Abstract
Introduction
Multicomponent interventions improve physical function and frailty in older adults, but their long-term benefit remains uncertain.
Methods
This prospective non-randomised study was conducted in 383 older Koreans (mean age, 76.8 years; female 72.3%) who were living alone or receiving medical aid. Of these, 187 individuals chose to receive a 24-week intervention that consisted of group exercise, nutritional supplements, depression management, deprescribing and home hazard reduction. The remaining 196 individuals received usual care. We compared the short physical performance battery (SPPB) score (0–12 points), frailty phenotype scale (0–5 points) and deficit-accumulation frailty index (0–1) at baseline, 6, 18 and 30 months.
Results
After 1:1 propensity score matching (n = 117 per group), the mean SPPB scores for the intervention and comparison groups were 7.6 versus 7.6 at baseline, 10.7 versus 7.1 at 6 months (mean difference, 3.5; 95% confidence interval [CI], 2.8–4.2), 9.1 versus 7.8 at 18 months (1.3; 95% CI, 0.6–2.0) and 8.6 versus 7.5 at 30 months (1.1; 95% CI, 0.4–1.8). The intervention group had lower frailty phenotype scale (1.1 versus 1.8; difference, −0.7; 95% CI −1.0 to −0.3) and frailty index (0.22 versus 0.27; difference, −0.04; −0.06 to −0.02) at 6 months, but similar scores at 18 and 30 months. The 30-month mean institutionalisation-free survival time was 28.5 months in the intervention group versus 23.3 months in the comparison group (difference, 5.2 months; 95% CI, 3.1–7.4).
Conclusions
The 24-week multicomponent intervention showed sustained improvement in physical function, temporary reduction in frailty and longer institutionalisation-free survival over 30 months.
Keywords: clinical trial, community health services, frailty, mortality, physical fitness, older people
Key Points
A 24-week multicomponent intervention programme provided sustained improvement in physical performance for up to 30 months.
Reduction in mortality and institutionalisation was seen in the intervention group.
Our results can inform design and implementation of a public health intervention for frail older adults in rural communities.
Introduction
The number of people living longer is increasing worldwide [1, 2]. As the number of ageing individuals increases, it leads to the burden of people with functional impairments and geriatric conditions including frailty and sarcopenia [3]. This burden affirms the importance of functioning independently in daily life, and multifactorial interventions to prevent or delay geriatric conditions or to improve function are ongoing [4].
Varying types of intervention studies have been conducted to examine the effect on older adults with frailty [5–7], and practical guidelines were made based on these studies [8, 9]. Some studies showed that exercise alone showed a modest improvement in physical function [10, 11]. Other studies of multicomponent interventions that included exercise, nutritional supplementation, and other interventions such as cognitive training and mental health support, showed a moderate improvement in physical function and frailty [12–14], whereas others found limited benefits [10, 11, 15]. This heterogeneity is possibly due to differences in the intervention types, adherence, target populations or follow-up periods.
However, most studies did not follow participants after the end of intervention period and usually focused on the short-term effect of intervention rather than the possible long-term health benefits [9–15]. These limitations might be due to limited resources, relatively subordinated research funding or difficulties in establishing a stable academic–public health collaborative model in communities. In resource-limited rural community settings, systematic long-term follow-up with intact initial population characteristics is difficult with frequent withdrawals of participants during both the intervention and observation period [16]. Previously, we showed that after a 24-week multicomponent intervention programme consisting of group exercise, protein supplementation, depression management, home hazard reduction and discontinuation of potentially inappropriate medications, targeting socioeconomically vulnerable community-dwelling older Koreans, the benefits lasted up to 6 months after cessation of the intervention [5]. However, it remains uncertain how long the benefit is sustained after 6 months.
In this study, we report the 30-month outcomes associated with the intervention programme by comparing with a usual care comparison group using propensity score matching to minimise bias of the non-randomised design.
Methods
Study design
The Aging Study of Pyeongchang Rural Area-Intervention Study (ASPRA-IS) is a prospective, single-arm intervention study, which delivered a 24-week multicomponent intervention in three geographical regions in Pyeongchang County, South Korea [5]. The intervention took place one region at a time for 24 weeks (region A: August 2015–January 2016; region B: February–July 2016; region C: August 2016–January 2017). The study protocol was approved by the Institutional Review Boards of Asan Medical Center and registered in 2015 (NCT02554994). Written informed consent was obtained from all participants before the study entry.
As the individuals who declined to participate in the intervention programme had same comprehensive geriatric assessment as part of the observational cohort, ASPRA cohort, we were able to collect information for both individuals who received intervention (intervention group) and individuals who declined to participate the intervention programme (comparison group). This study aimed to compare physical function and institutionalisation-free survival time over 30 months between intervention group and comparison group.
