Abstract
Background
Care home (CH) residents are mainly inactive, leading to increased dependency and low mood. Strategies to improve activity are required.
Design and setting
Cluster randomised controlled feasibility trial with embedded process and health economic evaluations. Twelve residential CHs in Yorkshire, United Kingdom, were randomised to the MoveMore intervention plus usual care (UC) (n = 5) or UC only (n = 7).
Participants
Permanent residents aged ≥65 years.
Intervention
MoveMore: a whole home intervention involving all CH staff designed to encourage and support increase in movement of residents.
Objectives and measurements
Feasibility objectives relating to recruitment, intervention delivery, data collection and follow-up and safety concerns informed the feasibility of progression to a definitive trial. Data collection at baseline, 3, 6 and 9 months included: participants’ physical function and mobility, perceived health, mood, quality of life, cognitive impairment questionnaires; accelerometry; safety data; intervention implementation.
Results
300 residents were screened; 153 were registered (62 MoveMore; 91 UC). Average cluster size: MoveMore: 12.4 CHs; UC: 13.0 CHs. There were no CH/resident withdrawals. Forty (26.1%) participants were unavailable for follow-up: 28 died (12 MoveMore; 16 UC); 12 moved from the CH. Staff informant/proxy data collection for participants was >80%; data collection from participants was <75%; at 9 months, 65.6% of residents provided valid accelerometer data; two CHs fully, two partially and one failed to implement the intervention. There were no safety concerns.
Conclusions
Recruiting CHs and residents was feasible. Intervention implementation and data collection methods need refinement before a definitive trial. There were no safety concerns.
Keywords: staff training, physical activity, older people, long-term care, cluster randomised feasibility trial
Key Points
Many care home (CH) residents are inactive, leading to increased dependency and low mood.
There are known benefits of maintaining/increasing levels of physical activity/decreasing sedentary behaviour in CH residents.
We developed a whole-home intervention (MoveMore) designed to encourage and support CH residents’ movements in daily routines.
We undertook a cluster-randomised trial to explore the feasibility of trial processes and delivering MoveMore.
Introduction
There are over 400,000 residents of care homes (CHs) in the United Kingdom [1]. Research suggests that the majority of residents spend their time inactive (79–87% of the time sedentary) [2, 3], despite the known benefits of maintaining (or increasing) levels of physical activity (PA) and decreasing sedentary behaviour [4]. Sedentary behaviour may have a detrimental effect on a number of parameters related to health [5], including cardiovascular risk [6], physical function [7, 8] and quality of life [9, 10]. For CH residents in particular, substantial levels of sedentary behaviour may lead to pressure sores, contractures, cardiovascular deconditioning, urinary infections and increased dependence on staff. Our extensive review [11] reports the feasibility of implementing programmes focused on enhancing PA in CHs but many were resource intensive and provided by external agents (for example exercise classes). An alternative approach would be to create a whole-home initiative to enhance routine activity among residents.
We have undertaken a programme of research to develop and preliminarily test strategies to enhance PA in the daily life routines of CH residents to improve their physical, psychological and social well-being: the Research Exploring Physical Activity in Care Homes (REACH) programme. We report here the final study: a cluster randomised controlled feasibility trial (cRCT), including a summary of the embedded process evaluation. A cost-effectiveness evaluation and detailed exploration of the PA and sedentary behaviour data are not reported here. The overall aims were to explore the feasibility of delivering a whole-home intervention (‘MoveMore’), designed to encourage and support CH residents to move more in daily routines, and to explore the feasibility of trial processes to inform the design of a future definitive trial [12].
A full description of trial objectives can be found in Forster et al. [12]. Briefly, this paper reports trial objectives around: CH and resident recruitment; follow-up rates; feasibility of collecting outcome data through the use of questionnaires (physical function, mobility and physiological well-being) and accelerometers (PA and sedentary behaviour); preliminary estimate of effectiveness of the intervention in improving PA levels; intervention delivery; residents’ outcomes and safety data.
Methods
A full description of trial procedures is provided in Forster et al. [12].
Trial design
A feasibility parallel-group cRCT comparing CHs (clusters) randomised to either MoveMore plus usual care (UC) or UC only. A cRCT was chosen as MoveMore was a whole-home intervention designed to increase movement levels of all residents. The study was reviewed and approved by the UK National Research Ethics Service (REC reference 15/EE/0125).
Study setting/clusters
We aimed to recruit 12 residential CHs (or units of CHs) within North and West Yorkshire through different recruitment strategies (reported in [13]).
Participants
Following screening of all residents, through discussions with CH manager and staff, baseline data were collected from all eligible (aged ≥65 years, permanent resident within the home, not terminally ill or bed-bound/cared for in bed, not taking part in, or planning to take part in, another trial that conflicted with the MoveMore intervention or data collection during the course of their involvement in the trial) and consenting residents. An assessment of capacity to consent to taking part in the study for eligible residents was undertaken by the manager or nominated deputy or by the researcher if capacity was unknown. Written informed consent for data collection was sought from those with capacity. Assent was sought from a personal consultee, or nominated consultee, where no personal consultee could be identified, for those lacking capacity [12].
Randomisation and allocation concealment
Randomisation was undertaken once residents within a CH had consented and were registered, and all baseline assessments were completed. CHs were randomised in a 1:1 ratio using a computer-generated minimisation program incorporating a random element, stratified on characteristics expected to be correlated with intervention delivery and outcome evaluation: CH size (small/medium ≤40 residents; large >40 residents); presence/absence of an activity co-ordinator.
CH staff were not blinded to allocation but researchers administering and collecting the outcome measures had no role in the intervention and were not informed of CHs’ allocation. Efforts were made to ensure that they remained blind to allocation, including maintaining separate office locations for ‘blinded’ and ‘unblinded’ researchers and requesting that CHs did not disclose their allocation to these researchers.
Intervention
The intervention was developed through a systematic process of intervention mapping [14] (Appendix 1 is available in Age and Ageing online). During this process ‘physical activity’ was conceptualised as ‘movement’ as a more appropriate term for CHs and their residents. MoveMore was designed to encourage and support residents to move more in their daily life, facilitated by changing the organisational routines and practices of the CH. Implementation involves a systematic approach to embedding the intervention in routine care, remaining flexible to be adapted to each CH’s needs.
The MoveMore programme (Appendix 1 is available in Age and Ageing online) was to be implemented over 3 months and required staff to: review current practice (observations); develop goals and action plans to effect change (reflection and action planning); act (pursue action plans) and review and evaluate progress. Implementation was supported through several strategies including identification of an intervention lead and core team in each home; provision of a manual, including an ‘Ideas Bank’ of resources to assist staff in getting started and keeping going; a series of three interactive workshops provided individually to each home.
Intervention delivery
Details of the workshops (including date, length of time, location, attendance, designation of staff) were recorded, a contemporaneous record of the monthly and ad hoc contact with the CHs (implementation enhancement) was kept and a review of documentary data relating to the cyclical process of change over time (observation, action planning and review sheets) was undertaken at each follow-up. Details of the intervention implementation process were also collected as part of the process evaluation, which utilised a mixed-methods comparative case study design [15–18] (Appendix 2 is available in Age and Ageing online).
Usual care
UC, defined as normal care delivered within the setting, continued in both arms. No restrictions were imposed on current practices or on homes undertaking additional development or training as part of UC.
Researchers looked for evidence in the homes for display of materials (posters, leaflets, etc.) related to movement. As part of the process evaluation, ethnographic observations were conducted to record patterns of movement in all homes. We also recorded changes in staff profile and other aspects of the CH context to understand changes in UC over the trial period.
