Abstract
Background
end-of-life care is not always in line with end-of-life preferences, so patients do not always die at their preferred place of death (PPD). This study aims to identify factors associated with patients’ PPD and changes in PPD.
Methods
we prospectively collected data on PPD at four time points within 6 months from 230 acutely hospitalised older patients who were part of the control group in a stepped-wedge randomised controlled trial. Associations between patient characteristics and preferences were calculated using multivariable (multinomial) logistic regression analysis.
Results
the mean age of participants was 80.7 years. 47.8% of the patients had no PPD at hospital admission. Patients previously admitted to hospital preferred to die at home (home versus no preference: odds ratio [OR] 2.38, 95% confidence interval [CI] 1.15–4.92; home versus healthcare facility: OR 3.25, 95% CI 1.15–9.16). Patients with more chronic diseases preferred the healthcare facility as their PPD (healthcare facility versus no preference: OR 1.33, 95% CI 1.09–1.61; healthcare facility versus home: OR 1.21, 95% CI 1.00–1.47). 32 of 65 patients changed their preference during follow-up, and most of these had no PPD at hospital admission (home versus no preference: OR 0.005, 95% CI ≤0.001–0.095) and poorer self-rated well-being (OR 1.82, 95% CI 1.07–3.08).
Conclusions
almost half of the patients had no PPD at baseline. Previous hospital admission, having more chronic diseases and living alone are associated with having a PPD. Introducing PPD could make older people aware of PPD and facilitate optimal palliative care.
Keywords: palliative care, preferred place of death, older people
Key Points
Patients are often unaware of their preferred place of death until someone introduces this topic for consideration.
Due to unawareness, some patients die before they consider their end-of-life preferences.
Alone living patients, patients with more chronic diseases and patients who were not admitted to hospital are more often unaware.
Background
Palliative care sets out to preserve the best possible quality of life (QoL) until death. One of the common values of palliative care is patients’ autonomy. Ideally, patients should be empowered to make decisions about their place of care, treatment options and access to specialist palliative care [3]. To make end-of-life decisions, patients should be provided with adequate information on diagnosis, prognosis and treatment options. Another important goal of palliative care is to preserve the patients’ dignity.
Facilitating care in line with end-of-life preferences is important when providing palliative care [4, 5]. However, end-of-life preferences are not always in congruence with end-of-life care. As a result, patients can experience unwanted care transitions at the end of life [6], high symptom burden [7], reduced QoL and not dying in their preferred place of death (PPD) [4, 6, 8–11]. These unfulfilled end-of-life preferences are caused by several factors. Patients’ palliative care needs are often not identified in a timely manner [12], and healthcare professionals may find it hard to initiate end-of-life conversations [13]. As a result, healthcare professionals can be unaware of patients’ end-of-life preferences.
An important end-of-life preference is the place where patients want to receive end-of-life care and eventually die. Dying at the PPD is an important indicator of good palliative care [14]. Several patient characteristics have been associated with the PPD. These include demographic factors such as age, gender, marital status, education and income level, physical and mental health, and concerns and beliefs about dying [10]. Living arrangements and wishes of family and loved ones were also associated with preferences [15, 16]. However, the factors associated with not having a PPD and changes in PPD are not well known. Previous retrospective studies found that changes in health condition, symptoms and performance status, family’s wishes and the fear of being a burden to relatives were associated with changes in PPD [17].
Knowing which factors influence older patients’ PPD and changes in PPD, especially in those patients who have no preference, could help healthcare professionals to identify which patients should be introduced to the concept of a PPD. This may eventually help patients to die in their preferred place. The aim of this study is to provide insight into the association of demographic, illness-related and environmental factors with PPD and changes in the PPD of older patients in the Netherlands.
Methods
We identified factors associated with PPD and changes in the PPD in an exploratory quantitative study using data from the care-as-usual phase of the PalliSupport study [18]. The PalliSupport study is a pragmatic multicentre stepped-wedge randomised controlled trial in which five hospitals and surrounding regions participated. The primary objective of this study was to evaluate the PalliSupport care pathway, which intends to improve care for older patients with palliative care needs who are acutely admitted to hospital [19]. From January 2019 to March 2020, we approached eligible patients for participation (Box 1).