Study population
All participants were recruited from the ASPRA cohort [3], which was a population-based prospective cohort study of 1,267 community-dwelling adults aged 65 and older in the Pyeongchang County, Korea, established in 2014. Among them, ASPRA-IS invited participants of the ASPRA cohort who were living alone or receiving medical aid at the time of recruitment. Excluded were those who were unable to walk 100 m, admitted to long-term care hospitals or nursing homes in the last 6 months, diagnosed with end-stage heart failure, end-stage renal disease or metastatic cancer, cognitively impaired, as defined by Mini-Mental State Examination-Dementia Screening score ≤18 points, and had a plan to move out of the study area within the next 6 months [3]. These eligibility criteria were discussed with the local public health department in Pyeongchang County, which prioritised allocation of resources to socioeconomically vulnerable residents. Participants made their own decisions on the receipt of the multicomponent intervention programme. If they were willing to participate, we allocated them in the intervention group and those who declined were allocated to the comparison group.
We screened 1,267 ASPRA cohort participants and found 383 eligible individuals. Of the 383 individuals, 187 individuals chose to receive the multicomponent intervention and 196 individuals declined participation (Supplementary Material A1).
A multicomponent intervention
The 24-week multicomponent intervention programme consisted of group exercise, nutritional supplementation, depression management, medication review and home hazard reduction. Group exercise programme and nutritional supplement were provided to all participants. Group exercise sessions were provided 60 min, twice a week and commercial nutritional supplements (125 ml liquid formula containing 200 kcal, 24.5 g carbohydrate, 13 g protein, 5.63 g essential amino acid and 7 g fat) were provided twice a day [17–19]. Depression management programme [20], deprescribing for potentially inappropriate medications for older adults [21] and home hazard evaluation and reduction [22], were provided for eligible participants based on each criterion (Table 1).
Table 1 .
Description of multicomponent intervention programme
| Focus | Description of intervention |
|---|---|
| Exercise [17] | • Intervention: 60-min group exercise session led by licensed trainers focussing on the following types. The intensity started from low-intensity exercise and increased intensity every month 1. Resistance (20 min): squat, plank, side plank, straight leg raises 2. Balance (20 min): one-leg standing, shifting from side to side, heel-to-toe walk 3. Aerobic/endurance (20 min): step up and down, quick pace, dancing 4. Exercise trainer was given instructions not to exceed 60–70% of the maximal exercise capacity based on perceived exertion scale • Target: all participants • Frequency: twice a week |
| Nutrition [18, 19] | • Intervention: administration of 125 ml commercial liquid formula containing 200 kcal of energy, 24.5 g carbohydrate, 13 g protein, 5.63 g essential amino acid and 7 g fat • Target: all participants • Frequency: twice a day |
| Depression [20] | • Intervention: evaluation by a geriatrician or a psychiatrist and administration of supportive psychotherapy or antidepressant medication as clinically indicated • Target: participants with the CES-D score >20 points at baseline • Frequency: monthly |
| Polypharmacy [21] | • Intervention: medication review by a geriatrician, and dose reduction or discontinuation of potentially inappropriate medications according to the 2012 Beer’s criteria • Target: participants taking five prescription medications at baseline • Frequency: monthly |
| Home hazards [22] | • Intervention: evaluation of home environment by a visiting nurse and a social worker using the Home Fall Prevention Checklist by Centers for Disease Control and Prevention and modification of the environment to eliminate any identified hazard • Target: all participants with any identified home hazard at baseline • Frequency: trimonthly |
From August 2015 to January 2017, a 24-week multicomponent intervention was implemented 6 months after the baseline assessment. During the 6 months pre-intervention period, participants received usual care from local public health centres. After finishing the 24-week multicomponent programme, the intervention group received usual care as the comparison group. A detailed protocol of the multicomponent programme was previously reported [5]. Adherence rates were 83.7% for group exercise attendance, 87.8% for nutritional supplements consumed (self-report), 88.4% for monthly visits for depression management, 91.3% for home hazard correction and 88.5% for monthly visits to evaluate polypharmacy. Among 187 participants, 125 (66.8%) completed at least 80% of the group exercise sessions and 80% nutritional supplements [23]. The comparison group received usual care during the entire study period.
Baseline and follow-up assessments
Individuals were followed up every 3 months for self-reported functional status via telephone interview. In-person comprehensive geriatric assessment were done every year: baseline (6 months before the start of the intervention programme), 6 months (at the end of the intervention), 18 and 30 months (Supplementary Material A1). Trained nurses who were not aware of the intervention status performed comprehensive geriatric assessment. Additional comprehensive geriatric assessment at the start of the intervention programme (month 0) was done for the intervention group. Information were collected on demographic characteristics, the number of household members, medical aid, multimorbidity, polypharmacy, current alcohol drinking status, number of falls, emergency room visits and days of hospitalisation in the last year. Detailed methods of the assessment were described previously [3, 5, 24].
