Abstract
Background
despite rapid population ageing, few studies have investigated frailty in older people in sub-Saharan Africa. We tested a cumulative deficit frailty index in a population of older people from rural South Africa.
Methods
analysis of cross-sectional data from the Health and Ageing in Africa: Longitudinal Studies of an INDEPTH Community (HAALSI) study. We used self-reported diagnoses, symptoms, activities of daily living, objective physiological indices and blood tests to calculate a 32-variable cumulative deficit frailty index. We fitted Cox proportional hazards models to test associations between frailty category and all-cause mortality. We tested the discriminant ability of the frailty index to predict one-year mortality alone and in addition to age and sex.
Results
in total 3,989 participants were included in the analysis, mean age 61 years (standard deviation 13); 2,175 (54.5%) were women. The median frailty index was 0.13 (interquartile range 0.09–0.19); Using population-specific cutoffs, 557 (14.0%) had moderate frailty and 263 (6.6%) had severe frailty. All-cause mortality risk was related to frailty severity independent of age and sex (hazard ratio per 0.01 increase in frailty index: 1.06 [95% confidence interval 1.04–1.07]). The frailty index alone showed moderate discrimination for one-year mortality: c-statistic 0.68–0.76; combining the frailty index with age and sex improved performance (c-statistic 0.77–0.81).
Conclusion
frailty measured by cumulative deficits is common and predicts mortality in a rural population of older South Africans. The number of measures needed may limit utility in resource-poor settings.
Keywords: frailty index, older people, global health
Key Points
Few studies have examined frailty in older people in sub-Saharan Africa.
We successfully derived a cumulative deficits frailty index from a large community-based cohort of older South Africans.
The frailty index predicted mortality independent of age and sex in this population.
The frailty index showed similar discriminant ability for 1-year mortality to the Fried score in this population.
Lack of recorded data may make routine use of a frailty index challenging in resource-poor settings.
Introduction
Systematic reviews have shown frailty to be associated with increased rates of emergency hospital admissions [1], care home placement [2], disability [3], lower quality of life [4] and higher mortality [5]. Frailty is thus a key concept underpinning identification of older people at risk of adverse outcomes and in shaping the design of healthcare and broader social responses to the ageing population [6, 7]. Consideration of frailty in a global context is essential given the rapid increase in both the proportion, and absolute number, of older people in low- and middle-income countries (LMICs) [8].
Although there is a wealth of frailty research from high-income countries (HICs), there has been considerably less focus on frailty identification or management in LMICs including sub-Saharan Africa [9]. Frailty in these countries is likely to have substantial social implications as older people frequently play vital roles within their communities, including as workers, farmers and carers for grandchildren. South Africa is a country of particular interest regarding ageing and frailty—its adult life expectancy has risen steeply since 2004, largely following the successes of anti-retroviral therapy roll-out for HIV [10]. The country’s population of over-60s has been predicted to double between 2012 and 2050 [11]. Establishing the current and future prevalence of frailty is essential if health and social care services are to meet the needs of South Africa’s ageing population. Tools to enable clinical identification of frailty and to facilitate research need to be developed and validated in LMICs to enable targeting of interventions to those most in need.
Frailty is commonly operationalised using two different models, which measure distinct constructs. The phenotype model measures frailty through physical indicators reflecting strength and energy homeostasis. The Fried criteria quantify this using a score composed of five items (walk speed, grip strength, activity levels, exhaustion or unintentional weight loss) [12]. In contrast, the cumulative-deficits model operationalises frailty as an incremental accumulation of age-related physiological deficits, integrating a broader range of information [13]. Both models have advantages and disadvantages, and the choice of model needs to fit both the intended purpose and the feasibility of use in the target environment. Both have been well-validated in HICs but few studies have examined their use in sub-Saharan Africa. Our previous study used data from the Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa (HAALSI) study—a large cohort of older people living in rural South Africa, to derive Fried frailty scores, demonstrating positive associations between frailty and all-cause mortality [14]. A small number of studies using frailty indices to measure frailty in sub-Saharan African populations exist [15–17], one of which showed an association between a higher frailty index and a higher risk of death.
