed Sci Technol. 2017;228:1–12. [Google Scholar]
- Moneda A., Carro M.D., Weisbjerg M.R., Roleda M.Y., Lind V., Novoa-Garrido M., Molina-Alcaide E. Variability and potential of seaweeds as ingredients of ruminant diets: an in vitro study. Animals. 2019;9:1–19. doi: 10.3390/ani9100851. 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J.L., Allen V.G., Pond K.R., Miller M.F., Wester D.B., Brown C.P., Evans R., Bagley C.P., Ivy R.L., Fontenot J.P. Tasco-Forage: IV. Influence of a seaweed extract applied to tall fescue pastures on sensory characteristics, shelf life, and vitamin E status in feedlot-finished steers. J Anim Sci. 2001;79:884–894. doi: 10.2527/2001.794884x. [DOI] [PubMed] [Google Scholar]
- Morais T., Inácio A., Coutinho T., Ministro M., Cotas J., Pereira L., Bahcvandziev K. Seaweed potential in the animal feed: a review. J Mar Sci Eng. 2020;8:559. [Google Scholar]
- Morgavi D., Forano E., Martin C., Newbold C.J. Microbial ecosystem and methanogenesis in ruminants. Animals. 2010;4:1024–1036. doi: 10.1017/S1751731110000546. [DOI] [PubMed] [Google Scholar]
- Mueller-Harvey I. Unravelling the conundrum of tannins in animal nutrition and health. J Sci Food Agric. 2006;86:2010–2037. [Google Scholar]
- Myhre G., Shindell D., Breon F.M., Collins W. Cambridge University Press; Cambridge and New York: 2013. Anthropogenic and natural radiative forcing in climate change: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate. etc. [Google Scholar]
- NASEM . The National Academies Press; Washington: 2018. National Academies of Science Engineering and Medicine. Improving characterization of anthropogenic methane emissions in the United States. [PubMed] [Google Scholar]
- Neethu P.V., Suthindhiran K., Jayasri M.A. The antioxidant and anti-life activity of Asparagopsis taxiformis. Pharmacogn Res. 2017;9:238–246. doi: 10.4103/pr.pr_128_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norzagaray-Valenzuela C.D., Valdez-Ortiz A., Shelton L.M., Jiménez-Edeza M., Rivera-López J., Valdez-Flores M., Germán-Báez J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J Appl Phycol. 2017;29:189–198. [Google Scholar]
- Norziah M.H., Ching C.Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000;68:69–76. doi: 10.1016/S0308-8146(99)00161-2. [DOI] [Google Scholar]
- NRC (National Academy of Science) National Academy Press; Washington, D.C.: 1980. Mineral tolerance of domestic animals. [Google Scholar]
- NRC (National Academy of Science) 6th rev. edn. National Academy Press; Washington, DC: 1985. Nutrient requirements of sheep. [Google Scholar]
- NRC (National Academy of Science) 7th ed. National Academy Press; Washington, DC: 1996. Nutrient requirements for beef cattle. [Google Scholar]
- NRC (National Academy of Science) 10th ed. National Academy Press; Washington, DC: 1998. Nutrient requirements of swine; pp. 128–129. [Google Scholar]
- NRC (National Academy of Science) 7th rev. edn. National Academy Press; Washington, DC: 2001. Nutrient requirements of dairy cattle. [Google Scholar]
- NRC (National Research Council) 2nd rev. ed. Natl. Acad. Press; Washington, DC: 2005. Mineral tolerance of animals. [Google Scholar]
- Nunes N., Valente S., Ferraz S., Barreto M.C., Carvalho M.A.A.P. Nutraceutical potential of Asparagopsis taxiformis extracts and assessment of a downstream purification strategy. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00957. 1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R.S., Capone D.G. Use of specific inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol. 1998;10:285–383. doi: 10.1007/978-1-4684-5409-3_8. [DOI] [Google Scholar]
- Orpin C.G., Greenwood Y., Hall F.J., Paterson I.W. The rumen microbiology of seaweed digestion in Orkney sheep. J Appl Microbiol. 1985;58:585–596. doi: 10.1111/j.1365-2672.1985.tb01715.x. [DOI] [PubMed] [Google Scholar]
- Ortega-Calvo J.J., Mazuelos C., Hermosı´n B., Sa´iz-Jime´nez C. Chemical composition of Spirulina and eucaryotic algae food products marketed in Spain. J Appl Phycol. 1993;5:425–435. [Google Scholar]
