Highlights
-
•
IL-24 can decrease the expression of stem cell markers in CD133+ osteosarcoma CSCs.
-
•
IL-24 can inhibit CD133+ osteosarcoma CSCs to form tumors and develop further.
-
•
IL-24 inhibits tumorigenicity of osteosarcoma CSCs via inhibiting Notch and Wnt/β-Catenin Signaling.
Abbreviations: CSCs, cancer stem cells; IL-24, interleukin-24; JNK, c-Jun N-terminal kinase (JNK; bFGF, basic fibroblast growth factor; EGF, Epidermal Growth Factor; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; NS, nucleostemin; HDAC6, histone deacetylase 6
Keywords: IL-24, Cancer stem cell, Osteosarcoma, Notch signaling, Wnt/β-catenin signaling
Abstract
Osteosarcoma frequently presents as recurrence and metastasis, even if the primary lesion was eradicated and/or radiotherapy and chemotherapy were administered. Osteosarcoma cancer stem cells (CSCs) are one of the key factors for the recurrence and metastasis of osteosarcoma. We have shown that interleukin-24 (IL-24) inhibits osteosarcoma cell proliferation, migration and invasion in vitro. In the current study, we investigated the role of IL-24 in inhibiting the growth of osteosarcoma CSCs. IL-24 inhibited proliferation and invasion and decreased the stemness of osteosarcoma CSCs in vitro. In a nude mouse xenograft model, IL-24 significantly inhibited the growth of tumors originating from osteosarcoma CSCs. Moreover, we found that IL-24 was able to inactivate both Notch and Wnt/β-Catenin signaling, which are important for the development of the biological characteristics of CSCs. These data demonstrate that IL-24 is able to kill not only cancer cells but also CSCs in osteosarcoma, suggesting that IL-24 might eradicate osteosarcoma and enhance long-term cure rates in patients with osteosarcoma.
1. Introduction
Osteosarcoma, originating from mesenchymal tissue, is a primary malignant bone tumor with a high degree of malignancy and is prone to early metastasis [1], [2]. Despite the combination of radiotherapy, chemotherapy and surgery, it is difficult to prevent the osteosarcoma recurrence and metastasis, which is one of the main causes of death in most patients [3], [4]. Osteosarcoma cancer stem cells (CSCs) are closely associated with the recurrence and metastasis of osteosarcoma [5]. Gibbs et al. first isolated osteosarcoma CSCs from osteosarcoma tissues and osteosarcoma cell lines using serum-free culture method [6]. Fujii et al. isolated osteosarcoma CSCs from various sarcoma cells, showing strong drug resistance, self-renewal, proliferation, differentiation and other stemness characteristics [7]. At present, osteosarcoma CSCs have been successfully isolated from a variety of osteosarcoma cell lines, including MG63, Hu09, SAOS-2, 143B, and U2-OS [8], [9], [10]. The discovery of osteosarcoma CSCs and targeted therapy have stimulated new ideas for the treatment of osteosarcoma.
Il-24, a melanoma differentiation-associated gene cloned from human melanoma HO1 cells, is a tumor suppressor gene with cytokine properties and belongs to the IL-10 family [11], [12], [13]. Subsequent studies have shown that IL-24 not only has direct antitumor effects, but also inhibits tumor metastasis and angiogenesis, enhances sensitization of cancer cell to radiation, chemotherapy and immunotherapy and elicits antitumor bystander effect [14], [15], [16], [17]. Given the broad-spectrum antitumor effect of IL-24, INGN241 (a replication-defective adenovirus carrying the IL-24 gene, Ad.IL-24) is currently being tested in the clinic Phase I trial in patients with advanced cancers [18], [19]. We have shown that IL-24 inhibits osteosarcoma cell proliferation, migration and invasion through the c-Jun N-terminal kinase (JNK)/c-Jun signaling pathway [20]. In the present study, we investigated the effects of IL-24 on osteosarcoma CSCs and attempted to elucidate the potential mechanisms.