Outcomes
Residents
The following were administered at baseline, 3, 6 and 9 months post CH randomisation by a blinded researcher and are reported here:
Resident self-reported data
Six-item cognitive impairment test: (6-CIT) [19]
Geriatric Depression Scale (GDS) [20]
Perceived health: EuroQol EQ-5D-5L questionnaire [21]
Quality of life: Dementia Quality Of Life questionnaire [22]; World Health Organization Quality of Life - OLD questionnaire (three questions) [23]
With staff informant
Physical function and mobility: Physical activity and mobility in residential care (PAM-RC) [24],1 Barthel index (BI) [25, 26],1 Functional Ambulation Classification (FAC) [27]; Elderly Mobility Scale (EMS) [28] (includes two physical assessments)
Charlson Comorbidity Index [29]
Perceived health: EuroQol EQ-5D-5L proxy-version questionnaire [21]
Quality of life: DEMQoL proxy-version [22] (only if resident unable to complete)
Health care resource use
The researchers also collected data on deaths, moves out of the CH, hospitalisations (also collected through NHS Digital data) and falls on a monthly basis via a telephone call with the home.
Accelerometry
To allow for the objective measurement of PA, residents were asked, at each data collection time point, to wear an ActiGraph wGT3X-BT accelerometer [Actigraph, Pensacola, Florida] on the hip, during waking hours for 7 days [12].
CH level data
CH managers were asked to provide information on CH demographics, the staff and resident profile of the home and anonymous home-level data relating to hospital admissions, general practitioner (GP) call-outs, mortality rates and falls.
Sample size
Although formal power calculations for feasibility studies are not usually undertaken, 12 CHs with an average of 8–12 residents provides sufficient statistical power to detect a standardised effect size of 0.50 across the outcome measures, assuming a Type I error rate of 0.20. An increased Type I error rate acknowledges that we are making a preliminary and non-definitive randomised comparison of the intervention with UC while providing the ability to detect that the intervention is promising and warrants further evaluation.
Statistical methods
All analyses and data summaries were conducted using SAS v9.4 on the intention-to-treat population, defined as allocation at randomisation, regardless of non-compliance with the protocol or withdrawal from the study. Recruitment uptake and follow-up, intervention delivery, compliance with accelerometer wear, assessment of outcome measures and safety were summarised using descriptive statistics and confidence interval (CI) estimation rather than by using formal hypothesis testing, with the exception of the planned preliminary estimate of effectiveness.
For outcome data, questionnaire outcomes and levels of PA and sedentary behaviour, cluster-level analysis was used to account for the small number of clusters and small sample size per cluster [30]. Point estimates were calculated in each arm and used to obtain a difference estimate of the unadjusted intervention effect. Corresponding 95, 80 and 67% CIs were also estimated. Point estimates for accelerometer data were only estimated for participants who provided valid data (i.e. ≥ 8 h 25 min on ≥4 days) with non-wear being defined as periods of at least 120 min of consecutives zero counts [12].
Progression to a definitive trial
Thresholds for specific outcomes were pre-defined to inform the feasibility of progressing to a definitive cRCT (Table 1).
Table 1 .
Pre-specified progression criteria and observed results
| Feasibility outcome | Pre-specified progression criteria | Feasibility trial | |||
|---|---|---|---|---|---|
| Green | Amber | Red | Observations | Status | |
| Recruitment | |||||
| • Number of CHs recruited | 12 | 10 | <10 | 12 | Green |
| • Number of screened residents eligible and who consent to take part in the trial | ≥20% | ≥10% | <10% | 159 consented (53% of 300 screened) | Green |
| • Average number of residents in each CH recruited to the trial | ≥10 | ≥8 | <8 | 12.75 | Green |
| Intervention delivery Proportion of intervention CHs completing the series of three workshops and completing at least one observation review and one action plan review |
≥75% | ≥50% | <50% | 60% | Amber |
| Data collection and follow-up at 9 months | |||||
| • Proportion of residents providing usable accelerometer data | ≥75% | Not specified | Not specified | 65.6% | Amber/Red |
| • Proportion of residents having reported outcome measures from either themselves or a proxy | ≥75% | ≥65% | <65% | Staff informant/proxy: >75% Resident-reported: <55% |
Green Red |
| • Loss to follow-up at 9 months | ≤25% | ≤35% | <35% | 26.1% | Amber |
| Safety concerns in the view of the Programme Steering Committee | None | None | Major | None | Green |
Green = proceed to randomised controlled design; amber = review randomised controlled design and/or intervention delivery, then proceed; red = stop and do not proceed with current trial design and/or intervention and implementation).
Results
Recruitment rate and baseline characteristics
392 CHs were screened, recruited and randomised over a 16-month period, between June 2015 and September 2016: 13 consented; 12 (7.0% of eligible) were randomised (5 MoveMore; 7 UC) (Figure 1).
Figure 1 .
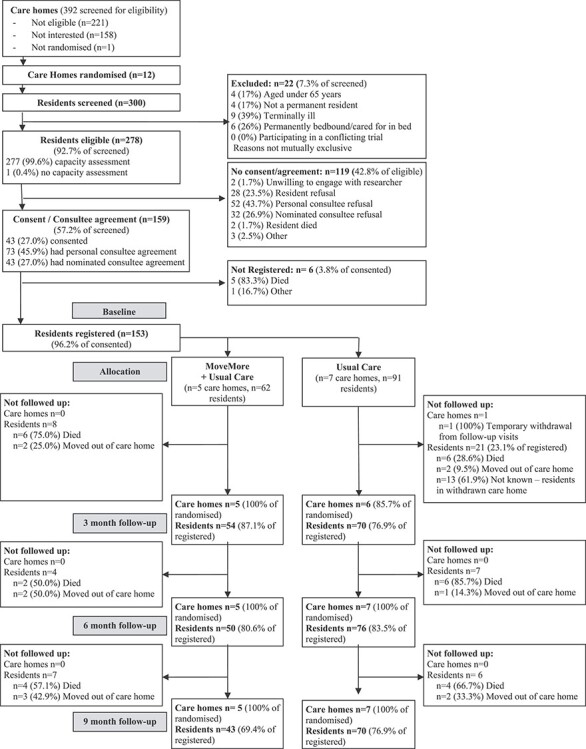
CH and resident screening, recruitment and follow-up.
300 residents were screened for eligibility between October 2015 and August 2016. 278 residents were eligible, 159 (57.2%) consented/had consultee agreement and 153 were registered to the study (Figure 1). The mean (SD) length of time between the process of starting resident screening and completing the registration of residents was 64.3 (13.5) days (range 50–97 days). The average number of residents recruited per CH was 12.75 (range 6–22; 12.4 MoveMore; 13.0 UC). The proportion of eligible residents who were registered was also similar between the arms (54.9% MoveMore; 55.2% UC).
We recruited similar proportions of residents with (43/72–59.7%) and without (116/203—57.1%) capacity to consent (Figure 1). The proportions of residents without capacity to consent recruited via personal and nominated consultees were similar (57.9% and 56.6% respectively).
Participants’ baseline characteristics (Table 2) reflected the poorer physical function and increased proportion with history of stroke in the MoveMore arm observed at screening. Additionally, participants in the MoveMore arm had greater cognitive impairment, and there was a higher proportion with at least one comorbidity (Table 2). Despite differences between arms in screening characteristics, there were no differences in the characteristics of eligible residents who did and did not consent, with the exception of a higher proportion of residents with dementia consenting (69.8% compared to 59.3%) (data not shown), though the proportion of consenting residents with dementia was balanced between arms (Table 2).
