Abstract
Background
the aim of this study was to examine the cross-sectional and longitudinal associations of different multimorbidity patterns with physical frailty in older adults.
Methods
we used data from the Swedish National study on Aging and Care in Kungsholmen to generate a physical frailty measure, and clusters of participants with similar multimorbidity patterns were identified through fuzzy c-means cluster analyses. The cross-sectional association (n = 2,534) between multimorbidity clusters and physical frailty was measured through logistic regression analyses. Six- (n = 2,122) and 12-year (n = 2,140) longitudinal associations were determined through multinomial logistic regression analyses.
Results
six multimorbidity patterns were identified at baseline: psychiatric diseases; cardiovascular diseases, anaemia and dementia; sensory impairments and cancer; metabolic and sleep disorders; musculoskeletal, respiratory and gastrointestinal diseases; and an unspecific pattern lacking any overrepresented diseases. Cross-sectionally, each pattern was associated with physical frailty compared with the unspecific pattern. Over 6 years, the psychiatric diseases (relative risk ratio [RRR]: 3.04; 95% confidence intervals [CI]: 1.59–5.79); cardiovascular diseases, anaemia and dementia (RRR 2.25; 95% CI: 1.13–4.49) and metabolic and sleep disorders (RRR 1.99; 95% CI: 1.25–3.16) patterns were associated with incident physical frailty. The cardiovascular diseases, anaemia and dementia (RRR: 4.81; 95% CI: 1.59–14.60); psychiatric diseases (RRR 2.62; 95% CI: 1.45–4.72) and sensory impairments and cancer (RRR 1.87; 95% CI: 1.05–3.35) patterns were more associated with physical frailty, compared with the unspecific pattern, over 12 years.
Conclusions
we found that older adults with multimorbidity characterised by cardiovascular and neuropsychiatric disease patterns are most susceptible to developing physical frailty.
Keywords: multimorbidity, frailty, older people, longitudinal population-based study, personalised medicine
Key Points
Older multimorbid adults characterised by cardiovascular and neuropsychiatric disease patterns are at higher frailty risk.
Multimorbidity patterns are differentially associated with incident physical frailty.
Not all older adults with multimorbidity are physically frail.
Introduction
Multimorbidity is generally understood to be the presence of two or more chronic conditions in one individual [1]. Its prevalence in older adults varies from 55 to 98%, depending on the definition utilised and population studied [2]. Multimorbidity is associated with lowered quality of life [2], disability [2] and mortality [3], as well as extensive healthcare utilisation [2], prolonged hospital stays [4] and complex pharmacological regimes [5, 6].
Along with multimorbidity, frailty limits health and survival in old age. Frailty is characterised by an accelerated ageing of organs and bodily systems [7]. When confronted with minor stressors, frail persons experience disproportionate negative health outcomes [7]. Frail individuals are at increased risk for mortality [8, 9], hospitalisation [7], falls [10], long-term care [8], loneliness [11] and reduced quality of life [12]. A 2020 meta-analysis found physical frailty’s prevalence to be 12% in adults aged 50 and above [13].
An association between multimorbidity and frailty has been previously established [14–20]. A 2019 meta-analysis found that 72% of frail individuals exhibit multimorbidity, but only 16% of multimorbid persons have frailty [21]. It is unclear why some with multimorbidity maintain robust health states while others experience frailty. An explanation could be that most studies have defined multimorbidity by disease count, which is a poorly discriminative measure [2]. Psychosocial, biological, pharmacological and other health factors heighten one’s likelihood of developing certain diseases and can result in systematic disease clustering [22, 23]. Defining multimorbidity through clusters accounts for disease patterns and diversity, which are differently associated with negative outcomes such as functional decline [24] and mortality [25]. A cross-sectional study used cluster analysis to examine chronic diseases that exist in robust and frail populations of multimorbid individuals [26]. However, there have not been investigations into the association between multimorbidity patterns and incident physical frailty. This study aims to determine the cross-sectional and longitudinal associations between different multimorbidity patterns and physical frailty in older Swedish adults.
Methods
Study design and population
Data were used from The Swedish National study on Aging and Care in Kungsholmen (SNAC-K): an ongoing population-based cohort study, following older adults (60+ years), living in Kungsholmen; a district of Stockholm, Sweden [27]. Participants were randomly selected from 11 age cohorts (60, 66, 72, 78, 81, 84, 87, 90, 93, 96 and 99+ years [27]). At baseline (2001–2004), 73% (n = 3,363) of all eligible, invited persons enrolled [28]. Since baseline, participants have been followed every 6 years if younger than 78 and every 3 years if 78 or older [28]. This study was approved by the Ethics Committee at Karolinska Institutet and the Regional Ethics Review Board in Stockholm. Participants, or proxy decision-makers for those with cognitive impairment, provided informed consent.