The study aims were firstly to develop a cumulative deficits frailty index using a large population-based cohort from South Africa, and secondly to test whether such a frailty index is associated with increased mortality and worsened subjective wellbeing.
Methods
We analysed baseline data from the HAALSI study [18]. HAALSI was created in 2014 to investigate the social, economic and biological determinants of age-related health within the Bushbuckridge sub-district in Mpumulanga province, northeast South Africa. The Agincourt Health and socio-Demographic Surveillance System (HDSS) has collected information on key life events (births, deaths and in and out-migrations) for all households in the area on an annual basis since 1992, as well as sociodemographic information on alternate years [19]. The HAALSI cohort was created in close association with HDSS cohort data, allowing for a wide range of health and socioeconomic data to be available to the study. Further details on sampling and enrolment to the HAALSI study are given in the Supplementary Methods (Supplementary data are available in Age and Ageing online). Ethical approval for HAALSI was given from the University of the Witwatersrand Human Research Ethics Committee, the Mpumalanga provincial Research and Ethics Committee and the Harvard T.H. Chan School of Public Health Office of Human Research Administration.
Creation of the frailty index
In total, 32 variables were selected from HAALSI cohort data, including symptoms, biological measurements, self-reported disease diagnoses and functional impairments including basic activities of daily living. Full details of the method for deriving the frailty index [20] are given in Supplementary Methods (Supplementary data are available in Age and Ageing online).
Establishing population-specific frailty severity categories
Cohort-specific cutoffs were established by following an identical method to that used to develop the UK electronic frailty index (eFI) [21]. The upper 99th percentile of the cohort’s frailty scores was established; this value was then divided into four categories of equal width. Participants above the 99th percentile were included in the fourth (severe) frailty category.
Outcome variables
All-cause mortality was ascertained using annual census data from the Agincourt HDSS. The occurrence and date of death was based on reports from the family of the deceased. If a household reported the death of a HAALSI participant during the study, detailed information was then collected by the census team, including the date of death, as described previously [19]. Data on deaths were collected during the 2016 HDSS census round (conducted between August and December 2016). Due to variability in the order of follow-up, time between enrolment in HAALSI and date of census follow-up varied from a minimum of 12 months to a maximum of 23 months.
As an additional test of validity, subjective wellbeing was also examined as an outcome variable in cross-sectional analysis. This construct was measured by asking the question ‘how satisfied are you with your life as a whole these days?’ using an integer scale from 0 (dissatisfied) to 10 (satisfied). Subjective wellbeing and quality of life are inherently similar measures [22], so we hypothesised that this variable would have a negative association with frailty (as has been shown in previous studies [14]). Most other outcomes that would be expected to correlate with frailty were either already present in the frailty index (e.g. activity impairment), were not collected as part of HAALSI (e.g. prospective falls data and hospital admissions), or were not relevant to the population (e.g. care home admission, as care homes are rare in rural South Africa).
Data analysis
Full details of the data analysis are shown in the Supplementary Material (Supplementary data are available in Age and Ageing online).
Results
Of the 5,059 individuals undergoing assessment in HAALSI, 3,989 participants were included in the main analyses presented here. A flowchart depicting who was excluded at each stage is shown in Figure 1. The mean age (standard deviation [SD]) of the analysis population was 61.3 (12.6) years; 2,175 (54.5%) were women. A total of 135 participants (3.4% of the cohort) had died by the time of data censoring; the mean follow-up time for participants was 17 months.
Figure 1 .
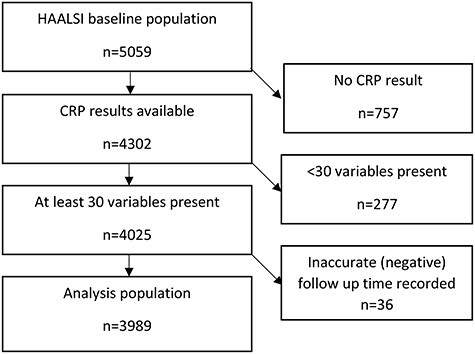
Flowchart of participants included in the analysis.