Table 2 .
Baseline characteristics of participating CHs and residents
| MoveMore + UC | UC | |
|---|---|---|
| CHs | (n = 5) | (n = 7) |
| Overall home size (number of beds): mean (SD) | 30.0 (2.92) | 38.0 (26.26) |
| Number of beds taking part: mean (SD) | 16.0 (8.51) | 20.0 (13.84) |
| Location | ||
| Urban | 0 (0%) | 2 (28.6%) |
| Suburban | 3 (60.0%) | 3 (42.9%) |
| Semi-rural | 2 (40.0%) | 1 (14.3%) |
| Rural | 0 (0%) | 1 (14.3%) |
| Ownership | ||
| Local authority | 1 (20.0%) | 0 (0%) |
| Independent | 2 (40.0%) | 3 (42.9%) |
| Chain | 1 (20.0%) | 2 (28.6%) |
| Not-for-profit | 1 (20.0%) | 2 (28.6%) |
| CH care provision | ||
| Residential | 3 (60.0%) | 5 (71.4%) |
| Residential/nursing | 1 (20.0%) | 0 (0%) |
| Residential/nursing/dementia/respite | 0 (0%) | 1 (14.3%) |
| Residential/nursing/dementia/intermediate care | 0 (0%) | 1 (14.3%) |
| Residential/dementia/respite/intermediate care | 1 (20.0%) | 0 (0%) |
| Participating CH/unit care provision | ||
| Residential | 3 (60.0%) | 5 (71.4%) |
| Residential/nursing | 1 (20.0%) | 0 (0%) |
| Residential/dementia | 1 (20.0%) | 2 (28.6%) |
| Rehabilitation/intermediate care facilitya | 1 (20.0%) | 1 (14.3%) |
| Telemedicine facility | 3 (60.0%) | 1 (14.3%) |
| Activity co-ordinator in post | 3 (60.0%) | 5 (71.4%) |
| Taking part in initiatives to enhance resident care | 1 (20.0%) | 2 (28.6%) |
| Resident profile (mean; SD) | ||
| Number of permanent residents | 26.0 (7.45) | 34.1 (23.32) |
| Number of permanent self-funded residents | 10.8 (8.58) | 15.3 (16.76) |
| Number of temporary residents | 2.2 (4.38) | 1.0 (1.15) |
| Staff profile (mean; SD) | ||
| Number of permanent staff | 34.2 (11.39) | 33.0 (16.05) |
| Number of agency staffb | 0.0 (0.00) | 0.6 (1.51) |
| Number of bank staffb | 2.2 (2.95) | 1.8 (1.94) |
| Number of staff who have face-to-face contact with residents | 36.4 (13.52) | 24.71 (18.12) |
| Residents | (n = 62) | (n = 91) |
| Age (years) (mean (SD)) | 87.1 (6.59) | 85.7 (7.35) |
| Gender: female | 51 (82.3%) | 71 (78.0%) |
| Diagnosis of dementia | 43 (69.4%) | 64 (70.3%) |
| Previous history of stroke | 14 (22.6%) | 10 (11.0%) |
| Registered blind | 1 (1.6%) | 5 (5.5%) |
| Ethnicity | ||
| White | 61 (98.4%) | 91 (100%) |
| Asian | 1 (1.6%) | 0 (0%) |
| Other ethnic group | 0 (0%) | 0 (0%) |
| Length of stay in the CH (months) | ||
| Mean (SD) | 31.2 (36.82) | 29.2 (33.77) |
| Median (IQR) | 16.5 (8.0, 42.0) | 17.0 (7.0, 37.0) |
| Funding typec | ||
| Continuing Healthcare | 0 (0%) | 1 (1.3%) |
| Local authority | 26 (59.1%) | 39 (51.3%) |
| Local authority and self-funded | 2 (4.5%) | 0 (0%) |
| Self-funded | 16 (36.4%) | 36 (47.4%) |
| FACd | ||
| 0—Non-functional ambulation | 14 (22.6%) | 18 (20.0%) |
| 1—Ambulatory dependent for physical assistance (level II) | 8 (12.9%) | 3 (3.3%) |
| 2—Ambulatory dependent for physical assistance (level I) | 7 (11.3%) | 8 (8.9%) |
| 3—Ambulatory dependent for supervision | 11 (17.7%) | 4 (4.4%) |
| 4—Ambulatory independent level surfaces only | 12 (19.4%) | 32 (35.6%) |
| 5—Ambulatory independent | 10 (16.1%) | 25 (27.8%) |
| EMS (score 0–20)d | ||
| Mean (SD) | 9.3 (6.81) | 10.7 (6.69) |
| Median (IQR) | 12.0 (3.0, 15.0) | 13.0 (4.0, 16.0) |
| Barthele (score 0–20) mean (SD) | 9.2 (4.87) | 11.6 (5.96) |
| PAM-RCf (score 0–21) mean (SD) | ||
| Total score | 9.1 (4.92) | 11.5 (5.45) |
| Ability domain score | 5.9 (2.75) | 7.1 (2.95) |
| Activity domain score | 3.2 (2.42) | 4.5 (2.91) |
| GDSg | ||
| Mean (SD) | 4.1 (3.11) | 3.9 (3.07) |
| Median (IQR) | 4.0 (2.0, 6.0) | 3.0 (1.5, 5.0) |
| WHOQoLh mean (SD) | ||
| Q1 | 3.5 (1.04) | 3.1 (1.43) |
| Q2 | 3.7 (0.77) | 3.3 (1.19) |
| Q3 | 3.5 (0.90) | 2.9 (1.19) |
| DEMQoLi mean (SD) | ||
| Resident completed (score 0–112); 29th score | 90.5 (13.56); 2.3 (0.58) |
93.3 (11.35); 2.2 (0.88) |
| Proxy completed (score 0–124); 32nd score | 96.6 (9.31); 2.0 (0.66) |
97.2 (8.70); 2.1 (0.63) |
| EQ5D-5Lj mean (SD) | ||
| Resident completed; visual analogue score | 0.75 (0.23); 70.6 (18.74) |
0.80 (0.22); 69.5 (21.07) |
| Proxy completed; visual analogue score | 0.60 (0.26); 73.8 (16.62) |
0.69 (0.24); 71.3 (20.02) |
| 6-CITk | 18.6 (8.12) | 16.4 (8.27) |
| Medical historyl | ||
| Dementia or Alzheimer’s | 45 (72.6%) | 64 (70.3%) |
| Cerebrovascular disease or transient ischemic disease | 16 (25.8%) | 10 (11.0%) |
| Rheumatic or connective tissue disease | 18 (29.0%) | 0 (0.0%) |
| Diabetes | 12 (19.4%) | 8 (8.8%) |
| Cancer (lymphoma, leukaemia, solid tumour) | 10 (16.1%) | 5 (5.5%) |
| Congestive heart failure | 9 (14.5%) | 6 (6.6%) |
| Renal disease | 11 (17.7%) | 4 (4.4%) |
| Pulmonary disease | 7 (11.3%) | 6 (6.6%) |
| Gastric or peptic ulcer | 5 (8.1%) | 1 (1.1%) |
| Peripheral vascular disease or bypass | 3 (4.8%) | 3 (3.3%) |
| Hemiplegia | 5 (8.1%) | 0 (0.0%) |
| Metastatic solid tumour | 3 (4.8%) | 1 (1.1%) |
| Myocardial infarction | 2 (3.2%) | 2 (2.2%) |
| Diabetes with end organ damage | 2 (3.2%) | 0 (0.0%) |
| HIV or AIDS | 0 (0.0%) | 1 (1.1%) |
| Mild liver disease | 1 (1.6%) | 0 (0.0%) |
| Comorbiditiesl | ||
| 0 comorbidities | 3 (4.8%) | 15 (16.5%) |
| 1 comorbidity | 18 (29.0%) | 50 (54.9%) |
| 2 comorbidities | 15 (24.2%) | 20 (22.0%) |
| 2+ comorbidities | 26 (41.9%) | 6 (6.6%) |
Numbers and percentages are presented unless otherwise stated aThe CH in the MoveMore+UC arm at baseline which offered rehabilitation/intermediate care had four beds, while the home in the UC arm offering this facility had 35 beds.