The cross-sectional analyses included 2,534 participants after excluding 190 institutionalised persons, 432 with less than two diseases and 207 missing frailty information. Those missing baseline frailty status were older, more likely to be female and, on average, affected by a higher number of diseases (Supplementary Table S1, Supplementary data are available in Age and Ageing online). The same participants were included in the longitudinal analyses, after excluding those with baseline frailty. In total, 2,122 participants were included in the 6-year analysis (94 missing frailty status) and 2,140 in the 12-year analysis (76 missing frailty status; Supplementary Figure S1, Supplementary data are available inAge and Ageing online).
Data collection
The following data were collected at each visit: (i) a physical functioning assessment and social interview, conducted by a trained nurse; (ii) a clinical examination involving a physician conducting neurological, geriatric and psychiatric assessments and (iii) a psychologist-administered cognitive assessment [28].
Chronic disease assessment
Clinical diagnoses in SNAC-K were ascertained by physicians through clinical examinations, self-reported health, review of medical journals, clinical lab parameters, anamnestic data and medication-use. All four-digit level International Classification of Diseases, 10th Revision (ICD-10) codes were designated as chronic or non-chronic, and a list of 60 categories of chronic diseases was identified using baseline SNAC-K data, considering disease prognosis, prevalence, pathophysiology and treatment [29].
Physical frailty assessment
Frailty was operationalised in accordance with a modified version of the frailty phenotype developed by Fried et al., which identifies an individual as physically frail if they exhibit at least three of the following: unintentional weight loss, low energy expenditure, self-reported exhaustion, slow gait speed and weak grip strength [30]. Unintentional weight loss was defined as loss of at least 1 kg within the last 3 months. Those exercising three times per month or less were said to have low energy expenditure. Self-reported exhaustion was defined as reported fatigue within the last 3 months. Gait speed (meters/second) was timed as participants walked 6 meters (m), or 2.4 m for those who considered themselves slow walkers. Slow gait speed was defined as the slowest 20%, adjusted by height and sex. Participants missing gait speed were classified as slow walkers if they: could not stand up or walk without assistance, used a wheelchair, could not move around without assistance, or could not walk 100–200 m without great difficulty. Those able to walk 1 km without difficulty were said to have non-slow gait speed. Grip strength (Newtons) was measured in both hands with an electronic dynamometer (Grippit®), using the strongest value of the two. Weak grip strength was classified as the lowest 20% of participants, adjusted by sex and body mass index. Those missing a grip strength measure were said to have weak grip strength if they could not open jars with lids. Individuals missing some of the five frailty criteria were not excluded if their available variables could classify them as frail (at least three frail criteria) or robust (at least three non-frail criteria).
Covariates
Education was categorised into elementary (<8 years), high school (8–12 years) and university (≥13 years). Civil status was categorised into partnered, widowed, unmarried and divorced. Smoking history was categorised as: never, former or current. Alcohol consumption was categorised as: never or occasional, light to moderate (1–14 standard drinks/week in men, 1–7 in women) or heavy (>14 standard drinks/week in men, >7 in women).
Statistical analyses
To identify multimorbidity patterns, all non-institutionalised participants with at least two chronic diseases were included. Upon performing a dimensionality reduction (i.e. multiple correspondence analysis) accounting for all diseases displaying a prevalence >2%, a fuzzy c-means cluster algorithm was employed to generate clusters of individuals (based on their diseases). This allowed individuals to belong to more than one cluster, as all participants were assigned a membership probability to each cluster. To obtain the optimal cluster number, validation indices and various degrees of fuzzification were tested. The soft clustering analysis was repeated 100 times to account for the random nature of cluster solutions and generate an average final outcome. Participants were then assigned to the cluster in which they had the highest membership probability, making it possible that participants in different clusters could share common diseases. To characterize the clusters of individuals in terms of diseases, observed/expected ratios were used, comparing disease prevalence in the cluster with that of the sample population. Additionally, disease exclusivity was calculated as the fraction of individuals with the disease in a cluster divided by the total number of individuals with the disease. Diseases were recognised to be associated with specific clusters when the exclusivity was ≥25% or the observed/expected ratio was ≥2 (Supplementary Table S2, Supplementary data are available inAge and Ageing online). Diseases with both an exclusivity ≥25% and observed/expected ratio ≥ 2 were represented in the cluster’s name. Further details on this methodology have been published [25, 31].
Participants’ baseline characteristics were analysed by multimorbidity pattern through chi-square tests and one-way analysis of variance. Logistic regression was utilised to assess the cross-sectional association between the multimorbidity patterns and baseline frailty. Multinomial logistic regression was used to assess the longitudinal (6- and 12-year) association between the multimorbidity patterns and frailty, and the outcome was categorised into non-frail (reference group), frail, dropouts and deceased (using data from the Swedish Cause of Death Register). All models were first adjusted for sociodemographic characteristics, and then for additional demographic (e.g. civil status) and behavioural factors (e.g. smoking history and alcohol consumption). Stratified analyses by age and sex were performed to test for a potential modifier effect. A P-value <0.05 was considered statistically significant in each analysis. Stata/IC 15.1 and R 4.0.0 were employed for the analyses.
