Abstract
Objectives
Due to the rapid spread of COVID-19 worldwide, many countries have designed clinical trials to find efficient treatments. We aimed to critically report the characteristics of all the registered and published randomized clinical trials (RCTs) conducted on COVID-19, and summarize the evaluation of potential therapies developed in various regions.
Evidence acquisition
We comprehensively searched PubMed, Cochrane Library, Web of Science, Scopus, and Clinicaltrial.gov databases to retrieve all the relevant studies up to July 19, 2021, in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. We included all English-language published/registered RCTs on COVID-19, and excluded non-RCT, in-vitro/in-vivo, editorials, and review studies. Two reviewers independently evaluated all the records, and then analyzed by using SPSS 17.
Results
Within 3018 included studies, 2801 (92.8%) and 217 (7.2%) were registered or published RCTs consisting of about 600 synthetic drugs. Herbal medicines have been studied in 23 trials (10.6%) among the published RCTs and in 357 registered RCTs (12.7%). Hydroxychloroquine 23 (10.6%) and convalescent plasma 194 (6.9%) alone or in combination with other agents were the most frequently used interventions in published and registered RCTs, respectively. Most published RCTs have been conducted in Western Pacific Region (WPRO) (50 trials, 23.0%) including 45 trials from China. Also, a greater proportion of registered RCTs have been conducted in the Region of the Americas (PAHO) (885 trials, 31.6%) including 596 RCTs from the United States (U.S). Globally, 283 registered trials have been conducted to assess new developed vaccines for COVID or previously established for other disorders.
Conclusion
The present study highlighted the wide range of potential therapeutic agents in published and registered COVID-19 clinical trials across a wide range of regions. However, it is urgently required to global coordination in order to conduct more well-designed trials and progress in discovering safe and effective treatments.
Graphical abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s40199-021-00422-8.
Keywords: SARS-CoV-2, Controlled trials, Interventions, Treatment, Systematic review
Objectives
On March 11, 2020, COVID-19 or the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) disease outbreak was verified as a global pandemic by the World Health Organization (WHO) [1]. The high infectivity and transmissibility of SARS-CoV-2 resulted in a high mortality and morbidity rate [2]. As of August 30, 2021, more than 214 million proven cases of COVID-19, including about 4.5 million deaths, were identified and reported by WHO [3]. A bibliometric analysis indicates that COVID-19-related studies have become the major topic of scientific centers around the world. No doubt, the pandemic has triggered a global request for relevant research to investigate novel therapeutic options [4, 5].
There were two other epidemics from the Coronaviridae family, including SARS-CoV and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV). In response to the current emergency, physicians draw on their previous experiences. To the best of our knowledge, there are few effective therapies for COVID-19 [6, 7]; as a result, a large number of clinical trials are currently underway to evaluate the efficacy of hundreds of drugs.
Several studies have described the registered COVID-19-related randomized clinical trials (RCTs), most of which are in reference to the "Clinicaltrials.gov" database. After meticulously reviewing the relevant literature, we did not come across any study analyzing both published and registered RCTs [8–10]. Thus, in this systematic review, we aimed to critically assess the characteristics of all the registered and published RCTs that have been conducted on COVID-19, summarize the evaluation of potential therapies developed in various regions, and discriminate the ongoing and completed RCTs. The results of this study may lead to a better review of the current scientific practices in the field and also help investigators avoid trial duplication and find the gaps in the scientific efforts.
Evidence acquisition
Data sources and Search
Public records of clinical trials are stored in a standardized format in the registries that would help researchers share their protocols. While providing accessible and reliable data, trial registry databases have the potential to reduce publication bias and promote the transparency and validity of studies [11]. By utilizing registry platforms and other databases, we present the prospects of therapeutic clinical trials on COVID-19 from January 1, 2020 to July 19, 2021.
To retrieve all the registered and published relevant RCTs, we searched PubMed, Cochrane Library, Web of Science, Scopus databases, Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrial.gov registry by using the following search terms: “COVID‐19”, (“therapeutics”, “herbal medicine”), “Randomized Controlled Trials”, their medical subject heading (MeSH), and equivalents (Table S1).
Data collection
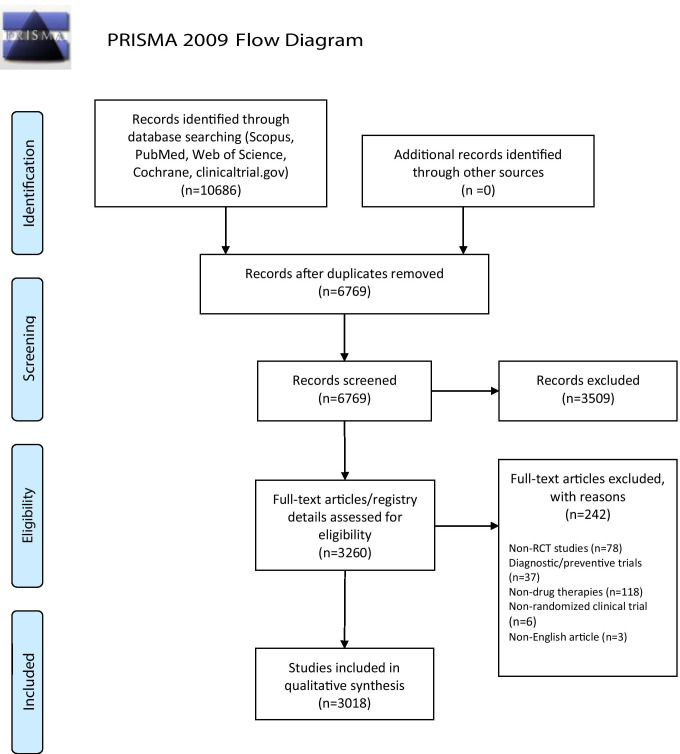
We extracted all the relevant studies up to July 19, 2021, in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Fig. 1). After data extraction and removal of duplicates, we screened the titles and abstracts of all the remaining papers/registry entries in reference to the inclusion and exclusion criteria. The inclusion criteria were:
Randomized controlled trials have been conducted/ registrated to treat COVID-19 by herbal, biological, or conventional treatments
Studies accessible in English
Studies published/registered from January 1, 2020 up to July 19, 2021
Fig. 1.
PRISMA flow diagram
The exclusion criteria were observational, experimental, editorial, review, or systematic review studies have been conducted on COVID-19.
These steps were performed by two independent researchers. Any discrepancy was resolved by a third researcher. The bibliographic details of the included studies were exported to an Excel file. Then, two reviewers retrieved the following main outcomes: types of treatment, target enrollment, trial phase, recruitment status, recruitment country, and recruitment region. Meanwhile, we grouped the countries into various regions based on the WHO regional classification: Region of the Americas (PAHO), African Region (AFRO), European Region (EURO), South-East Asia Region (SEARO), Western Pacific Region (WPRO), and Eastern Mediterranean Region (EMRO) [1]. For published studies, if the phase of the trials could not be extracted from the articles, we referred to the registry databases of clinical trials. In the final step of data collection, target interventions were classified into two major groups: herbal and synthetic agents. The synthetic drugs were categorized based on their therapeutic use, and then the most frequent medicines from each group were reported.
Data analysis
We classified the entries into an Excel file. For categorical variables, descriptive statistics including frequency and percentages are reported. For continuous variables, the median and interquartile are also reported. Then, we used SPSS 17 to report the frequencies, percentages, median, and interquartile of the variables for published and registered clinical trials, separately.
Results
From January 1, 2020 to July 19, 2021, a total of 10,686 records were retrieved. After removing the duplicates and irrelevant entries, we entered 3018 records into this study, including 2801 (92.8%) registered and 217 (7.2%) published RCTs. Overall, about 600 pharmaceutical medicines and more than 50 herbal agents were the targeted interventions of the studies. Details are shown in Table 1. Antivirals were the most common drug class under investigation in two regions (WPRO and EMRO). Immunomodulators were the most notable one in two regions (PAHO and EURO). Convalescent plasma was the most common evaluated treatment in the SEAR region. Considerably, the second common intervention class in the three regions (EURO, WPRO, and PAHO) was COVID-19 vaccines. Additionally, antivirals were the second common drugs' category in two regions (AFRO and SEAR).
Table 1.