Frailty index construction and distribution
Table 1 shows the prevalence of each individual variable used in the frailty index. The median frailty index for all participants was 0.13 (interquartile range [IQR] 0.09–0.19). A histogram of frailty indices showed the expected approximation to a gamma distribution (Supplementary Figure 1, Supplementary data are available in Age and Ageing online). The cohort-specific cutoffs were 0.00–0.09 for non-frail (n = 777, 19.5%), >0.09–0.19 for mild frailty (n = 2,391, 60.0%), >0.19–0.28 for moderate frailty (n = 557, 14.0%) and >0.28 for severe frailty (n = 263, 6.6%).
Table 1 .
Baseline details and prevalence of frailty index variables in HAALSI (n = 3,989)
| Variable | Prevalence |
|---|---|
| Mean age (SD) | 61.3 (12.6) |
| 40–49 (%) | 716 (17.9) |
| 50–59 (%) | 1,135 (28.5) |
| 60–69(%) | 1,080 (27.1) |
| 70–79 (%) | 692 (17.3) |
| 80+ (%) | 366 (9.2) |
| Male sex (%) | 1,814 (45.5) |
| Comorbidities | |
| Depression (%) | 633 (5.9) |
| HIV (%) | 942 (23.6) |
| Previous stroke (%) | 94 (2.4) |
| Bronchitis (%) | 25 (0.6) |
| Hypertension (%) | 2,542 (63.7) |
| Diabetes mellitus (%) | 461 (11.6) |
| Tuberculosis (%) | 362 (9.1) |
| Myocardial infarction (%) | 16 (0.4) |
| Heart failure (%) | 27 (0.7) |
| Kidney disease (%) | 175 (4.4) |
| Angina (%) | 381 (9.6) |
| Symptoms and signs | |
| Underweight (%) | 203 (5.1) |
| Weak grip strength (%) | 748 (18.8) |
| Slow gait (%) | 793 (19.3) |
| Cognitive impairment (%) | 691 (17.3) |
| Hearing problems (%) | 1,865 (46.8) |
| Visual problems (%) | 944 (23.7) |
| Pain in last 24 h (%) | 396 (9.9) |
| Worry (%) | 488 (12.2) |
| Sleep quality (%) | 231 (5.8) |
| Blood tests | |
| Elevated C-reactive protein (%) | 1,524 (38.2) |
| Anaemia (%) | 1,747 (43.8) |
| Hypercholesterolaemia (%) | 239 (6.0) |
| Hypertriglyceridaemia (%) | 788 (19.8) |
| Activities of daily living | |
| Difficulty walking (%) | 216 (5.4) |
| Difficulty dressing (%) | 60 (1.5) |
| Difficulty bathing (%) | 66 (1.7) |
| Difficulty eating by self (%) | 28 (0.7) |
| Difficulty going to toilet by self (%) | 96 (2.4) |
| Difficulty getting in and out of bed (%) | 117 (2.9) |
| Other | |
| Poor self-reported health (%) | 682 (17.1) |
| Low physical activity (%) | 690 (17.3) |
Prediction of all-cause mortality
Table 2 shows the results of Cox proportional hazards models, both with and without inclusion of age and sex in the models. A strong relationship between frailty index and time to death was seen, which was slightly attenuated by the inclusion of age and sex in the model. Including participants with missing C-reactive protein results but still with data for at least 30 variables showed similar results as did inclusion of participants with any level of missing data. Supplementary Table 2 shows the results of an analysis using cohort-specific cutoffs for frailty category; similar patterns were seen after categorisation of the frailty index.
Table 2 .