Sensitivity analyses
Missing data could have resulted in decreased statistical power and biased estimations of the association between the multimorbidity patterns and physical frailty. As such, multiple imputation using chained equations (MICE) was conducted as a sensitivity analysis [32]. Covariates and physical frailty status with missing observations were imputed, under the assumption that these observations were missing at random. Five imputed datasets were generated, using demographic data and disease status as auxiliary variables. Under Rubin’s rule, estimates were generated by pooling the results of the imputed datasets [33].
Results
The mean age of the 2,534 participants in the cross-sectional analyses was 74.3 ± 10.3 years and 64% were female. Six multimorbidity patterns were identified at baseline: psychiatric diseases (n = 149; 5.9%); cardiovascular diseases, anaemia and dementia (n = 195; 7.7%); sensory impairments and cancer (n = 287; 11.3%); metabolic and sleep disorders (n = 291; 11.5%); musculoskeletal (MSK), respiratory and gastrointestinal (GI) diseases (n = 402; 15.9%); and an unspecific (n = 1,210; 47.8%) pattern lacking overrepresented diseases (Supplementary Tables S2 and S3 for details supplementary data are available in Age and Ageing online). Table 1 displays the baseline characteristics of the total population (n = 2,534) according to the multimorbidity patterns. Participants in the cardiovascular diseases, anaemia and dementia pattern (83.4 ± 8.6 years), followed by the sensory impairments and cancer (83.2 ± 8.5 years), were the oldest. Those in the cardiovascular diseases, anaemia and dementia pattern presented with the highest number of chronic diseases (7.6 ± 2.4). The unspecific pattern was relatively healthier, characterised by a younger average age (71.2 ± 9.2) and the lowest number of chronic diseases (3.0 ± 1.1).
Table 1 .
Baseline characteristics of complete cases by multimorbidity pattern
| Characteristics | Unspecific (n = 1,210, 47.8%) | MSK, resp. & GI (n = 402, 15.9%) | Metabolic & sleep (n = 291, 11.5%) | Sensory & cancer (n = 287, 11.3%) | Cardio., anaemia & dementia (n = 195, 7.7%) | Psych. (n = 149, 5.9%) | Total (n = 2,534) |
|---|---|---|---|---|---|---|---|
| Age | 71.2 ± 9.2 | 74.8 ± 10.2 | 73.0 ± 8.8 | 83.2 ± 8.5 | 83.4 ± 8.6 | 71.2 ± 9.6 | 74.3 ± 10.3** |
| Sex (female) | 760 (62.8) | 310 (77.1) | 135 (46.4) | 189 (65.9) | 128 (65.6) | 109 (73.2) | 1,631 (64.4)** |
| Education | |||||||
| Elementary | 176 (14.6) | 66 (16.4) | 49 (16.8) | 69 (24.0) | 56 (28.9) | 21 (14.1) | 437 (17.3)** |
| High school | 589 (48.7) | 202 (50.3) | 155 (53.3) | 151 (52.6) | 102 (52.6) | 71 (47.7) | 1,270 (50.2) |
| University | 444 (36.7) | 134 (33.3) | 87 (29.9) | 67 (23.3) | 36 (18.6) | 57 (38.3) | 825 (32.6)** |
| Civil status | |||||||
| Partnered | 637 (52.7) | 167 (41.7) | 147 (50.5) | 84 (29.3) | 63 (32.3) | 60 (40.3) | 1,158 (45.7)** |
| Widowed | 242 (20.0) | 107 (26.7) | 66 (22.7) | 127 (44.3) | 86 (44.1) | 36 (24.2) | 664 (26.2)** |
| Unmarried | 172 (14.2) | 63 (15.7) | 40 (13.8) | 44 (15.3) | 30 (15.4) | 22 (14.8) | 371 (14.7) |
| Divorced | 158 (13.1) | 64 (16.0) | 38 (13.1) | 32 (11.2) | 16 (8.2) | 31 (20.8) | 339 (13.4)* |
| Alcohol consumption | |||||||
| Never/occasional | 341 (28.3) | 160 (40.1) | 100 (34.5) | 151 (53.4) | 108 (56.5) | 53 (35.6) | 913 (36.3)** |
| Light/moderate | 651 (54.0) | 170 (42.6) | 147 (50.7) | 97 (34.3) | 66 (34.6) | 55 (36.9) | 1,186 (47.1)** |
| Heavy | 214 (17.7) | 69 (17.3) | 43 (14.8) | 35 (12.4) | 17 (8.9) | 41 (27.5) | 419 (16.6)** |
| Smoking status | |||||||
| Never | 559 (46.5) | 197 (49.4) | 114 (39.3) | 160 (56.1) | 99 (50.8) | 58 (39.5) | 1,187 (47.1)* |
| Former | 457 (38.0) | 152 (38.1) | 131 (45.2) | 94 (33.0) | 79 (40.5) | 55 (37.4) | 968 (38.4) |
| Current | 187 (15.5) | 50 (12.5) | 45 (15.5) | 31 (10.9) | 17 (8.7) | 34 (23.1) | 364 (14.5)* |
| Number of diseases | 3.0 ± 1.1 | 4.5 ± 1.8 | 5.1 ± 1.8 | 5.7 ± 1.9 | 7.6 ± 2.4 | 5.4 ± 2.0 | 4.3 ± 2.1** |
| Physically frail | 56 (4.6) | 66 (16.4) | 25 (8.6) | 69 (24.0) | 76 (39.0) | 26 (17.5) | 318 (12.6)** |
Notes: MSK, resp. & GI = musculoskeletal, respiratory & gastrointestinal diseases; metabolic & sleep = metabolic & sleep disorders; sensory & cancer = sensory impairments & cancer; cardio., anaemia & dementia = cardiovascular diseases, anaemia & dementia; psych. = psychiatric. Missing variables: civil status (n = 2), education (n = 2), smoking status (n = 15) and alcohol consumption (n = 16). Values are presented as absolute number and column percentage (%) or mean ± standard deviation.
