Abstract
CDC37 encodes a 50-kDa protein that targets intrinsically unstable oncoprotein kinases including Cdk4, Raf-1, and v-src to the molecular chaperone Hsp90, an interaction that is thought to be important for the establishment of signaling pathways. CDC37 is required for proliferation in budding yeast and is coexpressed with cyclin D1 in proliferative zones during mouse development, a finding consistent with a positive role in cell proliferation. CDC37 expression may not only be required to support proliferation in cells that are developmentally programmed to proliferate but may also be required in cells that are inappropriately induced to initiate proliferation by oncogenes. Here we report that mouse mammary tumor virus (MMTV)-CDC37 transgenic mice develop mammary gland tumors at a rate comparable to that observed previously in MMTV-cyclin D1 mice. Moreover, CDC37 was found to collaborate with MMTV–c-myc in the transformation of multiple tissues, including mammary and salivary glands in females and testis in males, and also collaborates with cyclin D1 to transform the female mammary gland. These data indicate that CDC37 can function as an oncogene in mice and suggests that the establishment of protein kinase pathways mediated by Cdc37-Hsp90 can be a rate-limiting event in epithelial cell transformation.
Extracellular signals act to coordinate proliferation during the first gap (G1) phase of the cell division cycle. These signals typically act through receptor tyrosine kinases to activate protein kinase signaling pathways that direct the expression of genes required for proliferation. Recent studies have implicated components of the ras pathway in regulating the expression of D-type cyclins, a central component of mitogen-dependent cell cycle entry (1, 41). Ras activation leads to engagement of the Raf/MEK/MAPK pathway (47, 60, 65, 70, 72), and each of these components is necessary and sufficient to induce cyclin D expression (1, 2, 21, 27, 41, 69). D-type cyclins are essential activator subunits of Cdk4 and Cdk6, and holoenzyme complexes of these kinases have been implicated in cell cycle entry through multiple mechanisms. Cyclin D-Cdk4 complexes directly phosphorylate retinoblastoma protein (Rb) and initiate inactivation of its growth suppressor function (9, 12, 20, 34, 36). In addition, cyclin D-Cdk4 complexes may contribute to the activation of cyclin E-Cdk2 by titrating the Cdk inhibitor p27KIP1 from Cdk2 complexes (8, 19, 35, 45, 46, 55). Consistent with the central role of cyclin D in ras-dependent proliferation is the finding that Cdk4 inhibitors of the p16 class can inhibit ras-mediated proliferation in an Rb-dependent manner (30, 37, 41, 52).
The assembly of cyclin D-Cdk4 complexes is complex and appears to involve multiple steps, including a mitogen-dependent step (7, 8, 24, 34, 36). Previously, we cloned a mammalian homolog of the budding yeast and avian CDC37 gene (4, 15) and demonstrated that p50Cdc37 binds to Cdk4 and Cdk6 but not to Cdc2 and Cdk2 (58). In budding yeast, CDC37 is an essential gene and is required for formation of Cdc28-Cln complexes through an unknown mechanism (14). We and others have demonstrated that mammalian Cdc37 assembles with Cdk4 in high-molecular-weight complexes that also contain the molecular chaperone Hsp90 (11, 25, 58). Molecular analysis revealed that the CDC37 gene encodes the Hsp90-associated p50 protein (42, 58), previously seen in complexes with v-src (5, 6, 18, 66) and Raf (57) but whose identity was unknown. Cdc37 associates with Hsp90 independently of protein kinases and appears to function at least in part as a protein kinase-targeting subunit of Hsp90 (58). Genetic and biochemical data in several systems suggest that particular protein kinases are intrinsically unstable and their association with the Cdc37-Hsp90 chaperone is important for folding and/or activation of the targeted kinase (10, 14, 16, 38, 58, 71). Once Cdk4 is stabilized by the Cdc37 complex, it is released in a step that is not characterized and can then assemble with either inhibitors such as p16 or with cyclin D. Assembly with cyclin D requires a member of the p21 class of Cdk inhibitors, possibly in addition to a mitogen-dependent step (7, 24, 40).
CDC37 is expressed primarily in proliferative zones during embryonic development and in adult tissues, and its pattern of expression closely corresponded to that of cyclin D1 (58). Interestingly, CDC37 is not expressed in several adult tissues including virgin mammary duct epithelial cells but, like cyclin D1, is induced during pregnancy, consistent with a positive role in proliferation (58). These data, coupled with the fact that CDC37 is required for proliferation in budding yeast and Drosophila cells (10), suggest that CDC37 expression may be required to support proliferation in those cells that are developmentally programmed to proliferate but may also be required in those cells that are inappropriately induced to initiate proliferation by oncogenes. If this were the case, then CDC37 would be predicted to collaborate with transforming oncogenes. Standard tissue culture-based assays that measure oncogenic collaboration employ fibroblasts which already express high levels of Cdc37 (58), suggesting that this approach may not reveal the collaborative potential of Cdc37. Therefore, we sought to examine the effects of Cdc37 in vivo by targeting its expression to cells in the mammary gland and other tissues where it is normally not present in the adult animal. Mouse mammary tumor virus (MMTV)-CDC37 transgenic mice were found to develop mammary gland tumors at a rate comparable to that observed in MMTV-cyclin D1 mice. Moreover, CDC37 was found to collaborate with MMTV–c-myc in the transformation of multiple tissues, including mammary and salivary glands in females and testis in males, and with cyclin D1 in the mammary gland. In a parallel study (58a), we found that Cdc37 is absent from normal human prostate but is abundant in human prostate cancer. Interestingly, selective expression of CDC37 in the prostate leads to hyperplasia in transgenic mice (58a). Taken together, these data indicate that Cdc37 can function as an oncogene in mice and suggest that the establishment of protein kinase pathways mediated by Cdc37-Hsp90 can be a rate-limiting event in epithelial cell transformation.
MATERIALS AND METHODS
Generation of transgenic mice.
