Abstract
Background
after hospitalisation for cardiac disease, older patients are at high risk of readmission and death.
Objective
the cardiac care bridge (CCB) transitional care programme evaluated the impact of combining case management, disease management and home-based cardiac rehabilitation (CR) on hospital readmission and mortality.
Design
single-blind, randomised clinical trial.
Setting
the trial was conducted in six hospitals in the Netherlands between June 2017 and March 2020. Community-based nurses and physical therapists continued care post-discharge.
Subjects
cardiac patients ≥ 70 years were eligible if they were at high risk of functional loss or if they had had an unplanned hospital admission in the previous 6 months.
Methods
the intervention group received a comprehensive geriatric assessment-based integrated care plan, a face-to-face handover with the community nurse before discharge and follow-up home visits. The community nurse collaborated with a pharmacist and participants received home-based CR from a physical therapist. The primary composite outcome was first all-cause unplanned readmission or mortality at 6 months.
Results
in total, 306 participants were included. Mean age was 82.4 (standard deviation 6.3), 58% had heart failure and 92% were acutely hospitalised. 67% of the intervention key-elements were delivered. The composite outcome incidence was 54.2% (83/153) in the intervention group and 47.7% (73/153) in the control group (risk differences 6.5% [95% confidence intervals, CI −4.7 to 18%], risk ratios 1.14 [95% CI 0.91–1.42], P = 0.253). The study was discontinued prematurely due to implementation activities in usual care.
Conclusion
in high-risk older cardiac patients, the CCB programme did not reduce hospital readmission or mortality within 6 months.
Trial registration
Netherlands Trial Register 6,316, https://www.trialregister.nl/trial/6169
Keywords: cardiac rehabilitation, cardiology, case management, disease management, transitional care
Key Points
The CCB program combined case management, disease management and home-based cardiac rehabilitation.
This intervention did not reduce readmission or mortality within six months following hospitalization.
The selected patient population may not be responsive to high-intensity preventive strategies.
Introduction
The incidence of readmission and mortality in older patients with cardiovascular disease is rising [1, 2]. Hospital treatment of older cardiac patients is commonly disease-oriented with interventions based on disease-specific guidelines. However, geriatric conditions such as functional impairment, fall risk and malnutrition [3] often go unrecognised although they increase the risk of adverse events [4, 5].
The transitional phase, when patients transfer from hospital to home, is a high-risk period for adverse events [6]. Medication-related problems are common [7] and symptoms of physical deterioration often stay unrecognised [8]. Furthermore, participation in cardiac rehabilitation (CR) programmes is low [9]. As CR is effective in older patients [9], non-participation could increase the risk of recurrent cardiovascular events and mortality [10].
Transitional care has been shown effective in reducing hospital readmission and mortality [11–13]. However, results are inconclusive in older cardiac patients [14–17]. Most transitional care interventions are provided from a case management perspective, delivering interventions with a broad focus on patients’ needs [6, 17]. The integration of disease management and tailored home-based CR into transitional care interventions may be necessary.
The purpose of this study was to evaluate the effects on unplanned hospital readmission and mortality of the nurse-coordinated ‘cardiac care bridge (CCB) transitional care programme’ which combines case management, disease management and home-based CR in high-risk older hospitalised cardiac patients.
Methods
Study design and setting
We tested the CCB programme in a parallel single-blind multicentre randomised trial, performed between 5 June 2017 and 31 March 2020 in six hospitals surrounding Amsterdam, the Netherlands. Community nurses (CNs) and community-based physical therapists (PT) continued care post-discharge. The trial design has been published [18]. The study was approved by the Medical Ethics Committee of the Amsterdam University Medical Centre (Protocol ID: MEC2016_024) and registered in the Dutch Trial Register (NTR6316, April 6, 2017). The results are reported according to the Consort (Consolidated Standards of Reporting Trials) statement [19].
Study population
Cardiac patients of ≥70 years, admitted to the departments of cardiology or cardiothoracic surgery and admitted ≥48 h were eligible if they were at high risk of functional loss according to the screening instrument for frail older people of the Dutch Safety Management System (DSMS) [20]. Four geriatric conditions (limitation in activities of daily living [ADL], falls, malnutrition and delirium) are part of this frailty tool, and the DSMS-score ranges between 0 and 4 (Appendix 1, Supplementary data are available in Age and Ageing online). Patients were considered at high risk with a DSMS-score ≥ 2 in patients aged 70–79 years or DSMS-score ≥ 1 in patients aged ≥80 years [21]. Regardless of the DSMS-score, we also included patients with an unplanned hospital admission in the prior 6 months as this is associated with increased risk for adverse events [22].
Exclusion criteria were (i) inability to provide consent and follow instructions due to severe cognitive impairment (mini-mental state examination, MMSE < 15) or delirium as confirmed by the treating physician, (ii) congenital heart disease, (iii) life expectancy of ≤3 months as estimated by the treating physician, (iv) transfer from or planned discharge to a nursing home, (v) planned discharge to another department or hospital not participating in this study and (vi) inability to communicate.
Randomisation
The consent procedure and randomisation were performed ≤72 h after admission. According to the postponed informed consent procedure of Boter et al. [23], study participants were blinded to the specific study aims to prevent a potential Hawthorne effect [24]. At the end of the study, participants were fully informed about the intervention and treatment allocation. Stratified block randomisation to the intervention or control group, allocation ratio 1:1, was used with pre-stratification by study site and cognitive status (MMSE 15–23 vs ≥24). Allocation concealment was ensured by a web-based data management programme (Research Manager, https://my-researchmanager.com/en/) and random permuted blocks of two, four and six were used.