* P < 0.01.
** P < 0.001
Overall, 318 (13%) participants with physical frailty were identified at baseline, with the highest prevalence in the cardiovascular diseases, anaemia and dementia pattern (39%) and the lowest in the unspecific pattern (5%); prevalence was higher in older age cohorts across all patterns (Figure 1). In the fully adjusted cross-sectional analyses, all patterns presented statistically significant associations with frailty compared to the unspecific pattern, with odds ratios ranging from 1.74 (95% CI: 1.03–2.91) for the metabolic and sleep disorders pattern to 5.23 (95% CI: 3.38–8.09) for the cardiovascular diseases, anaemia and dementia pattern (Table 2).
Figure 1 .
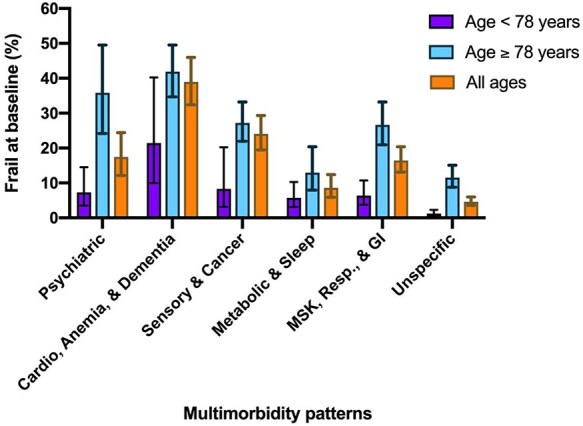
Baseline physical frailty prevalence and 95% confidence intervals by multimorbidity pattern. Notes: psychiatric = psychiatric diseases; cardio, anaemia & dementia = cardiovascular diseases, anaemia & dementia; sensory & cancer = sensory impairments & cancer; metabolic & sleep = metabolic & sleep disorders; MSK, resp. & GI = musculoskeletal, respiratory & gastrointestinal diseases.
Table 2 .
Cross-sectional association between multimorbidity patterns and physical frailty in complete cases at baseline (n = 2,534)
| Pattern | Cases | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| n /N | % | OR | 95% CI | OR | 95% CI | |
| Unspecific | 56/1,210 | 4.6 | 1 (ref) | – | 1 (ref) | – |
| MSK, resp. & GI | 66/402 | 16.4 | 3.13 | 2.10–4.66 | 3.13 | 2.08–4.70 |
| Metabolic & sleep | 25/291 | 8.6 | 1.81 | 1.09–3.01 | 1.74 | 1.03–2.91 |
| Sensory & cancer | 69/287 | 24.0 | 2.59 | 1.71–3.93 | 2.39 | 1.56–3.66 |
| Cardio., anaemia & dementia | 76/195 | 39.0 | 5.57 | 3.63–8.53 | 5.23 | 3.38–8.09 |
| Psychiatric | 26/149 | 17.5 | 4.96 | 2.90–8.47 | 4.86 | 2.80–8.43 |
Notes: MSK, resp. & GI = musculoskeletal, respiratory & gastrointestinal diseases; metabolic & sleep = metabolic & sleep disorders; sensory & cancer = sensory impairments & cancer; cardio., anaemia & dementia = cardiovascular diseases, anaemia & dementia; OR = odds ratio. Missing observations: civil status (n = 2), education (n = 2), smoking status (n = 15) and alcohol consumption (n = 16). Model 1: adjusted for age, sex and education. Model 2: adjusted for age, sex, education, civil status, smoking status and alcohol consumption.