Data were collected from electronic medical records (EMRs) and patient questionnaires (filled in either by the patients themselves or with the help of a researcher).
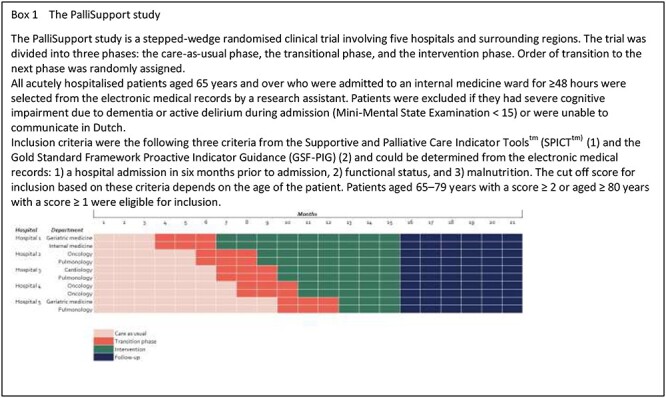
Ethical considerations
The PalliSupport study was approved by the Institutional Review board of the Amsterdam University Medical Centre at the Academic Medical Centre in the Netherlands (Protocol ID: METC2018_216). All participants provided written informed consent.
Dependent variables
The dependent variables in this study were PPD and changes in PPD. We asked the patient what their PPD was in a structured interview conducted during hospital admission. We categorised PPD into three groups: home, healthcare facility (hospital hospice, nursing home) and no preference. Changes in PPD were monitored at four points during follow-up (at 2 weeks, 1 month, 3 months and 6 months after hospital discharge). Patients were asked if their PPD had changed since the last interview, and if so, what the new PPD was.
Patients with no or unknown preference were analysed as one group because patients seemed to find it difficult to distinguish between not having a preference and not knowing their preference. Although some of these patients may have indeed had no PPD, we did not find differences in patient characteristics between these groups so decided to combine these patients into one group.
Independent variables
We selected independent factors a priori, based on the model of Gomes and Higginson [16], which describes demographic, illness-related and environmental factors.
Illness-related factors contained health-related QoL outcomes using the EuroQol-5D-5L, which measures mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [20] and the EQ-VAS, which measures patients’ self-rated health on a scale from 0 to 100. We also used the McGill QoL Questionnaire, which measures QoL in physical, psychological, support and existential domains in patients with a life-threatening illness [21]. Finally, we used the Dutch version of the Edmonton Symptom Assessment Scale to measure self-rated symptom burden [22]. All other illness-related factors were obtained through the EMR. These included emergency room (ER) visits and hospital admissions in the 6 months before admission; chronic conditions measured by the Charlson comorbidity index [23]; and the main diagnosis, which was not necessarily the reason for admission. We categorised the diagnoses into three groups: ‘cancer’, ‘organ failure’ and ‘frailty and neurological problems’. Medication use was dichotomised into <5 and ≥5 medicines (with ≥5 medicines identified as polypharmacy).
Demographic factors included age and gender. Ethnicity was not analysed since almost all patients were born in the Netherlands.
Environmental factors were obtained from the participant. We recorded marital status (which was dichotomised into married/living together or unmarried/divorced/widowed) and living arrangements (which was registered as living independently and living with home care).
Statistical analysis
We calculated frequencies and percentages. To analyse differences between these categories and independent variables, we used one-way ANOVA, Fisher’s exact test, and Kruskal–Wallis test. If a statistically significant difference was detected (P value ≤0.05), we analysed the association between patient characteristics and PPD using multinomial logistic regression. To determine whether factors were independently associated with PPD, we performed multivariable multinomial logistic regression including all factors with P values <0.10. We chose this cut-off point based on the sample size [24].
We calculated frequencies and percentages for changes in PPD and analysed differences between groups that did and did not change preference using independent T-test, Fisher’s exact test and Mann–Whitney U test. To estimate associations between significantly different independent variables (P value ≤0.05) and changing preferences, we did a logistic regression analysis. Multivariable logistic regression included all variables with P values <0.10 in logistic regression to identify which variables were independently associated with changes in PPD.
For both outcomes, we reported odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Statistical analysis was performed using SPSS version 26.
Results
We included 230 patients from the care-as-usual phase of the stepped-wedge randomised trial PalliSupport (Table 1). For 178 patients, we collected data on the PPD at baseline.