2. Materials and methods
2.1. Cell lines, antibodies and agents
The human osteosarcoma cell lines 143B and MG63, analyzed in this study was preserved in our laboratory and cultured as described [20], [21]. Antibodies used were from as follows: anti-CD133 (eBioscience, San Diego, USA); anti-Notch1, anti-HES1, anti-β-catenin, anti- HDAC6 and anti-β-Actin (Cell Signaling Technology, Inc. Danvers, MA, USA). The recombinant human IL-24 protein was preserved in our laboratory [22]. DAPT (Notch signaling Inhibiter) and XAV939 (Wnt/β-catenin signaling Inhibiter) were purchased from abcam (Cambridge, UK).
2.2. Sphere culture assay
1,000 suspended single cells were seeded in ultra-low attachment 6-well plates consisting serum-free sphere formation medium with basic fibroblast growth factor (bFGF; 20 ng/ml; Sigma), Epidermal Growth Factor (EGF; 20 ng/ml; Sigma), and Noggin (10 ng/ml; Sigma) and then incubated for 10 days.
2.3. RT-qPCR
The total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, USA) based on the manufacturer's instructions. RNA reverse-transcribed to cDNA with HiScript II Q RT SuperMix (Vazyme). cDNA was subjected to PCR for 40 cycles of 94 °C for 30 sec, 60 °C for 30 sec and 72 °C for 45 sec. RT-PCR was performed on the LightCycler 96real-time PCR system (Roche) using AceQ qPCR SYBR Green Master Mix kit (Vazyme). Experiments were performed in triplicate. The relative mRNA expression of targeted genes was analyzed based on the expression of GAPDH.
2.4. Immunofluorescence
Spheres were harvested, washed, fixed in 4% paraformaldehyde for 30 min, blocked with 0.5% (w/v) BSA in blocking buffer at room temperature for 30 min, incubated with the appropriate primary antibody containing 0.05% Tween 20 overnight at 4 °C, then wash and incubated with fluorescent secondary antibodies (Invitrogen) for 2 h at room temperature. Spheres were observed by using a ZEISS LSM 880 confocal fluorescence microscope.
2.5. Cell viability
Cell viability was analyzed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 1 × 104 cells per well were seeded in a 96-well plate and incubated in medium. The cells were continued or treated with IL-24 for the incubation. At the indicated time, the cells were harvested and analyzed by the standard MTT assay, as described previously [20].
2.6. Flow cytometry
The cells were harvested, washed, incubated with fluorochrome-conjugated antibodies for 20 min at 4 °C, and washed twice with PBS, then the samples were analyzed by the FACS CantoII (BD). The data were analyzed by FlowJo software (FlowJo 9.3.2).
2.7. Colony formation assay
100 single living cells were seeded in soft agar culture medium onto a 35-mm Petri dish, and incubated at 37 °C for 10 days. Colonies were photographed and counted with an optical microscope.
2.8. Invasion assay
The cells invasion assay was performed using 24-well Transwell unit coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, cells were seeded in the upper chambers and treated with IL-24. The lower chambers of the Transwell units were filled with medium supplemented with 10% FBS. After 48 h incubation, the cells that had passed through the filter into the lower wells were stained with 2% crystal violet and counted.
2.9. Western blotting
Western blot assay was performed as previously described [20]. The experiments were performed in triplicate, and the results were recorded as the mean of these experiments.
2.10. Animal experiment
6 to 8 weeks old male nude mice were used for the experiments. The protocol for the animal experiment was approved by the Xuzhou Medical University Institutional Animal Care and Use Committee, according to the ARRIVE guidelines. For nude mouse tumorigenicity assay:2 × 105 IL-24-treated CD133+ osteosarcoma CSCs were subcutaneously injected into the right flank of the mouse. The tumor formation and growth were monitored and measured every three days, and the tumor volumes were analyzed base on the formula: volume (mm3) = D × d2/2, where D and d are the longest and the shortest diameters, respectively. After 5 weeks, all of the mice were sacrificed, and tumor tissues were harvested and analyzed. For nude mouse tumor growth inhibition assay: Briefly, 143B cells (2 × 105 cells diluted in 100 μl PBS) were subcutaneously injected into the right flank of 10 nude mice. The mice were randomly divided into PBS, or IL-24 groups at about 2 weeks following injection of 143B cells, and then injected with 10 μg/g IL-24 or equal volume of PBS in the tail vein every 3 days, and meanwhile, the tumor volume of nude mice were determined. After 3 weeks, all of the mice were sacrificed, and tumor tissues were harvested and analyzed.