More common drugs’ categories based on registered/published RCTs conducted in each region
| Regions of trials | More common drugs’ categories based on registered/published RCTs | |||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | ||
| African Region | Antiparasite | Antiviral | Antimalaria | Covid-19 vaccine | Convalescent plasma | |
| Count | 12 | 10 | 9 | 7 | 4 | |
| % Within region | 24% | 20.0% | 18.0% | 14.0% | 8.0% | |
| Region of Americas | Immunomodulator | Covid-19 vaccine | Convalescent plasma | Antiviral | Immunoglobulin | |
| Count | 94 | 85 | 84 | 70 | 59 | |
| % Within location | 10.1% | 9.1% | 9.0% | 7.5% | 6.3% | |
| South East Asia Region | Convalescent plasma | Antiviral | Antiparasitic | Mineral | Covid-19 vaccine | |
| Count | 20 | 18 | 17 | 15 | 15 | |
| % Within location | 6.9% | 6.2% | 5.9% | 5.2% | 5.2% | |
| European Region | Immunomodulator | Covid-19 vaccine | Antiviral | Antimalaria | Convalescent plasma | |
| Count | 100 | 77 | 72 | 58 | 50 | |
| % Within location | 13.8% | 10.6% | 10.0% | 8.0% | 6.9% | |
| Eastern Mediterranean Region | Antiviral | Minerals | Immunomodulators | Antiparasite | Antimalaria | |
| Count | 69 | 51 | 42 | 42 | 36 | |
| % Within location | 12.1% | 8.9% | 7.3% | 7.3% | 6.3% | |
| Western Pacific Region | Antiviral | Covid-19 vaccine | Immunomodulator | Antimalaria | Stem cell | |
| Count | 81 | 75 | 47 | 28 | 23 | |
| % Within location | 19.1% | 17.7% | 11.1% | 6.6% | 5.4% | |
| Collaboration Between Regions | Antiviral | Immune modulator | Covid-19 vaccine | Immunoglobulin | Antineoplasm | |
| Count | 23 | 17 | 9 | 7 | 7 | |
| % Within location | 29.9% | 22.1% | 11.7% | 9.1% | 9.1% | |
Characteristics of the included clinical trials
Published trials
Overall, 217 published RCTs were included in the present study. The details of the evaluated agents for each region are separately presented in Table 2. The 217 published trials have conducted in WPRO (50, 23.0%), EURO (50, 23.0%), PAHO (45, 20.7%), EMRO (47, 21.7%), SEARO (15, 6.9%) and AFRO (one article, 0.5%). Intraregional cooperation has been made in nine (4.1%) studies. A majority of the published RCTs have been conducted in China (45 studies), Iran (33 studies), the United States (U.S) (22 studies), and Brazil (17 studies).
Table 2.
Target interventions of published COVID-19 RCTs for each region
| African Region | ||
|---|---|---|
| ChAdOx1 nCoV-19 vaccine | ||
| Region of Americas | ||
|
HCQ (*6) convalescent plasma (*5) bamlanivimab (*3) ACE-Inh or ARB (*3) remdesivir (*3) tocilizumab (*3) HCQ|¥HCQ + azithromycin (*2) azithromycin canakinumab dexamethasone methylprednisolone fluvoxamine NAC pentoxifylline proxalutamide |
ivermectin stem cell therapy enoxaparin rivaroxaban sulodexide ravulizumab vitamin D3 HCQ|ivermectin vitamin C + zinc HCQ|LPV/r|HCQ + LPV/r HCQ|azithromycin dutasteride|azithromycin|nitazoxanide Herbal agents: brazilian green propolis |
|
| South East Asia Region | ||
|
convalescent plasma (*2) CQ remdesivir favipiravir ivermectin tocilizumab itolizumab vitamin D3 stem cell therapy |
azithromycin|LPV/r|LPV/r + azithromycin|LPV/r + doxycycline Herbal agents: ayurvedic agents siddha drugs chyawanprash curcumine + piperine |
|
| European Region | ||
|
HCQ (*4) tocilizumab (*3) remdesivir (*2) favipiravir (*2) hydrocortisone (*2) convalescent plasma (*2) ivermectin (*2) azithromycin chlorpromazine budesonide immunoglobulin camostat mesilate auxora dexamethasone LPV/r ramipril umifenovir anakinra |
colchicine vitamin D3 SNG001 valsartan NAC vilobelimab HCQ + azithromycin tenofovir + emtricitabine azithromycin|lincomycin darunavir-ritonavir|LPV/r colchicine|ruxolitinib|secukinumab methylprednisolone + tacrolimus remdesvir|LPV/r|IFN|HCQ|AZD7442 BCG vaccines BNT162b2 vaccine (*2) ChAdOx1 nCoV-19 vaccine (*3) Herbal agents: quercetin (*2) |
|
| Eastern Mediterranean Region | ||
|
sofosbuvir + daclatasvir (*4) ivermectin (*4) convalescent plasma (*3) favipiravir (*2) HCQ (*2) IFN (*2) umifenovir (*2) immunoglobulin (*2) methylprednisolone azithromycin bromhexine febuxostat dexamethasone diphenhydramine multivitamin mometasone NAC L-cysteine levamisole |
omega-3 vitamin C vitamin D3 melatonin sofosbuvir + ledipasvir sofosbuvir + daclatasvir + ribavirin vitamin C + NAC HCQ + LPV/r| IFN β-1A|IFN β-1B IFN β-1A + LPV/r sofosbuvir + daclatasvir + ribavirin vitamin C + NAC losartan|amilodipine methylprednisolone|dexamethasone methylprednisolone|methylprednisolone + tocilizumab enoxaparin|heparin|atorvastatin Herbal agents: zinger + echinacea hyoscyamus niger |
|
| Western Pacific Region | ||
|
COVID-19 vaccine (*5) HCQ or CQ (*3) IFN (*3) convalescent plasma (*3) LPV/r (*2) rhG-CSF (*2) favipiravir (*2) azvudine bromhexine leflunomide remdesivir ruxolitinib triazavirin stem cell therapy telmisartan zinc LPV/r|CQ (*2) IFN + TFF2 |
darunavir|cobicistat ribavirin + LPV/r + IFN ribavirin + IFN + LPV/r baloxavir marboxil|favipiravir favipiravir|tocilizumab|favipiravir + tocilizumab Herbal agents: xuebijing (*2) shenhuang granule (*2) xuanfei baidu keguan-1 lianhuaqingwen coconut oil huoxiangzhengqi + lianhuaqingwen|lianhuaqingwen shuanghuanglian oil xiyanping honeysuckle + gardenia + abrotani traditional chinese herbal formula |
|
| Collaboration between regions | ||
|
remdesivir (*3) HCQ or CQ (*2) remdesivir|LPV/r + IFN|IFN|HCQ|LPV/r |
bromhexine convalescent plasma hydrocortisone |
|
(*N): Number of repeated items
¥: different arms of RCTs are separated by "|"
Abbreviations: HCQ hydroxychloroquine, ACE-Inh angiotensin-converting enzyme (ACE) inhibitors, ARB angiotensin receptor blockers, NAC N-acetyl cysteine, LPV/r lopinavir-ritonavir, BCG bacille Calmette-Guerin, CQ chloroquine, IFN interferon, rhG-CSF the recombinant human granulocyte colony-stimulating factor, TFF2 trefoil peptide
Chloroquine (CQ) or hydroxychloroquine (HCQ) (n = 28), convalescent plasma (n = 15), and lopinavir-ritonavir (LPV/r) (n = 14) were the most common examined medicines in the studies. Also, multiple combinations have been examined such as antiviral combinations (LPV/r plus umifenovir or sofosbuvir-daclatasvir plus ribavirin), and the combination of interferons (IFNs) and different antivirals such as remdesivir, ribavirin, umifenovir, and LPV/r.
Twenty-three studies (10.6%) investigated the efficacy of herbal medicines, four Indian traditional medicine, 12 Chinese herbal medicines, and seven studies from Iran, Italy, Brazil and Philippines. The exact names of herbal agents are mentioned in Table 2.
Other significant features of the trials including the sample size, phase, and geographical distributions of the studies are presented in Table 3. The sample sizes in published studies ranged from 8 to 503,875, with a median of 111 people per trial (interquartile range (IQR), 55–240). A major part of the studies (116 trials, 53.5%) incorporated more than 100 participants. Altogether, about 628,000 entrants joined the trials as intervention or control groups. As shown in Table 3, 19 published studies (8.8%) have been reported as phase 0 trials consisting of seven RCTs on herbal medicine and 12 trials on synthetic drugs. Furthermore, a small percentage of the trials (15 trials, 6.9%) have been carried out as the phase 1 or 1/2 RCT. Overall, 34.6% (75 trials) of the published RCTs have been conducted on phase 0 + phase1 or 1/2 + phase 2.
Table 3.