Table 3 displays the longitudinal association between the multimorbidity patterns and incident frailty. Of the 2,122 participants included in the 6-year analyses, 236 (11.1%) developed frailty, with those belonging to the psychiatric diseases (relative risk ratio [RRR]: 3.04; 95% CI: 1.59–5.79), cardiovascular diseases, anaemia and dementia (RRR: 2.25, 95% CI: 1.13–4.49), and metabolic and sleep disorders (RRR: 1.99; 95% CI 1.25–3.16) patterns having statistically significant increased relative risks of developing frailty within 6 years compared with those in the unspecific pattern, in the fully-adjusted model. Of the 2,140 participants included in the 12-year analyses, 435 (20.3%) developed frailty. In the fully adjusted model, those in the cardiovascular diseases, anaemia and dementia (RRR: 4.81; 95% CI: 1.59–14.60), psychiatric diseases (RRR: 2.62, 95% CI: 1.45–4.72) and sensory impairments and cancer (RRR: 1.87; 95% CI 1.05–3.35) patterns had statistically significant increased relative risks of developing frailty within 12 years compared with those in the unspecific pattern. See Supplementary Table S4 for the fully expanded and adjusted longitudinal analyses.
Table 3 .
Longitudinal association between multimorbidity patterns and physical frailty in complete cases at six (n = 2,122) and 12 (n = 2,140) years
| Pattern | Cases | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| n/N | % | RRR | 95% CI | RRR | 95% CI | |
| 6-year | ||||||
| Unspecific | 90/1,121 | 8.0 | 1 (ref) | – | 1 (ref) | – |
| MSK, resp. & GI | 38/321 | 11.8 | 1.28 | 0.82–1.99 | 1.22 | 0.78–1.92 |
| Metabolic & sleep | 35/260 | 13.5 | 1.99 | 1.26–3.15 | 1.99 | 1.25–3.16 |
| Sensory & cancer | 37/193 | 19.2 | 1.31 | 0.80–2.15 | 1.36 | 0.82–2.25 |
| Cardio., anaemia & dementia | 20/112 | 17.9 | 2.34 | 1.18–4.62 | 2.25 | 1.13–4.49 |
| Psychiatric | 16/115 | 13.9 | 3.03 | 1.60–5.74 | 3.04 | 1.59–5.79 |
| 12-year | ||||||
| Unspecific | 190/1,119 | 17.0 | 1 (ref) | – | 1 (ref) | – |
| MSK, resp. & GI | 75/323 | 23.2 | 1.46 | 1.00–2.13 | 1.38 | 0.94–2.03 |
| Metabolic & sleep | 55/260 | 21.2 | 1.49 | 0.97–2.28 | 1.43 | 0.93–2.19 |
| Sensory & cancer | 61/204 | 29.9 | 1.99 | 1.12–3.52 | 1.87 | 1.05–3.35 |
| Cardio., anaemia & dementia | 27/116 | 23.3 | 5.31 | 1.75–16.13 | 4.81 | 1.59–14.60 |
| Psychiatric | 27/118 | 22.9 | 2.74 | 1.54–4.89 | 2.62 | 1.45–4.72 |
Notes: MSK, resp. & GI = musculoskeletal, respiratory & gastrointestinal diseases; metabolic & sleep = metabolic & sleep disorders; sensory & cancer = sensory impairments & cancer; cardio., anaemia & dementia = cardiovascular diseases, anaemia & dementia. Missing observations in 6-year analysis: education (n = 1), civil status (n = 2), alcohol consumption (n = 8) and smoking status (n = 11). Missing observations in 12-year analysis: education (n = 1), civil status (n = 1), alcohol consumption (n = 9) and smoking status (n = 10). Model 1: adjusted for age, sex and education. Model 2: adjusted for age, sex, education, civil status, smoking status and alcohol consumption.
There were 2,741, and 2,423, participants included in the cross-sectional and longitudinal analyses, respectively, after employing MICE. The results of the imputed analyses align with the complete-case analyses (Supplementary Table S5, Supplementary data are available in Age and Ageing online).
Stratified analyses were conducted and the presence of an interaction between the patterns and incident physical frailty with age and sex was tested. No interaction was found; based on the stratified analyses, the association tended to be higher in females, and when stratified by age, significance was lost except for in the psychiatric diseases pattern, which is likely due to power (Supplementary Table S6, Supplementary data are available inAge and Ageing online).
Discussion
Our results indicate that distinct multimorbidity patterns are differentially associated with incident physical frailty in older adults. Compared with the unspecific one, all patterns exhibited a statistically significant cross-sectional association with frailty. The psychiatric diseases; cardiovascular diseases, anaemia and dementia; and metabolic and sleep disorders patterns were significantly associated with frailty development over 6 years compared with the unspecific pattern. Over 12 years, the cardiovascular diseases, anaemia and dementia; psychiatric diseases; and sensory impairments and cancer patterns were significantly associated with incident frailty compared with the unspecific pattern.