Table 1.
Baseline characteristics
| PPD at hospital admission | |||||
|---|---|---|---|---|---|
| Total (n = 178) | Home (n = 72) | Healthcare facility (n = 21) | No preference/not considered (n = 85) | P-value | |
| Age, mean (SD) | 80.7 (8.4) | 79.9 (9.0) | 78.2 (7.3) | 81.9 (7.9) | 0.18a |
| Gender male, N (%) | 77 (43.3) | 36 (50) | 10 (47.6) | 31 (36.5) | 0.28b |
| Marital status, N (%) | 0.21b | ||||
| Married/living together | 76 (42.7) | 36 (50.0) | 8 (38.1) | 32 (37.6) | |
| Unmarried/divorced/widowed | 100 (56.2) | 34 (47.2) | 13 (61.9) | 53 (62.4) | |
| Living arrangement, N (%) | 0.47b | ||||
| Independent | 89 (50.0) | 34 (47.2) | 13 (61.9) | 42 (49.4) | |
| With home care | 89 (50.0) | 38 (52.8) | 8 (38.1) | 43 (50.6) | |
| Informal caregiver involved, N (%) | 83 (46.6) | 38 (52.8) | 7 (33.3) | 38 (44.7) | 0.72b |
| Primary diagnose, N (%) | 0.45b | ||||
| Cancer | 56 (31.5) | 26 (36.1) | 9 (42.9) | 21 (24.7) | |
| Organ failure | 59 (33.1) | 25 (34.7) | 6 (28.6) | 28 (32.9) | |
| Frailty/neurological problems | 52 (29.8) | 16 (22.2) | 6 (28.6) | 31 (36.5) | |
| Polypharmacy, N (%) | 159 (89.3) | 61 (84.7) | 20 (95.2) | 78 (91.8) | 0.46b |
| Hospital admission in the last half year, N (%) | 87 (48.9) | 44 (62.9) | 9 (42.9) | 34 (40.0) | 0.01b |
| ER visit in the last half year, N (%) | 117 (65.7) | 51 (70.8) | 11 (52.4) | 55 (64.7) | 0.19b |
| Charlson comorbidity index, median [IQR] | 3 [1–6] | 3 [0–11] | 4[2–7.5] | 2[2–4] | 0.02c |
| KATZ risk score ≥2, N (%) | 106 (59.6) | 41 (56.9) | 14 (66.7) | 51 (60.0) | 0.56b |
| Delirium risk score ≥1, N (%) | 88 (49.4) | 33 (45.8) | 12 (57.1) | 43 (50.6) | 0.37b |
| Nutrition risk score ≥2, N (%) | 84 (47.2) | 31 (43.1) | 12 (57.1) | 41 (48.2) | 0.51b |
| Falls In the past half year, N (%) | 64 (36.0) | 25 (34.7) | 8 (38.1) | 31 (36.5) | 0.97b |
| EQ-VAS, mean (SD) | 52.9 (18.5) | 52.4 (18.1) |
50.9 (14.9) | 53.9 (19.9) | 0.64a |
| McGill overall QoL score, mean (SD) | 6.1 (1.9) | 6.2 (1.9) | 5.5 (1.6) | 6.2 (2.1) | 0.17a |
| ESAS, median [IQR] | |||||
| Pain | 4 [0–6] | 3 [0–6] | 5 [1.5–6] | 3[0–6] | 0.45c |
| Tiredness | 6 [3–8] | 5 [2.8–7.3] | 5 [2–8.5] | 6 [3–8] | 0.57c |
| Nausea | 0 [0–2] | 0 [0–3] | 0 [0–4.5] | 0 [0–1] | 0.23c |
| Depression | 0 [0–4] | 0 [0–4] | 2 [0–3.5] | 0 [0–5] | 0.91c |
| Anxiety | 0 [0–5] | 1 [0–5] | 1 [0–4] | 0 [0–5] | 0.66c |
| Drowsiness | 0 [0–4] | 0 [0–4] | 1 [0–4.8] | 0 [0–4] | 0.82c |
| Appetite | 5 [2–7] | 5 [1–7.5] | 5 [4–8] | 5 [2–7] | 0.79c |
| Feeling of well-being | 5 [3–6] | 5 [3–6] | 5 [3–7.5] | 5 [3–6] | 0.75c |
| Shortness of breath | 3 [0–6] | 2 [0–5] | 3 [0–7.5] | 3 [0–6] | 0.41c |
| Obstipation | 0 [0–4] | 0 [0–5] | 0 [0–4] | 0 [0–3] | 0.24c |
| Vomiting | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–03] | 0.15c |
| Sleeping problems | 4 [1–7] | 4 [0–7] | 5 [3–7.5] | 3 [2–7] | 0.53c |
| Ability to move around | 6 [4–8] | 6 [4–8] | 8 [5–8] | 6 [4–8] | 0.39c |
| Confusion | 0 [0–0] | 0 [0–0] | 0 [0–0.5] | 0 [0–0] | 0.43c |
| Dry mouth | 6 [2–8] | 5 [8] | 6 [0–9] | 6 [2–8] | 0.56c |
| Changed PPD, N (%) | 32 (49.2) | 2 (3.7) | 5 (62.5) | 22 (88) | <0.001b |