Usual care
All patients received a comprehensive geriatric assessment (CGA) at baseline by a cardiac research nurse. The control group continued with usual care including consultation by other disciplines during hospitalisation, outpatient visits to the cardiologist and cardiac nurse specialist and centre-based CR if indicated. In addition, standard care was provided by the family physician. The Dutch healthcare system is described in Appendix 2 (Supplementary data are available in Age and Ageing online).
Intervention
The CCB programme was performed in three phases (Figure 1): the clinical, discharge and post-clinical phase. The intervention consisted of three care components: (i) case management, (ii) disease management and (iii) home-based CR. The intervention key-elements are described below and in Appendix 3 (Supplementary data are available in Age and Ageing online). All involved healthcare professionals received a post-Bachelor-level training in case management, disease management and CR (Appendix 4, Supplementary data are available in Age and Ageing online). Informal caregivers were involved in the intervention if they were present.
Figure 1 .
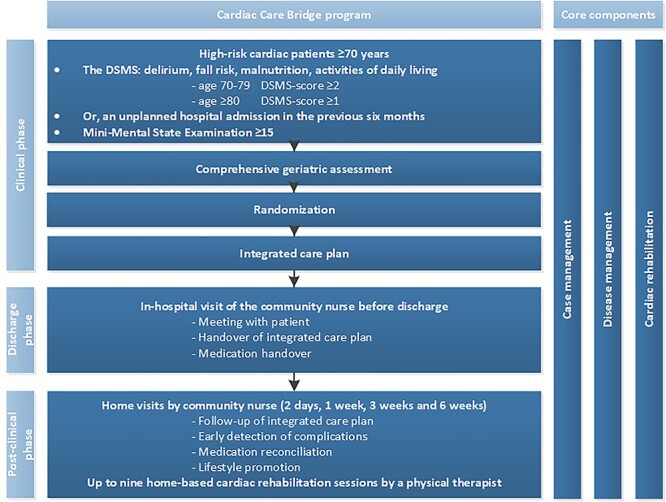
Overview of the CCB programme.
In the clinical phase, health issues identified by the CGA were discussed and prioritised by the cardiac research nurse and the participant. An integrated care plan based on patients’ goals was formulated, which was leading during the intervention. A geriatrician and other disciplines (e.g. dietician) were consulted based on CGA findings.
The discharge phase started when the discharge date was set. The cardiac research nurse contacted the CN and PT to arrange the post-clinical phase. In hospital, the CN visited the participant and the cardiac research nurse for a handover of the integrated care plan, and information about participants’ medical condition and treatments. In addition, the medical discharge letter was sent to all post-discharge CCB healthcare professionals.
In the post-discharge phase the CN planned home visits within 3 days, and 1, 3 and 6 weeks after discharge and an additional home visit within 12 weeks if necessary. During home visits, the CN reviewed the integrated care plan, participants’ health status, medication and potential drug-related problems (DRPs) including side-effects and inappropriate use. Together with the CCB pharmacist, medication reconciliation was performed during the first home visit. DRPs were signalled by the CN using the Red Flag instrument [25]. Issues were discussed with the pharmacist who proposed adjustments. For questions regarding participants’ health status, the CN contacted e.g. the general practitioner or cardiologist based on indication.
Furthermore, the PT provided one or two home-based CR sessions per week, with a maximum of nine sessions during the first 6 weeks post-discharge according to the Dutch CR guideline [26]. The first home visit by the PT was a joint intake with the CN and the participant to discuss goals and desired activities, which led to a rehabilitation plan. Depending on participants’ functional status a stepwise graded exercise approach was followed, including improving functional activities (e.g. rising from chair, walking and climbing stairs) and increasing muscle strength.
Primary and secondary outcomes
The primary outcome was a composite of first all-cause unplanned readmission or mortality within 6 months after randomisation. We defined an unplanned readmission as a non-elective admission ≥1 night. Secondary outcomes included the composite outcome at three and 12 months after randomisation and the incidence of the first all-cause unplanned hospital readmission and mortality separate at 3, 6 and 12 months. Mortality data were collected from medical files and the Dutch National Personal Records Database [27]. Data on readmissions were collected from medical files in the participating hospitals and supplemented with participants’ self-reported readmissions to other hospitals. Data collection were performed by research nurses who were blinded to the treatment allocation.
Sample size calculation
The sample size calculation was based on a comparable study of 101/674 hospitalised cardiac patients ≥65 years at high risk of functional loss [13]. Based on a 6 month incidence of 44% (readmission and mortality combined) in the usual care group and a minimal important difference of 12.5% in absolute risk reduction (from 44 to 31.5%) in participants in the intervention arm (chi-square test and a 2-sided alpha of 0.05; power of 80%), a sample size of 235 participants per group was required. To compensate for an assumed 5% loss to follow-up, the total intended sample size per group was 250.
Statistical analyses
Analyses were performed according to a predefined statistical analyses plan based on the intention-to-treat principle (Appendix 5, Supplementary data are available in Age and Ageing online).
We reported univariable outcomes and presented the multivariable models in the appendices as both analyses revealed comparable results. The treatment effect of the primary and secondary outcomes was expressed as risk differences (RD) and risk ratios (RR) and the corresponding 95% confidence intervals (CIs) based on a chi-square test.