bAgency and bank staff who have worked in the CH for a minimum of 1 month in the past 6 months.
cFunding type is not known for 33 registered residents (18 in the Move More arm and 15 in the UC arm).
dFAC and EMS score were not available for one resident in the UC group. Higher EMS scores indicate greater mobility. In the presence of missing item scores, overall EMS scores have been prorated if 50% or more of the 7 items were complete.
eHigher Barthel scores indicate greater self-care ability. In the absence of missing items scores, overall individual scores have been prorated if 50% or more items were complete.
fHigher PAM-RC scores indicate greater physical ability and activity. The ability domain (max score 10) comprises two questions; one around mobility and one around balance. The activity domain (max score 11) comprises three questions: walking frequency, outdoor mobility and wandering.
gScores 0–5 classed as normal with scores 5–15 indicative of depression. In the presence of missing items score, overall scores have been prorated if 50% or more of items were complete. Scores were not available for 20 (32.3%) residents in MoveMore+UC (MM + UC) and 47 (51.6%) in UC (UC).
hThree items from the WHOQoL-OLD were used in the trial and are rated on a five-point scale with higher scores indicating better quality of life. Q1 scores were not available for 27 (43.5%) residents in MM + UC and 52 (53.6%) in UC. Q2 and Q3 scores were not available for 28 (45.2%) residents in MM + UC and 55 (56.7%) in UC.
iHigher scores indicate better quality of life. In the presence of missing items scores, overall scores have been prorated if 50% or more of items were complete. DEMQoL proxy results are provided only for those with no resident completed results. 88 residents do not have resident completed scores (31 (50.0%) in MM + UC; 57 (62.6%) in UC); 82 (26 in MM + UC, 56 in UC) of these residents had proxy completed scores.
jIndex scores range from −0.281 indicating worst health state to 1.000 which indicates perfect health. Scores were not available for 20 (32.3%) residents in MM + UC and 43 (47.3%) residents in UC. The Visual Analog score represents overall rated health and was not available for 29 residents in MM + UC and 49 in UC. Proxy scores were not available for one resident in MM + UC.
kHigher scores indicate greater impairment. Scores were not available for 29 (46.8%) of residents in MM + UC and 55 (60.4%) of residents in UC.
lBased on the Charlson Comorbidity Index. Number (percentage) of residents with a confirmed diagnosis of the condition is reported.
Implementation of and adherence to the intervention (intervention delivery)
The five MoveMore CHs received three (or more) scheduled implementation workshops with content largely as planned. Three intervention CHs (60%) completed at least one observation review and action plan. Intervention CHs were categorised as full (n = 2), partial (n = 2) or failed (n = 1) implementers. In the two homes which proceeded to full adoption of MoveMore, following the initial workshop, observations were undertaken in the CH environment; reflected upon in the subsequent workshop to inform action planning; a range of action plans were tried out in the home, and then reviewed. Implemented action plans included incorporating movement in a review of the content of care plans; introducing systems for communicating action on movement, and training and supervision for all staff. Two homes partially implemented the intervention, undertaking some of the process over a lengthy period in fits and starts. In one CH, slow progress was made towards full implementation, in the other action was undertaken by committed care staff in their areas rather than at the level of the CH. One CH failed to implement: although workshops were provided, the CH lead ultimately did not recognise the need for change. The care staff in the team held a contrary view but they lacked the legitimacy and power to take it forward (Appendix 2 is available in Age and Ageing online).
Usual care (context)
CHs in the MoveMore arm were on average smaller than the UC homes, although there was greater variation in resident numbers across UC homes during the trial. They were, however, less likely to provide rehabilitation or intermediate care and have telemedicine facilities (Table 2). At baseline, more UC homes had an activity coordinator in place but by the end of the trial proportions were similar in both arms (Table 1, Appendix 3 is available in Age and Ageing online). See Appendix 3 available in Age and Ageing online for more details of UC.
Follow-up (attrition)
CH and resident retention during the study period were high (73.9% of residents followed-up at 9 months) with no CH or participant protocol violations or withdrawals, although one CH in the UC arm temporarily withdrew from researcher visits at 3 months due to renovations within the home (Figure 1).
Residents not completing follow-up were more likely to be male, have dementia, have no history of stroke, have lower physical function and have greater cognitive impairment (Appendix 4 is available in Age and Ageing online).
Assessment of outcome measures
Completion level
Questionnaires undertaken with staff informants had high completion levels that were similar between arms at all time points (Table 1; Appendix 5 is available in Age and Ageing online). Completion levels for the EMS, in particular the timed walk and functional reach items, were lower and differed between arms over time (data not shown). The most common reasons for non-completion of these two physical assessment items were that the resident declined, or did not understand what they were being asked to do, or the resident was too frail, unwell or tired to complete them.
Resident questionnaire completion rates were lower than those completed with staff informants, variable (33–74%) and differed between the arms (higher in the MoveMore arm for all questionnaires at all time points) (Table 2; Appendix 5 is available in Age and Ageing online). Lack of completion generally related to lack of capacity or cognitive impairment at the time of assessment. The DEMQOL and the 6-CIT had slightly poorer completion rates than the GDS, the WHOQOL-OLD and the EQ-5D-5L.
Staff proxy questionnaire completion rates were high (Table 3; Appendix 5 is available in Age and Ageing online).
Table 3 .