| Deceased within 6 months after hospitalisation, N (%) | 55 (30.9) | 22 (30.6) | 8 (38.1) | 25 (29.4) | 0.66b |
aOne-way ANOVA, bFisher’s exact test, cKruskal–Wallis test. IQR, interquartile range; SD, standard deviation.
Patients had a mean age of 80.7 years (SD 8.4), and there were slightly more females (56.7%). Most participants had organ failure as their main diagnosis (33.1%) and used more than five drugs (89.3%). Most patients were unmarried/living alone/widowed (56.2%) and half of the patients received home care (50%). Half of the patients were admitted to the hospital in the half year before admission and 65.7% had visited the ER. In total, 55 (30.9%) patients died within the 6-month follow-up after hospital discharge. Most patients (47.8%) had no PPD at hospital admission, and 40.4% preferred to die at home. Only 11.8% preferred to die in a healthcare facility (hospital, hospice, nursing home) (Table 1).
Factors associated with the PPD
We found statistically significant differences in Charlson comorbidity score index and prior hospitalisation based on the patients’ PPD at admission. Patients who had no preference had lower Charlson comorbidity index scores, and these patients with more chronic diseases were most likely to prefer to die in a healthcare facility (healthcare facility versus no preference: OR 1.33, 95% CI 1.09–1.61; healthcare facility versus home: OR 1.21, 95% CI 1.00–1.47). Patients who were hospitalised in the past half year were more likely to prefer to die at home (home versus no preference: OR 2.38, 95% CI 1.15–4.92; home versus healthcare facility: OR 3.25, 95% CI 1.15–9.16). In all three PPD groups, the proportion of patients who died within 6 months after hospitalisation was between 30 and 38%. No statistically significant difference was found between these groups (Table 2).
Table 2.
Multinomial logistic regression for PPD at hospital admission
| Home versus no preference/not considered yet | Home versus healthcare facility | Healthcare organisation versus no preference/not considered | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR | Adjusted OR | Unadjusted OR | Adjusted OR | Unadjusted OR | Adjusted OR | |||||||||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Charlson comorbidity index | 1.13 | 0.99–1.29 | 0.08 | 1.1 | 0.97–1.27 | 0.14 | 1.13 | 0.95–1.34 | 0.17 | 0.82 | 0.68–0.99 | 0.05 | 1.33 | 1.09–1.61 | <0.01 | 1.35 | 1.11–1.64 | <0.01 |
| Prior hospital admission | 2.52 | 1.31–4.85 | <0.01 | 2.38 | 1.15–4.92 | 0.02 | 2.80 | 1.03–7.68 | 0.04 | 3.25 | 1.15–9.16 | 0.03 | 0.89 | 0.33–2.39 | 0.83 | 0.74 | 0.26–2.08 | 0.57 |
Change in PPD
For 65 patients (37%), changes in PPD were observed at least once during follow-up. Eleven patients died during hospitalisation so were not followed up. All other missing data were due to patients not responding to follow-up. In our study sample, 32 patients changed their PPD during follow-up. Most patients who changed their PPD had no preference at admission and decided that their PPD was at home during follow-up (Figure 1).
Figure 1.