An MMTV-CDC37 transgene was generated by cloning a XhoI fragment containing the 1.6-kb mouse CDC37 open reading frame (ORF) into a plasmid containing an MMTV promoter, beta-globin splice sequences, and bGH polyadenylation sequences. The 4.63-kb transgene fragment was released from the plasmid by digesting with NotI/KpnI and then purified. Transgene DNA was microinjected into male pronuclei of B6D2F1 mouse embryos in the Baylor College of Medicine transgenic core facility. Resulting pups were screened by Southern analysis of genomic DNA isolated from mouse tails digested with BamHI. To establish lines of transgenic mice, founders were continuously mated with ICR mice. Nontransgenic littermates of heterozygous parents were used as controls. MMTV-CDC37 heterozygous females were mated with MMTV–c-myc (Charles River Laboratory) or MMTV-cyclin D1 homozygous transgenic males (64). Both MMTV–c-myc and MMTV-cyclin D1 mice were on a inbred FVB genetic background. Resulting progeny carried either both transgenes (c-myc+CDC37 or cyclin D1+CDC37) or a single transgene (c-myc or cyclin D1). Both groups of animals were monitored for tumor formation for comparison. For nontransgenic controls, MMTV-CDC37 heterozygous females were crossed with nontransgenic FVB males. The copy number was determined by quantitative Southern blotting of mixtures of tail DNA from nontransgenic and transgenic mice, followed by phosphorimager analysis. This analysis gave 8 and 5 copies for the MMTV-Cdc37.1 and MMTV-Cdc37.2 lines, respectively.
Northern analysis.
Total RNA was prepared from mouse tissues, separated on an 1% agarose gel, transferred to Hybond N+ (Amersham) membrane, and blotted with a 32P-labeled CDC37 cDNA probe to detect endogenous and transgene derived transcripts, or a 5′+3′ probe consisting of rabbit beta-globin splice site sequences and bovine polyadenylation signal DNA, which was used to detect only exogenous CDC37 transcripts. Blots were stripped and reprobed with a GAPDH (glyceraldehyde-3-phosphate dehydrogenase) probe to control for RNA levels. In some experiments, blots were also probed with a c-myc cDNA probe provided by M. Cole.
Histology and immunohistochemistry.
For histological analysis, mouse tissues were excised and fixed in 4% formaldehyde–phosphate-buffered saline PBS overnight at 4°C prior to being embedded in paraffin. Embedded tissues were sectioned at a thickness of 5 μm and stained with hematoxylin and eosin (H&E). For immunohistochemistry, 5-μm sections were stained with rabbit polyclonal affinity-purified Cdc37 antibodies or with anti-c-myc antibodies (NeoMarkers) as described previously (58).
Western blot analysis.
Frozen tumor specimens were used for preparation of protein lysates by homogenization in NP-40 buffer (58), followed by centrifugation and determination of protein concentration by Bradford assays. For Western blotting, 200 μg of extract was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12.5% PAGE gel and then transferred to nitrocellulose. Blotting was performed using polyclonal Cdc37 antibodies (58), Cdk4, Erk1 and Erk2, and c-myc antibodies from Santa Cruz or anti-phospho-ERK from New England Biolabs. Detection was accomplished by using horseradish peroxidase-conjugated secondary antibodies in combination with enhanced chemiluminescence (Amersham).
Whole-mount analysis.
Inguinal fat pads were excised from the animals, spread on a glass surface and fixed in 10% formalin for 10 to 12 h, and washed in acetone for 48 h, followed by washing in 100 and 95% ethanol (EtOH) for 1 h each. Tissues were stained with hematoxylin for 12 h (0.3% [wt/vol] hematoxylin and 0.34% [wt/vol] FeCl in 0.06 N HCl–80% EtOH). Stained tissues were washed for 1 h in distilled water and increasing concentrations of EtOH (70 to 100%) and finally in xylene. Tissues were stored in glass vials, covered with methyl salicylate.
RESULTS
MMTV-CDC37 transgenic mice.
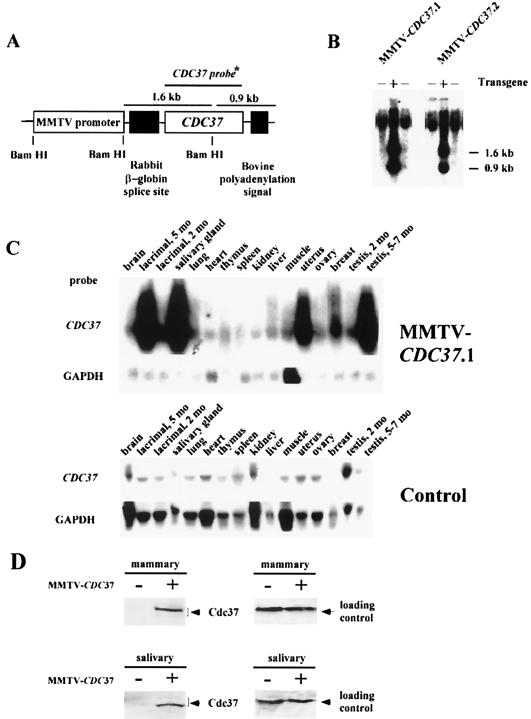
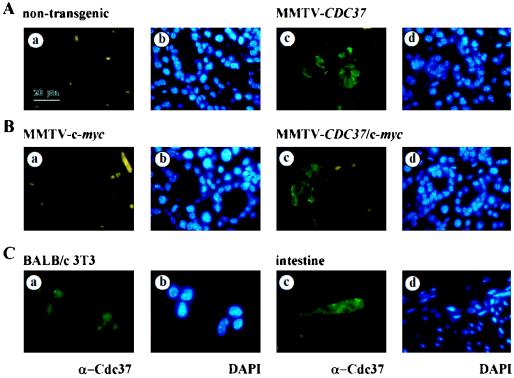
To assess the possible role of CDC37 in promoting neoplastic transformation, transgenic mice expressing mouse CDC37 under the control of the MMTV promoter (Fig. 1A) were generated. Two transgenic founders (Fig. 1B) were produced which transmitted the transgene to their progeny in a Mendelian fashion. Lines of transgenic animals (MMTV-CDC37.1 and MMTV-CDC37.2) were established by mating each founder with outbred ICR mice. Quantitative analysis of copy number revealed eight and five transgenes, respectively, for MMTV-Cdc37.1 and MMTV-Cdc37.2 strains (see Materials and Methods). The expression of Cdc37 was examined by Northern blotting, immunoblotting, and immunofluorescence, with an emphasis on tissues known to express MMTV-driven transgenes. CDC37 mRNA was high in the lacrimal, mammary, and salivary glands, uterus, and testis, using both the CDC37 cDNA (Fig. 1C) and transgene-specific regulatory sequences (5′+3′) (data not shown) as probes, compared to the low levels found in these tissues in nontransgenic animals. The levels of mRNA in the MMTV-CDC37.2 strain was ∼50% of those in the MMTV-CDC37.1 line (data not shown), a finding consistent with the lower copy number. Consistent with this, immunoblot analysis revealed that the Cdc37 protein was undetectable in extracts from normal salivary and virgin mammary glands but was readily detectable in extracts from transgenic mice (Fig. 1D). We previously reported that Cdc37 sometimes migrates as a doublet by SDS-PAGE (58). In normal virgin mammary gland, the more slowly migrating form of Cdc37 is predominant, while the more rapidly migrating form is predominant in salivary tissue. Cdc37 is a phosphoprotein (7), and we have shown that it is phosphorylated by casein kinase in vitro at sites that are also modified in vivo (data not shown). Thus, these isoforms may reflect differential phosphorylation in different tissues. To quantitatively address Cdc37 levels relative to those found normally in cycling cells, we examined Cdc37 protein by immunofluorescence and compared the levels with that found in sites of known Cdc37 expression in vivo. Transgenic Cdc37 was found in the majority of epithelial cells in the salivary gland (Fig. 2A) and Leydig cells in the testis (see Fig. 5) but was not detected in these cell types in nontransgenic animals. In the virgin mammary gland, Cdc37 was present in ∼30% of ductal epithelial cells (data not shown). Although CDC37 mRNA appears to be quite abundant, when examined at the single cell level, the levels of Cdc37 protein in all three tissues examined was similar to that found in proliferative cells in the intestine and in cycling BALB/c fibroblasts in culture (Fig. 2C). It is possible that translational and/or posttranslational events may control the total level of Cdc37 achievable in these tissues.