Measurement of physical performance
The primary outcome was the change of short physical performance battery (SPPB) score (range, 0–12 points; higher scores indicate better performance; minimal clinically important difference = 1), which is a subjective measure to estimate the magnitude and change in physical performance in older adults [25]. SPPB score consisted of three parts: time to complete five chair stands, standing balance and usual gait speed (each range, 0–4 points) [26]. Validity of SPPB in classifying frailty status has been reported previously in the Korean population [27]. Due to the protocol update in ASPRA cohort study after baseline assessment in region A, SPPB measurement were added from region B. SPPB chair stand score and balance score were not measured from 99 individuals (33 in intervention, 66 in comparison group) both arms in regionA.
Other measurements
Change of frailty phenotype scale (range, 0–5 points) [28], deficit-accumulation frailty index [29], and death and institutionalisation-free survival were assessed. A deficit-accumulation frailty index (range, 0–1; higher values indicate greater frailty) was calculated based on 47 items of our comprehensive geriatric assessment (Supplementary Material A2). The assessment included medical comorbidities (14 items), self-reported functional status and disability (21 items), physical performance (five items), mood (three items), cognition (one item), nutritional status (one item), polypharmacy (one item) and social interaction (one item) [3, 30–35] (Supplementary Material A2). Death and institutionalisation were captured every 3 months by nurses. The exact month of the event and reasons of loss to follow-up were recorded from the study participants or their family members.
Statistical analysis
All analysis compared outcome variables between individuals who enrolled in the intervention programme and who denied programme participation at baseline. Missing values were imputed using multivariable imputation by chained equation [36]. Variables included in the imputation model were outcome variables at each timepoint, reasons for loss to follow-up, town and baseline covariates listed above. We conducted imputation of baseline variables of two missing Center for Epidemiologic Studies Depression Scale (CES-D) scores, and SPPB chair stand score and balance score, which were not measured from 99 individuals (33 in intervention, 66 in comparison group) both arms in region A. Outcome variables not measured after loss to follow-up (institutionalisation/nursing home care or non-medical reason) were also imputed (Supplementary Material A3).
After imputation, we conducted a 1:1 propensity score matching using a nearest-neighbour method with a caliper width of 0.2 standard deviation of the logit propensity score [37]. A propensity score model was developed using logistic regression with baseline characteristics. Intervention status was specified as the dependent variable, and characteristics at baseline including age, sex, enrolled year, living alone, CES-D score, number of chronic conditions, number of falls in the last year, emergency room visit or admission in the last year, SPPB score, frailty phenotype and frailty index were used as independent variables. The balance in baseline characteristics between the two groups was assessed using standardised mean difference (SMD) [38].
We summarised the mean and standard deviation or proportions of baseline characteristics for both groups before and after propensity score matching. We used a linear mixed model with random intercept to determine the effect of the intervention on the SPPB score, frailty phenotype scale and frailty index at 6, 18 and 30 months. This model included indicator variables for intervention status, times as categorical variable and their interaction terms. The mean differences (MDs) in SPPB score, frailty phenotype scale and frailty index between two groups at 6, 18 and 30 months and its 95% confidence interval (CI) were calculated from a linear mixed model, and 95% CI of the SPPB score, frailty phenotype scale, and frailty index at 6, 18 and 30 months for the intervention and comparison group were calculated with 1,000 times resampling bootstrap. Generalised estimating equation generalised linear model was used to evaluate changes in CES-D score (Poisson distribution and log link), proportion of individuals with polypharmacy and proportion of individuals who had a fall in the last year (binomial distribution and logit link). Exchangeable correlation structure was used for above models.
Institutionalisation-free survival was obtained with Kaplan–Meier estimate. Log-rank test and Cox proportional hazard model was used to examine the statistical difference of survival and hazard between the intervention and comparison group. Restricted mean survival time was estimated for institutionalisation-free survival time at 30 months.
Two-sided P-value of <0.05 was used for all analysis for the statistical significance. Statistical analyses were performed with Stata Release 16 (StataCorp LLC, College Station, TX) and SAS software, version 9.4 (SAS Institute Inc.).