General characteristics of published/registered RCTs
| Phase | Registered RCTs | Published RCTs | |||||
|---|---|---|---|---|---|---|---|
| Herbal agents | Synthetic agents | Herbal and synthetic agents | Overall | Herbal agents | Synthetic agents | Overall | |
| 0 | 24 | 30 | 0 | 54(%1.9) | 7 | 12 | 19(8.8%) |
| 1 or ½ | 19 | 429 | 1 | 449(%16.0) | 0 | 15 | 15(%6.9) |
| 2 | 68 | 753 | 1 | 822(%29.3) | 2 | 39 | 41(%18.9) |
| 2/3 | 47 | 237 | 0 | 284(%10.1) | 2 | 31 | 33(%15.2) |
| 3 | 80 | 589 | 0 | 669(%23.9) | 2 | 53 | 55(%25.3) |
| 4 | 25 | 175 | 0 | 200(%7.1) | 1 | 13 | 14(%6.5) |
| Not applicable | 85 | 201 | 1 | 287(%10.2) | 6 | 14 | 20(%9.2) |
| Missing | 6 | 30 | 0 | 36(%1.3) | 3 | 17 | 20(%9.2) |
| Sample size | |||||||
| ≤ 50 | 58 | 654 | 1 | 713(25.5%) | 5 | 43 | 48(%22.1) |
| 51–100 | 160 | 571 | 1 | 732(26.1%) | 7 | 46 | 53(%24.4) |
| > 100 | 136 | 1219 | 1 | 1356(48.4%) | 11 | 105 | 116(%53.5) |
| Region | |||||||
| African Region | 10 | 39 | 0 | 50(1.8%) | 0 | 1 | 1(%0.5) |
| Region of Americas | 25 | 860 | 1 | 885(31.6%) | 1 | 44 | 45(%20.7) |
| South East Asia Region | 121 | 152 | 0 | 273(9.7%) | 4 | 11 | 15(%6.9) |
| European Region | 14 | 658 | 0 | 672(24.0%) | 2 | 48 | 50(%23.0) |
| Eastern Mediterranean Region | 117 | 362 | 1 | 480(17.1%) | 3 | 44 | 47(%21.7) |
| Western Pacific Region | 66 | 306 | 1 | 373(13.3%) | 13 | 37 | 50(%23.0) |
| Collaboration Between Regions | 1 | 67 | 0 | 68(2.4%) | 0 | 9 | 9(%4.1) |
| Sum | 354 | 2444 | 3 | 2801(100%) | 23 | 194 | 217(100%) |
Registered trials
We included 2801 registered RCTs from 7025 records in the registry platforms. The registered clinical trials were scheduled in various designs; 2638 with a single intervention group and 163 with multi-armed groups. Furthermore, a greater part of studies (2444 trials) has been conducted with synthetic agents containing pharmaceutical agents, stem cell therapies, convalescent plasma, and vaccines. The categories of synthetic agents and some common sub-groups of each type are presented in Table 4. COVID-19 vaccines were the most common studied intervention (258, 9.2%) have been evaluated in registered RCTs. Convalescent plasma (194 trials), HCQ or CQ (185), stem cell therapy (103), ivermectin (92), favipiravir (71), azithromycin (68), tocilizumab (67), LPV/r (62), and IFNs (49) are the other frequent synthetic agents have been evaluated in registered clinical trials (Table 4). Besides, 170 combinations of drug categories are under investigation in registered trials (Table 5), including 50 (29.4%) studies on antimalarials plus antibiotics, and 23 (13.5%) studies on antivirals plus antimalarials. The combinations of HCQ with azithromycin (37 trials), and LPV/r (11 trials) are more common than other combinations in registered studies. Additionally, IFNs have been examined in combination with other medicines (12 trials). Overall, 283 studies focused on vaccination; 258 trials have explored COVID-19 vaccine, and others have evaluated previously established vaccines for other disorders such as BCG (Bacillus Calmette-Guérin), modified BCG vaccine (9 trials), or MMR (Measles, Mumps, and Rubella) vaccine (3 trials). A greater part of the studies on COVID-19 vaccines has assessed vaccines based on messenger ribonucleic acid (mRNA) (71 trials) or viral vector vaccines (64 trials).
Table 4.
Categories of drugs in registered RCTs
| Drugs’ category | Number |
|---|---|
| Antimalarials | 198 |
|
• HCQ or CQ • artesunate • artemisinin • mefloquine |
185 5 4 2 |
| Antibiotics / Antifungals | 100 |
|
• azithromycin • doxycycline • co-trimoxazole • itraconazole • clarithromycin |
68 15 4 3 3 |
| Glucocorticoids | 108 |
|
• dexamethasone • methylprednisolone • budesonide • ciclesonide • prednisolone • prednisone |
34 31 10 10 7 6 |
| Immunoglobulins and monoclonal antibodies | 143 |
|
• IVIG • bamlanivimab • clazakizumab • casirivimab + imdevimab • bevacizumab • mavrilimumab |
24 13 6 6 4 3 |
| Antihypertensive agents | 72 |
|
• losartan • ACE-inh |
18 9 |
| Mucolytics | 36 |
|
• NAC • dornase alfa • bromhexine hydrochloride |
18 13 10 |
| Anticoagulants /Antiplatelets | 128 |
|
• heparin • enoxaparin • aspirin • nafamostat mesylate • rivaroxaban • dipyridamole |
29 26 15 10 10 3 |
| Hormones | 33 |
|
• melatonin • thymosin • estradiol • liothyronine • oxytocin |
14 7 3 2 1 |
| Other synthetic drugs | 318 |
|
• colchicine • atorvastatin • surfactant • famotidine • pirfenidone • isotretinoin • deferoxamine • aviptadil |
38 14 8 7 7 6 4 3 |
| Stem cell therapies | 103 |
| Antivirals | 315 |
|
• favipiravir • LPV/r • remdesivir • sofosbuvir + daclatasvir • umifenovir • ribavirin • oseltamivir • molnupiravir • sofosbuvir + ledipasvir • ASC09 • azvudine |
71 62 39 24 18 8 7 6 6 5 4 |
| Other Antiparasitics | 140 |
|
• ivermectin • nitazoxanide • niclosamide |
92 24 19 |
| Immunomodulators | 287 |
|
• tocilizumab • IFNs • anakinra • baricitinib • sarilumab • cyclosporine • leflunomide • interleukin-7 • sirolimus • adalimumab |
67 49 22 14 11 10 8 6 6 6 |
| Antineoplastic agents | 112 |
|
• ruxolitinib • acalabrutinib • imatinib • tofacitinib • lenzilumab • ibrutinib |
19 7 6 5 4 4 |
| Antihyperglycemic agents | 21 |
|
• pioglitazone • linagliptin • metformin |
4 3 5 |
| Antifibrinolytics | 42 |
|
• camostat mesylate • ulinastat • tranexamic acid |
29 4 3 |
| Mineral/Vitamins | 172 |
|
• vitamin C • vitamin D3 • zinc • vitamin A |
48 45 27 9 |
| Other biologic interventions | 229 |
| • convalescent plasma | 194 |
| Vaccines | 283 |
|
• COVID-19 vaccine - mRNA vaccine 71 - vector vaccines 64 - subunit vaccine 59 - inactivated vaccine 39 - DNA vaccine 13 - virus-like particle 6 - live attenuated vaccine 5 |
258 |
|
• BCG vaccine • mycobacterium vaccine • measles vaccine • polio vaccine |
6 5 3 2 |
*Studies may evaluate more than one intervention drug from different categories; accordingly, the sum of categories/drugs number may exceed more than 100%
Abbreviations: HCQ Hydroxychloroquine, CQ chloroquine, LPV/r lopinavir-ritonavir, IFN interferon, IVIG intravenous immunoglobulin, ACE-inh angiotensin converting enzyme inhibitors, NAC N-acetyl-cysteine, mRNA messenger ribonucleic acid, DNA deoxyribonucleic acid, BCG bacillus Calmette–Guérin
Table 5.