Multivariable logistic and Cox regression analyses were performed and resulting adjusted odds ratios (OR) were transformed into RRs [28]. We adjusted for frailty status, study site, age, sex, any admissions in the previous 6 months, Charlson comorbidity score, MMSE, cardiovascular diagnosis and living arrangement. In addition, we checked for treatment interaction with the following predefined subgroup analyses: age, frailty status, any unplanned hospital admission in the previous 6 months, cognitive impairment and diagnosis at index admission. Correction for (semi-)competing risk was performed by a unidirectional transition multistate model (illness-death model; [29, 30]).
All statistical tests were 2-sided. P-values < 0.05 were considered statistically significant. Analyses were performed with SPSS 25.0 (SPSS Inc., Chicago, IL, USA) and Stata Statistical Software: Release 13 (College Station, TX: StataCorp LP).
Intervention fidelity
Fidelity to key-elements of the intervention was registered by CCB healthcare professionals and evaluated by quality indicators [31]. For each participant, the denominator of the intervention key-elements was set to the number of feasible key-elements. Key-elements missed due to e.g. hospital readmission, death or disabilities that precluded participants from taking part in any key-element, were not deemed feasible and not counted in the denominator. The mean fidelity rate was calculated per intervention key-element and in addition for each participant, we calculated the mean fidelity percentage across all key-elements that a participant was entitled to. The overall adherence percentage across all 153 participants was calculated as an unweighted average of the participant-specific percentages.
Results
We screened 6,857 patients for enrolment, 623 patients (9%) were eligible for participation (Figure 2). Most exclusions were due to low DSMS-scores (59%). In total, 306 eligible patients provided informed consent (49%) and were randomised (153/153). Inclusion was discontinued prematurely on 31 March 2019 because of contrast problems between intervention and control due to an increase in implementation activities in usual care (e.g. home-based follow-up and the Red Flag instrument) [25]. Outcome data were complete for all included participants (follow-up until 31 March 2020).
Figure 2 .
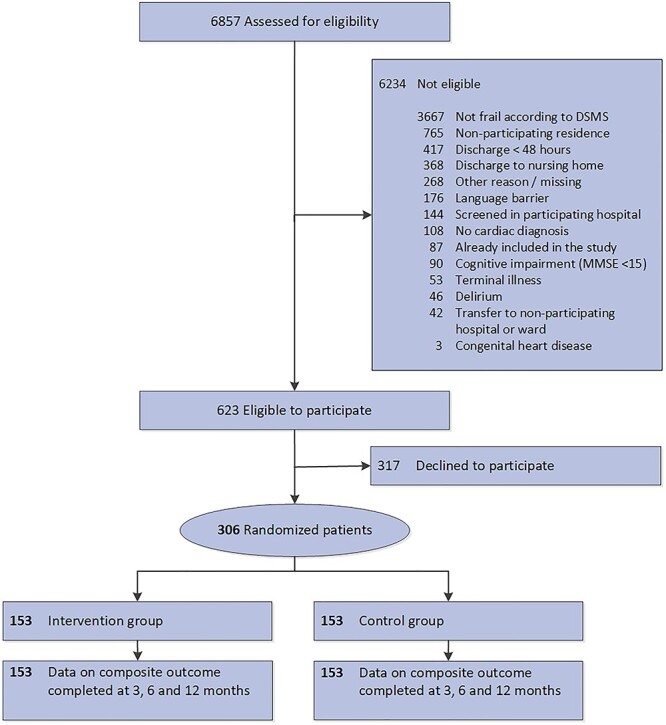
Flowchart CCB study.
On average, participants were 82.4 years old (SD 6.3) and 51% were male. Participants were mostly admitted for HF (58%) and 45% had had an unplanned hospital admission in the previous 6 months. In total, 56% were at risk of delirium, 47% had fallen in the 6 months prior to admission, 39% had ADL-limitations and 33% had malnutrition (Table 1).
Table 1 .