Comparison of questionnaire scores by arm at each time point
| Time Point | MoveMore +UC | UC | Mean difference (CI: 95%, 80%, 67%)a | |||
|---|---|---|---|---|---|---|
| n/Nb | Mean (SD) | n/Nb | Mean (SD) | |||
| Questionnaires completed by the researcher with staff informants | ||||||
| Elderly Mobility Scalec | Baseline | 62/62 | 9.3 (6.81) | 90/91 | 10.7 (6.69) | 2.10 (−2.75, 6.95) (−0.89, 5.09) (−0.13, 4.33) |
| 3 months | 52/54 | 9.3 (6.76) | 70/70 | 10.1 (6.74) | 1.42 (−2.62, 5.47) (−1.05, 3.90) (−0.42, 3.26) |
|
| 6 months | 50/50 | 8.8 (7.13) | 71/76 | 10.6 (6.89) | 3.23 (−1.62, 8.08) (0.25, 6.22) (1.00, 5.46) |
|
| 9 months | 42/43 | 8.9 (7.21) | 68/70 | 9.8 (6.55) | 3.31 (−2.66, 9.28) (−0.37, 6.99) (0.56, 6.05) |
|
| Barthel Indexd | Baseline | 62/62 | 9.2 (4.87) | 91/91 | 11.6 (5.96) | 3.07 (−0.11, 6.26) (1.11, 5.04) (1.61, 4.54) |
| 3 months | 52/54 | 9.6 (5.54) | 70/70 | 10.1 (6.05) | 1.41 (−2.59, 5.40) (−1.03, 3.85) (−0.41, 3.23) |
|
| 6 months | 50/50 | 8.2 (5.92) | 73/76 | 10.4 (6.26) | 3.40 (−1.20, 8.01) (0.57, 6.24) (1.29, 5.52) |
|
| 9 months | 42/43 | 8.4 (5.92) | 69/70 | 9.5 (6.55) | 2.55 (−2.29, 7.38) (−0.43, 5.52) (0.33, 4.77) |
|
| Physical Activity and Mobility in Residential Care Scalee | Baseline | 62/62 | 9.1 (4.92) | 91/91 | 11.5 (5.45) | 2.67 (−0.70, 6.03) (0.60, 4.74) (1.12, 4.21) |
| 3 months | 51/54 | 9.4 (5.12) | 69/70 | 11.4 (6.24) | 1.70 (−2.01, 5.42) (−0.57, 3.98) (0.01, 3.40) |
|
| 6 months | 50/50 | 8.9 (5.55) | 75/76 | 10.6 (5.81) | 1.88 (−1.53, 5.28) (−0.22, 3.97) (0.31, 3.44) |
|
| 9 months | 42/43 | 9.1 (5.40) | 63/70 | 10.4 (6.31) | 2.06 (−1.75, 5.87) (−0.28, 4.41) (0.31, 3.81) |
|
| Physical Activity and Mobility in Residential Care Scale (ability)f | Baseline | 62/62 | 5.9 (2.75) | 91/91 | 7.1 (2.95) | 1.18 (−0.39, 2.75) (0.21, 2.15) (0.46, 1.90) |
| 3 months | 51/54 | 5.8 (2.64) | 69/70 | 6.6 (3.02) | 0.77 (−0.92, 2.47) (−0.27, 1.81) (−0.001, 1.54) |
|
| 6 months | 50/50 | 5.7 (2.94) | 75/76 | 6.6 (3.20) | 0.87 (−0.99, 2.74) (−0.28, 2.02) (0.02, 1.73) |
|
| 9 months | 42/43 | 5.9 (3.08) | 63/70 | 6.2 (3.32) | 0.77 (−1.28, 2.81) (−0.49, 2.03) (−0.17, 1.71) |
|
| Physical Activity and Mobility in Residential Care Scale (activity)g | Baseline | 62/62 | 3.2 (2.42) | 91/91 | 4.5 (2.91) | 1.49 (−0.56, 3.54) (0.23, 2.75) (0.55, 2.43) |
| 3 months | 51/54 | 3.6 (2.75) | 69/70 | 4.8 (3.51) | 0.93 (−1.25, 3.12) (−0.40, 2.67) (−0.06, 1.93) |
|
| 6 months | 50/50 | 3.2 (2.90) | 75/76 | 4.0 (3.02) | 1.00 (−0.87, 2.87) (−0.15, 2.15) (0.14, 1.86) |
|
| 9 months | 42/43 | 3.2 (2.55) | 63/70 | 4.2 (3.34) | 1.29 (−0.62, 3.21) (0.11, 2.47) (0.41, 2.17) |
|
| Questionnaires completed by the researcher with the resident | ||||||
| Geriatric Depression Scaleh | Baseline | 42/62 | 4.1 (3.11) | 44/91 | 3.9 (3.07) | 0.26 (−3.14, 3.66) (−1.82, 2.34) (−1.29, 1.81) |
| 3 months | 40/54 | 4.5 (3.70) | 33/70 | 3.9 (3.11) | −1.21 (−3.71, 1.28) (−2.71, 0.28) (−2.32, −0.11) |
|
| 6 months | 37/50 | 4.2 (3.34) | 37/76 | 3.5 (3.08) | −1.06 (−3.16, 1.03) (−2.33, 0.21) (−2.00, −0.12) |
|
| 9 months | 29/43 | 3.2 (2.36) | 32/70 | 2.2 (1.94) | −0.82 (−2.14, 0.51) (−1.62, −0.01) (−1.41, −0.22) |
|
| World Health Organization Quality of Life questionnaire for elderly persons (Question 1)i | Baseline | 35/62 | 3.5 (1.04) | 39/91 | 3.1 (1.43) | −0.41 (−1.30, 0.48) (−0.95, 0.13) (−0.81, −0.01) |
| 3 months | 36/54 | 2.9 (1.30) | 26/70 | 3.0 (1.48) | 0.12 (−0.59, 0.82) (−0.30, 0.54) (−0.19, 0.43) |
|
| 6 months | 33/50 | 2.9 (1.28) | 33/76 | 3.4 (1.48) | 0.55 (−0.50, 1.60) (−0.08, 1.19) (0.08, 1.02) |
|
| 9 months | 30/43 | 3.3 (1.26) | 32/70 | 3.4 (1.36) | 0.28 (−0.49, 1.04) (−0.19, 0.74) (−0.07, 0.62) |
|
| World Health Organization Quality of Life questionnaire for elderly persons (Question 2)i | Baseline | 34/62 | 3.7 (0.77) | 36/91 | 3.3 (1.19) | −0.22 (−0.95, 0.51) (−0.67, 0.23) (−0.55, 0.12) |
| 3 months | 38/54 | 3.2 (1.17) | 26/70 | 3.5 (1.10) | 0.40 (−0.30, 1.10) (−0.02, 0.82) (0.09, 0.71) |
|
| 6 months | 36/50 | 3.1 (1.17) | 35/76 | 3.4 (1.35) | 0.49 (−0.34, 1.33) (−0.01, 1.00) (0.12, 0.87) |
|
| 9 months | 30/43 | 3.4 (1.10) | 33/70 | 3.5 (1.42) | 0.33 (−0.29, 0.95) (−0.05, 0.71) (0.05, 0.61) |
|
| World Health Organization Quality of Life questionnaire for elderly persons (Question 3)i | Baseline | 34/62 | 3.5 (0.90) | 36/91 | 2.9 (1.19) | −0.32 (−1.18, 0.55) (−0.85, 0.22) (−0.71, 0.08) |
| 3 months | 37/54 | 3.2 (1.01) | 28/70 | 3.1 (1.18) | 0.11 (−0.56, 0.78) (−0.29, 0.51) (−0.19, 0.41) |
|
| 6 months | 36/50 | 3.1 (1.19) | 33/76 | 3.2 (1.21) | 0.14 (−0.41, 0.69) (−0.19, 0.47) (−0.11, 0.39) |
|
| 9 months | 30/43 | 3.2 (0.97) | 33/70 | 3.6 (1.27) | 0.49 (0.03, 0.95) (0.21, 0.77) (0.29, 0.70) |
|
| Dementia Quality of Life toolj | Baseline | 31/62 | 90.5 (13.56) | 34/91 | 93.3 (11.35) | −1.24 (−20.62, 18.15) (−13.08, 10.61) (−10.06, 7.59) |
| 3 months | 35/54 | 88.2 (12.41) | 28/70 | 93.6 (11.39) | 5.16 (−4.33, 14.65) (−0.52, 10.84) (−0.96, 9.36) |
|
| 6 months | 36/50 | 89.4 (11.85) | 34/76 | 94.1 (9.89) | 5.24 (−0.94, 11.42) (1.49, 8.98) (2.46, 8.02) |
|
| 9 months | 29/43 | 92.1 (11.35) | 28/70 | 94.1 (10.59) | 1.83 (−3.26, 6.92) (−1.25, 4.92) (−0.46, 4.12) |
|
| EuroQol EQ5D-5Lk | Baseline | 42/62 | 0.75 (0.23) | 48/91 | 0.80 (0.22) | 0.05 (−0.13, 0.23) (−0.06, 0.16) (−0.03, 0.13) |
| 3 months | 38/54 | 0.69 (0.22) | 30/70 | 0.78 (0.23) | 0.12 (−0.07, 0.31) (−0.001, 0.23) (0.03, 0.20) |
|
| 6 months | 37/50 | 0.71 (0.24) | 36/76 | 0.79 (0.24) | 0.10 (−0.05, 0.25) (0.01, 0.19) (0.03, 0.17) |
|
| 9 months | 29/43 | 0.77 (0.22) | 33/70 | 0.78 (0.27) | 0.05 (−0.11, 0.21) (−0.05, 0.15) (−0.02, 0.13) |
|
| Six-Item Cognitive Impairment Testl | Baseline | 33/62 | 18.6 (8.12) | 36/91 | 16.4 (8.27) | −0.29 (−7.21, 6.64) (−4.52, 3.94) (−3.44, 2.86) |
| 3 months | 27/54 | 18.5 (7.87) | 23/70 | 14.0 (7.49) | −3.57 (−9.41, 2.27) (−7.06, −0.07) (−6.15, −0.98) |
|
| 6 months | 31/50 | 17.6 (7.59) | 26/76 | 13.6 (9.50) | −2.76 (−8.47, 2.94) (−6.22, 0.69) (−5.33, −0.20) |
|
| 9 months | 26/43 | 17.1 (7.98) | 23/70 | 14.6 (7.94) | −2.02 (−7.38, 3.34) (−5.27, 1.23) (−4.43, 0.39) |
|
a80 and 67% CIs are narrower than 95% CI because as the precision of the CI increases (i.e. the CI width decreases) the reliability of the CI containing the true mean difference decreases. Differences and CIs not adjusted for baseline scores.