2.11. Immunohistochemistry
The mice tumor samples were collected, and 4 µm-thick formalin-fixed and paraffin-embedded sections were stained with anti-Ki67 (Invitrogen) or anti-PCNA mAb (Invitrogen). Images were acquired by a digital scanner (Aperio ScanScope, Leica Biosystem) and analyzed slides via an image analysis workstation (Image Pro Plus 6.0, Media Cybernetics).
2.12. Statistical analysis
The values were presented as the mean ± standard deviation (S.D.) by using GraphPad Prism and Excel software and analyzed by Student's t test. p value equal or less than 0.05 was considered to be statistically significant.
3. Results
3.1. 143B cells form CD133high tumor spheres and express stem cell markers in the suspension culture system
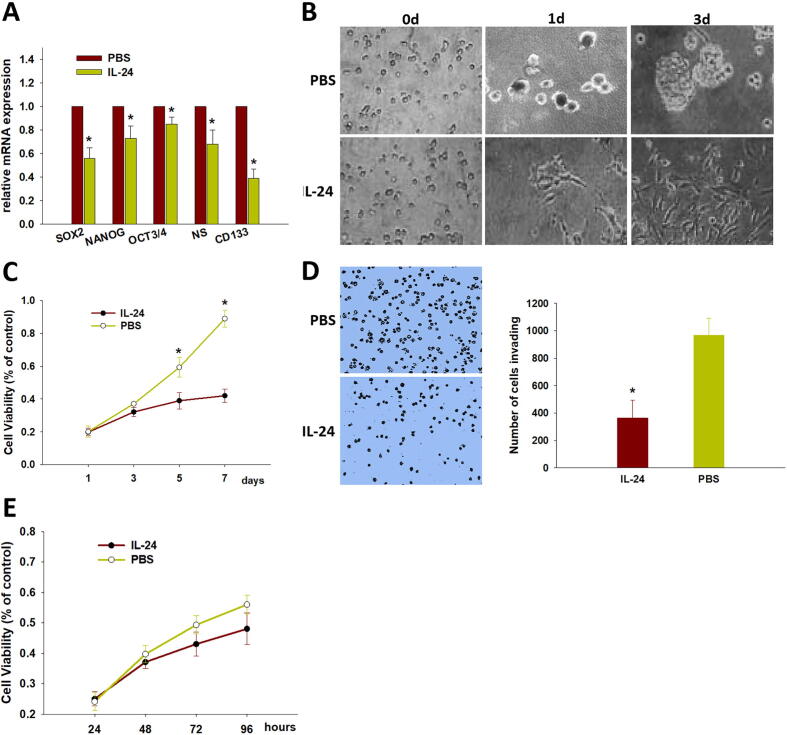
143B cells cultured by cell passage method in DMEM supplemented with 10% FBS grew with adherence and exhibited a fibroblast-like phenotype (Fig. 1A). At the beginning of growth in the suspension culture system with stem cell-specific serum-free medium containing a variety of growth factors, most of 143B cells adhered to the wall, and few cells were in suspension. After 48 h, small cell masses gradually aggregated and formed cell spheres, and at 10 days, typical tumor spheres had formed in the suspension culture system (Fig. 1B). We checked the expression level of several stem cell markers with quantitative real-time PCR. As shown in Fig. 1C, the expression of SOX2, NANOG, OCT3/4, nucleostemin (NS), and CD133 was significantly upregulated. By measuring the protein expression of CD133, one of the most well-characterized markers of cancer stem cell by immunofluorescence, we found that the tumor spheres expressed high levels of CD133 (Fig. 1D). Subsequently, CD133+ cells were purified using immunomagnetic separation miniMACS (Miltenyi Biotec, Germany) and analyzed with flow cytometry (Fig. 1E). The MTT assay showed that CD133-positive cells had a stronger proliferation ability (Fig. 1F), and the sphere formation assay showed that CD133-positive cells formed larger spheres and more spheres than CD133-negative cells (Fig. 1G). We observed the similar results in MG63 osteosarcoma cells (Supplemental Fig. 1A–G).