Baseline characteristics
| Intervention (n = 153) | Control (n = 153) | ||||
|---|---|---|---|---|---|
| Sociodemographics | Measurement | ||||
| Age | 82.5 | (6.1) | 82.3 | (6.5) | |
| 70–79 years | 40 | 26.1% | 51 | 33.3% | |
| ≥ 80 years | 113 | 73.9% | 102 | 66.7% | |
| Sex | Male | 70 | 45.8% | 86 | 56.2% |
| Country of origin | Netherlands | 135 | 88.2% | 138 | 90.2% |
| Level of educationa | Primary education | 66 | 43.1% | 61 | 39.9% |
| Secondary education | 52 | 34.0% | 44 | 28.8% | |
| Higher education | 35 | 22.9% | 47 | 30.7% | |
| Cohabitating | 66 | 43.1% | 68 | 44.4% | |
| Socioeconomic statusb | Low (< 1 SD) | 25 | 16.3% | 27 | 17.6% |
| Intermediate | 83 | 54.2% | 81 | 52.9% | |
| High (> 1 SD) | 45 | 29.4% | 45 | 29.4% | |
| Index hospitalisation | |||||
| Acute hospitalisation | 139 | 90.8% | 141 | 92.2% | |
| Length of stay | Days | 7 | [4–10] | 7 | [4.5–10] |
| Diagnosis on admission | Heart failure | 86 | 56.2% | 91 | 59.5% |
| Rhythm or conduction disorder | 27 | 17.6% | 20 | 13.1% | |
| Acute coronary syndrome | 19 | 12.4% | 24 | 15.7% | |
| Valve deficits | 14 | 9.2% | 12 | 7.8% | |
| Other | 7 | 4.6% | 6 | 3.9% | |
| Treatment during admission | Medical treatment only | 115 | 75.2% | 116 | 75.8% |
| PCI | 13 | 8.5% | 15 | 9.8% | |
| TAVR | 15 | 9.8% | 11 | 7.2% | |
| Device implantation | 12 | 7.8% | 10 | 6.5% | |
| Other | 1 | 0.7% | 4 | 2.6% | |
| Inclusion criteria | Measurement | ||||
| Previous hospital admission | ≤ 6 months prior to index event | 66 | 43.1% | 73 | 47.7% |
| Delirium | DSMS delirium risk score | 94 | 61.4% | 77 | 50.3% |
| Activities of daily living | DSMS impairment in ADL (KATZ-6) | 65 | 42.5% | 54 | 35.3% |
| Activities of daily living | Median (KATZ-6) | 1 | [0–3] | 0 | [0–2] |
| ADL-functioning | ALDS-score (0–100) | 72 | [58–84] | 76 | [63–86] |
| Malnutrition | DSMS malnutrition (SNAQ) | 57 | 37.3% | 43 | 28.1% |
| Fall risk | DSMS fall ≤6 months | 67 | 43.8% | 78 | 51.0% |
| Fear of falling | NRS ≥ 4 | 63 | 41.2% | 66 | 43.1% |
| DSMS scorec | DSMS 0 | 13 | 8.5% | 13 | 8.5% |
| DSMS 1 | 49 | 32.0% | 59 | 38.6% | |
| DSMS 2 | 50 | 32.7% | 57 | 37.3% | |
| DSMS 3 | 33 | 21.6% | 19 | 12.4% | |
| DSMS 4 | 8 | 5.2% | 5 | 3.3% | |
| Medical history | |||||
| Heart failure | 105 | 68.6% | 110 | 71.9% | |
| Hypertension | 95 | 62.1% | 94 | 61.4% | |
| Acute coronary syndrome | 57 | 37.3% | 53 | 34.6% | |
| Atrial fibrillation | 54 | 35.3% | 59 | 38.6% | |
| Diabetes mellitus | 52 | 34.0% | 47 | 30.7% | |
| Renal failure | 51 | 33.3% | 59 | 38.6% | |
| Chronic obstructive pulmonary disease | 29 | 19.0% | 24 | 15.7% | |
| Peripheral vascular disease | 29 | 19.0% | 21 | 13.7% | |
| Cerebrovascular accident | 23 | 15.0% | 27 | 17.6% | |
| Lifestyle factors | Measurement | ||||
| Current smoker | Self-reported | 16 | 10.5% | 14 | 9.2% |
| Body mass index | Kg/m2 | 26.8 | (5.9) | 25.8 | (4.6) |
| Geriatric conditions | Measurement | ||||
| Cognitive impairment | MMSE 15–23 | 47 | 30.7% | 48 | 31.4% |
| Comorbidities | Charlson Comorbidity Score | 3 | [1–4] | 3 | [1–4] |
| Depressive symptoms | GDS ≥ 6 | 22 | 14.6% | 18 | 11.8% |
| Anxiety | HADS-A ≥ 8 | 18 | 11.9% | 24 | 15.7% |
| Dyspnoea | Self-reported | 125 | 81.7% | 123 | 80.4% |
| Fatigue | NRS ≥ 4 | 114 | 74.5% | 114 | 74.5% |
| Dizziness | Self-reported | 65 | 42.5% | 76 | 49.7% |
| Urine incontinence | Self-reported | 42 | 27.5% | 41 | 26.8% |
| Polypharmacy | ≥ 5 (from medication overview) | 141 | 92.2% | 144 | 94.1% |
| Medication side effects | Self-reported | 34 | 22.2% | 35 | 22.9% |
| Functional status | SPPB | 4 | [2–6] | 5 | [3–7] |
| Handgrip strengthd | Male (norm >30 kg) | 26.4 | 9.2 | 27.0 | (7.8) |
| Female (norm >18 kg) | 16.1 | (5.8) | 15.3 | (4.7) | |
Note: (SD), [25–75 percentile].
Abbreviations: ALDS, Amsterdam Linear Disability Scale; CABG, Coronary Artery Bypass Grafting; DSMS, Dutch Safety and Management System; GDS, Geriatric Depression Scale; HADS-A, Hospital Anxiety and Depression Scale-Anxiety; MMSE, Mini-Mental State Examination; NRS, numeric rating scale; PCI, Percutaneous Coronary Intervention; SNAQ, Short Nutritional Assessment Questionnaire; SPPB, Short Physical Performance Battery; TAVR, Transcatheter Aortic Valve Replacement.
aPrimary education: elementary or primary school. Secondary education: pre-vocational, senior general or pre-university. Higher education: higher professional or university.
bSocioeconomic status score was calculated from the postal code of patients’ residence by the Netherlands Institute for Social Research (SCP) and based on income, employment and educational level.
cDutch Safety Management System [20]: the score between 0 and 4 points, based on four domains of frailty (malnutrition, risk of impairments in daily functioning, risk on delirium and fall risk). A higher score on the DSMS indicates a higher risk of functional loss.
dDominant hand highest value.