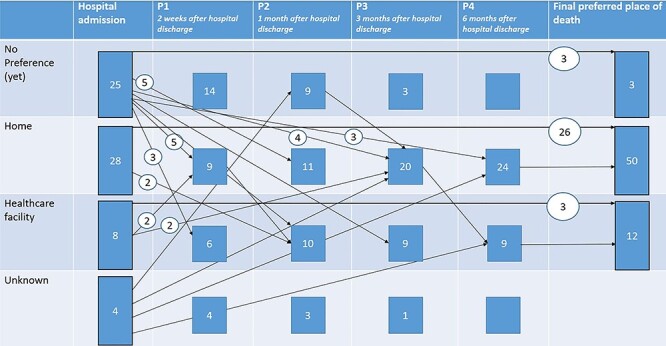
Changes in preference during follow-up. This figure represents the course of change in PPD for all patients for whom data on change in PPD were collected. Final PPD represents the last reported PPD, after which patient did not change their preference anymore.
Factors associated with changes in the PPD
Characteristics of patients who changed their preference were significantly different from those of patients who did not change their preference. These characteristics were marital status, ER visits and hospital admission in the past half year, self-rated well-being and self-rated dry mouth complaints. Logistic regression showed that patients who were unmarried/living alone/widowed were more likely to change their preference over time (OR 3.65, 95% CI 1.3–10.23). Patients who visited the ER in the past 6 months (OR 0.23, 95% CI 0.07–0.75) and patients who were admitted to hospital in the past half year (OR 0.29, 95% CI 0.11–0.83) were less likely to change their preference over time. However, these variables did not remain statistically significant after multivariable logistic regression analysis. Multivariable logistic regression identified self-rated well-being and an initial PPD as variables that were independently associated with a changing preference. Patients with worse well-being were more likely to change their preference over time (OR 1.82, 95% CI 1.07–3.08). Patients who had no preference at baseline were most likely to change their preference, whereas patients who preferred to die at home were very unlikely to change their preference (home versus no preference: OR 0.005, 95% CI <0.001–0.095). After multivariable logistic regression, the association between healthcare facility versus no preference was not statistically significant (healthcare facility versus no preference: OR 0.19, 95% CI 0.015–2.37) (Table 3).
Table 3.
Logistic regression for factors associated with changing PPD
| Changing preference over time | Changing preference over time | |||||
|---|---|---|---|---|---|---|
| Unadjusted OR | Adjusted ORa | |||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Marital status Reference category: married/living together |
3.65 | 1.30–10.23 | 0.01 | 1.36 | 0.13–14.0 | 0.79 |
| Prior hospital admission | 0.29 | 0.11–0.83 | 0.02 | 0.32 | 0.032–3.07 | 0.32 |
| ER visit in the past half year | 0.23 | 0.07–0.75 | 0.02 | 0.34 | 0.035–3.16 | 0.34 |
| ESAS wellbeing | 1.46 | 1.11–1.92 | <0.01 | 1.82 | 1.07–3.08 | 0.03 |
| ESAS dry mouth | 1.14 | 0.98–1.34 | 0.10 | |||
| Place of preference Home Healthcare facility Reference category: no preference |
0.005 0.24 |
0.001–0.057 0.04–1.55 |
<0.01 0.13 |
0.005 0.19 |
<0.001–0.095 0.015–2.37 |
<0.01 0.20 |
All variables except ESAS dry mouth were included in the adjusted multivariable logistic regression analysis. ESAS, Edmonton Symptom Assessment Scale.
Discussion
In this study, we explored factors associated with PPD and changes in PPD in acutely hospitalised older patients living at home in the Netherlands using EMRs and questionnaires. Our results suggest that most patients have no PPD when asked about it for the first time followed by the preference to die at home. Patients with multiple chronic diseases and patients who were admitted to hospital in the past half year were more likely to have a PPD at hospital admission. Patients with poorer self-rated health and patients who had no PPD were most likely to change their preferences over time, in most cases choosing home as their PPD. Neither having a PPD nor the PPD itself was associated with death within 6 months, indicating that some patients died before choosing their PPD.