Fig. 1.
CD133+ osteosarcoma CSCs were harvested, purified, and identified. (A) Normal 143B cells were grown in DMEM with 10 % FBS. (B) 143B cells were able to form spheres in suspension culture system. (C) The mRNA expression of stem cell markers (OCT3/4, NANOG, SOX2, nucleostemin (NS) and CD133) were determined in spheres formed from 143B in suspension culture system by real-time PCR. (D) CD133 protein expression was detected in sphere by immunofluorescence. (E) CD133+ osteosarcoma CSCs proliferation was assessed by MTT at day 1, 3, 5, 7 and contrasted with CD133- osteosarcoma cells. (F) The percentage of CD133+ cells were analyzed in adherence cells, sphere cells, and purification cells by Flow cytometry. (G) CD133+ osteosarcoma CSCs formed spheres in suspension culture system, while CD133- osteosarcoma cells had no sphere formation. Data are shown as mean ± SD from four independent experiments (n = 4); *p < 0.05.
3.2. IL-24 attenuates stem cell-like properties of CD133+ 143B cells
We previously determined that overexpression of IL-24 inhibits 143B cell proliferation, migration and invasion in vitro. In this study, we further investigated whether IL-24 affects the biological behavior of osteosarcoma CSCs. We first detected whether c influences stem cell-like phenotypes in CD133+ osteosarcoma CSCs. As shown in Fig. 2A, stem cell markers (SOX2, NANOG, OCT3/4, nucleostemin, and CD133) were significantly reduced in IL-24–treated CD133+ osteosarcoma CSCs. Next, we examined whether IL-24 impairs CD133+ osteosarcoma CSC survival. To evaluate the effect of IL-24 on colony formation, we cultured single CD133+ osteosarcoma CSCs in a nonadhesive culture system containing 50 pg/ml IL-24. As shown in Fig. 2B, the cells treated with IL-24 failed to form spheres, while those cultured without IL-24 were able to form spheres again. As shown in Fig. 2C and D, IL-24 significantly inhibited CD133+ osteosarcoma CSC proliferation and invasion. To characterize whether IL-24 have an impact on CD133- osteosarcoma cells, we used CD133- 143B cell to perform cytotoxicity assays. As shown in Fig. 2E, IL-24 have no effect on CD133- osteosarcoma cells proliferation. We observed a similar result that IL-24 can downregulate the gene expression of stem cell markers and inhibit cell proliferation in CD133+ MG63 osteosarcoma CSC (Supplemental Fig. 2A–C). These results suggested that IL-24 represses the tumor-initiating potential of CD133+ osteosarcoma CSCs.
Fig. 2.
IL-24 inhibits stemness properties of CD133+ osteosarcoma CSCs. (A) IL-24 downregulated the mRNA expression of OCT3/4, NANOG, CD133, nucleostemin (NS) and SOX2 of CD133+ osteosarcoma CSCs. IL-24 inhibited CD133+ osteosarcoma CSCs colony formation (B), proliferation (C), and invasion (D). Data are shown as mean ± SD from four independent experiments (n = 4); *p < 0.05. (E) CD133- 143B cells were treated with the dose of 20 μg/ml of IL-24 for 24, 48, 72, and 96 h, cells were subjected to the MTT assay. IL-24 have no effect on CD133- osteosarcoma cells proliferation. Data are shown as mean ± SD (n = 3).
3.3. IL-24 inhibits CD133+ 143B cells’ ability to form tumors and develop further in vivo
Furthermore, we investigated whether IL-24 could suppress CD133+ osteosarcoma CSC-induced tumorigenesis in vivo. We cultured CD133+ osteosarcoma CSCs and treated them with IL-24. The cells were then injected subcutaneously into the flanks of nude mice. As shown in Fig. 3A and B, only 50% of IL-24–treated CD133+ osteosarcoma CSCs formed tumors in nude mice (3/6), whereas control group cells led to tumor formation in 100% of mice (6/6), which suggests that IL-24 can effectively inhibit CSC-induced tumorigenicity in osteosarcoma. We also found that the expression of the cell proliferation markers Ki-67 (Fig. 3C and D) and PCNA (Fig. 3E and F) was remarkably reduced in tumors from the IL-24-treated group. These results imply that IL-24 could inhibit CD133+ osteosarcoma CSCs’ ability to form tumors and develop further.