Results
Characteristics of study population
Intervention participants were older than individuals receiving usual care (mean age, 77.1 [interquartile range (IQR) 74–80] versus 75.6 years [IQR 71–79]), and more likely to be women (75.9 versus 68.4%). Intervention group had lower grip strength, which is below the cutoff value for the definition of sarcopenia (17.3 versus 20.2 kg; cutoff is <26 kg for men and <18 kg for women, definition from Asian Working Group for Sarcopenia) [39], more at risk of malnutrition (Mini Nutritional Assessment-Short Form score ≤11, 52.9 versus 41.8%), had higher prevalence of polypharmacy (31.0 versus 23.5%), risk of depression (CES-D score ≥16, 23.0 versus 21.6%) and fall in the last year (21.9 versus 13.8%). The intervention group also had worse SPPB score (7.4 versus 8.4) and were more frail (frailty phenotype scale, 2.3 versus 1.8; frailty index, 0.27 versus 0.23) (Table 2).
Table 2 .
Comparison of baseline characteristics before and after propensity score matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Intervention (N = 187) |
Comparison (N = 196) |
SMD | Intervention (N = 117) |
Comparison (N = 117) |
SMD | |
| Age, mean (SD) | 77.1 (5.1) | 75.6 (6.2) | 0.27 | 76.3 (4.9) | 76.3 (6.5) | 0.01 |
| Female, n (%) | 142 (75.9) | 134 (68.4) | 0.17 | 85 (72.6) | 90 (76.9) | −0.10 |
| Enrolled year, mean (SD) | ||||||
| 2014 | 33 (17.6) | 66 (33.7) | 0.39 | 23 (19.7) | 19 (16.2) | 0.10 |
| 2015 | 88 (47.1) | 66 (33.7) | 50 (42.7) | 50 (42.7) | ||
| 2016 | 66 (35.3) | 64 (32.7) | 44 (37.6) | 48 (41.0) | ||
| Medical aid, n (%) | 48 (25.7) | 30 (15.3) | 0.26 | 26 (22.2) | 22 (18.8) | 0.08 |
| Living alone, n (%) | 144 (77) | 175 (89.3) | −0.33 | 100 (85.5) | 98 (83.8) | 0.05 |
| ASM/height2, kg/m2, mean (SD) | 5.9 (1.1) | 6.2 (1.1) | −0.19 | 6.0 (1.1) | 6.0 (1.1) | 0.03 |
| Grip strength, kg, mean (SD) | 17.3 (7.1) | 20.2 (9.2) | −0.36 | 18.0 (7.5) | 17.3 (7.4) | 0.10 |
| No. chronic conditions, mean (SD) | 1.6 (1.0) | 1.4 (1.0) | 0.22 | 1.5 (1.0) | 1.5 (1.1) | 0.03 |
| Polypharmacy, n (%) | 58 (31.0) | 46 (23.5) | 0.17 | 34 (29.1) | 33 (28.2) | 0.02 |
| ADL or IADL disability, n (%) | 90 (48.1) | 78 (39.8) | 0.17 | 56 (47.9) | 60 (51.3) | −0.07 |
| Decreased food intake in the last 3 months, n (%) | 77 (41.2) | 58 (29.6) | 0.24 | 41 (35.0) | 42 (35.9) | 0.02 |
| CES-D score, mean (SD) | 9.5 (9.5) | 9.8 (9.9) | −0.03 | 10.2 (10.0) | 9.9 (9.5) | 0.03 |
| MMSE-DS < 24, n (%) | 78 (41.7) | 59 (30.7) | −0.03 | 45 (38.5) | 43 (37.1) | 0.03 |
| Emergency room visit or admission in the last year, n (%) | 36 (19.3) | 35 (17.9) | 0.04 | 22 (18.8) | 22 (18.8) | 0.00 |
| Fall in the last year, n (%) | 41 (21.9) | 27 (13.8) | 0.21 | 24 (20.5) | 23 (19.7) | 0.02 |
| SPPB total score, mean (SD) | 7.4 (2.7) | 8.4 (2.8) | −0.34 | 7.6 (2.6) | 7.6 (2.8) | 0.02 |
| Gait speed, m/s | 0.7 (0.2) | 0.8 (0.3) | −0.38 | 0.7 (0.2) | 0.7 (0.2) | −0.05 |
| Chair stand, score | 2.5 (1.3) | 2.7 (1.3) | −0.15 | 2.5 (1.3) | 2.5 (1.3) | 0.01 |
| Balance test, score | 2.4 (1.5) | 2.8 (1.3) | −0.25 | 2.6 (1.4) | 2.5 (1.4) | 0.07 |
| Frailty phenotype, mean (SD) | 2.3 (1.3) | 1.8 (1.2) | 0.37 | 2.2 (1.2) | 2.2 (1.1) | −0.04 |
| Frailty index, mean (SD) | 0.27 (0.10) | 0.23 (0.11) | 0.33 | 0.25 (0.09) | 0.26 (0.12) | −0.03 |
ADL, activities of daily living; ASM, appendicular skeletal muscle; BMI, body mass index; IADL, instrumental activities of daily living; MNA-SF, Mini Nutritional Assessment-Short Form; MMSE-DS, Mini-Mental State Examination Dementia Screening.