Drug combinations in registered RCTs
| Drug Combinations in registered/published RCTs | Number of trials N |
|---|---|
| Antimalaria + Antibiotic with/without following combinations | 50 |
| HCQ + azithromycin | 37 |
| HCQ + azithromycin + doxycycline | 1 |
| artesunate-atovaquone + azithromycin | 1 |
| artesunate-amodiaquine + azithromycin | 2 |
| HCQ + azithromycin + zinc | 2 |
| HCQ + azithromycin + zinc + vitamin C + vitamin D3 | 1 |
| HCQ + azithromycin + LPV/r | 1 |
| HCQ + azithromycin + tocilizumab | 2 |
| HCQ + azithromycin + oseltamivir | 2 |
| mefloquine + azithromycin + tocilizumab | 1 |
| Antiviral combinations | 11 |
| ASC09 + ritonavir | 2 |
| ASC09 + ritonavir + oseltamivir | 1 |
| LPV/r + favipiravir | 2 |
| LPV/r + oseltamivir | 1 |
| LPV/r + ribavirin | 1 |
| sofosbuvir + ledipsavir | 1 |
| oseltamivir + ritonavir | 1 |
| oseltamivir + darunavir + ritonavir | 1 |
| favipiravir + maraviroc | 1 |
| Antimalaria + Antiviral | 23 |
| HCQ + LPV/r | 11 |
| HCQ + favipiravir | 4 |
| HCQ + sofosbuvir + daclatasvir | 2 |
| HCQ + remdesivir | 1 |
| HCQ + ribavirin | 1 |
| CQ + darunavir-ritonavir + favipiravir | 1 |
| oseltamivir + CQ | 2 |
| oseltamivir + HCQ | 1 |
| tenofovir + LPV/r + CQ | 1 |
| Antiviral + Immunomodulator with/without following combinations | 13 |
| IFN + remdesivir | 1 |
| IFN + LPV/r | 3 |
| IFN + LPV/r + ribavirin | 1 |
| IFN + ribavirin | 1 |
| IFN + danoprevir + ritonavir | 1 |
| IFN + umifenovir | 1 |
| IFN + ASC09 + ritonavir | 1 |
| tocilizumab + favipiravir | 1 |
| IFN + HCQ + LPV/r | 3 |
| tocilizumab + remdesivir | 1 |
| Antiparasitic with/without following combinations | 17 |
| ivermectin + doxycycline | 4 |
| ivermectin + HCQ | 1 |
| ivermectin + losartan | 1 |
| ivermectin + nitazoxide | 2 |
| nitazoxanide + ribavirin | 1 |
| nitazoxanide + ribavirin + ivermectin | 1 |
| nitazoxanide + favipiravir | 1 |
| nitazoxanide + HCQ | 1 |
| levamisole + inosine pranobex | 1 |
| levamisole + formoterol + budesonide | 2 |
| niclosamide + camostat mesylate | 1 |
| nitazoxanide + HCQ + ribavirin | 1 |
| Immunomodulator with/without following combinations | 8 |
| tacrolimus + methylprednisolone | 1 |
| tocilizumab + pembrolizumab | 1 |
| tocilizumab + ruxolitinib | 1 |
| IFN + dexamethasone + IVIG | 1 |
| IFN + HCQ | 1 |
| IFN + bromhexine + umifenovir | 1 |
| IFN-kappa + TFF2 | 1 |
| ruxolitinib + anakinra | 1 |
| Other combinations | 48 |
Abbreviations: HCQ hydroxychloroquine, CQ chloroquine, IFN interferon, LPV/r lopinavir-ritonavir, IVIG intravenous immunoglobulin, TTF2 trefoil factor 2
Herbal medicines have been studied in 357 registered RCTs (12.7%). The research studies on herbal medicine are centralized in India (114, 31.9%), China (61, 17.1%), and Iran (106, 29.7%), constituting 78.7% of the registered studies on herbal medicines. Curcumin (14 trials), Nigella Sativa (12 trials), licorice extract (14 trials), ginger (11 trials), AYUSH-64 (9 trials), and artemisinin (8 trials) are the most popular herbal medicines in registered RCTs.
Ninety-five countries have participated in the planning of 2690 (96.0%) registered clinical trials individually and 111 (4.0%) trials in collaboration with other countries. Out of 111 international RCTs, 66 (59.5%) studies were inter-regional. Other studies included 24 (21.6%) investigations in collaboration with other countries in the EURO, 17 (15.3%) studies across the PAHO, and four (3.6%) studies in the AFRO. Approximately one-third of all the studies (885 trials; 31.6%) have scheduled to begin in the PAHO and one-fourth in the EURO (672 trials; 24.0%). Among 357 herbal RCTs, a majority of trials have been planned in the SEARO (121 studies), EMRO (117 studies), and WPRO (67 studies). The geographical distribution of studies is presented in Table 3. The U.S conducted more than one-fifth (596 trials; 21.3%) of registered RCTs with the highest number of trials with immunomodulators (62 trials, 10.4%).
The sample sizes in registered studies ranged from four to 500,000. The scheduled RCTs involved a median of 100 enrollments per trial (interquartile range (IQR), 50–294). Considerably, 1445 trials (51.6%) were designed with the involvement of 100 or fewer participants. Nearly 2.6 million individuals participated in the trials. Moreover, 1325 trials (47.3%) were designed on early phases (phase 0, 1, 2), and there were 54 (1.9%) registered trials on phase 0. Besides, 200 (7.1%) clinical trials were scheduled in the post-marketing research phase (phase 4). No information was obtained from the phases of 36 studies. Details are shown in Table 3. Overall, 584 (20.8%) trials had not yet begun enrollment until the beginning date of current systematic review, and about half of all the registered trials (1397 trials; 49.9%) were recruiting participants. There were 496 (17.7%) trials that completed participant enrollment, but we did not identify any published results. Out of 93 (3.4%) terminated/withdrawn trials, 34 trials included the investigation of HCQ. The details of the studies are listed in Table 6.
Table 6.
Recruiting status of COVID-19 registered RCTs
| Region | Drug type | |||
|---|---|---|---|---|
| Herbal agents | Synthetic agents | Herbal and synthetic agents | Overall | |
| Not yet recruiting | 92 | 491 | 1 | 584(20.8%) |
| Enrolling by invitation | 4 | 33 | 0 | 37(1.3%) |
| Recruiting | 98 | 1296 | 3 | 1397(49.9%) |
| Active, not recruiting | 3 | 170 | 0 | 173(6.2%) |
| Suspended | 3 | 19 | 0 | 22(0.8%) |
| Terminated | 1 | 26 | 0 | 27(1.0%) |
| Withdrawn | 1 | 65 | 0 | 66(2.4%) |
| Enrollment completed | 152 | 344 | 0 | 496(17.7%) |
| Sum | 354 | 2444 | 3 | 2801(100%) |
Discussion
In this study, we included 2801 (92.8%) registered and 217 (7.2%) published RCTs. HCQ/CQ, convalescent plasma and LPV/r were the most common trailed medicines in published studies. Moreover, COVID-19 vaccines, convalescent plasma, HCQ, stem cell therapy, ivermectin, favipiravir, and azithromycin were the commonly targeted interventions in registered RCTs. The combinations of HCQ and azithromycin or LPV/r were more frequent than other combinations in the registered studies.
Findings of this study show the characteristics of global scientific activities during the outbreak. A previous study on COVID-19-related registered trials of Clinicaltrial.gov revealed that 92 drugs or plasma were being investigated until March 26, 2020 [8], while we report about 600 synthetic drugs up to July 19, 2021, which indicates progress in evaluating various medications during the COVID-19 pandemic. In Mehta et al.’s study, antivirals, antimalarials, and immunomodulators were commonly trialed, respectively [8]. However, in our study, the proportion of immunomodulatory drugs and convalescent plasma was significantly increased, showing a different result.
Synthetic drugs
Different drugs have been investigated in RCTs, mostly by repurposing available therapeutics from various categories such as antiviral, antimalarial, immunomodulator, anticoagulant, antibiotic, anti-parasite, and minerals. According to the guidelines from national and international organizations several medications have been more discussed for COVID-19 treatment. For instance, plasma therapy, LPV/r, tocilizumab, remdesivir, sofosbuvir-daclatasvir, corticosteroids, IFNs, ivermectin, and anti-thrombotics are frequently discussed in guidelines [12].
Plasma or immunoglobulins obtained from donors who recovered from the disease may include high levels of polyclonal antibodies. These pathogen-specific antibodies can neutralize virus particles and cause passive immunity in recipients [13]. Fifteen articles assessed the safety and efficacy of convalescent plasma for SARS-CoV-2 infection. Furthermore, about 194 RCTs have been registered to assess the beneficial effects of convalescent plasma. Overall, multiple RCTs have reported no meaningful efficacy of convalescent plasma transfusion on the 28-day mortality rate of infected patients with COVID-19 [14–18]. Based on Food and Drug Administration (FDA) authorization on February 4, 2021, high-titer COVID-19 convalescent plasma only meet the criteria for emergency use authorization (EUA) in the management of hospitalized patients in the early stages of the disease, as well as hospitalized patients with humoral immune deficiencies [19].
LPV/r and darunavir-cobicistat are anti-human immunodeficiency virus (HIV) drugs, which have been employed to treat COVID-19. Their main antiviral mechanism is inhibiting viral proteases. Ritonavir inhibits the cytochrome P450 metabolism of lopinavir and increases the plasma concentration of lopinavir. LPV/r exhibited in vitro antiviral activity against SARS-CoV-2 proteases including 3-chymotrypsin-like serine protease and papain-like protease [20]. RCTs conducted on these antivirals failed to show any significant clinical benefit against COVID-19 [21].
Remdesivir is another antiviral that developed during the Ebola virus outbreak. Remdesivir inhibits RNA-dependent RNA polymerase (RdRP) enzyme. RNA viruses encode RdRP or RNA replicase that is involved in the replication of the virus genome. Hence RdRP has no homolog in the host cell. This feature allows development of antivirals while decreasing the potential risk of injury to the human host cells. A broad range of RdRP inhibitors has been investigated as potential therapeutics against SARS-CoV-2 [22]. Remdesivir was the first drug found to be effective against SARS-CoV-2. Two large RCTs showed different efficacy of remdesivir. The RCT has been conducted by The Adaptive COVID-19 Treatment Trial (ACTT) showed shortened duration of disease course and mortality reduction in patients who needed supplemental oxygen [23]. Conversely, another large international, multi-arm, RCT named "Solidarity" [24] and other RCTs [25, 26] has not reported an overall reduction in mortality of COVID-19 hospitalized patients who received remdesivir. Overall, Solidarity has not reported mortality benefit, shortened time to discharge or ventilation requirement reduction following the administration of remdesivir. Nevertheless, ACTT and Solidarity employed different main outcomes. The ACTT was powered for clinical improvement, while the primary endpoint for Solidarity was the reduction of mortality rate. On May 1, 2020, FDA issued an EUA for remdesivir among hospitalized patients older than 12 years old with severe COVID-19 that furthermore expanded to EUA for adults and pediatrics weighing ≥ 3.5 kg [27].