- Ortiz J., Romero N., Robert P., et al. Dietary fiber, amino acid, fatty acids and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006;99:98–104. [Google Scholar]
- Pal A., Kamthania M.C., Kumar A. Bioactive compounds and properties of seaweeds- a review. Open Access Libr J. 2014;1:1–17. e752. [Google Scholar]
- Park Y.W., Juarez M., Ramos M., Haenlein G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rum Res. 2007;68:88–113. [Google Scholar]
- Patra A.K. Enteric methane mitigation technologies for ruminant livestock: a synthesis of current research and future directions. Environ Monit Assess. 2012;184:1929–1952. doi: 10.1007/s10661-011-2090-y. [DOI] [PubMed] [Google Scholar]
- Patra A.K. Recent advances in measurement and dietary mitigation of enteric methane emissions in ruminants. Front Vet Sci. 2016;3:1–17. doi: 10.3389/fvets.2016.00039. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N., de Nys R., Steinberg P. Chemical defense against bacteria in the red alga Asparagopsis armata: linking structure with function. Mar Ecol Prog Ser. 2006;306:87–101. [Google Scholar]
- Pirian K., Jeliani Z.Z., Sohrabipour J., Arman M., Faghihi M.M., Yousezadi M. Nutritional and bioactivity evaluation of common seaweed species from the Persian Gulf. Iran J Sci Technol. 2017;42:1795–1804. doi: 10.1007/s40995-017-0383-x. [DOI] [Google Scholar]
- Plaza M., Santoyo S., Jaime L., Garcia-Blairsy R.G., Herrero M., Senorans F.J., Ibãnez E. Screening for bioactive compounds from algae. J Pharmaceut Biomed Anal. 2010;51:450–455. doi: 10.1016/j.jpba.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Pompeu L.B., Williams J.E., Spiers D.E., Weaber R.L., Ellersieck M.R., Sargent K.M., Feyerabend N.P., Vellios H.L., Evans F. Effect of Ascophyllum nodosum on alleviation of heat stress in dairy cows. Prof Anim Sci. 2011;27:181–189. [Google Scholar]
- Prayitno C.H., Utami F.K.U., Nugroho A., Widyastuti T. The effect of seaweed (Gracilaria sp.) supplementation in sheep feed on methanogenesis inhibition in vitro. 1st Int Conf Anim Sci Technol (ICAST). 2018;247:1–7. [Google Scholar]
- Ragan M.A., Glombitza K.W. In: Progress in phycological research. Round F.E., Chapman D.J., editors. BioPress Ltd.; Bristol, UK: 1986. Phlorotannins, brown algal polyphenols; pp. 129–141. [Google Scholar]
- Ragan M.A., Jensen A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.) J Exp Mar Biol Ecol. 1978;34:245–258. doi: 10.1016/S0022-0981(78)80006-9. [DOI] [Google Scholar]
- Rao P.V.S., Periyasam C., Kumar K.S., Rao A.S., Anantharaman P. In: Bioprospecting of algae. Noor M.N., Bhatnagar S.K., Sinha S.K., editors. University of Alahabas Press; Allahabad, India: 2018. Seaweed: distribution, production and uses; pp. 59–78. [Google Scholar]
- Rindi F., Soler-Vila A., Guiry M.D. In: Marine bioactive compounds: sources, characterization, and applications. Hayes M., editor. Springer Science and Business Media, LLC, Berlin/Heidelberg; 2011. Taxonomy of marine macroalgae used as sources of bioactive compounds; pp. 232–259. [Google Scholar]
- Rizk A.M. Fatty acid composition of twelve algae forms the coastal zone of Qatar. Plant Foods Hum Nutr. 1997;51:27. doi: 10.1023/a:1007980227542. [DOI] [PubMed] [Google Scholar]
- Robic A., Rondeau-Mouro C., Sassi J.F., Lerat Y., Lahaye M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae) Carbohydr Polym. 2009;77:206–216. [Google Scholar]
- Rochfort S., Parker A.J., Dunshea F.R. Plant bioactives for ruminant health and productivity. Phytochemistry. 2008;69:299–322. doi: 10.1016/j.phytochem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Rogers H.J., Perkins H.R. In: Cell walls and membranes. Rogers H.J., Perkins H.R., editors. F. N. Spon Ltd. London; 1968. Microbial cell walls and membranes; pp. 114–134. Published by E. [Google Scholar]
- Roque B.M., Salwen J.K., Kinley R., Kebreab E. Inclusion of Asparagopsis armata in lactating dairy cow's diet reduces enteric methane emission by over 50 percent. J Clean Prod. 2019;234:132–138. [Google Scholar]