Primary outcome
The incidence of the 6-month composite outcome of first all-cause readmission or mortality was 54.2% (83/153) in the intervention group and 47.7% (73/153) in the control group (RD 6.5%, 95% CI −4.7–18%, RR 1.14, 95% CI 0.91–1.42, P = 0.341; Table 2 and Figure 3). The multivariable analysis showed similar results (Appendix 6, Supplementary data are available in Age and Ageing online).
Table 2 .
Primary and secondary outcomes in the CCB study
| Intervention n = 153 (%) | Control n = 153 (%) | Risk difference (%) (95% CI) | Risk ratio (95% CI) | P-value risk ratio | |
|---|---|---|---|---|---|
| Composite outcome | |||||
| 3 months | 63 (41.2) | 59 (38.6) | 2.6 (−8.4–13.6) | 1.07 (0.81–1.41) | 0.641 |
| 6 months | 83 (54.2) | 73 (47.7) | 6.5 (−4.7–18.0) | 1.14 (0.91–1.42) | 0.253 |
| 12 months | 101 (66.0) | 88 (57.5) | 8.5 (−2.4–19.3) | 1.15 (0.96–1.37) | 0.126 |
| Unplanned readmission a | |||||
| 3 months | 45 (29.4) | 48 (31.4) | −1.9 (−12.2–8.3) | 0.94 (0.67–1.32) | 0.709 |
| 6 months | 60 (39.2) | 59 (38.6) | 0.7 (−10.3–11.6) | 1.02 (0.77–1.35) | 0.907 |
| 12 months | 73 (47.7) | 70 (45.8) | 1.9 (−0.2–13.1) | 1.04 (0.82–1.32) | 0.731 |
| Mortality | |||||
| 3 months | 26 (17.0) | 20 (13.1) | 3.9 (−4.1–12.0) | 1.30 (0.76–2.23) | 0.337 |
| 6 months | 36 (23.5) | 28 (18.3) | 5.2 (−3.9–14.3) | 1.29 (0.83–2.00) | 0.261 |
| 12 months | 59 (38.6) | 41 (26.8) | 11.8 (1.3–22.2) | 1.44 (1.04–2.00) | 0.028 |
aResults are not corrected for (semi-)competing risk. Appendix 8 of supplementary data presents the for (semi-)competing risk corrected outcomes in a multi-state (illness-death) model.
Figure 3 .
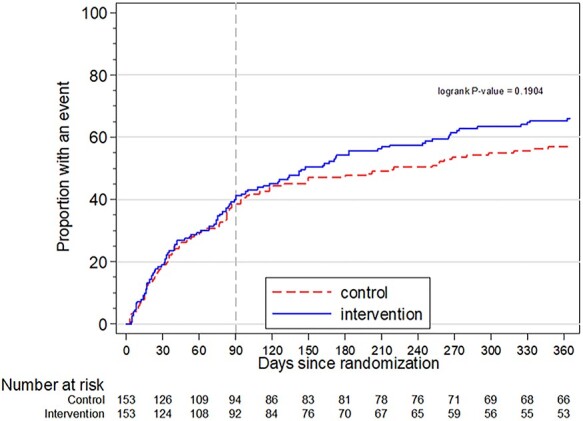
Kaplan–Meier curve of the composite outcome within 12 months. Dashed line at 90 days marks the end of the intervention period. The curves of the intervention and control group in the primary outcome diverged after the intervention was completed at 90 days follow-up.
In the univariable subgroup analyses of the primary outcome, interaction seemed present in participants who had been admitted in the previous 6 months (Appendix 7, Supplementary data are available in Age and Ageing online). No treatment interactions were found for age, DSMS-score, cardiovascular diagnosis and cognitive impairment on the composite outcome.
Secondary outcomes
At three and 12 months after randomisation, statistically non-significant differences were found on the composite outcome (Table 2). In addition, we did not find statistically significant differences on readmission (3, 6 and 12 months) and mortality (on 3 and 6 months). However, at 12 months follow-up, 38.6% of participants in the intervention group and 26.8% participants in the control group had died (RD 11.8%, 95% CI 1.3–22.2%, RR 1.44, 95% CI 1.04–2.00, P = 0.028). Multivariable regression analyses of all secondary outcomes showed comparable results (Appendix 6, Supplementary data are available in Age and Ageing online). Uni- and multivariable Cox regression analyses are presented in Appendix 6 (Supplementary data are available in Age and Ageing online). Appendix 8 of supplementary data shows the results of the multi-state illness-death models up to 12 months.
Intervention fidelity
In total, the mean participant fidelity percentage across all key-elements that a participant entitled to was 67%. However, the fidelity rates varied widely across the various key-elements (median 60%, IQR [41–69], range [17–100]). Table 3 presents the measures of intervention fidelity per key-element. In total, 75% of all intervention key-elements in the clinical phase were performed, 37% in the discharge phase and 64% in the post-clinical phase.
Table 3 .