Sofosbuvir is a uridine analog that inhibits the NS5B protein of hepatitis C virus (HCV). NS5B plays a key role in virus replication [22]. Sofosbuvir in combination with daclatasvir or ledipasvir has been investigated in multiple RCTs with sample sizes ranging from 48 to 82 participants. The available evidence from RCTs showed that sofosbuvir-daclatasvir or sofosbuvir-ledipasvir, in comparison with the comparator arm may enhance clinical recovery. However, no statistically significant reduction in mortality rate has been reported [28–31]. Further RCTs with larger sample sizes are required to support the results.
It has been revealed that antimalarial agents may exhibit various properties including antiviral effects against some types of RNA viruses, selective anti-inflammatory effects against some chronic autoimmune diseases, as well as immunomodulatory effects. Antimalarial agents potentially could inhibit lysosomal activity and autophagy in host cells through signaling via cytokines [20]. Among antimalarial agents, HCQ is the most common examined drug against SARS-CoV-2. A large-enrollment, open-label, multi-arm RCT named “Randomized Evaluation of COVID-19 Therapy” (RECOVERY) was conducted in the United Kingdom to compare potential treatments with the standard of care. In the RECOVERY trial, 4716 COVID-19 patients were recruited to investigate the clinical benefit of HCQ in comparison with the standard treatment. There was no significant difference in 28-day mortality rates between the two groups. Also, participants in the HCQ group had a longer hospitalization [32]. The FDA issued that HCQ is unlikely to be effective in COVID-19 treatment. In addition, it has some potentially harmful side effects including ventricular arrhythmias, prolonged QT interval, and Torsade-de-Pointes [33, 34]. On June 15, 2020, the FDA revoked the EUA for HCQ and CQ in the treatment of certain hospitalized COVID-19 patients [35].
According to the role of tumor necrosis factor-alpha (TNF-α), IFN-γ, interleukin (IL)-1β, IL-2, IL-6, IL-10, and other pro-inflammatory cytokines in the pathogenesis of COVID-19 pneumonia and lung damage, the modulation of immune response plays an essential role in limiting the morbidity and mortality of COVID-19 [36, 37]. In severe stages of the disease, the inflammatory response of the lungs increases that may lead to greater gas exchange between alveolar air and blood of capillaries, causing respiratory distress [38]. Consequently, several drugs with immunomodulatory and anti-inflammatory mechanisms are being studied for COVID-19 treatment.
Corticosteroids have anti-inflammatory properties via binding to intracellular receptors and blocking pro-inflammatory genes' promoters [39]. The anti-inflammatory effect of the corticosteroids in COVID-19 patients has been assessed in several trials [40, 41]. A reduction of mortality rate has been observed in the RECOVERY trial after 10-day treatment with low-dose dexamethasone [41]. Meta-analysis of seven trials has shown a significant reduction in mortality rate (odds ratio (OR), 0.66; 95% CI, 0.53 to 0.82) in critically ill COVID-19 patients who received corticosteroids in comparison with patients who received placebo or usual care [42]. Accordingly, WHO strongly recommended corticosteroid administration in critically ill COVID-19 patients [43].
High levels of pro-inflammatory cytokines, which cause cytokine storm, have been discovered in COVID-19 patients. Elevated levels of IL-6 are considered to be one of the main causes of the cytokine storm. One methodology to alter the aggressive stage of the COVID-19 may be the control of related pro-inflammatory cytokines [44]. Tocilizumab, sarilumab and siltuximab are monoclonal antibodies that are known for blocking IL-6 receptors with high affinity. Monoclonal antibodies are among promising therapies against SARS-CoV-2 which play an important role in viral attachment and cell entry [45]. On June 24, 2021, tocilizumab received an EUA for hospitalized COVID-19 patients aged ≥ 2 years old. The tocilizumab is indicated for patients who are receiving systemic corticosteroids and need supplemental oxygen or breathing support [46]. The FDA recommendation was in the line with the results from RECOVERY [47] and other RCTs [48–50] that assessed the safety of tocilizumab for COVID-19 treatment. RECOVERY has reported a lower mortality rate (29%) for patients who received tocilizumab over four weeks compared with standard care (33%) (RR 0.86, 95% CI 0.77–0.96) [47]. In contrast to the result of RECOVERY, multiple RCTs have reported no significant difference in the 28-day mortality rate between patients among the tocilizumab group or control group [50–53].
A clinical trial has recommended the use of monoclonal antibodies, which are specifically designed to neutralize the spike protein of SARS-CoV-2, in outpatients with mild to moderate severity of COVID-19 [54, 55]. Novel monoclonal antibody therapies (casirivimab, imdevimab, and bamlanivimab) received an EUA from the U.S. FDA in progressive mild to moderate COVID-19 outpatients [56].
IFNs exhibit immunomodulatory and antiviral properties. IFNs are a group of cytokine signaling molecules that are induced in response to the detection of viral RNA. When proteins’ sensors located in endosomes (toll-like receptors) detect a viral RNA, IFNs attach to receptors of the cell membrane, causing phosphorylation of a diverse array of transcription factors and inhibition of viral replication [57, 58]. WHO Solidarity trial could not approve the clinical benefit of IFN-β1 in hospitalized COVID-19 patients [24]. In contrast, multiple RCTs have reported different results [49, 59–61]. For instance, nebulized IFN-1β in a phase two RCT has increased the recovery rate in the hospitalized COVID-19 patients [61]. Moreover, another RCT has observed a significant reduction of mortality rate (OR, 13.5; 95% CI, 1.5 to 118) after treatment with IFN [60]. Nevertheless, several RCTs are still ongoing to investigate the advantages of IFNs and confirm the results.
Ivermectin is an antiparasitic agent that demonstrated its antiviral activity against RNA viruses such as West Nile, dengue virus, influenza, and HIV-1. It has been supposed that ivermectin may inhibit SARS-CoV-2 replication by inhibition of the importin α/β receptor. Importin α/β delivers virus integrate proteins into the nucleus of the host cell [62]. Multiple RCTs have studied ivermectin use in COVID-19 patients. Some RCTs could not found any clinical efficacy of ivermectin [63–65], while others have reported faster time to recovery of COVID-19 disease [66–70], a remarkable decrease of cytokines and inflammatory markers [67, 68], faster viral clearance [71], or decrease in mortality rate [67, 68] in participants who received ivermectin in comparison with the standard treatment protocol. Nevertheless, the majority of these RCTs had methodological issues like small sample sizes, various ivermectin dosages, and different concomitant drugs given to the patients.
Several investigations have demonstrated coagulopathy associated with COVID-19 disease [72]. Early coagulopathy of COVID-19 is characterized by a substantial increase in D-dimer levels and fibrinogen-degradation products. Viral infection triggers innate immune responses like systemic inflammatory responses. When the defense system of the host activates, thrombin produces and coagulation activates as essential communication components between cellular and humeral amplification networks. This is defined as immunothrombosis or thromboinflammation. Anticoagulant agents like low molecular weight heparin (enoxaparin) are indicated for prevention or treatment of disseminated intravascular coagulopathy, thromboembolism or sepsis-induced coagulopathy [73]. In a retrospective study of 4389 hospitalized patients, prophylactic and therapeutic anticoagulant therapy was associated with reduced mortality rate and mechanical ventilation. Among patients who received therapeutic anticoagulation, an estimated 47% reduction of in-hospital mortality has been observed [74]. Given the result of an RCT, the use of therapeutic-dose anticoagulant increased bleeding and did not enhance the clinical outcome in comparison with prophylactic-dose anticoagulants [75].
Herbal agents
Since the beginning of the pandemic, herbal medicines and natural products have been repurposed for the management of COVID-19 [76]. As a result, multiple RCTs have been conducted on this topic in different countries. Curcumin, Nigella Sativa, and licorice extract were the more common drugs investigated in RCTs. Results from a systematic review of seven clinical trials on herbal medicines demonstrated the potential role of combined herbal medicines with Western medicine on symptom relief [77]. Licorice is a plant that has been used to control COVID-19 with anti-inflammatory properties. Glycyrrhizin, a frequent component of licorice, provides anti-inflammatory activity through antagonism of toll-like receptor 4. Besides, both glycyrrhizin and glycyrrhetinic acid can reduce virus transmission, which may happen by a reduction in expression of type 2 transmembrane serine protease (TMPRSS2). TMPRSS2 play a critical role in virus uptake [78, 79]. Licorice also exhibits immunomodulatory, anti-oxidant, and antibacterial activity. Components of the plant can bind to viral fusion proteins inhibiting viral entry to the host cells, they also can decrease expression of ACE2 [80]. A molecular docking study showed that nigellidine and α-hederin from Nigella Sativa have better energy scores toward 6LU7 and 2GTB, which are the main proteases found in CoVs, active sites rather than HCQ, CQ, and favipiravir [81].