Fig. 3.
IL-24 inhibits CD133+ osteosarcoma CSCs induced tumorigenesis in vivo. (A and B) IL-24-treated CD133+ osteosarcoma CSCs reduced the capacity of tumor formation in nude mice (tumor formation rate: 50%; 3/6). Immunohistochemistry analysis of Ki-67 (C and D) and PCNA (E and F) in tumor tissues from the mice xenografts. Scale bar, 200 μm. (G) Establishment of the 143B-mouse tumor model and treatment regimen. (H) Solid tumors excised from mice injected with IL-24 or PBS (n = 5). (I) Tumor volume over the course of treatment. Data are shown as mean ± SD; *p < 0.05.
To characterize whether IL-24 have in vivo impact on osteosarcoma development, we used a right flank 143B osteosarcoma mouse model, in which IL-24 was administered (Fig. 3G). We found that the rate of tumor growth in the IL-24 group was inhibited when compared with the PBS group, and the tumor volume of the IL-24 group was significantly reduced when compared with the PBS groups (Fig. 3H and I). These results demonstrated that IL-24 have impact on osteosarcoma development.
3.4. IL-24 regulates Notch and Wnt/β-Catenin signaling to inhibit osteosarcoma CSCs traits
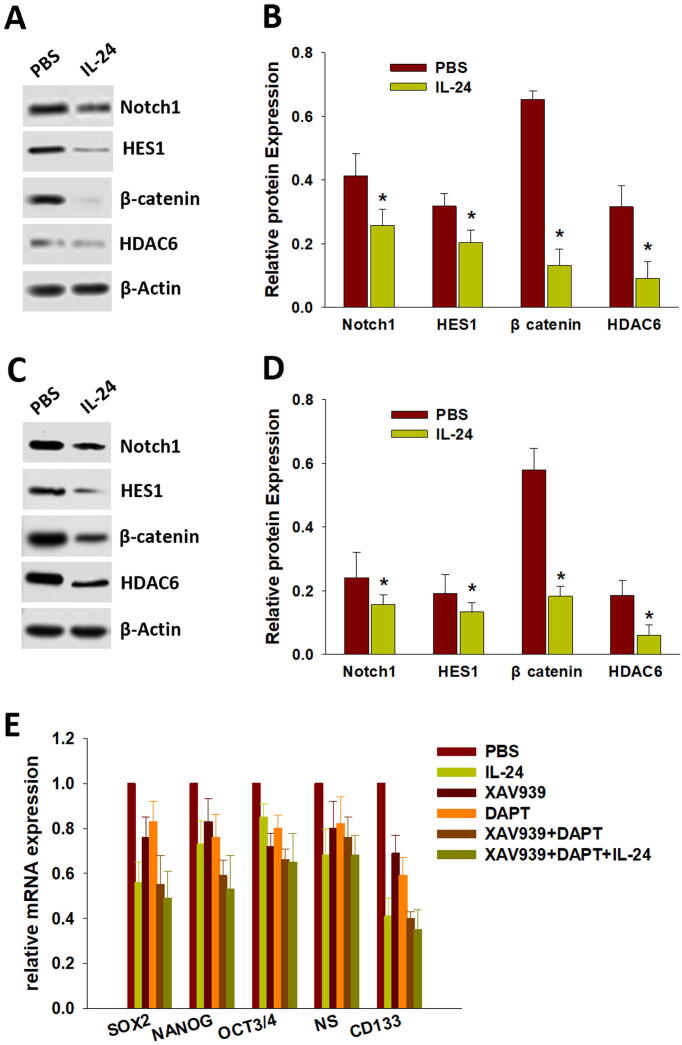
To elucidate the mechanism of IL-24 in the inhibition of osteosarcoma cell stemness, we evaluated the regulation of IL-24 in Notch and Wnt/β-catenin signaling, which was confirmed to be involved in the maintenance of cell stemness pathways. As shown in Fig. 4A and B, IL-24 significantly decreased the expression of β-catenin and Notch1, as well as the β-catenin downstream protein histone deacetylase 6 (HDAC6) and Notch1 downstream protein HES1 in CD133+ osteosarcoma CSCs. Consistent with the in vitro data, the expression of Notch1, HES1, β-catenin and HDAC6 was also decreased in IL-24–treated xenograft tumor tissues compared to the control (Fig. 4C and D). Next, we evaluated whether inactivated Notch (DAPT, Notch signaling inhibitor) and Wnt/β-catenin (XAV939, Wnt/β-catenin signaling inhibitor) signaling affects the function of IL-24 in CD133+ osteosarcoma CSCs. As shown in Fig. 4E, after inhibiting both Notch1 and β-catenin, IL-24 had almost no effect on the stemness of CD133+ osteosarcoma CSCs. These results imply that IL-24 inhibits CD133+ osteosarcoma CSC stemness and tumorigenesis by inactivating both the Notch and Wnt/β-catenin signaling pathways.