- Roque B.M., Brooke C.G., Ladau J., Polley T., March L.J., et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim Microb. 2019;2019:1–14. doi: 10.1186/s42523-019-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque B.M., Venegas M., Kinley R., deNys R., Neoh T.L., Duarte T.L., Yang X., Salwen J.K., Kebrean E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by 80 percent in beef steers. bioRxiv. 2020 doi: 10.1101/2020.07.15.204958doi. The preprint service for Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saker K.E., Allen V.G., Fontenot J.P., Bagley C.P., Ivy R.V., Evans R.R., Wester D.B. Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J Anim Sci. 2001;79:1022–1031. doi: 10.2527/2001.7941022x. [DOI] [PubMed] [Google Scholar]
- Salo-Vaananen P.P., Koivistoinen P.E. Determination of protein in foods: comparison of net protein and crude protein (N × 6.25) values. Food Chem. 1996;57:27–31. [Google Scholar]
- Salvador N., Gómez Garreta A., Lavelli L., Ribera M.A. Antimicrobial activity of Iberian macroalgae. Sci Mar. 2007;71:101–114. [Google Scholar]
- Samarakoon K., Jeon Y.J. Bio-functionalities of proteins derived from marine algae—a review. Food Res Int. 2012;48:948–960. [Google Scholar]
- Sarojini Y., Lakshminarayana K., Rao P.S. Variations in the distribution of flavonoids in some seaweed of Visakhapatnam coast of India. Sch Res Lib. 2012;4:1481–1484. [Google Scholar]
- Sawyer M.S., Hoover W.H., Sniffen C.J. Effects of a ruminal methane inhibitor on growth and energy metabolism in the ovine. J Anim Sci. 1974;38:908–914. doi: 10.2527/jas1974.384908x. [DOI] [PubMed] [Google Scholar]
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. doi: 10.1016/0031-9422(91)83426-L. [DOI] [Google Scholar]
- Scholten J.C.M., Conrad R., Stams A.J.M. E¡ect of 2-bromo-ethane sulfonate, molybdate and chloroform on acetate consumption by methanogenic and sulfate-reducing populations in freshwater sediment. FEMS Microbiol Ecol. 2000;32:35–42. doi: 10.1111/j.1574-6941.2000.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Schone F., Rajendram R. In: Comprehensive handbook of iodine. Preedy Victor R., Burrow Gerard N., Watson Ronald., editors. Academic Press; Oxford: 2009. Iodine in farm animals; pp. 151–170. [Google Scholar]
- Singh B.K., Chopra R.C., Rai S.N., Verma M.P., Mohanta R.K. Nutritional evaluation of seaweed on nutrient digestibility, nitrogen balance, milk production and composition in Sahiwal cows. Proc Natl Acad Sci India. 2015;87:437–443. [Google Scholar]
- Spears K. Developments in food colorings: the natural alternatives. Trends Biotechnol. 1988;6:283–288. [Google Scholar]
- Stams A.J., Plugge C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol. 2009;7:568–577. doi: 10.1038/nrmicro2166. [DOI] [PubMed] [Google Scholar]
- Stengel D.B., Connan S., Popper Z. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol Adv. 2011;29:483–501. doi: 10.1016/j.biotechadv.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Tayyab U., Novoa-Garrido M., Roleda M.Y., Lind V., Weisbjerg M.R. Ruminal and intestinal degradability of various seaweed species measured in situ in dairy cows. Anim Feed Sci Technol. 2016;213:44–54. [Google Scholar]
- Thapa H.R., Lin Z., Yi D., Smith J.E., Schmidt W.E., Agarwal V. Genetic and biochemical reconstitution of bromoform synthesis in Asparagopsis lends insights into seaweed reactive oxygen species enzymology. ACS Chem Biol. 2020;15:1662–1670. doi: 10.1021/acschembio.0c00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N.V., Kim S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar Drugs. 2013;11:146–164. doi: 10.3390/md11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins N.W., Hunter R.A. Methane reduction in beef cattle using a novel antimethanogen. Anim Prod Aust. 2004;25 329-329. [Google Scholar]
- Tomkins N.W., Colegate S.M., Hunter R.A. A bromochloromethane formulation reduces enteric methanogenesis in cattle-fed grain-based diets. Anim Prod Sci. 2009;49:1053–1058. [Google Scholar]
- Trei J.E., Parish R.C., Singh Y.K., Scott G.C. Effect of methane inhibitors on rumen metabolism and feedlot performance of sheep. J Dairy Sci. 1971;54:536–540. doi: 10.3168/jds.s0022-0302(71)85882-4. [DOI] [PubMed] [Google Scholar]