The beneficial effects of herbal medicines are shown in several clinical trials. Anti-inflammatory and anti-thrombotic activities of curcumin besides its antiviral, antibacterial and antifungal properties of the compound can prevent secondary infections as well as reduce morbidity and mortality [82]. Administration of quercetin to outpatients significantly has reduced the need or the length of hospitalization, non-invasive oxygen therapy, progression to intensive care units, decrease virus clearance, and deaths without peculiar side effects [83, 84]. Recovery and improvement rate in patients suffering from COVID-19 has increased by prescription of Chinese herbal formulation adjuvant to usual treatment [85]. Administration of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules (traditional Chinese medicine, TCM) to COVID-19 patients showed no significant difference in the severity of the disease, while they caused a significant decrease in antibiotic utilization in patients [86]. Reduning injection, another formulation of TCM, resulted in a shorter median time to resolution of the clinical symptoms, hospital stay, defervescence as well as a shorter time of nucleic acid test turning negative in the COVID-19 patients [87]. Intravenous injection of xuebijing contains some plants with main components of amino acid, phenolic acid, flavonoid glycoside, and elysine. Xuebijing can also downregulate the expression of pro-inflammatory cytokines IL-6, IL-8 and TNF-α in severe COVID-19 patients and improves main clinical symptoms [88]. Echinacea tablet with Zingiber officinalis in the outpatient of COVID-19 improved cough, dyspnea, and muscle pain compared to HCQ, with no specific side effects [89]. Essential oil of thyme improved symptoms of patients such as fever, cough, dyspnea, dizziness, muscular pain, anorexia, weakness, lethargy, and fatigue. Significant increases in lymphocyte count and calcium, as well as a decrease in neutrophil count and blood urea nitrogen (BUN), were also reported in patients suffering from COVID-19 [90].
Although several studies have evaluated the beneficial effects of herbal preparations against COVID-19, these studies suffer from some drawbacks in study design like limited sample size, lack of clear primary and secondary outcomes, administration of some herbal drugs without providing any criteria regarding the quality and active components of them. Mostly, they have reported improvement of clinical signs or symptoms without considering the mechanism involved.
Vaccines
Currently, there is an increasing number of RCTs on COVID-19 vaccine candidates. These vaccines are based on three major strategies of vaccine design. Key differences include if they employ the entire microorganism (the whole microbe approach); just portions of the microbe that stimulate the immune system (the subunit approach), or only the genetic materials that contains information for producing particular proteins rather than the entire virus (the genetic approach).
There are three subtitles of vaccines, which are prepared with the whole microbe approach, including inactivated, Live-attenuated, and viral vector. Each type has some challenges in the injection. Inactivated vaccines typically fail to produce the cellular adaptive immune response and long-lasting immunity. Accordingly, they require additional booster doses and adjuvants, to induce sufficient immune response [91, 92]. Thirty-nine inactivate vaccines candidate have been initiated clinical trials, which eight vaccine candidates have been approved at least in one country:
QazVac by Kazakhstan RIBSP/ approved in Kazakhstan
Covaxin by Bharat Biotech/ or BBV152 approved in nine countries
COVIran Barekat inactivated Vaccine by Shifa Pharmed Industrial Co approved in Iran
Inactivated (Vero Cells by Sinopharm (Wuhan)) approved in China
SARS-CoV-2 Vaccine (Vero Cells) by Minhai Biotechnology Co approved in China
CoronaVac by Sinovac approved in 39 countries
BBIBP-CorV (Vero Cells) by Sinopharm (Beijing) approved in 60 countries
KoviVac by Chumakov Center approved in Russian Federation
The main challenge with live-attenuated vaccines is that they may regain wild-type virulence in some cases. Another challenge with these vaccines is that they cannot be administered to immunosuppressed people [92, 93]. The COVI-VAC by Codagenix Inc is the only live-attenuated vaccine candidate and now it is on phase one of the clinical trial (NCT04619628). Viral vector vaccines are mainly designed from a carrier virus-like adenovirus, poxvirus, or measles. Their main benefit is that the immunogen provokes the innate immune system. Subsequently, the innate immune system triggers adaptive T cell-mediated and humoral immune systems. Viral vectors like adenovirus type 5 (Ad5) vector, recombinant adenovirus type 5 (rAd5) vector, recombinant adenovirus type 26 (rAd26) vector, and chimpanzee adenovirus (ChAd) containing the spike gene of SARS-CoV-2, are among promising vaccine platforms. The following vaccines are approved viral vector vaccines:
Ad5-nCoV by CanSino approved in eight countries
Oxford/AstraZeneca by Serum Institute of India Covishield approved in 45 countries
Ad26.COV2.S by Janssen (Johnson & Johnson) approved in 59 countries
Sputnik Light by Gamaleya approved in 12 countries
AZD1222 by Oxford/AstraZeneca in 121 countries
Sputnik V by Gamaleya approved in 71 countries
Another approach, subunit vaccines originate from an immunogenic fraction, rather than the complete pathogen, and then induce potent immune responses [94]. This approach contains two types including protein subunit and virus-like particles. Protein subunit vaccine can be safely offered to immunocompromised patients, but it usually requires booster doses. Current clinical trials for protein subunit vaccines utilize different immunogens. Protein subunit vaccines usually employ spike protein or its receptor-binding domain (117). There are four approved protein subunit vaccines:
CIGB-66 by the Center for Genetic Engineering and Biotechnology (CIGB) approved in Cuba
Medigen/MVC-COV1901 approved in Taiwan
RBD-Dimer by Anhui Zhifei Longcom approved in Uzbekistan and China
EpiVacCorona by FBRI approved in Russian Federation and Turkmenistan
Deoxyribonucleic acid (DNA) and mRNA vaccines are two types of vaccines prepared by the genetic approach. DNA vaccines deliver DNA plasmids generated in bacteria, to the host cells by a special delivery platform. Currently, thirteen RCTs examine DNA vaccine candidates against SARS-CoV-2. The ZyCoV-D vaccine by Zydus Cadila is the only DNA vaccine that is approved in India. (CTRI/2021/01/030416).
mRNA vaccines require to enter the cytoplasm or endoplasmic reticulum. Since mRNA is an unstable polymer, for long-term storage of mRNA vaccines, temperatures ranging from − 70 ºC to − 20 ºC is required. Novel modifications to vaccine designs such as the addition of stabilizing compounds or particular mutations, enable the preservation of mRNA vaccines at temperatures ranging from 2 to 8 ºC for up to about six months [94]. There are three approved vaccine candidates against COVID-19:
BNT162b2 by Pfizer/BioNTech approved in 97 countries
TAK-919 by Takeda (Moderna formulation) approved in Japan
mRNA-1273 by Moderna approved in 69 countries
Some important aspects of immunization remain to be more explained. As larger populations, including those with compromised immunity, will be vaccinated, the durability of the protection made by the various vaccine approaches, and more details of induced immune responses will be revealed.
While poorly controlled studies and case series run a higher risk of bias, RCTs are the gold standard of research designs and may provide reliable evidence (129). RCTs, such as those included in our study, played a critical role in the recommendation for/recommendation against specific drugs (124, 130–132). Our study may help better recognize the completed and ongoing RCTs to avoid duplication and therapeutic failures.
As previously shown, 25.5% of trials involve ≤ 50 participants, which is a considerable percentage. On 18 March 2020, the director of WHO announced that “Multiple small trials with different methodologies may not give us the clear, strong evidence we need about which treatments help to save lives” and encouraged researchers through the countries to collaborate and join Solidarity, the largest international trial [95]. Moreover, regional collaborations are valuable; alongside, several international RCTs are being formed under the control of coordinating bodies; still, it is important to ensure that the process of the trials is well-targeted in low-income countries, and interventions must be affordable and available for patients and researchers [96]. Besides, there have been many parallel clinical trials designed to investigate similar hypotheses and drugs. Some trials are terminated due to the unavailability of the sample size. An international platform for investigators to collaborate in studies may be a good idea for preventing the waste of time and costs [97].
Our study had several strengths and limitations. The main strength was its sample size. This study systematically included trials that focused on COVID-19, conducted on potential vaccines, herbal agents, and synthetic agents and described the geographical distribution of RCTs. Therefore, its results may give insight into future research. As a limitation, in some cases, the status of completed clinical trials was outdated. One limitation of our study is the discrepancy and delay in the registry’s recruitment status. Meanwhile, there might be some completed clinical trials that we did not report as “completed” trials. Moreover, drugs can be classified into different categories, and there are some overlaps in the categories (for example, some medications can be classified into more than one category of immunomodulators, antineoplastics, and immunoglobulins).