Interpretation of findings
In previous studies, over 60% of patients preferred to die at home [16, 25, 26]. This proportion was lower in our study, possibly because we included the option ‘no preference’. In previous studies, patients with poor self-rated health were less likely to prefer to die at home [10, 27]. In support of this, we found that patients with more chronic diseases were more likely to prefer to die in a healthcare facility than at home. Having more chronic diseases is expected to negatively influence patients’ self-rated health, supporting our finding that patients with more chronic diseases choose a healthcare facility as their PPD.
Similar to previous studies [15, 16, 28], our patients changed their PPD over time, although in smaller proportions. Patients with more severe illness and patients who planned their care in advance are found to have more stable end-of-life preferences [29]. We believe that this was the first time many of our participants were asked about their PPD, which could explain the high proportion of patients with no PPD. The question might have prompted them to consider this topic, leading to a change in PPD over time. Our finding that patients without a PPD were not admitted to hospital in the past half year may support this reasoning, since the PPD might have already been discussed during previous hospital visits. Furthermore, previous hospital admissions may reflect a more advanced or severe stage of disease, meaning end-of-life options may already have been considered.
Patients who were unmarried/living alone/widowed were more likely to change their preference over time. Although this association did not remain significant after correcting for the PPD, we believe this finding is important because it indicates that patients without a PPD often lived alone. End-of-life preferences are often discussed and decided with loved ones [30], which patients who live alone cannot do.
The association we found between poorer self-rated well-being and changing preferences appears contradictory at first sight. According to our above-mentioned reasoning, we would expect patients with poor self-rated well-being to be more stable about their preferences. This discrepancy might be because this was the first time the question was asked. We asked patients about their preferences during an acute hospital admission, where they may not have had the energy or desire to answer this (potentially unexpected) question. Asking about the PPD when acute hospitalisation was over might have given patients more time and ease to consider the topic, especially those patients with poorer self-rated health. Although we do not know how patients rated their well-being at the moment of change, our findings suggest that well-being is related to the change in preferences and highlight the importance of monitoring patients’ well-being and symptom burden over time.
Our finding that patients can change their PPD over time highlights the importance of discussing patients’ preferences and following up on this to monitor any changes in their wishes. Proportions of deceased patients were similar for patients with and without a PPD, suggesting that some patients did not realise they were nearing the end-of-life and died before they could consider their preferences. This highlights the importance of providing adequate information concerning diagnosis and prognosis and discussing end-of-life preferences at an early stage. The fact that patients who primarily claimed to have no preference but changed their mind over time indicates a willingness to consider the topic. Knowing that patients who have not been admitted to hospital before, patients with less chronic diseases and patients who live alone are less likely to have a PPD, healthcare professionals could make an extra effort to discuss end-of-life preferences with these patients, giving them the chance to think about and discuss their preferences and ultimately to die where they want to.
Strengths and limitations
The strength of this study is that we collected data prospectively, in contrast to most previous studies which collected data retrospectively. We also included older patients with organ failure and included no preference as an outcome. Since many patients had not been asked about PPD before, including having no preference as an outcome was a valuable addition. This provides insight into factors associated with end-of-life preferences and highlights that patients are willing to consider their PPD.
Our study also has some limitations. First, although the longitudinal data collection was a strength, we do recognise that we measured sociodemographic and illness-related variables during hospital admission when the patients were sick. It is possible that some of these factors, such as daily functioning and symptom burden, changed after hospital admission and that this could have had an impact on the results. However, van Seben et al. concluded that many geriatric syndromes, such as mobility impairment, are likely to continue after hospital discharge [31].
Second, we faced known difficulties in studies concerning patients with palliative care needs [32]. We were not able to approach all eligible patients since some were considered not healthy enough to participate. This resulted in selection bias as patients with more severe symptoms were not included. Furthermore, response rates were low. Patients who reported more tiredness, drowsiness and appetite complaints were less likely to respond to follow-up. These symptoms reflect a need for palliative care and highlight the difficulties in following up on these patients. However, self-rated health was not different between groups, indicating that participants gave a proper reflection of the study population.
Third, almost all patients in our study sample were Dutch. This does not represent the total population of older people living in the Netherlands, 16% of whom are not Dutch [33], so our findings may not be generalizable to all older people living at home in the Netherlands. To improve generalisability, further research is warranted with a larger scope. Older people living at home and in care facilities as well as both acute and long-term care settings should be included. Furthermore, follow-up on baseline characteristics will provide more detailed information on PPD changes over time. More insight into the actual place of death would provide a more complete picture of the end-of-life phase and whether end-of-life preferences are achieved.