Propensity score matching resulted in 117 matched pairs. Baseline characteristics were adequately balanced between the two groups with the absolute SMD <0.1, including SPPB score (7.6 versus 7.6), frailty phenotype scale (2.2 versus 2.2) and frailty index (0.25 versus 0.26) (Table 2).
Outcomes
In the matched cohort, the intervention group had higher SPPB scores than the comparison group at 6 months (MD 3.5; 95% CI, 2.8–4.2; P < 0.001), 18 months (1.3; 95% CI, 0.6–2.0; P < 0.001) and 30 months (1.1; 95% CI, 0.4–1.8; P = 0.003) (Figure 1).
Figure 1 .
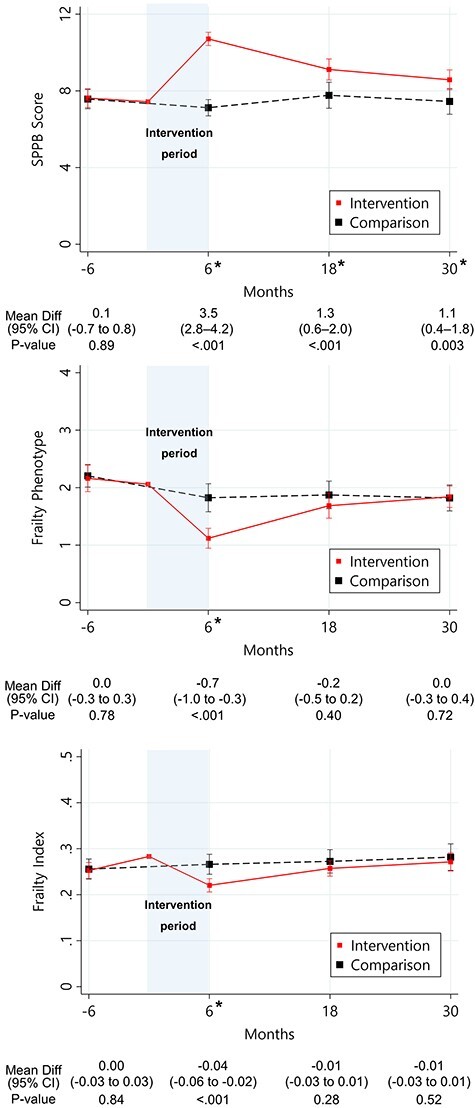
Change in SPPB score and frailty by intervention status. *P-value <0.05.
They had lower frailty phenotype scale (MD, −0.7; 95% CI, −1.0 to −0.3, P < 0.001) and frailty index (−0.04; −0.06 to −0.02; P < 0.001) only at 6 months, but similar scores at 18 months (frailty phenotype: MD, −0.2; −0.5 to 0.2; frailty index: MD, −0.01; −0.03 to 0.01) and 30 months (frailty phenotype: MD, 0.0; −0.3 to 0.4; frailty index: MD, −0.01; −0.03 to 0.01) (Figure 1).
Intervention group and comparison group have no statistically significant difference in CES-D score (8.0 [CI 7.5–8.5] versus 8.2 [CI 7.7–8.7]), prevalence in polypharmacy (Odds ratio (OR) 1.15; CI 0.66–2.01) and had no difference in number of falls in the last 6 months (Incidence rate ratios (IRR) 1.3; CI 0.9–1.9) at the end of 24-week intervention (Supplementary Material A4). Kaplan–Meier estimate of institutionalisation-free survival showed difference between the intervention and comparison group (log-rank P < 0.001) with a hazard ratio of 0.31 (95% CI, 0.17–0.56) (Figure 2). Institutionalisation-free survival at 30 months was 87.0% (95% CI, 79.4–92.0%) for the intervention group and 64.9% (95% CI, 55.2–73.0%) for the comparison group. The 30-month mean institutionalisation-free survival time was 28.5 months (95% CI, 27.6–29.4) in the intervention group and 23.3 months (95% CI, 21.3–25.2) in the comparison group, with a difference of 5.2 months (95% CI, 3.1–7.4; P < 0.001).
Figure 2 .

Kaplan–Meier estimate of death and institutionalisation-free survival.