The relation between multimorbidity and frailty has been reported in several studies [14–21], but others do not support this association [34, 35]. Varying definitions of multimorbidity might be a reason for these conflicting results. Despite its widespread recent use, and in being in line with the deficit accumulation frailty model, operationalizing multimorbidity through disease counts neglects the nature and interactions of diseases, which may differently affect frailty susceptibility. We assessed multimorbidity through patterns, recognizing its qualitative complexity and the tendency of certain diseases to coexist [22]. Although frailty has not been previously investigated as an outcome, other studies have demonstrated that multimorbidity patterns have a differential impact on healthcare utilisation [36], physical and cognitive function [24], as well as disability [37] and are associated with different risk factors [31, 38].
Cross-sectionally, each clinically specific multimorbidity pattern was significantly associated with physical frailty compared with the unspecific pattern. The magnitude of the odds ratios suggests that the cardiovascular diseases, anaemia, and dementia and psychiatric diseases patterns were most strongly associated with physical frailty at baseline, compared with the unspecific pattern. This could simply be an expression of the severity and functional impairment caused by the diseases in these patterns, such as dementia, depression and cerebrovascular diseases, which have systemic implications [39–41].
Longitudinally, the results indicate that multimorbidity patterns characterised by cardiovascular and neuropsychiatric diseases are most strongly associated with physical frailty. The cardiovascular diseases, anaemia and dementia pattern is characterised by cardiac diseases that impact organ perfusion, oxygenation, and thus, muscle performance, which is strongly implicated in frailty’s pathogenesis [42, 43]. Furthermore, dementia may impact one’s nutritional status, physical activity engagement and muscle fitness, promoting frailty development [39, 44]. The neuropsychiatric conditions in the psychiatric diseases pattern, such as depression and anxiety, are associated with fatigue as well as reduced appetite and physical activity, which are key components of frailty [30, 40]. Nguyen et al. found that neuropsychiatric multimorbidity was associated with increased mortality in robust, but even more so in frail, individuals [45]. Although the study only considered 10 chronic conditions to assess multimorbidity, it supports the idea of adverse health outcomes being related to the presence of multiple neuropsychiatric diseases [45].
Those in the metabolic and sleep disorders pattern had a significantly higher risk of developing frailty within 6, but not 12, years compared with the unspecific pattern. This pattern is characterised by conditions such as obesity, diabetes and sleep disorders, which impact physical activity, fatigue, and thus, frailty [46–48]. A reason for the lack of long-term association with frailty could be that these individuals have developed more severe cardiovascular diseases, which could lead them to change pattern membership, diluting the association with incident frailty. The sensory impairments and cancer pattern was significantly associated with frailty development compared with the unspecific pattern, over 12 years. Participants with these patterns likely had less severe cancers at baseline to be well enough to enrol in SNAC-K, which eventually could lead to frailty.
Understanding how different multimorbidity patterns are associated with frailty can inform guidelines, prognoses and preventive strategies for groups at higher frailty risk. Frailty results in increased healthcare utilisation and its management is costly [8]. An enhanced understanding of the features of older multimorbid persons at risk for frailty can allow public health authorities to plan community interventions and health resources accordingly. Generating homogeneous groups of individuals based on multimorbidity patterns also has important implications for research and clinical management. Clinicians can provide improved prognoses and treatment plans for patients based on their multimorbidity pattern, implementing treatment programs such as physical activity to prevent or delay frailty onset. Moreover, this calls for future research into the biological mechanisms responsible for these different health impacts.
This study is strengthened by its long follow-up, making it possible to investigate multimorbidity patterns and frailty-onset across several time-points. We took the novel approach of evaluating physical frailty-risk by pattern within a multimorbid population, rather than comparing multimorbid participants to a healthy reference group. Measuring multimorbidity through patterns, rather than disease count, provides a more nuanced classification of multimorbidity and a qualitative dimension that can be useful in clinical practice and research. Data regarding multimorbidity and frailty were high-quality, as SNAC-K involves comprehensive clinical assessments and participant interviews [49]. Moreover, the rigorous disease classification and advanced clustering methods provided a unique opportunity to investigate multimorbidity patterns in relation to frailty. The sensitivity analyses, involving imputations, also strengthened the results.
Nonetheless, there are limitations to consider. Selection bias could have been introduced through the 73% response rate. Information bias might have occurred, as the exposure and outcome assessments involved self-reported data. To limit this, multiple sources (questionnaires, hospital records, clinical assessments and proxies for those with cognitive impairment) were used. Given the complex and dynamic nature of multimorbidity in older adults, it is possible that participants’ multimorbidity pattern statuses changed since baseline. Many in the unspecific pattern would have changed to a pattern with a more burdensome disease status, possibly underestimating specific patterns’ associations with frailty. It is also likely that many older participants died before they could be captured as frail, possibly underestimating the association. Confounding might have impacted the relation between the multimorbidity patterns and physical frailty—as such, extensive covariate adjustment was performed. Lastly, the external validity should be critically appraised, as participants were limited to Kungsholmen, a relatively affluent area of Stockholm.