In conclusion, our study shows that asking patients about their PPD encourages them to consider their end-of-life options. This could improve healthcare as not knowing or refusing to discuss these preferences may increase the likeliness of being admitted to hospital for end-of-life care. Knowing whether a patient is willing to consider their end-of-life options will help healthcare professionals to initiate discussions about the PPD.
Acknowledgements
We are grateful to all the participants and all the nurses and physicians who were involved in the PalliSupport study and all the research students and nurses who were involved in the recruitment of patients and data collection, in particular Ans van Driel.
Contributor Information
Iris van Doorne, Section of Geriatric Medicine, Department of Internal Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands.
Marjon van Rijn, Section of Geriatric Medicine, Department of Internal Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; Faculty of Health, Center of Expertise Urban Vitality, Amsterdam University of Applied Science, Amsterdam, The Netherlands; Department of Medicine for Older People, Amsterdam Public Health Research Institute, Amsterdam UMC - Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Sjoerd M Dofferhoff, Section of Geriatric Medicine, Department of Internal Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands.
Dick L Willems, Section of Medical Ethics, Department of General Practice, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands.
Bianca M Buurman, Section of Geriatric Medicine, Department of Internal Medicine, Amsterdam Public Health Research Institute, Amsterdam University Medical Center, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; Faculty of Health, Center of Expertise Urban Vitality, Amsterdam University of Applied Science, Amsterdam, The Netherlands; Department of Medicine for Older People, Amsterdam Public Health Research Institute, Amsterdam UMC - Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This study was funded by ZonMw (The Netherlands Organisation for Health Research and Development), grant number 844001103. This study was part of a larger research project.
References
- 1. Highet G, Crawford D, Murray SA et al. Development and evaluation of the Supportive and Palliative Care Indicators Tool (SPICT): a mixed-methods study. BMJ Support Palliat Care 2014; 4: 285–90. [DOI] [PubMed] [Google Scholar]
- 2. Mudge AM, Douglas C, Sansome X et al. Risk of 12-month mortality among hospital inpatients using the surprise question and SPICT criteria: a prospective study. BMJ Support Palliat Care 2018; 8: 213–20. [DOI] [PubMed] [Google Scholar]
- 3. Radbruch L, & Payne S. White Paper on standards and norms for hospice and palliative care in Europe : part 2. Eur J Palliat Care 2010; 17: 22–33. http://www.ejpc.eu.com/ejpc/ejpcIssue.asp?Z=597622&IssueID=101. [Google Scholar]
- 4. Bell CL, Somogyi-Zalud E, Masaki KH. Factors associated with congruence between preferred and actual place of death. J Pain Symptom Manag 2010; 39: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weitzen S, Teno JM, Fennell M et al. Factors associated with site of death: a national study of where people die. Med Care 2003; 41: 323–35. [DOI] [PubMed] [Google Scholar]
- 6. Abarshi E, Echteld M, Van den Block L et al. Transitions between care settings at the end of life in the Netherlands: results from a nationwide study. Palliat Med 2010; 24: 166–74. [DOI] [PubMed] [Google Scholar]
- 7. Merchant SJ, Brogly SB, Booth CM et al. Palliative Care and Symptom Burden in the Last Year of Life: a population-based study of patients with gastrointestinal cancer. Ann Surg Oncol 2019; 26: 2336–45. [DOI] [PubMed] [Google Scholar]
- 8. Fried TR, van Doorn C, O'Leary JR et al. Older persons’ preferences for site of terminal care. Ann Intern Med 1999; 131: 109–12. [DOI] [PubMed] [Google Scholar]
- 9. Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med 2000; 3: 287–300. [DOI] [PubMed] [Google Scholar]
- 10. Foreman LM, Hunt RW, Luke CG et al. Factors predictive of preferred place of death in the general population of South Australia. Palliat Med 2006; 20: 447–53. [DOI] [PubMed] [Google Scholar]
- 11. Tang ST, McCorkle R. Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care 2003; 19: 230–7. [PubMed] [Google Scholar]
- 12. Glaudemans JJ, Moll van Charante EP, Willems DL. Advance care planning in primary care, only for severely ill patients? A structured review. Fam Pract 2015; 32: 16–26. [DOI] [PubMed] [Google Scholar]
- 13. Periyakoil VS, Neri E, Kraemer H. No easy talk: a mixed methods study of doctor reported barriers to conducting effective end-of-life conversations with diverse patients. PLoS One 2015; 10: e0122321. 10.1371/journal.pone.0122321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boddaert M. Kwaliteitskader palliatieve zorg Nederland. 2017. https://www.pallialine.nl/index.php?pagina=/richtlijn/item/pagina.php&id=41753&richtlijn_id=1078 (30 December 2020, date last accessed).