Conclusions
Our study highlighted the wide range of therapeutic agents that have been used in COVID-19 clinical trials, including vaccine candidates, herbal medicines, biological interventions, and pharmaceutical drugs from various groups, predominantly antivirals and immunomodulators. On account of the increasing number of RCTs in the current pandemic, it is valuable to be informed of other studies being conducted simultaneously to save time and avoid duplication. Based on the mentioned defects, we recommend that scientists share more details of trial registries such as the exact generic names of drugs and ingredients of plant extracts, and be more responsible for updating the trials’ status. Also, the WHO encouraged different territories to collaborate in order to facilitate global decision-making. Successful international cooperation such as “RECOVERY” and “Solidarity” provide considerable information about various therapeutic choices. The findings of our study will inform global health decisions. In some cases, conflicting findings have been reported about the efficacy of treatments. This may be due to various methodologies, sample sizes, dosage, and comparator arms that have been applied. Nevertheless, some drugs like corticosteroid, remdesivir, tocilizumab, and monoclonal antibodies demonstrated remarkable results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study is in-home study without any funding supports.
Abbreviations
- ACE-Inh
Angiotensin-converting enzyme (ACE) inhibitors
- ACTT
Adaptive COVID-19 Treatment Trial
- Ad5
Adenovirus type 5
- AFRO
African Region
- ARB
Angiotensin receptor blockers
- BCG
Bacillus Calmette-Guérin
- BUN
Blood urea nitrogen
- CENTRAL
Cochrane Central Register of Controlled Trials
- ChAd
Chimpanzee adenovirus
- CQ
Chloroquine
- DNA
Deoxyribonucleic acid
- EMRO
Eastern Mediterranean Region
- EUA
Emergency use authorization
- EURO
European Region
- FDA
Food and Drug Administration
- HCQ
Hydroxychloroquine
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- IFN
Interferon
- IL
Interleukin
- IQR
Interquartile range
- IVIG
Intravenous immunoglobulin
- LPV/r
Lopinavir-ritonavir
- MERS-CoV
Middle East Respiratory Syndrome Coronavirus
- MeSH
Medical Subject Heading
- MMR
Measles, Mumps and Rubella
- mRNA
Messenger ribonucleic acid
- NAC
N-acetyl cysteine
- PAHO
Region of the Americas
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- rAd
Recombinant adenovirus
- RCT
Randomized clinical trial
- RdRP
RNA-dependent RNA polymerase
- RECOVERY
Randomized evaluation of COVID-19 therapy
- rhG-CSF
The recombinant human granulocyte colony-stimulating factor
- SARS-CoV-2
Severe acute respiratory syndrome Coronavirus 2
- SEARO
South-East Asia Region
- TCM
Traditional Chinese medicine
- TFF2
Trefoil peptide
- TMPRSS2
Type 2 transmembrane serine protease
- TNF-α
Tumor necrosis factor-alpha
- U.S
United States of America
- WHO
World Health Organization
- WPRO
Western Pacific Region
Authors' contributions
OTM designed the study and interpreted data. PA, MM and OTM extracted data, wrote draft of the manuscript and interpreted data. PA, MM, AM, EH revised manuscript. MM, AM, OTM and BL helped in quality assessment and revised some sections. All authors read and approved the final manuscript.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pooria Asili and Maryam Mirahmad contributed equally to this work.
Contributor Information
Pooria Asili, Email: p-asili@alumnus.tums.ac.ir.
Maryam Mirahmad, Email: m-mirahmad@alumnus.tums.ac.ir.
Ozra Tabatabaei-Malazy, Email: tabatabaeiml@sina.tums.ac.ir.
Azadeh Manayi, Email: manayi@sina.tums.ac.ir.
Elahe Haghighat, Email: E-haghighat@razi.tums.ac.ir.
Mohammad Mahdavi, Email: mahdavi_chem@yahoo.com.
Bagher Larijani, Email: emrc@tums.ac.ir.
References
- 1.Organization WH. Coronavirus disease (COVID-2019) situation reports: Geneva, Switzerland; 2021. Available from: https://covid19.who.int/. Accessed 30 Oct 2021.
- 2.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. WHO Coronavirus Disease (COVID-19) Dashboard: Geneva, Switzerland; 2021. Available from: https://covid19.who.int/. Accessed 30 Oct 2021.
- 4.Chahrour M, Assi S, Bejjani M, Nasrallah AA, Salhab H, Fares M, et al. A bibliometric analysis of COVID-19 research activity: a call for increased output. Cureus. 2020;12(3):e7357-e. doi: 10.7759/cureus.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atlasi R, Noroozi Chakoli A, Ramezani A, Tabatabaei-Malazy O, Larijani B. Scientometric analyzing the output of researchers and organizations on COVID-19 for better conducting the scientific efforts: with a glance to endocrinology. J Diabetes Metab Disord. 2021;20(1):1–12. doi: 10.1007/s40200-020-00718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng VC, Chan JF, To KK, Yuen K. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100(2):407–419. doi: 10.1016/j.antiviral.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousefifard M, Zali A, Mohamed Ali K, Madani Neishaboori A, Zarghi A, Hosseini M, et al. Antiviral therapy in management of COVID-19: a systematic review on current evidence. Arch Acad Emerg Med. 2020;8(1):e45-e. doi: 10.22037/aaem.v8i1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta HB, Ehrhardt S, Moore TJ, Segal JB, Alexander GC. Characteristics of registered clinical trials assessing treatments for COVID-19: a cross-sectional analysis. BMJ Open. 2020;10(6):e039978. doi: 10.1136/bmjopen-2020-039978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabby MII, Hossain F. Study of ongoing registered clinical trials on COVID-19: a narrative review. Sao Paulo Med J. 2020;138:441–56. doi: 10.1590/1516-3180.2020.0208.r1.15062020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Zhou Q, Xu M, Kang J, Chen Y. Characteristics of clinical trials relating to COVID-19 registered at ClinicalTrials.gov. J Clin Pharm Ther. 2020;45(6):1357–62. doi: 10.1111/jcpt.13222. [DOI] [PubMed] [Google Scholar]
- 11.Aslam A, Imanullah S, Asim M, El-Menyar A. Registration of clinical trials: Is it really needed? N Am J Med Sci. 2013;5(12):713. doi: 10.4103/1947-2714.123266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie J, Wang Z, Liang J, Lin H, Yang Z, Wang Y, et al. Critical review of the scientific evidence and recommendations in COVID-19 management guidelines. Open Forum Infect Dis. 2021;8(8):ofab376. doi: 10.1093/ofid/ofab376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall A, Pirofski L-A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The RCG, Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021:2021.03.09.21252736; 10.1101/2021.03.09.21252736
- 15.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med. 2020;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell MR, Grinsztejn B, Cummings MJ, Justman J, Lamb MR, Eckhardt CM, et al. A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. medRxiv. 2021:2021.03.12.21253373; 10.1101/2021.03.12.21253373 [DOI] [PMC free article] [PubMed]
- 17.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA. Recommendations for investigational COVID-19 convalescent plasma: Food and Drug Administration; 2020 .Available from: https://www.fda.gov/media/141477/download. Accessed 30 Aug 2021.
- 20.Sarkar C, Mondal M, Torequl Islam M, Martorell M, Docea AO, Maroyi A, et al. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front Pharmacol. 2020;11(1428):1239. doi: 10.3389/fphar.2020.572870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Xia L, Liu L, Xu Q, Ling Y, Huang D, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect Dis. 2020;7(7):ofaa241. doi: 10.1093/ofid/ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vicenti I, Zazzi M, Saladini F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin Ther Pat. 2021;31(4):325–337. doi: 10.1080/13543776.2021.1880568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consortium WST Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10238):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan L, Singh AP, Gifty Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: a prospective randomised study. Indian J Anaesth. 2021;65(Suppl 1):S41-s6. doi: 10.4103/ija.IJA_149_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA. Fact sheet for health care providers: Emergency Use Authorization (EUA) of remdesivir: Food and Drug Administration; 2021. Available from: https://www.fda.gov/media/137566/download. Accessed 30 Aug 2021.
- 28.Khalili H, Nourian A, Ahmadinejad Z, Emadi Kouchak H, Jafari S, Dehghan Manshadi SA, et al. Efficacy and safety of sofosbuvir/ ledipasvir in treatment of patients with COVID-19; A randomized clinical trial. Acta Biomed. 2020;91(4):e2020102. doi: 10.23750/abm.v91i4.10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadeghi A, Ali Asgari A, Norouzi A, Kheiri Z, Anushirvani A, Montazeri M, et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020;75(11):3379–3385. doi: 10.1093/jac/dkaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbaspour Kasgari H, Moradi S, Shabani AM, Babamahmoodi F, Davoudi Badabi AR, Davoudi L, et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J Antimicrob Chemother. 2020;75(11):3373–3378. doi: 10.1093/jac/dkaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roozbeh F, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Merat S, Wentzel H, et al. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J Antimicrob Chemother. 2021;76(3):753–757. doi: 10.1093/jac/dkaa501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen LS, Dolladille C, Drici M-D, Fenioux C, Alexandre J, Mira J-P, et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization Pharmacovigilance Database. Circulation. 2020;142(3):303–305. doi: 10.1161/CIRCULATIONAHA.120.048238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine: Food and Drug Administration; 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and. Accessed 30 Aug 2021.