Association between frailty index and all-cause mortality at most recent follow-up in HAALSI
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) |
P | Hazard ratio (95% CI) |
P | ||
| At least 30 variables; CRP included (n = 3,989) | Frailty index (per 0.01 increment) | 1.06 (1.05, 1.08) | <0.001 | 1.06 (1.04, 1.07) | <0.001 |
| Male sex | – | – | 2.87 (1.99, 4.13) | <0.001 | |
| Age (per year) | – | – | 1.03 (1.02, 1.05) | <0.001 | |
| At least 30 variables; CRP optional (n = 4,285) | Frailty index (per 0.01 increment) | 1.06 (1.05, 1.08) | <0.001 | 1.05 (1.04, 1.07) | <0.001 |
| Male sex | – | – | 2.77 (1.94, 3.95) | <0.001 | |
| Age (per year) | – | – | 1.03 (1.02, 1.05) | <0.001 | |
| Allowing fewer than 30 variables (n = 5,014) | Frailty index (per 0.01 increment) | 1.08 (1.07, 1.09) | <0.001 | 1.07 (1.06, 1.08) | <0.001 |
| Male sex | – | – | 2.18 (1.68, 2.82) | <0.001 | |
| Age (per year) | – | – | 1.04 (1.03, 1.05) | <0.001 | |
Notes: Cox proportional hazards models. CRP: C-reactive protein.
Association between frailty index and subjective wellbeing
The relationship between subjective wellbeing scores and frailty index is shown in Figure 2. Participants with higher subjective wellbeing had lower frailty indices, although there was the suggestion of an inverted U-shaped association for those with the lowest subjective wellbeing scores.
Figure 2 .
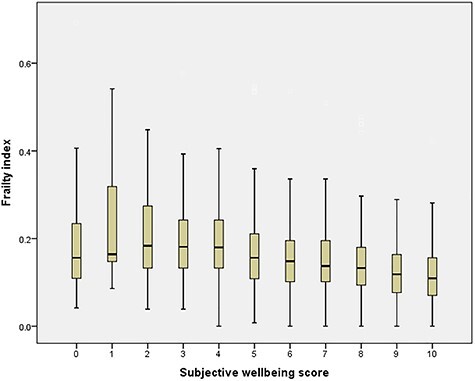
Association between Frailty Index and subjective wellbeing in HAALSI. Kruskal–Wallis test: P < 0.001.
Discriminant ability of the frailty index
Table 3 shows the c-statistic for the ability of the frailty index to predict death within a year from baseline. The frailty index alone was inferior to age and sex combined, but improved discriminant performance when added to age and sex. In sensitivity analyses allowing inclusion of those with fewer than 30 frailty variables (and hence allowing inclusion of those most likely to be frail), the discriminant performance of the frailty index improved, performing on its own as well as age and sex and showing good discriminant performance (c statistic of 0.81) when combined with age and sex. C-statistics derived from time to death showed similar values and patterns to those derived from one-year survival and are shown in Supplementary Table 3 (Supplementary data are available in Age and Ageing online).
Table 3 .
Discriminant ability for prediction of death by 1 year for frailty index in HAALSI
| Main analysis* (n = 3,910; 62 deaths) |
Allowing missing variables (n = 5,001; 133 deaths) |
|
|---|---|---|
| C-statistic (95% CI) | C-statistic (95% CI) | |
| Age and sex only | 0.728 (0.666–0.789) | 0.727 (0.684–0.770) |
| Frailty index only | 0.684 (0.617–0.751) | 0.763 (0.718–0.807) |
| Frailty index, age and sex | 0.771 (0.712–0.830) | 0.808 (0.770–0.846) |
*Excluding those with missing C-reactive protein or with fewer than 30 variables
Discussion
We were successful in creating a 32-variable frailty index using data from a wide range of variables in the HAALSI cohort. This number of variables met guidelines for frailty tools based on the cumulative deficits model [20]. The frailty index showed the expected gamma distribution, successfully predicted mortality and was associated with subjective wellbeing. The frailty index was able to discriminate between those alive and dead at 1-year follow-up and provided additional discriminant performance when added to age and sex; performance was similar to that of the Fried score in this cohort as previously reported [14]. Relaxing the constraints on the number of variables required to build the frailty index allowed a greater proportion of participants to be included in analyses (including more people with severe frailty) and improved its ability to predict mortality.