- Trei J.E., Scott G.C., Parish R.C. Influence of methane inhibition on energetic efficiency of lambs. J Anim Sci. 1972;34:510–515. doi: 10.2527/jas1972.343510x. [DOI] [PubMed] [Google Scholar]
- Ungerfeld E.M., Rust S.R., Boone D.R., Liu Y. Effects of several inhibitors on pure cultures of ruminal methanogens. J Appl Microbiol. 2004;97:520–526. doi: 10.1111/j.1365-2672.2004.02330.x. [DOI] [PubMed] [Google Scholar]
- Ushakova N.A., Kotenkova E.V., Kozlova A.A., Nifatov A.V. A study of the mechanism of probiotic effect of Bacillus subtilis strain 8130. Appl Biochem Microbiol. 2006;42:252–257. [PubMed] [Google Scholar]
- van Ginneken V.J.T., Helsper J.P.F.G., de Visser W., van Keulen H., Brandenburg W.A. Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids Health Dis. 2011;10:104. doi: 10.1186/1476-511X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nevel C.J., Demeyer D.I. In: Biotechnology and animal feeds and animal feeding. Wallace R.J., Chesson A., editors. VCH Publishers Inc.; New York: 1995. Feed additives and other interventions for decreasing methane emissions; pp. 329–349. [Google Scholar]
- van Nevel C.J., Demeyer D.I. Control of rumen methanogenesis. Environ Monit Assess. 1996;42:73–77. doi: 10.1007/BF00394043. [DOI] [PubMed] [Google Scholar]
- Visser A.M., Brok A.E., Westphal A.H., Hendriks W.H., Gruppen H., Vincken J.P. Resolubilisation of protein from water-insoluble phlorotannin-protein complexes upon classification. J Agric Food Chem. 2017;65:9595–9602. doi: 10.1021/acs.jafc.7b03779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucko M.J., Magnusson M., Kinley R.D., Villart C. The effects of processing on the in vitro antimethanogenic capacity and concentration of secondary metabolites of Asparagopsis taxiformis. J Appl Phycol. 2017;29:1577–1586. [Google Scholar]
- Wang Y., Xu Z., Bach S., McAllister T. Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim Feed Sci Technol. 2008;145:375–395. doi: 10.1016/j.anifeedsci.2007.03.013. [DOI] [Google Scholar]
- Wang Y., Alexander T.W., McAllister T.A. In vitro effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on rumen bacterial populations and fermentation. J Sci Food Agric. 2009;89:2252–2260. [Google Scholar]
- Wang Y., Xu Z., Bach S.J., McAllister T.A. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian-Australian J Anim Sci. 2009;22:238–245. [Google Scholar]
- Wijesinghea W.A.J.P., Jeona Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Car Poly. 2012;88:13–20. [Google Scholar]
- Williams J.E., Spiers D.E., Thompson-Golden L.N., Hackman T.J., Ellersleck M.R., Wax L., Colling D.P., Corners J.B., Lancaster P.A. Effects of Tasco in alleviation of heat stress in beef cattle. Prof Anim Sci. 2009;25:109–117. [Google Scholar]
- Williams Y.J., Popobski S., Rea S.M., Skillman L.C., Toovey A.F., Northwood K.S., Wright A.D.G. A vaccine against rumen methanogens can alter the composition of archaea populations. Appl Environ Microbiol. 2009;75:1860–1866. doi: 10.1128/AEM.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.M., Kennedy F.S., Wolf R.S. The reaction of multi-halogenated hydrocarbons with free and bound reduced Vitamin B12. Biochemist. 1968;7:1707–1713. doi: 10.1021/bi00845a013. [DOI] [PubMed] [Google Scholar]
- Yu Z., Smith G.B. Inhibition of methanogenesis by C1- and C2-polychlorinated aliphatic hydrocarbons. Environ Toxicol Chem. 2000;19:2212–2217. [Google Scholar]
- Zaki M.A., Nour A.M., Omar E., Tag El-Din A.E. The use of seaweed meal in feeding common carp. Asian-Aust. J Anim Sci. 1994;7(2):183. [Google Scholar]
- Zhou M., Hünerberg M., Chen Y., Reuter T., McAllister T.A., Evans F., Critchley A.T., Guana L. Air-dried brown seaweed, Ascophyllum nodosum, alters the rumen microbiome in a manner that changes rumen fermentation profiles and lowers the prevalence of foodborne pathogens. Appl Environ Sci. 2018;3:1–18. doi: 10.1128/mSphere.00017-18. e00017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M.B., Ito Y., Hess S.Y., Fujieda K., Molinari L. High thyroid volume in children with excess dietary iodine intakes. Am J Clin Nutr. 2005;81:840–844. doi: 10.1093/ajcn/81.4.840. [DOI] [PubMed] [Google Scholar]