FIG. 1.
Characterization of MMTV-CDC37 transgene expression. (A) Structure of the construct used to generate MMTV-CDC37 mice (see Materials and Methods for details). (B) Southern blot analysis of MMTV-CDC37.1 and MMTV-CDC37.2 transgenic lines. Tail DNA was digested with BamHI prior to Southern analysis with the CDC37 cDNA. The 1.6-kb band corresponds to the construct fragment containing rabbit β-globin splice site, and the 0.9-kb bands represent fragments containing bovine polyadenylation signal (see panel A). +, Mice containing the transgene; −, mice lacking the transgene. (C) Northern blot analysis of CDC37 expression in tissues derived from transgenic and control animals. Total RNA was hybridized with the CDC37 cDNA which detects both endogenous CDC37 and the transgene derived message. The GAPDH probe (GAPDH ORF) is used as a loading control. Muscle tissue has intrinsically higher levels of GAPDH mRNA. (D) Immunoblot analysis of Cdc37 in nontransgenic and MMTV-Cdc37.1 mice. Tissue extracts (100 μg) from the indicated tissues were separated by SDS-PAGE and blotted with affinity-purified anti-Cdc37 antibodies. A nonspecific cross-reacting band observed with monoclonal antibody 9E10 was used as a loading control.
FIG. 2.
Analysis of Cdc37 expression by immunofluorescence. (A and B) Salivary gland tissue sections from nontransgenic (Aa and b), MMTV-CDC37 (Ac and d), MMTV–c-myc (Ba and b), and MMTV–CDC37/c-myc (Bc and d) mice were stained with anti-Cdc37 antibodies and visualized with secondary antibodies labeled with fluorescein isothiocyanate (FITC). Nuclei were visualized with DAPI (4′,6′-diamidino-2-phenylindole). (C) BALB/c 3T3 cells (a and b) or intestinal sections from nontransgenic mice (c and d) were probed with anti-Cdc37 and nuclei identified by DAPI. In the intestine, Cdc37 expression is limited to a narrow band of proliferating cells (58). The same exposures were used for all figures.
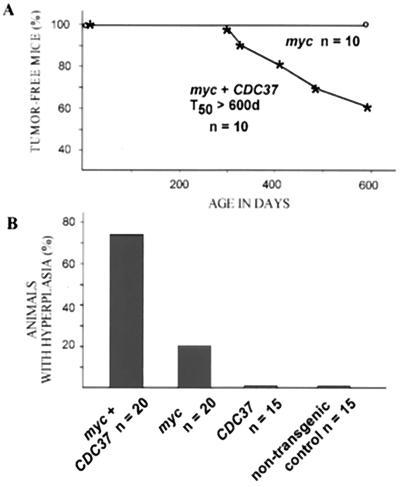
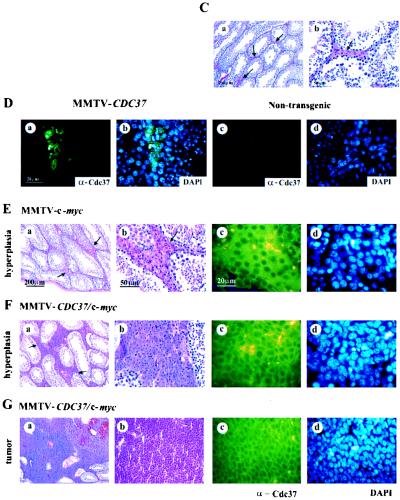
FIG. 5.
CDC37 cooperates with c-myc in the induction of the Leydig cell hyperplasia and transformation. (A) MMTV–CDC37/c-myc double transgenic males develop tumors, while male animals expressing a single transgene are unaffected. A plot of tumor-free mice over time is shown. Three of four tumors were Leydig cell tumors, while the fourth was a lymphoma. (B) MMTV–CDC37/c-myc double transgenic males display extensive Leydig cell hyperplasia compared to MMTV–c-myc and MMTV-CDC37 animals. Histological sections of testis derived from grossly unaffected males were analyzed at 400 days of age. The number of animals in each group is shown. (C) Tissue section of a normal testis with arrows indicating the positions of Leydig cells located between seminiferous tubules with active spermatogenesis: (a) ×100 magnification, H&E staining; and (b) ×400 magnification, H&E staining to show the usual number and morphology of Leydig cells. (D) Expression of CDC37 in the testis of MMTV-CDC37 transgenic (a and b) or nontransgenic (c and d) male mice at ×1,000 magnification: (a and c) CDC37 expression in the cytoplasm of Leydig cells; and (b and d) DAPI staining to identify nuclei. (E) Mild hyperplasia found in 20% of 400-day-old males expressing MMTV–c-myc. (F) High-grade hyperplasia found in 75% of 400-day-old MMTV–CDC37/c-myc mice. (G) Example of a Leydig cell tumor found in MMTV–CDC37/c-myc mice: (a) ×100 magnification, H&E staining; (b) ×400 magnification, H&E staining; (c) ×1,000 magnification field stained with anti-Cdc37 antibodies; and (d) ×1,000 magnification, DAPI staining of the same field as panel c to identify nuclei.
Ectopic expression of CDC37 in the mouse breast leads to transformation.
MMTV-CDC37 lines and control littermates were maintained as breeding colonies and monitored for developmental and transformation phenotypes for up to 2 years. Transgenic animals appeared normal at birth, and their growth was indistinguishable from their nontransgenic littermates. Their reproduction, number of pups per litter, and lactation in females were normal, although promiscuous male breast development was detected (see below).