A small number of studies have estimated the prevalence of frailty in older populations in sub-Saharan African countries, using either the Fried frailty score [14, 23, 24], or a variant of a frailty index [15–17]. The prevalence of frailty depends heavily on the tool used, the methods used to derive the cutoffs, and the population studied; the cutoffs derived for the HAALSI cohort are considerably lower than the cutoffs derived for the UK eFI [21] highlighting the importance of deriving population and method-specific cutoffs. In most studies however, prevalence of frailty among older populations is substantial (>10%) and frailty becomes increasingly common at advanced age [25]. Whilst there is some interest in being able to compare frailty prevalence and outcomes between populations, the real value of frailty measurement to patients, researchers and healthcare systems is in identifying those at risk within a cohort, population or healthcare system [26]. This provides the foundation for developing appropriate interventions and healthcare strategies to prevent frailty and its consequences, to care appropriately for those with frailty, and to improve health and functional status in those with frailty. Whether or not to apply cutoffs, and what those cutoffs should be, will depend on the use to which a frailty index is put; there may, for example, be scant benefit in identifying a large segment of the older population as having frailty if no interventions can be deployed to mitigate the adverse consequences of frailty in large numbers of people. In HICs, cumulative deficit indices have also been shown to be superior to the Fried frailty score in predicting death [27]. Frailty indices typically encompass the components of the Fried score in addition to multiple other components, many of which would be expected to provide additional prognostic information.
Strengths and limitations
A strength of this study was the range of variables available to construct the frailty index, ranging from symptoms and signs, through measurements of physiology, blood tests and activities of daily living. Such an approach accords with the original ethos underpinning the creation of frailty indices as a measure of a broad range of deficits across all organ systems. Several variables used objective measures and agreed diagnostic criteria. These features make comparison with results obtained in HICs easier.
Only a limited number of conditions were sought in HAALSI, and only a limited number of blood tests were measured. Some diagnoses (e.g. heart failure and kidney disease) are very likely to be underestimated; others (e.g. thyroid disease) could not be sought. The lack of easy to access primary and secondary healthcare in the Agincourt area will also contribute to underdiagnosis of other self-reported conditions, and even when a diagnosis has been made, self-report (as opposed to medical notes review) depends on the diagnosis being communicated to the patient and the patient reporting the diagnosis to the research team. Some variables (for instance cognition score, walk speed, hand grip strength and physical activity score) did not have pre-established points to determine deficit cutoffs. As a result, the bottom quintile of the full HAALSI cohort had to be used to mark the point between acceptable health and a ‘deficit’. These variables would therefore have been affected by a normalisation effect where ~20% of the cohort scored a deficit regardless of the population’s health. This does not necessarily affect the index’s ability to predict poor outcomes, though it would negatively affect the comparability of these results with future studies unless identical cutoffs are used.
A further potential limitation of the study was that several HAALSI participants did not have an adequate number of variables to be included in secondary analysis. Frailty index literature currently suggests that no fewer than 30 variables should compose a participant’s frailty score [20]. However, we were able to address this issue through sensitivity analysis, and inclusion of participants with lower variable counts actually improved the predictive ability of the frailty index. This is likely due to the fact that those with missing data in HAALSI behave as though they are frail; in previous work, those participants for whom a Fried score could not be constructed due to missing data had comparable survival to those with frailty on the Fried score [14].