Discussion
In this prospective study for a 24-week multicomponent intervention targeting frailty and geriatric syndrome in socioeconomically vulnerable community-dwelling older adults with mild to moderate frailty, we found that initial improvement in physical performance after the 24-week intervention period persisted up to 30 months Although improvement in frailty by either the phenotype model or deficit-accumulation model gradually disappeared and there was no significant improvement in CES-D, polypharmacy and fall, the rates of mortality and long-term care institutionalisation were lower in the intervention group. These results suggest that the benefit of our 24-week multicomponent intervention programme on physical function can last beyond the immediate post-intervention period and may delay death and institutionalisation in socioeconomically vulnerable older adults.
With accumulating evidence emphasising frailty as a strong predictor and risk factor of poor health outcomes in older adults, studies have sought to design and test multicomponent interventions to improve disability, physical performance or frailty [14, 15, 40, 41]. To date, studies have showed short-term benefits of intervention programmes, whereas long-term effects from interventions are not well established [42]. In this study, by combining group exercise, protein supplementation, depression management, deprescribing and home hazard reduction, we were able to improve physical performance and prevent mortality and institutionalisation over 2 years after completion of the intervention. There are several potential explanations for the success of our intervention. We targeted socioeconomically vulnerable older adults living alone or on lower income, who might have greater potential for improvement when provided with adequate health interventions. Moreover, group dynamics from group exercise sessions may have caused positive feedback [43] as well as reinforcement from celebration of high attendance may have maximised the adherence to the exercise programme. In contrast to previous studies, which largely focused on exercise and nutrition, our intervention targeted common geriatric syndromes of depression and polypharmacy and included home hazard reduction for individuals with identified risk factors at the initial assessment. We speculate that such patient-centred multimodal approach may be responsible for our positive results.
We observed a relatively large improvement of the SPPB score immediately after the intervention programme in comparison with previous studies [5]. Improvement in the SPPB score was sustained over 24 months, whereas improvement in frailty was temporary. The frailty phenotype includes parameters other than physical performance, such as grip strength, exhaustion and weight loss. Similarly, a deficit-accumulation frailty index is heavily weighted by the comorbidity burden. These components of frailty may be less likely to improve with our multicomponent intervention. As an alternative explanation, SPPB score might be subject to practice or learning effect from repeated assessments over time. However, stable trends in SPPB score among the comparison group make such possibility unlikely.
There are several challenges in conducting community-based interventions for vulnerable older populations, especially in underserved rural areas, such as communities in our study, in which there are limited resources and infrastructure to conduct a randomised controlled trial [44–47]. To deliver a frailty intervention programme in a cost-effective manner in resource-limited rural communities, it is important to know how long the effectiveness of the intervention lasts. Self-efficacy and health autonomy strategies are important determinants of sustained benefit [48]. However, our study intervention did not include cognitive behavioural interventions to improve self-efficacy or health autonomy, nor did we measure them in our study. In addition, it is crucial to evaluate the long-term effectiveness of the intervention while minimising loss to follow-up for both the intervention group and the usual care comparison group. We were able to overcome these challenges by delivering our intervention programme within an ongoing prospective cohort study of older adults in the study areas. Even for older adults in the usual care group who declined to receive the intervention, we were able to obtain sufficient information on health characteristics and study outcomes from the cohort assessments.
There are several limitations in the study. First, our results from socioeconomically vulnerable older adults in underserved rural areas in Korea may not be generalisable to other populations in resource-rich urban areas. However, the sustained benefit over 24 months after completion of the intervention in our study is informative to policymakers who are designing a public health intervention. Second, despite our effort to minimise bias by using multivariable imputation and propensity score matching, we acknowledge that our results do not establish causality. Nonetheless, in the dearth of clinical trials with long-term follow-up, our study provides useful information. Also, given that the intervention group was more frail and physically impaired, and the differential dropout of sicker people in the control group at baseline, the benefit of our intervention may have shown a larger benefit if residual bias was entirely adjusted. Third, we were unable to assess the optimal component and duration of our multicomponent programme. In our previous study, we have shown that effects of the 24-week intervention slowly showed diminished association from 24 months and beyond [23]. As a next step, a pragmatic cluster randomised controlled trial of public health centres is warranted to overcome limitations of the non-randomised design and determine the optimal intervention components to prevent frailty in vulnerable older populations.
In conclusion, our study suggests that a 24-week multicomponent intervention programme provides sustained improvement in physical performance and reduction in mortality and institutionalisation in socioeconomically vulnerable older adults with mild to moderate frailty. Our results can inform design and implementation of a public health intervention for the soaring number of older adults in resource-limited rural communities.
Supplementary Material
Acknowledgements
The authors are indebted to the public health professionals and nurses of the Pyeongchang County Hospital, Public Health Center and Community Health Posts for their administrative support and efforts in enrolment, retention and measurements. The authors also appreciate Maeil Diaries Co., Ltd (Seoul, Korea) for providing nutritional supplements (Selecs) free of charge. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle [49].