This study found specific multimorbidity patterns to be differentially associated with incident physical frailty in older Swedish adults. Persons in multimorbidity patterns characterised by cardiovascular and neuropsychiatric diseases are at increased risk of developing physical frailty. Further research should investigate the specific mechanisms responsible for this differential risk between different multimorbidity patterns and physical frailty.
Supplementary Material
Acknowledgements
We thank the SNAC-K participants and the SNAC-K Group for their collaboration in data collection and management.
Contributor Information
Clare Tazzeo, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
Debora Rizzuto, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden; Stockholm Gerontology Research Center, Stockholm, Sweden.
Amaia Calderón-Larrañaga, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
Albert Roso-Llorach, Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina (IDIAPJGol), Barcelona, Spain; Universitat Autònoma de Barcelona, Campus de la UAB, Bellaterra (Cerdanyola del Vallès), Barcelona, Spain.
Alessandra Marengoni, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden; Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
Anna-Karin Welmer, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden.
Graziano Onder, Department of Cardiovascular, Endocrine-Metabolic Diseases and Aging, Istituto Superiore di Sanità, Rome, Italy.
Caterina Trevisan, National Research Council-Neuroscience Institute, Aging Branch, Padova, Italy; Geriatric Unit, Department of Medicine (DIMED), University of Padova, Padova, Italy.
Davide Liborio Vetrano, Aging Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet and Stockholm University, Stockholm, Sweden; Centro Medicina dell'Invecchiamento, Fondazione Policlinico Universitario "A. Gemelli" IRCCS, and Università Cattolica del Sacro Cuore, Rome, Italy.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was supported by the funders of the Swedish National study on Ageing and Care (SNAC): the Ministry of Health and Social Affairs, Sweden; the participating County Councils and Municipalities; the Swedish Research Council for Medicine and Health [VR; 521-2013-8676, 2017-06088, 2016-00981]; and the Swedish Research Council for Health, Working life and Welfare [Forte; 2016-07175, 2017-01764]. This work was also supported by the Italian Ministry of Health [PE-2016-02364885]. The funders had no role in any step of the present study, from study protocol to manuscript preparation.
References
- 1. van den Akker M, Buntinx F, Knottnerus J. Comorbidity or multimorbidity: what’s in a name? a review of literature. Eur J Gen Pract 1996; 2: 65–70. [Google Scholar]
- 2. Marengoni A, Angleman S, Melis R et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10: 430–9. [DOI] [PubMed] [Google Scholar]
- 3. Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67: 130–8. [DOI] [PubMed] [Google Scholar]
- 4. Librero J, Peiró S, Ordiñana R. Chronic comorbidity and outcomes of hospital care: length of stay, mortality, and readmission at 30 and 365 days. J Clin Epidemiol 1999; 52: 171–9. [DOI] [PubMed] [Google Scholar]
- 5. Dumbreck S, Flynn A, Nairn M et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ 2015; 350: h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vetrano DL, Calderón-Larrañaga A, Marengoni A et al. An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci 2018; 73: 1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394: 1365–75. [DOI] [PubMed] [Google Scholar]
- 9. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev 2013; 12: 719–36. [DOI] [PubMed] [Google Scholar]
- 10. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc 2015; 16: 1027–33. [DOI] [PubMed] [Google Scholar]
- 11. Hoogendijk EO, Suanet B, Dent E, Deeg DJ, Aartsen MJ. Adverse effects of frailty on social functioning in older adults: results from the Longitudinal Aging Study Amsterdam. Maturitas 2016; 83: 45–50. [DOI] [PubMed] [Google Scholar]
- 12. Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health 2016; 70: 716–21. [DOI] [PubMed] [Google Scholar]
- 13. O’Caoimh R, Sezgin D, O’Donovan MR et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing 2021; 50: 96–104. [DOI] [PubMed] [Google Scholar]
- 14. Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018; 3: e323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alvarado BE, Zunzunegui M-V, Béland F, Bamvita J-M. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci 2008; 63: 1399–406. [DOI] [PubMed] [Google Scholar]
- 16. de Souto Barreto P, Greig C, Ferrandez A-M. Detecting and categorizing frailty status in older adults using a self-report screening instrument. Arch Gerontol Geriatr 2012; 54: e249–54. [DOI] [PubMed] [Google Scholar]
- 17. Castell M-V, Sánchez M, Julián R, Queipo R, Martín S, Otero Á. Frailty prevalence and slow walking speed in persons age 65 and older: implications for primary care. BMC Fam Pract 2013; 14: 86. doi: 10.1186/1471-2296-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong CH, Weiss D, Sourial N et al. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: a cross-sectional study. Aging Clin Exp Res 2010; 22: 54–62. [DOI] [PubMed] [Google Scholar]