Intervention fidelity
| Intervention key-elements | N a | % |
|---|---|---|
| Clinical phase | ||
| CGA and CGA-based integrated care plan | 153/153 | 100 |
| Geriatric consultation based on indicationb | 11/66 | 17 |
| Discharge phase | ||
| Handover | ||
| Face-to-face | 49/134 | 37 |
| Telephone Written |
19/134 66/134 |
14 49 |
| Post-clinical phase | ||
| Community nurse home visitsc | 82/133 | 62 |
| First home visit within 72 h after discharge | 76/133 | 57 |
| Number of community nurse home visits | Median 3 | IQR 2–4 |
| Medication reconciliation including the Red Flag instrument [25] | 118/133 | 89 |
| Follow-up of the integrated care plan | 71/132 | 54 |
| Lifestyle promotion | 91/132 | 69 |
| Joint home-visit of the physical therapist and community nurse | 33/81 | 41 |
| Home-based CRd | 70/116 | 60 |
| Number of home-based rehabilitation sessions | Median 4 | IQR 2–6 |
| Mean participant-specific fidelity percentage | 153 | 67 |
Abbreviations: CGA, comprehensive geriatric assessment; IQR, interquartile range.
aThe denominator is set on the number of eligible patients per intervention key-element.
bGeriatric team consultation was indicated in case of ≥1 problem within the psychological domain or ≥ 5 geriatric problems in total.
cFour home visits, according to the CCB protocol.
dMax. nine home-based rehabilitation session, according to the CCB protocol.
Discussion
In this study, we found that the CCB programme did not reduce hospital readmission or mortality within 6 months following hospitalisation. Similarly, for the secondary outcomes of unplanned hospital readmission and mortality alone, no statistically significant differences were found. Based on our findings, there is only a limited possibility that the CCB programme would be beneficial.
Systematic reviews on transitional care interventions in patients with HF found that high intensity interventions and (nurse) home visiting programmes reduce the incidence of readmission [11, 14, 15], mortality [11] and the composite endpoint of all-cause readmission and mortality [15]. The discrepancy of these reviews [11, 15] with our findings may be related to a higher mean age (82.4 years versus 70–74 years) and the frailty of the older cardiac population in our trial. In line with our findings, two recent randomised trials in patients with HF [16] and patients with AMI [17] reported no significant differences on readmission and mortality.
To our knowledge, our study is the first that combined case management, disease management and home-based CR in frail older patients with a variety of cardiac diagnoses. However, we did not find that integration of these intervention components improves outcomes. Several factors may have contributed to the results. First, we included a severely frail study population with a high mean age, many disabling comorbidities and geriatric conditions and an extensive medical history. In both groups, mortality rates were high. These factors suggest that the included population may have been beyond the reach of prevention programmes such as the CCB programme. Second, within the high-quality Dutch standard healthcare system many services are being offered to frail older patients which possibly diminished the contrast between the groups (Appendix 2, Supplementary data are available in Age and Ageing online). Third, we observed that real-world circumstances affected the fidelity of this intervention. Our intervention fidelity may have contributed to the lack of effect. A higher fidelity on the intervention key-elements could have resulted in a greater contrast between the intervention and control group. However, we cannot exclude the possibility that full fidelity would have led to even more deleterious effects on mortality due to the detrimental trend in the intervention group, through yet unexplained mechanisms.
An extended process evaluation was performed in parallel to the trial and addresses the barriers and facilitators for intervention fidelity [31]. In brief, low fidelity rates in healthcare professionals were mostly associated with time limits. For example, the short hospital stay and ad hoc discharge planning reduced the opportunity for geriatric consultation or an in-hospital handover of the integrated care plan to the community nurse. For future purpose, geriatric co-management interventions could be considered during hospitalisation in which the responsibility for the treatment is shared between the treating physician and the geriatric team. This kind of intervention intensifies collaboration and has proven to reduce mortality post-discharge [32, 33]. Furthermore, alternative communication routes such as a video call handover between the patient, the hospital and community nurse, may ensure continuity of care while less time-consuming than an in-hospital handover. We explored the unexpectedly high mortality rates in the intervention group. Baseline differences in the population regarding e.g. level of frailty were explored statistically. However, correction in the multivariable analysis yielded essentially the same results. Alternatively, our findings may be due to the play of chance considering the limited statistical power. Previously, Fan et al. [34] performed a comprehensive care programme to reduce hospitalisation in patients with pulmonary disease and found unexplained higher mortality rates among intervention patients.
In this frail older cardiac patients, other interventions with more focus on quality of life may be needed [35]. For example, advance care planning (ACP) may be more suitable as the CCB population seemed unresponsive to high intensity preventive interventions and event rates were high. ACP focuses on patient-centred preferences to increase comfort, quality of life and reduce readmission [36]. Future studies should carefully consider the population eligible for preventive interventions versus those who are eligible for palliative interventions.
Study limitations
The following limitations should be considered. First, only 9% (623/6857) of screened patients were considered eligible for the CCB programme. Most patients were excluded because of low DSMS-scores and having their residence in non-participating residential areas. In total, 49% of eligible patients provided informed consents. Patients more often refuse study participation when they experience mental and physical health problems [37]. Second, we were unable to continue the study until the planned 500 participants due to the quickly (and prematurely) developing regular transitional care for older cardiac patients in our region. This development illustrates that the high rates of readmission and mortality in this high-risk population were being recognised and that professionals seek effective preventive interventions. Unfortunately, as a result of these developments, we were unable to achieve the planned sample size and this clearly impacts on the power of the study.