- 15. Brogaard T, Neergaard MA, Sokolowski I et al. Congruence between preferred and actual place of care and death among Danish cancer patients. Palliat Med 2013; 27: 155–64. [DOI] [PubMed] [Google Scholar]
- 16. Gomes B, Higginson IJ, Calanzani N et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012; 23: 2006–15. [DOI] [PubMed] [Google Scholar]
- 17. Win MM, Fischer A, Good P. The pattern and timing of changes in preferred place of death for patients admitted to a community specialist palliative care service. Prog Palliat Care 2019; 27: 4–9. [Google Scholar]
- 18. van Rijn M, Flierman I, Willems D, Buurman B. PalliSupport-The development, feasibility and implementation of a transitional integrated care pathway for older patients with palliative care needs. Int J Integr Care 2019; 19: 412 doi: 10.5334/ijic.s3412. [DOI] [Google Scholar]
- 19. Flierman I, van Rijn M, de Meij M et al. Feasibility of the PalliSupport care pathway: results from a mixed-method study in acutely hospitalized older patients at the end of life. Pilot Feasibility Stud 2020; 6: 129. 10.1186/s40814-020-00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–43. [DOI] [PubMed] [Google Scholar]
- 21. Cohen SR, Mount BM, Strobel MG et al. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 1995; 9: 207–19. [DOI] [PubMed] [Google Scholar]
- 22. Bruera E, Kuehn N, Miller MJ et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991; 7: 6–9. [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 24. Peduzzi P, Concato J, Kemper E et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–9. [DOI] [PubMed] [Google Scholar]
- 25. Koekoek B. Regie over de plaats van sterven- een kwantitatieve en kwalitatieve verkenning 2014. https://www.vptz.nl/onderzoek-publicaties/regie-plaats-sterven-kwantitatieve-en-kwalitatieve-verkenning/ (25 January 2021, date last accessed).
- 26. Abarshi E, Onwuteaka-Philipsen B, Donker G et al. General practitioner awareness of preferred place of death and correlates of dying in a preferred place: a nationwide mortality follow-back study in the Netherlands. J Pain Symptom Manag 2009; 38: 568–77. [DOI] [PubMed] [Google Scholar]
- 27. Ohmachi I, Arima K, Abe Y, Nishimura T, Goto H, Aoyagi K. Factors influencing the preferred place of death in community-dwelling elderly people in Japan. Int J Gerontol 2015; 9: 24–8. [Google Scholar]
- 28. Evans R, Finucane A, Vanhegan L et al. Do place-of-death preferences for patients receiving specialist palliative care change over time? Int J Palliat Nurs 2014; 20: 579–83. [DOI] [PubMed] [Google Scholar]
- 29. Auriemma CL, Nguyen CA, Bronheim R et al. Stability of end-of-life preferences: a systematic review of the evidence. JAMA Intern Med 2014; 174: 1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abba K, Lloyd-Williams M, Horton S. Discussing end of life wishes – the impact of community interventions? BMC Palliat Care 2019; 18: 26. 10.1186/s12904-019-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Seben R, Reichardt LA, Aarden JJ et al. The course of geriatric syndromes in acutely hospitalized older adults: The Hospital-ADL Study. J Am Med Dir Assoc 2019; 20: 152–158.e2. [DOI] [PubMed] [Google Scholar]
- 32. Sherman DW, McSherry CB, Parkas V et al. Recruitment and retention in a longitudinal palliative care study. Appl Nurs Res 2005; 18: 167–77. [DOI] [PubMed] [Google Scholar]
- 33. Statline . Bevolking; geslacht, leeftijd en nationaliteit op 1 januari. 2020. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/03743/table?fromstatweb (30 December 2020, date last accessed).