- 36.Borcherding N, Jethava Y, Vikas P. Repurposing anti-cancer drugs for COVID-19 treatment. Drug Des Dev Ther. 2020;14:5045. doi: 10.2147/DDDT.S282252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saini KS, Lanza C, Romano M, de Azambuja E, Cortes J, de Las HB, et al. Repurposing anticancer drugs for COVID-19-induced inflammation, immune dysfunction, and coagulopathy. Br J Cancer. 2020;123(5):694–697. doi: 10.1038/s41416-020-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yousefi H, Mashouri L, Okpechi SC, Alahari N, Alahari SK. Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: a review describing drug mechanisms of action. Biochem Pharmacol. 2021;183:114296. doi: 10.1016/j.bcp.2020.114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Goly AMM. Lines of treatment of COVID-19 Infection. Covid-19 infections and pregnancy. 2021;p. 91–144; 10.1016/B978-0-323-90595-4.00002-9
- 41.Gendrot M, Duflot I, Boxberger M, Delandre O, Jardot P, Le Bideau M, et al. Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: in vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int J Infect Dis. 2020;99:437–440. doi: 10.1016/j.ijid.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Group TWREAfC-TW. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41; 10.1001/jama.2020.17023 [DOI] [PMC free article] [PubMed]
- 43.Organization WH. Corticosteroids for COVID-19: living guidance, 2 September 2020: Geneva, Switzerland; 2020 [Available from: https://apps.who.int/iris/handle/10665/334125] Accessed 30 Aug 2021.
- 44.Lam S, Lombardi A, Ouanounou A. COVID-19: A review of the proposed pharmacological treatments. Eur J Pharmacol. 2020;886:173451. doi: 10.1016/j.ejphar.2020.173451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbasifard M, Khorramdelazad H. The bio-mission of interleukin-6 in the pathogenesis of COVID-19: A brief look at potential therapeutic tactics. Life Sci. 2020;257:118097. doi: 10.1016/j.lfs.2020.118097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FDA. Fact sheet for health care providers Emergency Use Authorization (EUA) of Bamlanivimab: Food and Drug Administration; 2021 [Available from: https://www.fda.gov/media/143603/download] Accessed 30 Aug 2021.
- 47.Group RC Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of Tocilizumab vs Standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolf J, Abzug MJ, Wattier RL, Sue PK, Vora SB, Zachariah P, et al. Initial Guidance on Use of Monoclonal Antibody Therapy for Treatment of COVID-19 in Children and Adolescents. J Pediatric Infect Dis Soc. 2021:629–34. 10.1093/jpids/piaa175 [DOI] [PMC free article] [PubMed]
- 57.Meffre E, Iwasaki A. Interferon deficiency can lead to severe COVID. Nature. 2020;587(7834):374–376. doi: 10.1038/d41586-020-03070-1. [DOI] [PubMed] [Google Scholar]
- 58.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahmani H, Davoudi-Monfared E, Nourian A, Khalili H, Hajizadeh N, Jalalabadi NZ, et al. Interferon β-1b in treatment of severe COVID-19: A randomized clinical trial. Int Immunopharmacol. 2020;88:106903. doi: 10.1016/j.intimp.2020.106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davoudi-Monfared E, Rahmani H, Khalili H, Hajiabdolbaghi M, Salehi M, Abbasian L, et al. A randomized clinical trial of the efficacy and safety of Interferon β-1a in treatment of severe COVID-19. Antimicrob Agents Chemother. 2020;64(9):e01061–e1120. doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(2):196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chachar AZK, Khan KA, Asif M, Tanveer K, Khaqan A, Basri R. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. Int J Sci. 2020;9(09):31–5. doi: 10.18483/ijSci.2378. [DOI] [Google Scholar]
- 65.Chowdhury ATMM, Shahbaz M, Karim MR, Islam J, Guo D, He S. A randomized trial of ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID19 patients. Research Square. 2021. 10.21203/rs.3.rs-38896/v1
- 66.Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. 2020:2020.10.26.20219345; 10.1101/2020.10.26.20219345
- 67.Morteza Shakhsi N, Nematollah G, Peyman N, Abbas A, Leila Z, Amir J, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial. Research Square. 2021. 10.21203/rs.3.rs-109670/v1
- 68.Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, Orhan S, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aref ZF, Bazeed S, Hassan MH, Hassan AS, Rashad A, Hassan RG, et al. Clinical, biochemical and molecular evaluations of ivermectin mucoadhesive nanosuspension nasal spray in reducing upper respiratory symptoms of mild COVID-19. Int J Nanomed. 2021;16:4063–4072. doi: 10.2147/ijn.s313093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021;93(10):5833–5838. doi: 10.1002/jmv.27122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopes RD, de Barros ESPGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253–63. doi: 10.1016/s0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tabatabaei-Malazy O, Abdollahi M, Larijani B. Beneficial effects of anti-oxidative herbal medicines in diabetic patients infected with COVID-19: a hypothesis. Diabetes Metab Syndr Obes. 2020;13:3113–3116. doi: 10.2147/DMSO.S264824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ang L, Song E, Lee HW, Lee MS. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9(5):1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murck H. Symptomatic protective action of glycyrrhizin (licorice) in COVID-19 infection? Front Immunol. 2020;11:1239. doi: 10.3389/fimmu.2020.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Safa O, Hassani-Azad M, Farashahinejad M, Davoodian P, Dadvand H, Hassanipour S, et al. Effects of Licorice on clinical symptoms and laboratory signs in moderately ill patients with pneumonia from COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):1–3. doi: 10.1186/s13063-020-04706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomaa AA, Abdel-Wadood YA. The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomedicine Plus. 2021:100043. 10.1016/j.phyplu.2021.100043 [DOI] [PMC free article] [PubMed]
- 81.Bouchentouf S, Missoum N. Identification of Compounds from Nigella Sativa as New Potential Inhibitors of 2019 Novel Coronasvirus (Covid-19): Molecular Docking Study. Preprints. 2020. 10.26434/chemrxiv.12055716.v1
- 82.Pawar KS, Mastud RN, Pawar SK, Pawar SS, Bhoite RR, Bhoite RR, et al. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol. 2021;12:7. doi: 10.3389/fphar.2021.669362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Pierro F, Derosa G, Maffioli P, Bertuccioli A, Togni S, Riva A, Allegrini P, Khan A, Khan S, Khan BA, Altaf N, Zahid M, Din Ujjan I, Nigar R, Khushk MI, Phulpoto M, Lail A, Devrajani BR, Ahmed S. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med. 2021;14:2359–66. doi: 10.2147/IJGM.S318720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Pierro F, Iqtadar S, Khan A, Mumtaz SU, Chaudhry MM, Bertuccioli A, Derosa G, Maffioli P, Togni S, Riva A, Allegrini P, Khan S. Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial. Int J Gen Med. 2021;14:2807–16. doi: 10.2147/IJGM.S318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu K, Guan WJ, Bi Y, Zhang W, Li LJ, Zhang BL, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:9. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y, et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161:105126. doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu X, Zhang J, Zheng W, Yang Z, Zhao X, Wang C, et al. Efficacy and safety of Reduning injection in the treatment of COVID-19: a randomized, multicenter clinical study. Ann Palliat Med. 2021;10(5):5146–55. doi: 10.21037/apm-20-2121. [DOI] [PubMed] [Google Scholar]
- 88.Luo ZJ, Chen W, Xiang MQ, Wang H, Xiao W, Xu C, et al. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur J Integr Med. 2021;42:8. doi: 10.1016/j.eujim.2021.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mesri M, Esmaeili Saber SS, Godazi M, Roustaei Shirdel A, Montazer R, Koohestani HR, et al. The effects of combination of Zingiber officinale and Echinacea on alleviation of clinical symptoms and hospitalization rate of suspected COVID-19 outpatients: a randomized controlled trial. J Complement Integr Med. 2021 doi: 10.1515/jcim-2020-0283. [DOI] [PubMed] [Google Scholar]
- 90.Sardari S, Mobaien A, Ghassemifard L, Kamali K, Khavasi N. Therapeutic effect of thyme (Thymus vulgaris) essential oil on patients with covid19: a randomized clinical trial. J Adv Med Biomed Res. 2021;29(133):83–91. doi: 10.30699/jambs.29.133.83. [DOI] [Google Scholar]
- 91.Kumar A, Meldgaard TS, Bertholet S. Novel platforms for the development of a Universal Influenza Vaccine. Front Immunol. 2018;9:600. doi: 10.3389/fimmu.2018.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J, Peng Y, Xu H, Cui Z, Williams RO. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21(6):225. doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. 2021. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 6(1):28. 10.1038/s41541-021-00292-w [DOI] [PMC free article] [PubMed]
- 95.Organization WH. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020: Geneva, Switzerland; 2020 [Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---18-march-2020. Accessed 30 Oct 2021.
- 96.Cattani M. Global coalition to accelerate COVID-19 clinical research in resource-limited settings. Lancet. 2020;395(10233):P1322–P1325. doi: 10.1016/S0140-6736(20)30798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasford J. Impact of the COVID-19 pandemic on clinical trials with drugs. Expert Opin Drug Saf. 2020;19(11):1373–1375. doi: 10.1080/14740338.2020.1828861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.