- 19. Chang SS, Weiss CO, Xue Q-L, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci 2010; 65: 407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mendonça N, Kingston A, Yadegarfar M et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing 2020; 49: 974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vetrano DL, Palmer K, Marengoni A et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2019; 74: 659–66. [DOI] [PubMed] [Google Scholar]
- 22. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014; 67: 254–66. [DOI] [PubMed] [Google Scholar]
- 23. Calderón-Larrañaga A, Vetrano DL, Ferrucci L et al. Multimorbidity and functional impairment–bidirectional interplay, synergistic effects and common pathways. J Intern Med 2019; 285: 255–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vetrano DL, Rizzuto D, Calderón-Larrañaga A et al. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med 2018; 15: e1002503. doi: 10.1371/journal.pmed.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vetrano DL, Roso-Llorach A, Fernández S et al. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat Commun 2020; 11: 3223. doi: 10.1038/s41467-020-16780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machón M, Mateo-Abad M, Clerencia-Sierra M et al. Multimorbidity and functional status in older people: a cluster analysis. Eur Geriatr Med 2020; 11: 321–32. [DOI] [PubMed] [Google Scholar]
- 27. Santoni G. Data Description. SNAC-K Population Study. Updated September 26, 2019. https://www.snac-k.se/for-researchers/data-description/ (1 October 2020, date last accessed).
- 28. Äldrecentrum . Study Plan. SNAC-K Population Study. https://www.snac-k.se/about/study-plan/ (1 October 2020, date last accessed).
- 29. Calderón-Larrañaga A, Vetrano DL, Onder G et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol A Biol Sci Med Sci 2017; 72: 1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–57. [DOI] [PubMed] [Google Scholar]
- 31. Marengoni A, Roso-Llorach A, Vetrano DL et al. Patterns of multimorbidity in a population-based cohort of older people: sociodemographic, lifestyle, clinical, and functional differences. J Gerontol A Biol Sci Med Sci 2020; 75: 798–805. [DOI] [PubMed] [Google Scholar]
- 32. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011; 20: 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons, 1987. [Google Scholar]
- 34. Espinoza SE, Jung I, Hazuda H. Lower frailty incidence in older Mexican Americans than in older European Americans: the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc 2010; 58: 2142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jung H-W, Jang I-Y, Lee YS et al. Prevalence of frailty and aging-related health conditions in older Koreans in rural communities: a cross-sectional analysis of the Aging Study of Pyeongchang Rural Area. J Korean Med Sci 2016; 31: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juul-Larsen HG, Christensen LD, Bandholm T et al. Patterns of multimorbidity and differences in healthcare utilization and complexity among acutely hospitalized medical patients (≥65 Years) – a latent class approach. Clin Epidemiol 2020; 12: 245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci 2016; 71: 823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jackson CA, Dobson AJ, Tooth LR, Mishra GD. Lifestyle and socioeconomic determinants of multimorbidity patterns among mid-aged women: a longitudinal study. PLoS One 2016; 11: e0156804. doi: 10.1371/journal.pone.0156804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitchell SL, Teno JM, Kiely DK et al. The clinical course of advanced dementia. New Engl J Med 2009; 361: 1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 41. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 42. Feigenbaum MS, Welsch MA, Mitchell M, Vincent K, Braith RW, Pepine CJ. Contracted plasma and blood volume in chronic heart failure. J Am Coll Cardiol 2000; 35: 51–5. [DOI] [PubMed] [Google Scholar]
- 43. Kokkinos PF, Choucair W, Graves P, Papademetriou V, Ellahham S. Chronic heart failure and exercise. Am Heart J 2000; 140: 21–8. [DOI] [PubMed] [Google Scholar]
- 44. van Alphen HJ, Volkers KM, Blankevoort CG, Scherder EJ, Hortobágyi T, van Heuvelen MJ. Older adults with dementia are sedentary for most of the day. PLoS One 2016; 11: e0152457. doi: 10.1371/journal.pone.0152457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nguyen QD, Wu C, Odden MC, Kim DH. Multimorbidity patterns, frailty, and survival in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2019; 74: 1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Forhan M, Gill SV. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab 2013; 27: 129–37. [DOI] [PubMed] [Google Scholar]
- 47. Sinclair AJ, Rodriguez-Mañas L. Diabetes and frailty: two converging conditions? Can J Diabetes 2016; 40: 77–83. [DOI] [PubMed] [Google Scholar]
- 48. Buysse DJ. Insomnia. JAMA 2013; 309: 706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lagergren M, Fratiglioni L, Hallberg IR et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 2004; 16: 158–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