As it turned out, the overall event rate (51%) was higher than the 44% used in the sample size calculation. This proportion of outcome, which is a much stronger driver of power than sample size, is close to the statistically ideal 50% event rate. The RD point estimate of 6.5% indicates an untoward effect of intervention, leaving only a small tail of the statistical outcome distribution in the range of possible beneficial effects. The trial preserved sufficient statistical precision to render beneficial effects greater than −4.7% in RD (the primary outcome’s lower 95% confidence limit) unlikely. Last, we performed a complex intervention according to a standardised intervention protocol. We invested in an intensive training programme and organised regular follow-up meetings, however, variation in the intervention performance turned out to be inevitable. Our findings reflect the effectiveness and working mechanisms of the intervention under ‘real’ circumstances and the perceived barriers and facilitators showed some important lessons on organizing care for frail older cardiac patients [31].
Conclusion
A randomised trial of nurse-coordinated transitional care compared to usual care in The Netherlands found no reduction of unplanned hospital readmission or mortality within 6 months following hospitalisation in high-risk older cardiac patients. Although the suboptimal intervention fidelity prevented assessment of the effect of the full programme, large beneficial effects are unlikely with our findings. It is also conceivable that the patient population selected may not be responsive to high-intensity preventive strategies.
Supplementary Material
Contributor Information
Patricia Jepma, Amsterdam UMC, Department of Cardiology, Amsterdam, the Netherlands; Amsterdam University of Applied Sciences, Faculty of Health, Centre of Expertise Urban Vitality, Amsterdam University of Applied Sciences,Amsterdam, the Netherlands.
Lotte Verweij, Amsterdam UMC, Department of Cardiology, Amsterdam, the Netherlands; Amsterdam University of Applied Sciences, Faculty of Health, Centre of Expertise Urban Vitality, Amsterdam University of Applied Sciences,Amsterdam, the Netherlands.
Bianca M Buurman, Amsterdam University of Applied Sciences, Faculty of Health, Centre of Expertise Urban Vitality, Amsterdam University of Applied Sciences,Amsterdam, the Netherlands; Amsterdam UMC, Department of Internal Medicine, Section of Geriatric Medicine, Amsterdam, the Netherlands.
Michel S Terbraak, Amsterdam University of Applied Sciences, Faculty of Health, Centre of Expertise Urban Vitality, Amsterdam University of Applied Sciences,Amsterdam, the Netherlands.
Sara Daliri, OLVG Hospital, Department of Clinical Pharmacy, Amsterdam, the Netherlands.
Corine H M Latour, Amsterdam University of Applied Sciences, Faculty of Health, Centre of Expertise Urban Vitality, Amsterdam University of Applied Sciences,Amsterdam, the Netherlands.
Gerben ter Riet, Amsterdam UMC, Department of Cardiology, Amsterdam, the Netherlands; Amsterdam University of Applied Sciences, Faculty of Health, Centre of Expertise Urban Vitality, Amsterdam University of Applied Sciences,Amsterdam, the Netherlands.
Fatma Karapinar - Çarkit, OLVG Hospital, Department of Clinical Pharmacy, Amsterdam, the Netherlands.
Jill Dekker, Bovenij Medical Centre, Department of Cardiology, Amsterdam, the Netherlands.
Jose L Klunder, OLVG Hospital, Department of Cardiology, Amsterdam, the Netherlands.
Su-San Liem, Amstelland Hospital, Department of Cardiology, Amstelveen, the Netherlands.
Arno H M Moons, OLVG Hospital, Department of Cardiology, Amsterdam, the Netherlands.
Ron J G Peters, Amsterdam UMC, Department of Cardiology, Amsterdam, the Netherlands.
Wilma J M Scholte op Reimer, Amsterdam UMC, Department of Cardiology, Amsterdam, the Netherlands; HU University of Applied Sciences Utrecht, Research Group Chronic Diseases, Utrecht, the Netherlands.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was supported by the Netherlands Organisation for Health Research and Development (ZonMw) as part of the ‘From Knowledge to Action II programme’ (grant no. 520002002) and the Dutch Research Council (NWO) (grant nos. 023.008.024 to LV, 023.009.036 to PJ).