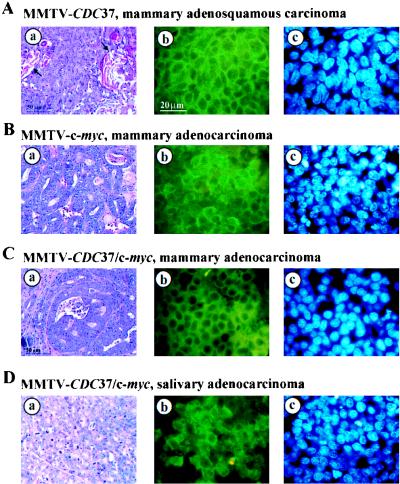
Malignant transformation of the mammary gland or other organs was not observed during first 1.5 years of life in CDC37 transgenic animals. However, as MMTV-CDC37 animals approached 18 months of age, a significant fraction of animals from both lines developed proliferative disorders, including mammary tumors and lymphomas (Table 1; Fig. 3 and 4A). Histopathological analysis indicated that mammary tumors were adenocarinomas and adenosquamous carcinomas (Fig. 3). By 22 months of age, 100% of MMTV-CDC37.1 females had developed tumors in the mammary or lymphoid compartments (Fig. 3A and 4A; Table 1). Mammary tumors arose as singular persistent masses adjacent to normal mammary epithelium. Mitotic figures were rare, indicative of slow-growing carcinomas. Histopathological examination also revealed enlarged nuclei and frequent keratin deposits which are indicative of squamous differentiation (Fig. 4A). Necrotic and apoptotic changes were minimal. Immunohistochemistry revealed Cdc37 protein expression in a large fraction of tumor cells (Fig. 4A). Lymphomas in transgenic females usually manifested themselves as an extreme weakness of the animals and obvious enlargement of the lymph nodes. Two cases of lymphomas were discovered in animals that already had developed mammary adenosquamous carcinomas. All lymphomas exhibited very low mitotic activity (data not shown), which could explain the slow progression of disease. Twenty animals of the MMTV-CDC37.2 line were autopsied at 17 months of age. Nine animals displayed evidence of proliferative disorders (Table 1), primarily mammary adenosquamous carcinomas and lymphomas, although one case of sarcoma was found. As in the first line examined, all tumors displayed a low mitotic index with little evidence of apoptosis. Nontransgenic control animals were subjected to a detailed pathological analysis either in parallel with CDC37 transgenic animals or at 17 to 22 months of age. No evidence of proliferative disturbances was found in nontransgenic animals (Fig. 3A and Table 1).
TABLE 1.
Neoplasms found in transgenic females carrying the MMTV-CDC37 transgene
| Pathology | Line | % Mice affected (no./total) |
|---|---|---|
| Mammary ductal adenosquamous carcinoma | MMTV-CDC37.1 | 60 (6/10) |
| MMTV-CDC37.2 | 20 (4/20) | |
| Lymphoma | MMTV-CDC37.1 | 50 (5/10) |
| MMTV-CDC37.2 | 20 (4/20) | |
| Mammary ductal adenocarcinoma | MMTV-CDC37.1 | 10 (1/10) |
| MMTV-CDC37.2 | 0 (0/20) | |
| Sarcoma | MMTV-CDC37.1 | 0 (0/10) |
| MMTV-CDC37.2 | 5 (1/20) | |
| Total affected | MMTV-CDC37.1a | 100 (10/10) |
| MMTV-CDC37.2b | 45 (9/20) | |
| Nontransgenicc | 0 (0/30) |
Tumors occurred between 18 and 22 months of age.
All animals were sacrificed and analyzed at 17 months of age without outward signs of transformation.
Animals were examined in parallel with MMTV-CDC37 mice at 17 to 22 months of age.
FIG. 3.
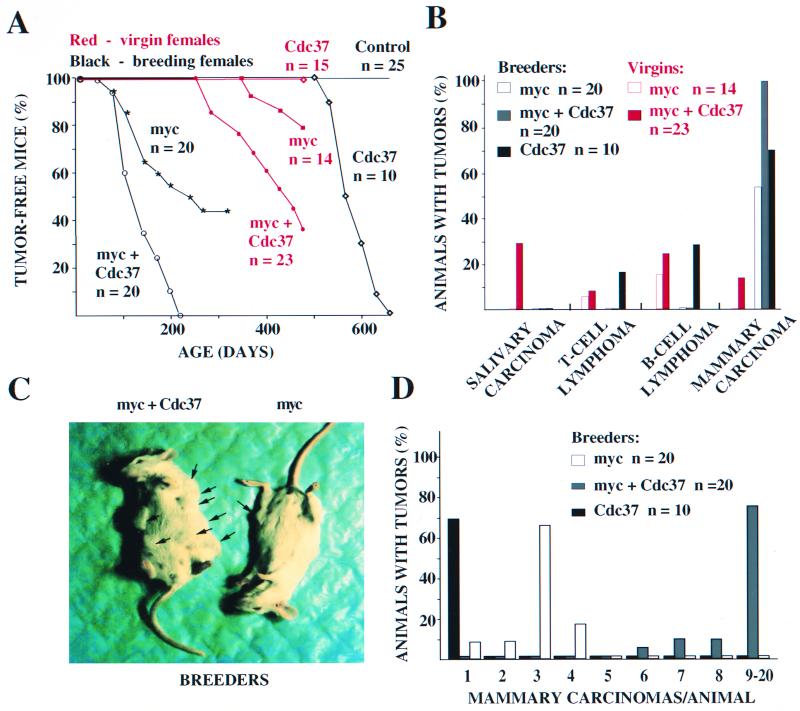
MMTV-CDC37 facilitates transformation of the mouse mammary epithelium and collaborates with c-myc to transform multiple tissues. (A) Quantitation of incidence of proliferative disorders. Tumor-free animals from breeding females are shown in black, while the tumor incidence in virgin animals is shown in red. n, number of animals in each group. Data shown for Cdc37 mice were from the MMTV-CDC37.1 line. Breeding and virgin MMTV–CDC37/c-myc females bore either MMTV-CDC37.1 or MMTV-CDC37.2 transgenes. (B) Types of tumors developed by virgin or breeding MMTV-CDC37, MMTV–c-myc, and double transgenic MMTV–CDC37/c-myc mice. The percentage of the animals developing each type of tumor from panel A is shown. Some of the animals developed more than one type of malignancy. The ages of breeding animals were as follows: MMTV-CDC37, 17 to 22 months; MMTV–c-myc, 3 to 12 months; and MMTV–c-myc/CDC37, 3 to 7 months. The ages of virgin animals were as follows: MMTV–c-myc, 12 to 16 months; and MMTV–c-myc/CDC37, 9 to 16 months. (C) Gross appearance of the breeding females expressing either MMTV–c-myc (right) or MMTV–CDC37/c-myc (left). The double transgenic females develop more tumors per animal than do single c-myc transgenics. The additional tumors, which were not visible by gross examination, were detected by detailed histopathological analysis. (D) Quantitation of tumor number per animal. The percentage of animals developing a given number of mammary adenocarcinomas is shown. MMTV-CDC37 animals developed only one tumor per animal. c-myc-expressing animals developed from 1 to 4 tumors/animal, while the majority of the double transgenics had between 9 and 20 tumors/animal. The number of tumors was estimated by counting foci on sections from fixed preparations of all mammary glands. The ages of animals are given in panel B.