Fig. 4.
IL-24 regulates Notch and Wnt/β-Catenin signaling to inhibit osteosarcoma CSCs traits. (A and B) IL-24 significantly downregulated the expression of β-catenin and Notch1, as well as the downstream protein HDAC6 and HES1 in CD133+ osteosarcoma CSCs, respectively. (C and D) The same results were observed on tumor tissue in vivo. (E) IL-24 almost have no effect on stemness of CD133+ osteosarcoma CSCs after inhibiting of both Notch1 and β-catenin. Data are shown as mean ± SD; *p < 0.05.
4. Discussion
CSCs with the capacity for infinite proliferation and multidirectional differentiation determine the occurrence, development, recurrence, metastasis and other characteristics of cancer [23]. However, the lack of efficacy of current treatment for CSCs leads to cancer recurrence and metastasis and poor prognosis of patients [24]. Osteosarcoma CSCs were found and closely related to the occurrence, development, recurrence and metastasis of osteosarcoma [5]. Therefore, finding novel methods to kill osteosarcoma CSCs will provide a new strategy for the effective treatment of osteosarcoma.
It is critical to isolate and characterize osteosarcoma CSCs for targeted treatment. The serum-free suspension culture system, containing serum-free culture medium with multiple growth factors, is a common method for isolating stem cells from osteosarcoma [25]. In this study, 143B or MG63 cells were suspended in multi-growth factor serum-free cell culture medium, and typical CD133-positive stem cell spheres were obtained. However, during cell culture, the spherical cells became increasingly cohesive, and it was difficult to obtain single osteosarcoma CSCs. In addition, some studies have found that cultured stem cells in serum-free suspension culture systems contain differentiated osteosarcoma non-stem cells. Thus, we further sorted CD133+ cells as osteosarcoma CSCs with the CD133 MicroBead Kit and observed that CD133+ osteosarcoma CSCs increased the expression of stem cell markers and sphere formation capacity.
We and others have confirmed that IL-24 has potential as an anticancer agent because of its significant antitumor effects and safety in clinical trials. Moreover, Fisher et al. found that IL-24 can inhibit the proliferation of breast cancer stem cells via induction of ER stress and apoptosis and inhibit the growth of tumors originating from breast cancer stem cells in vivo [26]. We also document that IL-24 inhibits osteosarcoma cell proliferation, migration and invasion in vitro. In this study, we found that IL-24 can decrease the expression of stem cell markers and sphere formation capacity in CD133+ osteosarcoma CSCs and inhibit tumorigenesis both in vitro and in vivo. These data imply that IL-24 is a potential agent against osteosarcoma CSCs.
The Wnt/β-catenin and Notch pathways are critical for the development processes and maintenance of stem cell compartments [27], [28]. The combination of Notch inhibitors and Wnt/β-catenin inhibitors significantly enhanced the anticancer effects in phase I clinical trials [29]. IL-24 inhibits the Wnt/β-catenin signaling pathway in breast cancer stem cells, via activation of GSK3β and Akt inhibition leading to downregulation of β-catenin phosphorylation and proteasome degradation [26]. Our current study shows that IL-24 can inhibit both Notch and the Wnt/β-catenin signaling pathways and leaded to dramatic inhibitory effects on osteosarcoma CSCs stemness and tumorigenesis in vitro and in vivo.