Contributor Information
Gahee Oh, Marcus Institute for Aging Research, Hebrew Senior Life, Boston, MA, USA.
Heayon Lee, Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; Division of Pulmonary, Critical Care and Sleep Medicine, Department of Internal Medicine, Eunpyeong St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
Chan Mi Park, Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; Harvard T.H.Chan School of Public Health, Boston, MA 02115, USA.
Hee-Won Jung, Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Eunju Lee, Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea.
Il-Young Jang, Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; PyeongChang Health Center and County Hospital, PyeongChang, Gangwon-Do, Republic of Korea.
Jack M Guralnik, Division of Gerontology, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, USA.
Dae Hyun Kim, Marcus Institute for Aging Research, Hebrew Senior Life, Boston, MA, USA; Division of Gerontology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
The Aging Study of Pyeongchang Rural Area, an intervention study, was funded by the Pyeongchang County Hospital, Pyeongchang County, Gangwon Province, Korea. This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI18C2383), and partially supported by a grant (2019IF0592) from the Asan Institute for Life Science, Asan Medical Center, Seoul, Korea. Dr D.H.K. is supported by grants (R01AG056368, R01AG062713, R21AG060227, P30AG031679 and P30AG048785) from the National Institute on Aging.
References
- 1. World Health Organization . World Health Statistics 2018: Monitoring Health for the SDGs, Sustainable Development Goals. Geneva: World Health Organization, 2018. [Google Scholar]
- 2. Jang I-Y, Lee HY, Lee E. Geriatrics Fact Sheet in Korea 2018 from National Statistics. Ann Geriatr Med Res 2019; 23: 50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung H-W, Jang I-Y, Lee YS et al. Prevalence of frailty and aging-related health conditions in older Koreans in rural communities: a cross-sectional analysis of the Aging Study of Pyeongchang Rural Area. J Korean Med Sci 2016; 31: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee H, Lee E, Jang I-Y. Frailty and comprehensive geriatric assessment. J Korean Med Sci 2020; 35: e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jang I-Y, Jung H-W, Park H et al. A multicomponent frailty intervention for socioeconomically vulnerable older adults: a designed-delay study. Clin Interv Aging 2018; 13: 1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang L, Gao Y, Liu X et al. Effects of whey protein nutritional supplement on muscle function among community-dwelling frail older people: a multicenter study in China. Arch Gerontol Geriatr 2019; 83: 7–12. [DOI] [PubMed] [Google Scholar]
- 7. Christie J. Progressive resistance strength training for improving physical function in older adults. Int J Older People Nurs 2011; 6: 244–6. [DOI] [PubMed] [Google Scholar]
- 8. Dent E, Lien C, Lim WS et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc 2017; 18: 564–75. [DOI] [PubMed] [Google Scholar]
- 9. Franse CB, van Grieken A, Alhambra-Borras T et al. The effectiveness of a coordinated preventive care approach for healthy ageing (UHCE) among older persons in five European cities: a pre-post controlled trial. Int J Nurs Stud 2018; 88: 153–62. [DOI] [PubMed] [Google Scholar]
- 10. Gill TM, Baker DI, Gottschalk M et al. A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Arch Phys Med Rehabil 2004; 85: 1043–9. [DOI] [PubMed] [Google Scholar]
- 11. LIFE Study Investigators, Pahor M, Blair SN, Espeland M et al. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci 2006; 61: 1157–65. [DOI] [PubMed] [Google Scholar]
- 12. Cameron ID, Fairhall N, Langron C et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med 2013; 11: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng TP, Feng L, Nyunt MS et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med 2015; 128: 1225–36.el. [DOI] [PubMed] [Google Scholar]
- 14. Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc 2016; 17: 426–33. [DOI] [PubMed] [Google Scholar]
- 15. Serra-Prat M, Sist X, Domenich R et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing 2017; 46: 401–7. [DOI] [PubMed] [Google Scholar]
- 16. Burton E, Hill AM, Pettigrew S et al. Why do seniors leave resistance training programs? Clin Interv Aging 2017; 12: 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bull F, Al-Ansari S, Biddle S et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020; 54: 1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Volkert D, Beck A, Cederholm T et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019; 38: 10–47. [DOI] [PubMed] [Google Scholar]
- 19. Jung HW, Kim SW, Kim IY et al. Protein intake recommendation for Korean older adults to prevent sarcopenia: expert consensus by the Korean Geriatric Society and the Korean Nutrition Society. Ann Geriatr Med Res 2018; 22: 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen D, Vu CM. Current depression interventions for older adults: a review of service deliverly approaches in primary care, home-based, and community based settings. Curr Tran Geriatr Gerontol Rep 2013; 2: 37–44. [Google Scholar]
- 21. Bloomfield H, Greer N, Linsky A et al. Deprescribing for community-dwelling older adults: a systematic review and meta-analysis. J Gen Intern Med 2020; 35: 3323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention (CDC) . A Home Fall Prevention Checklist for Older Adults. Available at https://www.cdc.gov/steadi/pdf/check_for_safety_brochure-a.pdf (21 June 2015, date last accessed).