FIG. 4.
Phenotypic analysis of tumors developed by MMTV-CDC37 transgenic mice. (Aa) Ductal adenosquamous carcinoma of the mammary gland derived from an MMTV-CDC37 mouse was stained with H&E. Arrows indicate squamous differentiation. (Ab and c) Adjacent tumor sections from Aa were stained with anti-Cdc37 antibodies and visualized with FITC (b), while nuclei were visualized with DAPI. H&E, ×400 magnification. Immunofluorescence, ×1,000 magnification. (B) Same as panel A except that the tumor was a mammary adenocarcinoma from an MMTV–c-myc mouse. (C) Same as panel A except that the tumor was a mammary adenocarcinoma from an MMTV–CDC37/c-myc mouse. (D) Same as panel A except that the tumor was a salivary gland adenocarcinoma from an MMTV–CDC37/c-myc mouse.
CDC37 cooperates with c-myc in induction of mammary tumors in breeding females.
CDC37 is expressed in proliferative zones in adult tissues and is coexpressed with cyclin D1 in several tissues, but it is absent in many differentiated cell types, including many epithelial cell types (58). We therefore hypothesized that CDC37 expression might be required to support transformation by oncogenic pathways. In this case, we would predict that inappropriate CDC37 expression might promote proliferative events dependent on oncogenic pathways.
To test this, we crossed MMTV-CDC37 heterozygous females with MMTV–c-myc and MMTV-cyclin D1 homozygous males. To control for differences in genetic backgrounds, we monitored heterozygous c-myc and cyclin D1 littermates alongside the double transgenics. Previously, it was shown that multiple rounds of pregnancy and lactation are able to promote expression of the c-myc transgene and accelerate tumorigenesis (56). We evaluated the influence of the level of expression of the transgene on tumorigenesis by dividing single and double transgenic females into two groups: one was kept virgin, and the other was kept in the presence of breeder males. Both lines of CDC37-expressing animals were used for these experiments. The approximately equal number of double transgenic females carried either MMTV-CDC37.1 or MMTV-CDC37.2 in combination with c-myc transgene. No differences between the two lines were observed in the kinetics of tumor appearance and tumor specificity in either breeding or virgin double transgenic females, and therefore the data for two lines were pooled together (Fig. 3A).
Tumors were observed in breeding MMTV–c-myc females as early as 3 months of age and 50% of females had developed tumors by 250 days of age in this genetic background (Fig. 3A and 4B). In contrast, breeding females carrying both c-myc and CDC37 transgenes developed tumors with accelerated kinetics, and 50% of females developed tumors by the age of 115 days (Fig. 3A and 4C). All tumors developed by breeding females were mammary ductal and alveolar adenocarcinomas (Fig. 3B). In addition to the acceleration of tumor incidence, we also observed a dramatic increase in the number of tumors/animal (Fig. 3C and D). This included both an increase in the number of glands affected as well as the number of tumors/gland (Fig. 3D). While MMTV–c-myc animals rarely had all of the glands affected, virtually all of the double transgenic animals were affected in every gland (Fig. 3C). While MMTV–c-myc females had on average three tumors per animal, MMTV–CDC37/c-myc approached 20 tumors per animal, on average (Fig. 3D). In many cases, the tumor masses were so abundant it prevented an exact determination of the number of tumor foci. On sections of both MMTV–CDC37/c-myc and MMTV–c-myc mammary glands all transitions from normal to transformed epithelium could be seen, including multiple areas of hyperplasia.
Altered tissue specificity of transformation in nonbreeding MMTV–CDC37/c-myc females.
CDC37 is normally not expressed in virgin mammary epithelium but is induced during pregnancy. c-myc has been shown to induce mammary transformation in virgin mice in some genetic backgrounds, although the extent of transformation is much lower than was observed with multiple pregnancies. To examine whether CDC37 can collaborate with c-myc in the absence of hormonal stimulation, we examined females maintained in the virgin state. In our strain background, MMTV–c-myc virgin females typically incurred B-cell lymphomas as opposed to mammary carcinomas (Fig. 3B). The kinetics of tumor development were very slow, and only 25% of females developed tumors by the age 500 days. In contrast, the kinetics of tumor incidence in double transgenics generated from both CDC37 lines were substantially accelerated (Fig. 3A). Relative to single c-myc transgenics, the spectrum of tumors was much wider (Fig. 3B), including both T- and B-cell lymphomas, as well as mammary and salivary gland adenocarcinomas. In double transgenic females, a prevalent tumor type was salivary adenocarcinoma (Fig. 3B). Salivary tumors from MMTV–CDC37/c-myc animals contain readily detectable Cdc37 (Fig. 4D) and c-myc (data not shown). This tumor type has never been reported in c-myc-expressing animals, although c-myc expression is readily observed in the salivary of phenotypically normal salivary glands in MMTV–c-myc mice (see Fig. 6B). Adenocarcinomas found in double transgenics appeared to be fast growing, with many mitotic figures (Fig. 4D). Taken together, these data indicate that MMTV-CDC37 can alter the rates and extent of transformation in both breeding and nonbreeding MMTV–c-myc mice and can also alter the specificity of transformation.
FIG. 6.