Use in practice
Frailty measurement has multiple potential uses in research and care for older people—including prognostication, and identification of at-risk groups for intervention. Where a large number of health and functional variables can be collected, creation of a cumulative deficits frailty index provides valuable information both for prognostic assessment and for population segmentation to target interventions at those most at risk of the consequences of the frailty syndrome. Such approaches have proven to be feasible in the UK and other HICs, with the use of country-specific cutoffs enabling this population segmentation to enable further assessment and intervention to be targeted to those above a given frailty index threshold. In resource-poor environments such as many LMIC healthcare systems, significant time and effort would be required to collect the multiple variables required for a frailty index and the marginally superior discriminant ability of the frailty index for mortality is unlikely to justify this additional effort. A more limited range of diagnoses may be able to be made in such settings, and construction of a frailty index with sufficient domains is likely to rely on self-reported diagnoses to a greater extent than would be the case in high-income settings. These limitations are likely to limit the applicability of a frailty index approach in practice. In such situations, simpler tools (for instance the Fried score or the Clinical Frailty Scale [28]) are more practical; local adaptations (an example being the B-FIT tool derived in a rural Tanzanian population; [29]) are available and provide an alternative approach. Further work is required however to test whether the frailty index offers superior discriminant performance over simple frailty tools for other important outcomes such as falls or future decline in physical function.
Future studies should focus on evaluation and comparison of frailty tools in other LMIC settings but also need to understand more about the lived experience of frailty in these environments. The cultural construct of frailty may vary across different societies [30], and this, along with other cultural differences (for instance in the role of older people, or views of exercise in maintaining health) will have a major impact on how those living with frailty can best be helped. At present, the strongest evidence for improving or mitigating frailty lies with exercise training [31], but methods to prevent and improve frailty for older people living in LMICs will require context-specific development, adaptation and testing. In parallel, healthcare systems in sub-Saharan Africa will need to adapt to deliver the care and interventions required by large numbers of older people living with frailty [32] to ensure that this rapidly growing sector of the population maintains health and function.
Supplementary Material
Acknowledgement
Dr Barker and Professor Witham acknowledge support from the NIHR Newcastle Biomedical Research Centre. ANW is supported by the Fogarty International Centre of the National Institutes of Health under Award Number K43TW010698. This paper describes the views of the authors and does not necessarily represent the official views of the National Institutes of Health (USA).
Contributor Information
Fred J Barker, AGE Research Group, NIHR Newcastle Biomedical Research Centre, Translational Clinical Research Institute, Newcastle University and Newcastle-upon-Tyne NHS Trust, Newcastle upon Tyne, UK.
Justine I Davies, Institute of Applied Health Research, University of Birmingham, Birmingham, UK; MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
F Xavier Gomez-Olive, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Kathleen Kahn, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Fiona E Matthews, Population Health Sciences Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK.
Collin F Payne, School of Demography, The Australian National University, Canberra, Australian Capital Territory, Australia.
Joshua A Salomon, Department of Medicine, Stanford University School of Medicine, Palo Alto CA, USA.
Stephen M Tollman, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Alisha N Wade, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Richard W Walker, Population Health Sciences Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK; Northumbria Healthcare NHS Foundation Trust, Tyne and Wear, UK.
Miles D Witham, AGE Research Group, NIHR Newcastle Biomedical Research Centre, Translational Clinical Research Institute, Newcastle University and Newcastle-upon-Tyne NHS Trust, Newcastle upon Tyne, UK; MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
National Institutes of Ageing (NIH) grant 1P01AG041710-01A1 National Dept of Science and Innovation (via South African Medical Research Council); Wellcome Trust (058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z).