- 23. Park CM, Oh G, Lee H et al. Multicomponent intervention and long-term disability in older adults: a nonrandomized prospective study. J Am Geriatr Soc 2021; 69: 669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jang IY, Jung HW, Lee CK et al. Comparisons of predictive values of sarcopenia with different muscle mass indices in Korean rural older adults: a longitudinal analysis of the Aging Study of Pyeong Chang Rural Area. Clin Interv Aging 2018; 13: 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perera S, Mody SH, Woodman RC et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–9. [DOI] [PubMed] [Google Scholar]
- 26. Jung HW, Jang IY, Lee CK et al. Usual gait speed is associated with frailty status, institutionalization, and mortality in community-dwelling rural older adults: a longitudinal analysis of the Aging Study of Pyeongchang Rural Area. Clin Interv Aging 2018; 13: 1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung HW, Jin T, Baek JY et al. Functional age predicted by electronic short physical performance battery can detect frailty status in older adults. Clin Interv Aging 2020; 15: 2175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 29. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007; 62: 738–43. [DOI] [PubMed] [Google Scholar]
- 30. S-N J, Kawachi I. Why do older Korean adults respond differently to activities of daily living and instrumental activities of daily living? A differential item functioning analysis. Ann Geriatr Med Res 2019; 23: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jang IY, Jung HW, Lee CK et al. Korean version of the Fatigue, Resistance, Ambulation, Illnesses and Loss of weight questionnaire versus the Modified Kihon Checklist for Frailty Screening in Community-Dwelling Older Adults: the Aging Study of Pyeong Chang Rural Area. Geriatr Gerontol Int 2017; 17: 2046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jang IY, Jung HW, Lee CK et al. Comparison between Korean version of Physical Activity Scale for the Elderly and International Physical Activity Questionnaire-Short Form in evaluation of frailty phenotype. Ann Geriatr Med Res 2017; 21: 101–7. [Google Scholar]
- 33. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977; 1: 385–401. [Google Scholar]
- 34. Park H, Jang IY, Lee HY et al. Screening value of social frailty and its association with physical frailty and disability in community-dwelling older Koreans: Aging Study of Pyeong Chang Rural Area. Int J Environ Res Public Health 2019; 16: 2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rabin R, Gudex C, Selai C et al. From translation to version management: a history and review of methods for the cultural adaptation of the EuroQol five-dimensional questionnaire. Value Health 2014; 17: 70–6. [DOI] [PubMed] [Google Scholar]
- 36. Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw 2011; 45: 1–20. [Google Scholar]
- 37. Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical Software Components S432001, Boston College Department of Economics, revised 01 Feb 2018.
- 38. Bayoumi AM. STDDIFF: Stata module to compute standardized differences for continuous and categorical variables. Statistical Software Components S458275, Boston College Department of Economics, revised 09 Mar 2021.
- 39. Chen LK, Liu LK, Woo J et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 40. Walston J, Buta B, Xue Q-L. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med 2018; 34: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Theou O, Stathokostas L, Roland KP et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011; 2011: 569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puts MTE, Toubasi S, Andrew MK et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017; 46: 383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Komatsu H, Yagasaki K, Saito Y et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr 2017; 17: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fougère B, Aubertin-Leheudre M, Vellas B et al. Clinical research for older adults in rural areas: the MINDED study experience. Age (Dordr) 2016; 38: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cohen GI. Clinical research by community oncologists. CA Cancer J Clin 2003; 53: 73–81. [DOI] [PubMed] [Google Scholar]
- 46. Maurer LH, Davis T, Hammond S et al. Clinical trials in a rural population: professional education aspects. J Cancer Educ 2001; 16: 89–92. [DOI] [PubMed] [Google Scholar]
- 47. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol 2002; 12: 248–56. [DOI] [PubMed] [Google Scholar]
- 48. Lachman M, Lipsitz L, Lubben J et al. When adults don’t exercise: behavioral strategies to increase physical activity in sedentary middle-aged and older adults. Innov Aging 2018; 2: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Haehling S, Morley JE, Coats AJS et al. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia, and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017; 8: 1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