bRepresents the number of completed questionnaires (fully completed or prorated where applicable) out of the number of residents available for follow-up.
cScore 0–20; higher score = greater mobility.
dScore 0–20; higher score = greater self-care ability.
eScore 0–21; higher score = greater physical ability and activity.
fScore 0–10.
gScore 0–11.
hScore 0–15; score ≥5 indicative of depression.
iScore 1–5; higher score = greater quality of life.
jScore 0–112; higher score = better quality of life.
kIndex value −0.281 to 1.000.
lScore 0–28; higher scores = greater impairment.
Outcome estimation
Residents in the UC arm had higher EMS scores at baseline indicating greater mobility, though there was no evidence that this was a significant difference. During the study, scores decreased in both arms with evidence of a difference emerging between the arms from 6 months with scores higher in the UC arm (diff = 3.23 (80% CI: 0.25, 6.22)). BI and PAM-RC scores were significantly higher in the UC arm at baseline. During the study, scores fluctuated in the MoveMore arm and decreased in the UC arm, such that by 9 months there was no evidence of a difference between the arms (Barthel: 2.55 (80% CI −0.43, 5.52); PAM-RC: 2.06 (80% CI −0.28, 4.41) (Table 3).
Accelerometer wear data
At baseline, the proportion of participants agreeing to wear the accelerometer was high in both arms (96.8% MoveMore; 93.4% UC). At 9 months, the proportion wearing the accelerometer in the MoveMore arm was maintained, while in the UC arm the proportion decreased to 71.4% (55% of registered residents at baseline).
The proportion of residents providing useable accelerometer data (i.e. met the minimum wear criteria) differed between the arms at baseline such that the proportion was higher in the UC arm (90.6 versus 81.7%). While the proportion decreased in both arms across the study period, the decrease was more marked in the UC arm with 60% of residents meeting the minimum wear criteria at 9 months compared with 72.5% in the MoveMore arm. Overall, 65.6% of residents provided usable accelerometer data at 9 months (Appendix 6 is available in Age and Ageing online).
The numbers of registered residents wearing an accelerometer and achieving the minimum wear time criteria required for analysis did not meet the pre-specified progression criterion and were deemed insufficient to conduct a formal cluster-level analysis to provide a robust preliminary estimate of effectiveness.
Levels of physical activity and sedentary behaviour
At baseline, residents in both arms spent more than 85% of their time sedentary (mean (SD): MoveMore arm 91.4% (4.7%); UC arm 86.6% (10.0%))—on average more than 11 and a half hours per day (Table 4). They therefore spent very little time undertaking any PA (Table 4): average 1 h 7 min (8.5% of accelerometer wear time) in the MoveMore arm and 1 h 53 min (13.4% of accelerometer wear time) in the UC arm.
Table 4 .
Time and proportion of time residents spent sedentary and in physical activity
| Time point | MoveMore + UC | UC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) time | 80% CI | Mean (SD) proportion of time | 80% CI | N | Mean (SD) time | 80% CI | Mean (SD) proportion of time | 80% CI | |
| Sedentary time | ||||||||||
| Baseline | 49 | 11 h 38 min (1 h 59 min) |
11 h 16 min to 12 h 0 min |
91.4% (4.7%) | 90.6–92.3% | 77 | 11 h 41 min (2 h 39 min) |
11 h 18 min to 12 h 5 min |
86.6% (10.0%) | 85.2–88.1% |
| 3 months | 39 | 11 h 50 min (1 h 58 min) |
11 h 25 min to 12 h 14 min |
90.5% (5.5%) | 89.4–91.7% | 41a | 11 h 56 min (2 h 19 min) |
11 h 27 min to 12 h 24 min |
89.1% (10.8%) | 87.0–91.3% |
| 6 months | 38 | 11 h 37 min (1 h 58 min) |
11 h 12 min to 12 h 2 min |
90.3% (5.5%) | 89.1–91.5% | 46 | 12 h 20 min (2 h 35 min) |
11 h 50 min to 12 h 49 min |
88.6% (7.7%) | 87.1–90.1% |
| 9 months | 29 | 11 h 31 min (1 h 42 min) |
11 h 6 min to 12 h 0 min |
89.1% (5.5%) | 87.8–90.5% | 30 | 12 h 33 min (2 h 35 min) |
11 h 56 min to 13 h 10 min |
87.4% (10.8%) | 84.8–89.9% |
| Time per day spent in physical activity | ||||||||||
| Baseline | 49 | 1 h 7 min (40 min) |
59 min to 1 h 14 min |
8.5% (4.7%) | 7.7–9.4% | 77 | 1 h 53 min (1 h 35 min) |
1 h 39 min to 2 h 7 min |
13.4% (10.0%) | 11.9–14.9% |
| 3 months | 39 | 1 h 18 min (54 min) |
1 h 7 min to 1 h 29 min |
9.5% (5.5%) | 8.3–10.6% | 41a | 1 h 32 min (1 h 34 min) |
1 h 13 min to 1 h 51 min |
10.8% (10.8%) | 8.7–13.0% |
| 6 months | 38 | 1 h 16 min (47 min) |
1 h 6 min to 1 h 26 min |
9.7% (5.5%) | 8.5–10.9% | 46 | 1 h 36 min (1 h 7 min) |
1 h 24 min to 1 h 49 min |
11.4% (7.7%) | 9.9–12.9% |
| 9 months | 29 | 1 h 25 min (47 min) |
1 h 14 min to 1 h 37 min |
10.9% (5.5%) | 9.6–12.2% | 30 | 2 h 0 min (2 h 16 min) |
1 h 27 min to 2 h 32 min |
12.6% (10.8%) | 10.1–15.2% |
aOne CH withdrew from data collection visits.
At 9 months, there was a decrease from baseline in the proportion of time spent sedentary among residents in the MoveMore arm, whereas there was no suggestion of an overall change among residents in the UC arm. Although there was no suggestion of a difference between the arms at 9 months, this equates to an average increase in time spent in any intensity of PA of 18 min in the MoveMore arm (10.9% of accelerometer wear time) and 7 min in the UC arm (12.6% of accelerometer wear time) (Table 4).