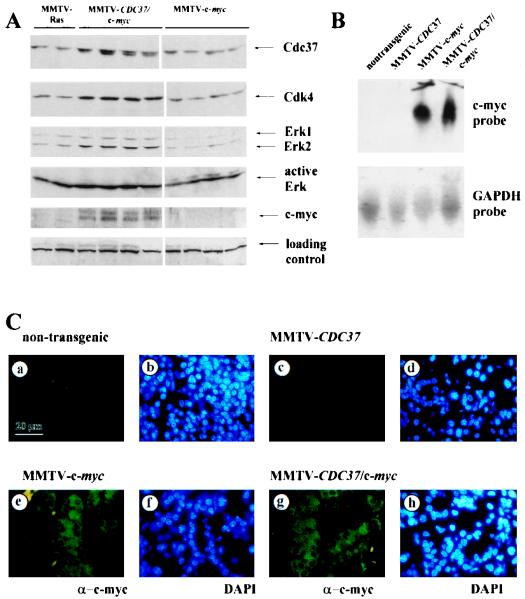
MMTV–CDC37/c-myc mammary tumors have higher levels of multiple signaling proteins than tumors from MMTV–c-myc animals. (A) Protein extracts (200 μg/lane) from individual tumors derived from the indicated animals were separated by SDS-PAGE, transferred to nitrocellulose, and probed with the indicated antibodies. (B) Northern blot analysis of c-myc mRNA in salivary tissue from nontransgenic, MMTV–c-myc, and MMTV–CDC37/c-myc mice. Blots were stripped and reprobed with GAPDH as a loading control. (C) Expression of c-myc in phenotypically normal salivary gland tissue from nontransgenic (a and b), MMTV-CDC37 (c and d), MMTV–c-myc (e and f), and MMTV–CDC37/c-myc (g and h) mice. (a, c, e, and g) Anti-c-myc. (b, d, f, and h) DAPI used to visualize nuclei.
Testicular hyperplasia and transformation in MMTV–CDC37/c-myc males.
MMTV–c-myc-expressing males are typically free of proliferative disorders (58). Therefore, we were surprised to find evidence of both overt Leydig cell tumors and testicular hyperplasia in double transgenic males (Fig. 5). Cdc37 is normally not detectable in the testis of an adult mouse but is readily apparent in Leydig cells in MMTV-CDC37 mice (Fig. 5D). Leydig cell tumors were observed in MMTV–CDC37/c-myc male mice at as young as 10 months (Fig. 5A, G). One of the four tumor-bearing animals had two distinct Leydig cell tumors, one in each testis. At an age of ∼400 days, about two-thirds of all apparently unaffected males were sacrificed, and their testes were subjected to detailed histological analysis. A significant fraction (75%) of double transgenic males displayed Leydig cell hyperplasia (Fig. 5F), a possible precursor to overt transformation. In contrast, only about 20% of MMTV–c-myc males displayed modest Leydig cell hyperplasia (Fig. 5B, C and E). Nontransgenic and MMTV-Cdc37 males did not display any hyperplasia.
Biochemical analysis of tumors derived from breeding MMTV–c-myc and MMTV–CDC37/c-myc transgenic females.
To begin to address how CDC37 and c-myc collaborate in transformation, we examined the levels of several protein kinases as well as c-myc in mammary carcinomas from MMTV–CDC37/c-myc and MMTV–c-myc animals (Fig. 6A). As a control, we also examined the levels of proteins in mammary tumors derived from an MMTV-ras mouse (59). As expected, Cdk4 levels were increased in tumors expressing MMTV-CDC37 (Fig. 6A, lanes 3 to 6), relative to that found with MMTV–c-myc alone (lanes 7 to 10), as were the Erk1 levels. We also found that activated Erk levels were higher in MMTV–CDC37/c-myc mice than in MMTV–c-myc mice (Fig. 6A). Unexpectedly, we found that c-myc levels were also increased in the presence of MMTV-CDC37 compared to animals expressing only MMTV–c-myc (Fig. 6A). The observed differences in protein levels cannot be explained by the increased number of dividing cells, since no significant difference was observed in the mitotic index of these tumors (data not shown). One explanation for increased c-myc abundance is that Cdc37 can affect expression from the MMTV promoter, thereby causing an indirect increase in c-myc levels. Analysis of c-myc mRNA in tissues derived from MMTV–c-myc and MMTV–c-myc/CDC37 mice, however, revealed similar levels of c-myc mRNA (Fig. 6B). Thus, Cdc37 does not indirectly influence c-myc expression from the MMTV-transgene promoter. An alternative explanation is that Cdc37 expression causes an alteration in the population of cells expressing c-myc. To test this, we examined c-myc expression in sections containing phenotypically normal tissue from various tissues. c-myc staining was not detected in nontransgenic animals (Fig. 6C) but was evident in the cytoplasm of all epithelial cells in the salivary and mammary glands from MMTV–c-myc mice (Fig. 6C and data not shown). The presence of Cdc37 had no discernible effect on the levels or extent of c-myc expression (Fig. 2B and 6C), ruling out increased numbers of c-myc-positive cells as an explanation for the observed increase in c-Myc protein levels. Tumors derived from MMTV-ras and MMTV–c-myc mice contained primarily the more slowly migrating Cdc37 isoform, while MMTV–CDC37/c-myc tumors contain both Cdc37 isoforms (Fig. 6A).
CDC37 cooperates with cyclin D1 in transformation of the mammary epithelium.
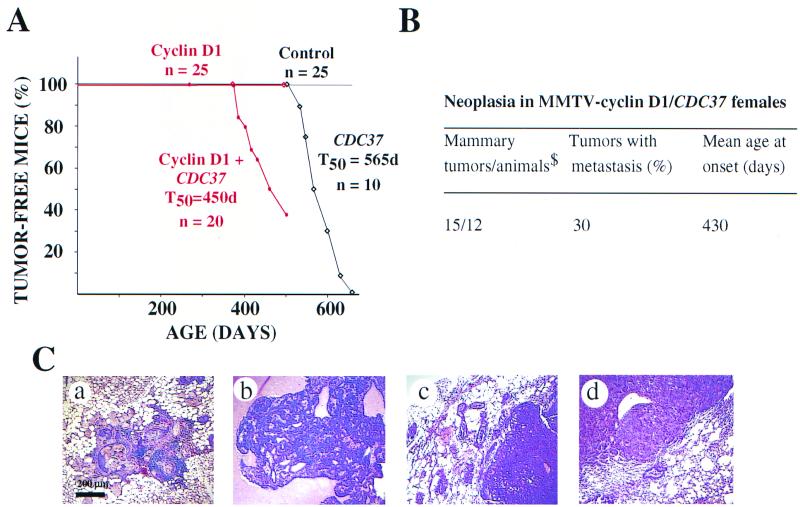
To further test the effect of simultaneous expression of CDC37 with other oncogenes, we created transgenic animals expressing both CDC37 and cyclin D1 under control of the MMTV promoter. Previous studies have demonstrated that MMTV-cyclin D1 mice develop mammary gland adenocarcinomas with an average age of onset of 534 days (64). In the genetic background of our study, no proliferative disturbances were found in MMTV-cyclin D1 mice for up to 650 days (Fig. 7). Similar results have been noted in other mixed genetic backgrounds with MMTV-cyclin D1 mice (E. V. Schmidt and A. Arnold, unpublished data). Animals expressing both cyclin D1 and CDC37 display evidence of mammary tumors at the age of 13 months, at which time control MMTV-CDC37 and MMTV-cyclin D1 mice had yet to display a transformation phenotype (Fig. 7A and B).