References
- 1. Virani SS, Alonso A, Benjamin EJ et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141: e139–596. [DOI] [PubMed] [Google Scholar]
- 2. Roth GA, Forouzanfar MH, Moran AE et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015; 372: 1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dodson J, Hajduk A, Murphy T et al. Thirty-day readmission risk model for older adults hospitalized with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2019; 12: e005320. doi: 10.1161/CIRCOUTCOMES.118.005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vitale C, Jankowska E, Hill L et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail 2019; 21: 1299–305. [DOI] [PubMed] [Google Scholar]
- 5. Bell SP, Orr NM, Dodson JA et al. What to expect from the evolving field of geriatric cardiology. J Am Coll Cardiol 2015; 66: 1286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naylor MD, Shaid EC, Carpenter D et al. Components of comprehensive and effective transitional care. J Am Geriatr Soc 2017; 65: 1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoonover H, Corbett CF, Weeks DL et al. Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J Patient Saf 2014; 10: 186–91. [DOI] [PubMed] [Google Scholar]
- 8. van Seben R, Reichardt LA, Essink DR et al. "I Feel Worn Out, as if I Neglected Myself": older patients' perspectives on post-hospital symptoms after acute hospitalization. Gerontologist 2019; 59: 315–26. [DOI] [PubMed] [Google Scholar]
- 9. O'Neill D, Forman DE. Never too old for cardiac rehabilitation. Clin Geriatr Med 2019; 35: 407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piepoli MF, Hoes AW, Agewall S et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016, 2016; 37: 2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Berre M, Maimon G, Sourial N et al. Impact of transitional care services for chronically ill older patients: a systematic evidence review. J Am Geriatr Soc 2017; 65: 1597–608. [DOI] [PubMed] [Google Scholar]
- 12. Verhaegh KJ, Mac Neil-Vroomen JL, Eslami S et al. Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Aff 2014; 33: 1531–9. [DOI] [PubMed] [Google Scholar]
- 13. Buurman B, Parlevliet J, Allore H et al. Comprehensive geriatric assessment and transitional care in acutely hospitalized patients - the transitional care bridge randomized clinical trial. JAMA Intern Med 2016; 176: 302–9. [DOI] [PubMed] [Google Scholar]
- 14. Van Spall HGC, Rahman T, Mytton O et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail 2017; 19: 1427–43. [DOI] [PubMed] [Google Scholar]
- 15. Feltner C, Jones CD, Cene CW et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med 2014; 160: 774–84. [DOI] [PubMed] [Google Scholar]
- 16. Van Spall HGC, Lee SF, Xie F et al. Effect of patient-centered transitional care services on clinical outcomes in patients hospitalized for heart failure: the PACT-HF randomized clinical trial. JAMA 2019; 321: 753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meisinger C, Stollenwerk B, Kirchberger I et al. Effects of a nurse-based case management compared to usual care among aged patients with myocardial infarction: results from the randomized controlled KORINNA study. BMC Geriatri 2013; 13: 115. doi: 10.1186/1471-2318-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verweij L, Jepma P, Buurman BM et al. The cardiac care bridge program: design of a randomized trial of nurse-coordinated transitional care in older hospitalized cardiac patients at high risk of readmission and mortality. BMC Health Serv Res 2018; 18: 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Hopewell S, Schulz KF et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010 Aug; 63: e1–37. [DOI] [PubMed] [Google Scholar]
- 20. Dutch Safety Management Program Practical guide for frail older patients [in Dutch]. Place unknown: Dutch Safety Management Program, 2009. [Google Scholar]
- 21. Heim N, van Fenema EM, Weverling-Rijnsburger AW et al. Optimal screening for increased risk for adverse outcomes in hospitalised older adults. Age Ageing 2015; 44: 239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou H, Della PR, Roberts P et al. Utility of models to predict 28-day or 30-day unplanned hospital readmissions: an updated systematic review. BMJ Open 2016; 6: e011060. doi: 10.1136/bmjopen-2016-011060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boter H, van Delden JJ, de Haan RJ et al. A modified informed-consent procedure in which the complete information is given retrospectively: no objection from participating patients. Ned Tijdschr Geneeskd 2005; 149: 29–32. [PubMed] [Google Scholar]
- 24. Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a ``trial effect''. J Clin Epidemiol 2001; 54: 217–24. [DOI] [PubMed] [Google Scholar]
- 25. Sino C, van Dooren A, Haverkamp A. Recognition of drug related problems by home healthcare employees: a Dutch observational study with self reports. J Nurs Educ And Pract 2013; 3: 41–9. [Google Scholar]
- 26. Dutch Society for Cardiology . Multidisciplinary guideline for cardiac rehabilitation. Utrecht: Dutch Society for Cardiology, 2011. [Google Scholar]
- 27. Government of the Netherlands . What information is in the Personal Records Database? Den Haag, Netherlands. Available at: https://www.government.nl/topics/personal-data/question-and-answer/what-information-is-in-the-personal-records-database. accessed 2 July 2020.
- 28. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280: 1690–1. [DOI] [PubMed] [Google Scholar]
- 29. Hinchliffe SR, Scott DA, Lambert PC. Flexible parametric illness-death models. Stata J 201312/01; 2021/04; 13: 759–75. [Google Scholar]
- 30. Crowther MJ, Lambert PC. Parametric multistate survival models: flexible modelling allowing transition-specific distributions with application to estimating clinically useful measures of effect differences. Stat Med 2017 Dec 20; 36: 4719–42. [DOI] [PubMed] [Google Scholar]
- 31. Verweij L, Spoon DF, Terbraak MS et al. The Cardiac Care Bridge randomized trial in high-risk older cardiac patients: a mixed-methods process evaluation. J Adv Nurs 2021; 77: 2498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deschodt M, Claes V, Van Grootven B et al. Comprehensive geriatric care in hospitals: the role of inpatient geriatric consultation teams – Synthesis. Brussels: Belgian Health Care Knowledge Centre (KCE), 2015. [Google Scholar]
- 33. Deschodt M, Flamaing J, Haentjens P et al. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med 2013; 11: 48. doi: 10.1186/1741-7015-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fan VS, Gaziano JM, Lew R et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012; 156: 673–83. [DOI] [PubMed] [Google Scholar]
- 35. Ponikowski P, Voors AA, Anker SD et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016, 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 36. Kernick LA, Hogg KJ, Millerick Y et al. Does advance care planning in addition to usual care reduce hospitalisation for patients with advanced heart failure: A systematic review and narrative synthesis. Palliat Med 2018; 32: 1539–51. [DOI] [PubMed] [Google Scholar]
- 37. Ecarnot F, Meunier-Beillard N, Quenot JP et al. Factors associated with refusal or acceptance of older patients (≥ 65 years) to provide consent to participate in clinical research in cardiology: a qualitative study. Aging Clin Exp Res 2020; 32: 133–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