Safety data
Review of falls, hospitalisations, visits to the Accident and Emergency Department (A&E) and deaths indicated no adverse effects of the intervention. Full details of the safety data are available from the authors.
Discussion
Generalisability and context
We fulfilled our CH recruitment target, recruiting a range of CHs [13]. Eligibility was inclusive as the intervention was designed to benefit most residents in the home. 92.7% of residents were eligible, of whom we recruited 57% (consistent with rates quoted in other studies [31–37]). The recruited residents can be considered a representative sample. Importantly, residents judged to lack capacity to consent—who form a high proportion of the CH population—were recruited equally to the study. There were no withdrawals. We found high levels of sedentary behaviour with concomitant low levels of PA in residents, commensurate with other studies [2, 3, 38].
Limitations
The stratified randomisation process did not achieve balance between arms in the number of CHs due to the small number of clusters randomised. Alternative methods for ensuring balance in sample size should be considered for a definitive trial. Further, there were differences in the populations of screened and recruited residents between the two arms. For the randomisation of CHs in a definitive trial, stratification by baseline stroke, physical function and cognitive impairment of residents should be considered.
Return rates for resident-completed outcomes were low but comparable with other CH studies [39–41]. While proxy returns were higher, these are not necessarily an accurate reflection of residents’ viewpoints [42, 43]. Further work is required to clarify the most appropriate measures for this group of people. It was feasible to use accelerometers to measure PA and sedentary behaviour in older CH residents using a tailored and robust data collection protocol and procedures. Administration of accelerometers to participants was greatly enhanced by the very skilled and experienced research staff who worked flexibly, including at weekends and evenings, leading to one of the largest ever data sets for this population. Residents’ compliance with accelerometer wear was similar to comparable studies both at baseline [2, 44, 45] and at follow-up [2] despite the comparative frailty of our population. However, as insufficient participants met the pre-determined minimum wear time criteria, we were unable to make a reliably informed decision on the most appropriate endpoint(s) for future use in a definitive trial.
Delivery of the intervention workshops took far longer than anticipated, although efficiency improved over time through increased contact with the CHs and refinement of the workshop timings. The workshops provided a forum to create a shared understanding of what needed to change and to generate ideas about goals, priorities and creative solutions from different perspectives, although solutions were sometimes difficult to put into practice.
Interpretation and implications for future research
Recruitment of CHs and an unbiased population of frail CH residents (including those deemed to lack capacity to consent) was feasible, although time-consuming (approx. 60 days to recruit residents per CH), as found by other CH studies [39, 46]. Our intervention was implemented, at least in part, in four of the five CHs, demonstrating an appropriate methodology for influencing the care environment. However, further optimisation is required to enhance implementation.
Loss to 9-month follow-up for recruited residents was 26.1% (18.3% died), comparable with other CH studies [34, 39, 40]. The frailty of the population leads to difficulties with longer follow-up periods, so alternative methods of evaluation could be considered [47].
Implications for progression
Progression criteria indicated that recruitment of CHs and residents to the study was feasible. Although intervention delivery was challenging and not achieved in all CHs, it was achievable and safe. Accelerometer and resident-reported outcome data collection rates were not at acceptable levels for progression.
Supplementary Material
Footnotes
Collected anonymously for all residents at screening to establish, at the level of the care home, the physical activity profile, participation in activities of daily living/self-care and ambulatory capacity.
Contributor Information
Anne Forster, Academic Unit for Ageing and Stroke Research, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK; Leeds Institute of Health Sciences, University of Leeds, Leeds, West Yorkshire, UK.
Jennifer Airlie, Academic Unit for Ageing and Stroke Research, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK.
Alison Ellwood, Centre for Dementia Studies, University of Bradford, Bradford, UK.
Mary Godfrey, Academic Unit for Ageing and Stroke Research, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK; Leeds Institute of Health Sciences, University of Leeds, Leeds, West Yorkshire, UK.
John Green, Academic Unit for Ageing and Stroke Research, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK.
Bonnie Cundill, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds UK.
Bryony Dawkins, Academic Unit of Health Economics, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK.
Nicola McMaster, NHS England and NHS Improvement, Leeds, UK.
Claire Hulme, Institute of Health Research, College of Medicine and Health, University of Exeter, Exeter, UK.
Robert Cicero, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds UK.
Vicki McLellan, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds UK.
Liz Graham, Academic Unit for Ageing and Stroke Research, Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK.
Bev Gallagher, NHS Bradford District and Craven Clinical Commissioning Group, Bradford, UK.
David R Ellard, Warwick Clinical Trials Unit, Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK.
Joan Firth, Patient and Public Involvement Contributor, Ilkley, UK.
Amanda Farrin, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds UK.
Acknowledgements
The authors wish to thank and acknowledge the help and support of all the CHs, CH staff and residents who participated in the study. The authors would also like to thank the members of the Programme Steering Committee for their very helpful contribution throughout the REACH programme of research.
This paper is written on behalf of the REACH Programme Team who met regularly to oversee the programme of research. We would like to acknowledge the expert support of the REACH Programme Team which comprised: Karen Birch, School of Biomedical Sciences, University of Leeds, UK; David Ellard, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick, UK; Amanda Farrin, Leeds Institute of Clinical Trials Research, University of Leeds; Joan Firth, Personal and Public Representative; Anne Forster, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK and Leeds Institute of Health Sciences, University of Leeds, UK; Bev Gallagher, NHS Bradford District and Craven Clinical Commissioning Group, UK; Mary Godfrey (deceased), Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK and Leeds Institute of Health Sciences, University of Leeds, UK; Elizabeth Graham, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK; John Green, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK; Rebecca Hawkins, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK; Claire Hulme, Health Economics Group, Institute of Health Research, University of Exeter, UK; Rebecca Lawton, School of Psychology, Faculty of Medicine and Health, University of Leeds, UK; Najma Siddiqi, Department of Health Sciences, Hull York Medical School, University of York, UK and John Young, Academic Unit for Ageing and Stroke Research, Bradford Institute for Health Research, Bradford Teaching Hospitals NHS Foundation Trust, UK.
Team members have reviewed and agreed the content of the final manuscript.
Declaration of Sources of Funding
This article presents independent research funded by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research programme (Development and preliminary testing of strategies to enhance routine physical activity in care homes, reference number RP-PG-1210-12017). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Declaration of Conflicts of Interest
None.
Registration
ISRCTN registry, ISRCTN16076575.
Protocol
Trials 2017; 18(1):1-14. https://doi:10.1186/s13063-017-1921-8.
References
- 1. Laing Buisson Care homes for older people Market Report. London: LaingBuisson July 2018.
- 2. Marmeleria J, Ferreira S, Raimundo A. Physical activity and physical fitness of nursing home residents with cognitive impairment: a pilot study. Exp Gerontol 2017; 100: 63–9. [DOI] [PubMed] [Google Scholar]
- 3. Barber SE, Forster A, Birch KM. Levels and patterns of daily physical activity and sedentary behavior measured objectively in older care home residents in the United Kingdom. J Aging Phys Act 2015; 23: 133–43. [DOI] [PubMed] [Google Scholar]
- 4. UK Chief Medical Officers’ Physical Activity Guidelines. https://www.gov.uk/government/publications/physical-activity-guidelines-uk-chief-medical-officers-report (Sept 2019). (accessed 4 June 2021).