FIG. 7.
Cooperation between CDC37 and cyclin D1 oncogenes in breeding female mice. (A) By 15 months of age, a significant number of MMTV-CDC37/cyclin D1 double transgenic breeding females developed mammary adenocarcinomas, while none of the single transgenics developed tumors. A plot of the number of tumor-free animals over the time is shown. (B) Neoplasms developed by MMTV-cyclin D1/CDC37 breeding females were all mammary adenocarcinomas with frequent methastasis to the lung. $, number of animals that developed palpable tumors. (C) Histological analysis of proliferative disorders (×100 magnification, H&E staining): (a) metaplastic and hyperplastic changes observed in both single MMTV-cyclin D1 and double MMTV-CDC37/cyclin D1 transgenic females; (b) well-differentiated secreting mammary adenocarcinoma, developed by MMTV-CDC37/cyclin D1 double transgenic female; (c) poorly differentiated mammary adenocarcinoma, developed by MMTV-CDC37/cyclin D1 female; and (d) lung metastasis from a double transgenic mouse.
Tumors developed by double transgenic animals appeared as rapidly dividing single mass adenocarcinomas. The majority of adenocarcinomas were well-differentiated carcinomas with high levels of secretion, although several cases of poorly differentiated adenocarcinomas without apparent secretion were also observed (Fig. 7C). The majority of animals developed one mammary tumor, but frequent cases of metastasis to the lung was observed during pathological analysis (Fig. 7Cd).
Each of the double and single transgenic animals subjected to the detailed pathological analysis also displayed several foci of hyper- and metaplastic mammary epithelia (Fig. 7a). The appearance of hyperplastic areas was reported previously for the single MMTV-cyclin D1 transgenics (64). In our experiment, the frequency of the appearance of the hyper- and metaplastic foci was similar in MMTV-cyclin D1 single and MMTV-CDC37/cyclin D1 double transgenic animals at a similar age.
Inappropriate mammary duct development in male MMTV-CDC37 mice.
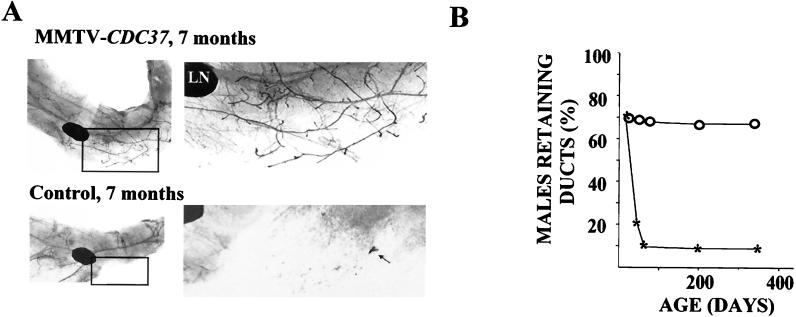
Phenotypic analysis of mammary glands during development failed to identify significant differences between female MMTV-CDC37 mice and their wild-type littermates, except for a 2- to 3-day delay in the rate of involution after lactation (data not shown). However, we did observe alterations in the development of male ductal systems, as assessed by whole-mount analysis. The development of rudimentary mammary ducts begins during embryonic development. Sexual dimorphism is already pronounced at embryonic day 14 when the male anlage undergoes significant cell death caused by androgens (22, 50). The degree of breast duct development varies in different mouse strains, ranging from the presence of the initial ductal sprout in some of the fat pads to a relatively well developed branching ductal tree. In the strain background used here, male mice do not develop a significant mammary duct structure, although the fat pad is well developed. In contrast, 60 to 70% of the adult MMTV-CDC37 male mice have well-formed breast ducts with different degrees of elaboration by the age of 7 months (Fig. 8). In the MMTV-CDC37.2 line, which has lower levels of expression, 30 to 40% of male animals developed breast ducts in the inguinal fat pad by the age of 7 months. In control nontransgenic littermates of the similar mixed background, only 10% of adult males have a nonbranching initial sprout structure (Fig. 8B).
FIG. 8.
Inappropriate mammary duct development in MMTV-CDC37 transgenic males. (A) Whole-mount analysis of the mammary glands from transgenic males and nontransgenic littermates at 7 months of age. Inguinal mammary glands were fixed in formalin, cleared with acetone, and stained with hematoxylin to visualize mammary ducts. By 7 months, a significant number of transgenic males developed an extensive system of breast ducts resembling that of a normal virgin female, while only 10% of the males in the control group had retained an initial sprout. LN, lymph node; black arrow, initial duct sprout in a nontransgenic male. (B) Percentage of transgenic and nontransgenic animals retaining breast structures as a function of age. For each time point, more than 10 inguinal mammary glands were autopsied and analyzed.
To monitor the age dependence of the effect, we performed whole-mount analysis of the male mammary glands at different ages (Fig. 8B). This analysis demonstrated that 70% of 4-week-old MMTV-CDC37.1 and control animals have a tiny initial breast sprout which later would give rise to breast ducts. During the first 6 weeks after birth, this ductal sprout regressed in most of the nontransgenic animals, and the fraction that maintained a ductal sprout (10%) did not change for up to 8 weeks and later (Fig. 8B). In contrast, the percentage of MMTV-CDC37 animals that maintain and elaborate ductal systems remained at ∼70%. At 6 weeks of age 70% of transgenic animals have about the same or somewhat better developed initial sprout, and by 8 weeks 70% of transgenic animals have a well-developed branching duct system resembling the structures found in older MMTV-CDC37 males. There was no significant change in breast duct development between the ages of 8 weeks and 7 months in both transgenic and control groups. The mechanism underlying this developmental alteration is not known at present but could reflect effects of CDC37 on the androgen receptor, as has been observed in budding yeast cells (13).