In conclusion, a combination strategy of isolation of osteosarcoma CSCs from 143B and MG63 cell lines was applied. We evaluated IL-24 as a potent agent inhibits the phenotype and tumorigenicity of osteosarcoma CSCs via downregulation both Notch and Wnt/β-Catenin Signaling. The data suggest further validation of the potential use of IL-24 as a novel agent for targeting CSCs and leading to a long-term cure rate in osteosarcoma.
CRediT authorship contribution statement
Baobiao Zhuo: Conceptualization, Supervision, Funding acquisition. Xihua Wang: Methodology, Data curation, Formal analysis. Yang Shen: Investigation, Data curation, Formal analysis. Jiayong Li: Methodology, Investigation. Shixian Li: Formal analysis, Investigation. Yuan Li: Conceptualization, Supervision. Rong Wang: Project administration, Supervision, Validation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81972511), Jiangsu Provincial Medical Talent (No. QNRC2016373) and the Science and Technology Department of Xuzhou (No. KC18026).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2021.100403.
Contributor Information
Baobiao Zhuo, Email: zhuobaobiao@163.com.
Rong Wang, Email: 3239511602@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Isakoff M.S., Bielack S.S., Meltzer P., Gorlick R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015;33:3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelan J.S., Davis L.E. Osteosarcoma, Chondrosarcoma, and Chordoma. J. Clin. Oncol. 2018;36(2):188–193. doi: 10.1200/JCO.2017.75.1743. [DOI] [PubMed] [Google Scholar]
- 3.Prudowsky Z.D., Yustein J.T. Recent Insights into Therapy Resistance in Osteosarcoma. Cancers (Basel) 2020;13 doi: 10.3390/cancers13010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luetke A., Meyers P.A., Lewis I., Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Brown H.K., Tellez-Gabriel M., Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189–195. doi: 10.1016/j.canlet.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs C.P., Kukekov V.G., Reith J.D., Tchigrinova O., Suslov O.N., Scott E.W., Ghivizzani S.C., Ignatova T.N., Steindler D.A. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7(11):967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii H., Honoki K., Tsujiuchi T., Kido A., Yoshitani K., Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int. J. Oncol. 2009;34:1381–1386. [PubMed] [Google Scholar]
- 8.Wang L., Park P., Lin C.-Y. Characterization of stem cell attributes in human osteosarcoma cell lines. Cancer Biol. Ther. 2009;8(6):543–552. doi: 10.4161/cbt.8.6.7695. [DOI] [PubMed] [Google Scholar]
- 9.Wilson H., Huelsmeyer M., Chun R., Young K.M., Friedrichs K., Argyle D.J. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Vet. J. 2008;175(1):69–75. doi: 10.1016/j.tvjl.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Martins-Neves S.R., Lopes A.O., do Carmo A., Paiva A.A., Simoes P.C., Abrunhosa A.J., Gomes C.M. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer. 2012;12:139. doi: 10.1186/1471-2407-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pestka S., Krause C.D., Sarkar D., Walter M.R., Shi Y., Fisher P.B. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 12.Jiang H., Lin J.J., Su Z.Z., Goldstein N.I., Fisher P.B. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- 13.Caudell Eva G., Mumm John B., Poindexter Nancy, Ekmekcioglu Suhendan, Mhashilkar Abner M., Yang Xiaohong Helena, Retter Mark W., Hill Paul, Chada Sunil, Grimm Elizabeth A. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J. Immunol. 2002;168(12):6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 14.Sauane Moira, Gopalkrishnan Rahul V, Sarkar Devanand, Su Zao-Zhong, Lebedeva Irina V, Dent Paul, Pestka Sidney, Fisher Paul B. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14(1):35–51. doi: 10.1016/S1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 15.Sauane M., Su Z.-z., Gupta P., Lebedeva I.V., Dent P., Sarkar D., Fisher P.B. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2008;105(28):9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dash Rupesh, Bhoopathi Praveen, Das Swadesh K., Sarkar Siddik, Emdad Luni, Dasgupta Santanu, Sarkar Devanand, Fisher Paul B. Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction. Cancer Res. 2014;74(2):563–574. doi: 10.1158/0008-5472.CAN-13-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emdad L., Bhoopathi P., Talukdar S., Pradhan A.K., Sarkar D., Wang X.Y., Das S.K., Fisher P.B. Recent insights into apoptosis and toxic autophagy: The roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic. Semin. Cancer Biol. 2020;66:140–154. doi: 10.1016/j.semcancer.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham C.C., Chada S., Merritt J.A., Tong A., Senzer N., Zhang Y., Mhashilkar A., Parker K., Vukelja S., Richards D., Hood J., Coffee K., Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol. Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Tong A.W., Nemunaitis J., Su D., Zhang Y., Cunningham C., Senzer N., Netto G., Rich D., Mhashilkar A., Parker K., Coffee K., Ramesh R., Ekmekcioglu S., Grimm E.A., van Wart Hood J., Merritt J., Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol. Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhuo B., Shi Y., Qin H., Sun Q., Li Z., Zhang F., Wang R., Wang X. Interleukin-24 inhibits osteosarcoma cell migration and invasion via the JNK/c-Jun signaling pathways. Oncol. Lett. 2017;13:4505–4511. doi: 10.3892/ol.2017.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuo Baobiao, Li Yuan, Li Zhengwei, Qin Haihui, Sun Qingzeng, Zhang Fengfei, Shen Yang, Shi Yingchun, Wang Rong. PI3K/Akt signaling mediated Hexokinase-2 expression inhibits cell apoptosis and promotes tumor growth in pediatric osteosarcoma. Biochem. Biophys. Res. Commun. 2015;464(2):401–406. doi: 10.1016/j.bbrc.2015.06.092. [DOI] [PubMed] [Google Scholar]
- 22.Liu Jun-Jie, Zhang Bao-Fu, Yin Xiao-Xing, Pei Dong-Sheng, Yang Zhi-Xia, Di Jie-Hui, Chen Fei-Fei, Li Hui-Zhong, Xu Wei, Wu Yong-Ping, Zheng Jun-Nian. Expression, purification, and characterization of RGD-mda-7, a His-tagged mda-7/IL-24 mutant protein. J. Immunoassay Immunochem. 2012;33(4):352–368. doi: 10.1080/15321819.2012.659782. [DOI] [PubMed] [Google Scholar]
- 23.Lytle Nikki K., Barber Alison G., Reya Tannishtha. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer. 2018;18(11):669–680. doi: 10.1038/s41568-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke Michael F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019;380(23):2237–2245. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 25.Palmini G., Zonefrati R., Mavilia C., Aldinucci A., Luzi E., Marini F., Franchi A., Capanna R., Tanini A., Brandi M.L. Establishment of Cancer Stem Cell Cultures from Human Conventional Osteosarcoma. J. Vis. Exp. 2016 doi: 10.3791/53884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhutia S.K., Das S.K., Azab B., Menezes M.E., Dent P., Wang X.Y., Sarkar D., Fisher P.B. Targeting breast cancer-initiating/stem cells with melanoma differentiation-associated gene-7/interleukin-24. Int. J. Cancer. 2013;133:2726–2736. doi: 10.1002/ijc.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyler C.E., Rich J.N. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong A.L.A., Bellot G.L., Hirpara J.L., Pervaiz S. Understanding the cancer stem cell phenotype: A step forward in the therapeutic management of cancer. Biochem. Pharmacol. 2019;162:79–88. doi: 10.1016/j.bcp.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Song S., Christova T., Perusini S., Alizadeh S., Bao R.Y., Miller B.W., Hurren R., Jitkova Y., Gronda M., Isaac M., Joseph B., Subramaniam R., Aman A., Chau A., Hogge D.E., Weir S.J., Kasper J., Schimmer A.D., Al-awar R., Wrana J.L., Attisano L. Wnt inhibitor screen reveals iron dependence of beta-catenin signaling in cancers. Cancer Res. 2011;71:7628–7639. doi: 10.1158/0008-5472.CAN-11-2745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.