- 5. de Rezende LF, Rey-López JP, Matsudo VKR, do Carmo Luiz O. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health 2014; 14: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Same RV, Feldman DI, Shah N et al. Relationship between sedentary behavior and cardiovascular risk. Curr Cardiol Rep 2016; 18: 6. doi: 10.1007/s11886-015-0678-5. [DOI] [PubMed] [Google Scholar]
- 7. Sardinha LB, Santos DA, Silva AM, Baptista F, Owen N. Breaking-up sedentary time is associated with physical function in older adults. J Gerontol A Biol Sci Med Sci 2015; 70: 119–24. [DOI] [PubMed] [Google Scholar]
- 8. Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc 2013; 45: 1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Neill C, Dogra S. Different Types of Sedentary Activities and Their Association With Perceived Health and Wellness Among Middle-Aged and Older Adults: A Cross-Sectional Analysis. Am J Health Promot 2016; 30: 314–22. [DOI] [PubMed] [Google Scholar]
- 10. Meneguci J, Sasaki JE, da Silva Santos Á, Scatena LM, Damião R. Socio-demographic, clinical and health behavior correlates of sitting time in older adults. BMC Public Health 2015; 15: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crocker T, Forster A, Young J et al. Physical rehabilitation for older people in long-term care. Cochrane Database Syst Rev 2013; 2: CD004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forster A, Airlie J, Birch K et al. Research Exploring Physical Activity in Care Homes (REACH): study protocol for a randomised controlled trial. Trials 2017; 18: 1–14. doi: 10.1186/s13063-017-1921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellwood A, Airlie J, Cicero R et al. Recruiting care homes to a randomised controlled trial. Trials 2018; 19: 535. doi: 10.1186/s13063-018-2915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartholomew L, Parcel G, Kok G, Gottlieb N, Fernandez M. Planning Health Promotion Program: an Intervention Mapping Approach. 3rd edition. San Francisco, CA: Joffey-Ball, 2011. [Google Scholar]
- 15. Ragin C, Becker H. What is a case? In: Ragin C, Becker H, eds. What Is a Case? Exploring the Foundations of Social Inquiry. Cambridge: Cambridge University Press, 1992; 1–18. [Google Scholar]
- 16. Stake R. The Art of Case Study Research. London: Sage, 1995. [Google Scholar]
- 17. Yin R. Case Study Research: Design and Methods. London: Sage, 2009. [Google Scholar]
- 18. Byrne D. Case-based methods: why we need them? What they are? How to do them? In: Byrne D, Ragin C, eds. The Sage Handbook of Case-Based Methods. London: Sage, 2013; 1–10. [Google Scholar]
- 19. Brooke P, Bullock R. Validation of a 6 item cognitive impairment test with a view to primary care usage. Int J Geriatr Psychiatry 1999; 14: 936–40. [PubMed] [Google Scholar]
- 20. Yesavage JA, Brink TL, Rose TL et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- 21. Herdman M, Gudex C, Lloyd A et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith S, Lamping D, Banerjee S et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007; 37: 737–46. [DOI] [PubMed] [Google Scholar]
- 23. Power M, Quinn K, Schmidt S, WHOQOL-OLD Group . Development of the WHOQOL-OLD module. Qual Life Res 2005; 14: 2197–214. [DOI] [PubMed] [Google Scholar]
- 24. Whitney JCJ, Lord SR, Jackson SHD. The physical activity and mobility in residential care (PAM-RC) scale. Pre-publication data – paper available from lead author 2013.
- 25. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965; 14: 61–5. [PubMed] [Google Scholar]
- 26. Collin C, Wade D, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988; 10: 61–3. [DOI] [PubMed] [Google Scholar]
- 27. Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther 1964; 64: 35–40. [DOI] [PubMed] [Google Scholar]
- 28. Smith R. Validation and reliability of the Elderly Mobility Scale. Phys Ther 1994; 80: 744–7 [Erratum published in Physiother 1994; 80:879.]. [Google Scholar]
- 29. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–51. [DOI] [PubMed] [Google Scholar]
- 30. Hayes RJ, Moulton LH. Cluster Randomised Trials. Second edition. Chapman & Hall/CRC Biostatistics Series. Boca Raton, Fl, 2017. [Google Scholar]
- 31. Kalinowski S, Budnick A, Kuhnert R et al. Nonpharmacologic pain management interventions in German nursing homes: a cluster randomized trial. Pain Manag Nurs 2015; 16: 464–74. [DOI] [PubMed] [Google Scholar]
- 32. Zermansky AG, Alldred DP, Petty DR et al. Clinical medication review by a pharmacist of elderly people living in care homes – randomised controlled trial. Age Ageing 2006; 35: 586–91. [DOI] [PubMed] [Google Scholar]
- 33. Underwood M, Lamb SE, Eldridge S et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 2013; 382: 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siddiqi N, Cheater F, Collinson M et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing 2016; 45: 652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flicker L, Mac Innis RJ, Stein MS et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc 2005; 53: 1881–8. [DOI] [PubMed] [Google Scholar]
- 36. Sackley CM, Walker MF, Burton CR et al. An occupational therapy intervention for residents with stroke related disabilities in UK care homes (OTCH): cluster randomised controlled trial. Br Med J 2015; 350: h468. doi: 10.1136/bmj.h468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandvik RK, Selbaek G, Seifert R et al. Impact of a stepwise protocol for treating on pain intensity in nursing home patients with dementia: a cluster randomized trial. Eur J Pain 2014; 18: 1490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sackley CM, Hoppitt T, Levin S, Cardoso K. Observations of activity levels and social interaction in a residential care setting. Int J Ther Rehabil 2006; 13: 370–3. [Google Scholar]
- 39. Graham L, Ellwood A, Hull K et al. A posture and mobility training package for care home staff: results of a cluster randomised controlled feasibility trial (the PATCH trial). Age Ageing 2020; 49: 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Surr CA, Holloway I, Walwyn RE et al. Dementia Care Mapping™ to reduce agitation in care home residents with dementia: the EPIC cluster RCT. Health Technol Assess 2020; 24: 1–172. doi: 10.3310/hta24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hurley MV, Wood J, Smith R et al. The feasibility of increasing physical activity in care home residents: Active Residents in Care Homes (ARCH) programme. Physiotherapy 2020; 107: 50–7. [DOI] [PubMed] [Google Scholar]
- 42. Devine A, Taylor SJC, Spencer A, Diaz-Ordaz K, Eldridge S, Underwood M. The agreement between proxy and self-completed EQ-5D for care home residents was better for index scores than individual domains. J Clin Epidemiol 2014; 67: 1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griffiths AW, Smith SJ, Martin A, Meads D, Kelley R, Surr CA. Exploring self-report and proxy-report quality-of-life measures for people living with dementia in care homes. Qual Life Res 2020; 29: 463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leung P, Ejupi A, van Schooten KS et al. Association between sedentary behaviour and physical, cognitive and psychosocial status among older adults in assisted living. Biomed Res Int 2017; 9160504. doi: 10.1155/2017/9160504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corcoran MP, Chui KK, White DK et al. Accelerometer assessment of physical activity and its association with physical function in older adults residing at assisted care facilities. J Nutr Health Aging 2016; 20: 752–8. [DOI] [PubMed] [Google Scholar]
- 46. Goodman C, Baron NL, Machen I et al. Culture, consent, costs and care homes: enabling older people with dementia to participate in research. Aging Ment Health 2011; 15: 475–81. [DOI] [PubMed] [Google Scholar]
- 47. Walwyn R, Farrin A, Surr C. Dealing with unavoidably high loss to follow-up in care home trials-The DCM-EPIC trial. Trials 2019; 20: 579. doi: 10.1186/s13063-019-3688-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