References
- 1. Vermeiren S, Vella-Azzopardi R, Beckwée D et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 2016; 17: 1163.e1–17. [DOI] [PubMed] [Google Scholar]
- 2. Kojima G. Frailty as a predictor of nursing home placement among community-dwelling older adults: a systematic review and meta-analysis. J Geriatr Phys Ther 2018; 41: 42–8. [DOI] [PubMed] [Google Scholar]
- 3. Kojima G. Frailty as a predictor of disabilities among community-dwelling older people: a systematic review and meta-analysis. Disabil Rehabil 2017; 39: 1897–908. [DOI] [PubMed] [Google Scholar]
- 4. Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health 2016; 70: 716–21. [DOI] [PubMed] [Google Scholar]
- 5. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing 2017; 47: 193–200. [DOI] [PubMed] [Google Scholar]
- 6. Clegg A, Young J, Iliffe S, Olde Rikkert MGM, Rockwood K. Frailty in elderly people. Lancet 2013; 382: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394: 1365–75. [DOI] [PubMed] [Google Scholar]
- 8. He W, Goodkind D, Kowal PUS. Census Bureau, International Population Reports, P95/16-1, An Aging World: 2015. Washington, DC: U.S. Government Publishing Office, 2016. [Google Scholar]
- 9. Gray WK, Richardson J, McGuire J et al. Frailty screening in low- and middle-income countries: a systematic review. J Am Geriatr Soc 2016; 64: 806–23. [DOI] [PubMed] [Google Scholar]
- 10. Bor J, Herbst AJ, Newell M-L, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013; 339: 961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNFPA and Help Age International. Ageing in the Twenty-First Century A Celebration and A Challenge. New York, London: 2012. https://www.unfpa.org/public/home/publications/pid/11584 (31 August 2020, date last accessed) [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 13. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payne CF, Wade A, Kabudula CW et al. Prevalence and correlates of frailty in an older rural African population: findings from the HAALSI cohort study. BMC Geriatr 2017; 17: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biritwum RB, Minicuci N, Yawson AE et al. Prevalence of and factors associated with frailty and disability in older adults from China, Ghana, India, Mexico, Russia and South Africa. Maturitas 2016; 91: 8–18. [DOI] [PubMed] [Google Scholar]
- 16. Lewis EG, Wood G, Howorth K et al. Prevalence of frailty in older community-dwelling Tanzanians according to comprehensive geriatric assessment. J Am Geriatr Soc 2018; 66: 1484–90. [DOI] [PubMed] [Google Scholar]
- 17. Gray WK, Orega G, Kisoli A et al. Identifying frailty and its outcomes in older people in rural Tanzania. Exp Aging Res 2017; 43: 257–73. [DOI] [PubMed] [Google Scholar]
- 18. Gómez-Olivé FX, Montana L, Wagner RG et al. Cohort profile: health and ageing in Africa: a longitudinal study of an INDEPTH Community in South Africa (HAALSI). Int J Epidemiol 2018; 47: 689–90j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kahn K, Collinson MA, Gómez-Olivé FX et al. Profile: agincourt health and socio-demographic surveillance system. Int J Epidemiol 2012; 41: 988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clegg A, Bates C, Young J et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016; 45: 353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skevington SM, Böhnke JR. How is subjective well-being related to quality of life? Do we need two concepts and both measures? Soc Sci Med 2018; 206: 22–30. [DOI] [PubMed] [Google Scholar]
- 23. Witham M, Davies J, Bärnighausen T et al. Frailty and physical performance in the context of extreme poverty: a population-based study of older adults in rural Burkina Faso. Wellcome Open Res 2019; 4: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis EG, Coles S, Howorth K et al. The prevalence and characteristics of frailty by frailty phenotype in rural Tanzania. BMC Geriatr 2018; 18: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Caoimh R, Sezgin D, O’Donovan MR et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 2021; 50: 96–104. [DOI] [PubMed] [Google Scholar]
- 26. Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age Ageing 2015; 44: 545–7. [DOI] [PubMed] [Google Scholar]
- 27. Kulminski AM, Ukraintseva SV, Kulminskaya IV et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the cardiovascular health study. J Am Geriatr Soc 2008; 56: 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis EG, Whitton LA, Collin H et al. A brief frailty screening tool in Tanzania: external validation and refinement of the B-FIT screen. Aging Clin Exp Res 2020; 32: 1959–67. [DOI] [PubMed] [Google Scholar]
- 30. Kaufman SR. The social construction of frailty: an anthropological perspective. J Aging Stud 1994; 8: 45–58. [Google Scholar]
- 31. Negm AM, Kennedy CC, Thabane L et al. Management of frailty: a systematic review and network meta-analysis of randomized controlled trials. J Am Med Dir Assoc 2019; 20: 1190–8. [DOI] [PubMed] [Google Scholar]
- 32. Onen BL, Harris C, Ignatowicz A et al. Ageing, frailty and resilience in Botswana: rapid ageing, rapid change. Findings from a national working group meeting and literature review. BMC Proc 2019; 13: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