DISCUSSION
Proliferation requires the coordinated activation of multiple signaling pathways, which ultimately converge on the cell cycle machinery to promote DNA replication and cell division. Studies in a variety of systems suggest that Cdc37 and Hsp90 are required to establish important signaling pathways through interaction with intrinsically unstable kinases, including the oncoprotein kinases Cdk4 and Raf-1 and src family members (16, 58, 62, 71). In this study, we have examined the proliferative role of CDC37 through analysis of MMTV-CDC37 transgenic mice. Remarkably, we found that expression of CDC37 alone promotes neoplastic transformation of both the mammary epithelium and cells of the lymphoid compartment in older females. In this context, CDC37 functions as a weak oncogene with rates of transformation similar to that observed previously in MMTV-cyclin D1 mice (onset at 18 to 22 months) (64). Mammary tumors from these animals displayed low mitotic activity, a finding consistent with their very slow development and growth. Two independent lines of MMTV-CDC37 mice both displayed transformation in tissues known to be transformed by MMTV-driven oncogenes, although the penetrance of the phenotype is not as severe in the MMTV-CDC37.2 strain as in the MMTV-CDC37.1 strain (Table 1). Transgenic mice expressing cyclin E, cyclin D, and ras also display variability in the extent and tissue specificity of transformation (3, 32, 61, 64). This variability may reflect the site of integration and/or the levels of expression. We consider it likely that the persistent expression of CDC37 may allow what would otherwise be silent somatic mutations occurring over time in these animals to give rise to transformation. CDC37 appears to have multiple targets, many of which can promote proliferation in various settings. Thus, it is not clear whether the multiple transformation events we have observed in MMTV-CDC37 mice reflect mutational activation of a single collaborating pathway or mutations in different pathways in different tumors that occur stochastically.
Because of the link between Raf-1, Cdk4, and Cdc37, we asked whether CDC37 could cooperate with c-myc-dependent transformation by breeding MMTV-CDC37 and MMTV–c-myc mice. In principle, stabilization and/or activation of Raf-1 by ectopic Cdc37, which has been observed in heterologous systems (15), could inappropriately activate the ras pathway, and this could be observed as collaboration with c-myc in vivo. c-myc can collaborate with ras to transform a variety of cell types both in vitro and in vivo (26, 56). The ability of ras to function as a growth promoter as opposed to a growth inhibitor may rely upon inactivation of the ARF/Mdm2/p53 pathway. In primary fibroblasts, ras can induce G1 arrest and a senescence-like state dependent upon p53 and p16INK4a, but this activity is lost with immortalization (29, 53). The selective pressure on c-myc-expressing cells to inactivate the ARF-p53 pathway or undergo apoptosis (73), therefore, provides a plausible model for collaboration between ras and myc in cellular transformation (reviewed in reference 54). c-myc may also promote proliferation by controlling Cdk activity. c-myc expression can induce Cdk4/cyclin D kinase activity in certain situations (33). There is also evidence that cyclin D1 and Cdk4 are required for the proliferative effects of c-myc (17, 48) and that the expression of cyclin D1 and c-myc could be interdependent in some systems (48). In addition, c-myc expression leads to cyclin E/Cdk2 kinase activation, at least in part through inactivation of p27 (28, 43, 49, 63).
We found that CDC37 and c-myc collaborate to transform multiple tissues in both breeding and nonbreeding females, as well as in males, and both MMTV-CDC37 lines behaved similarly in this regard. In breeding and virgin females, CDC37 enhanced both the rate and extent of mammary transformation by c-myc. Importantly, the number of tumor foci observed with c-myc in the presence of MMTV-CDC37 was dramatically increased (from an average of three tumors/animal to an average approaching 20 tumors/animal) in breeding females. This result suggests that in some cell types CDC37 expression may be rate limiting for transformation. In this regard, we have observed expression of CDC37 in c-myc and ras induced mammary tumors, despite the fact that CDC37 is not expressed in resting mammary epithelium. Interestingly, induction of Cdc37 is not a simple consequence of c-myc expression since phenotypically normal tissues expressing abundant c-myc lack detectable Cdc37 (Fig. 2). Thus, additional events that give rise to induction of Cdc37 are apparently occurring during the process of c-myc-dependent transformation. The increased rates of mammary transformation observed with pregnancy in MMTV–c-myc and MMTV-ras transgenic mice may reflect the fact that CDC37 is normally induced during pregnancy (58) and could provide a proliferation-permissive setting that allows for these oncogenes to promote transformation. We expect that other events, including inactivation of the ARF/p53 pathway (reviewed in reference 53), are also involved in c-myc-mediated transformation in MMTV-CDC37 mice.
Unexpected was the finding that Cdc37 expression allowed transformation by c-myc in cell types where it is normally not oncogenic. In virgin females, MMTV–CDC37/c-myc mice developed salivary tumors. Although MMTV-ras mice develop salivary tumors (31), MMTV–c-myc mice have not been reported to develop salivary tumors. The inability of c-myc to transform the salivary epithelium is considered a peculiarity of this oncogene. Our results suggest that the absence of CDC37 expression in adult salivary glands may contribute to the inability of c-myc to transform this tissue.
We also found that expression of CDC37 allows c-myc to transform Leydig cells in the testis. c-myc induced a very mild hyperplasia in a small fraction of the animals, but when CDC37 was coexpressed there was a dramatic increase in the extent and severity of Leydig cell hyperplasia. Moreover, 30% of the double transgenic animals examined displayed evidence of overt Leydig cell neoplasia. The effect of CDC37 on the extent of proliferation of Leydig cells is possibly due to its effect on Cdk4. Recent studies show that one of the phenotypes of Cdk4 knockout males is the reduction of the number of the Leydig cells and abnormalities in sperm maturation and infertility (44). Moreover, the expression of a mutant form of Cdk4 that cannot bind p16INK4a leads to an increased population of testicular Leydig cells (44). These studies point to the important role of Cdk4 kinase in the proliferation of this cell type. The cooperative behavior of the c-myc and CDC37 in the induction of hyperproliferation and transformation in Leydig cells may therefore be explained by the role of CDC37 in the stabilization of Cdk4 kinase (58) and c-myc in the induction of cyclin D1 expression (48). In contrast to c-myc, MMTV-CDC37 did not affect the rate of mammary transformation induced by MMTV-neu in nonbreeding animals, although a severalfold increase in mitotic index was observed (data not shown).
Biochemical data indicate that Cdk4 is a major target of the Cdc37-Hsp90 chaperone complex (11, 58). If ectopic expression could inappropriately stabilize Cdk4, then one might expect to see increased proliferation in response to coexpression of cyclin D1. CDC37 mRNA is coordinately regulated with cyclin D1 during breast development and in adult tissues, suggesting a functional link (58). Consistent with this, we found that MMTV-CDC37 can collaborate with MMTV-cyclin D1 in the transformation of mammary epithelial cells.
Although the phenotypic consequences of CDC37 expression and collaboration with c-myc and cyclin D1 are striking, the biochemical mechanisms underlying its action are likely to be complex, possibly involving multiple kinase pathways that function interdependently to promote proliferation. Stabilization and/or activation of Cdk4 or Raf could result in both activation of the ras pathway and activation of Cdks. In the latter case, increased Cdk4 levels could simultaneously sequester p16INK4a and promote proliferation via activation by cyclin D. This could, in turn, lead to activation of cyclin E-Cdk2 by both increasing cyclin E expression and by sequestration of p27. We have observed increased levels of both Cdk4 and the Erk1 kinase. Interestingly, we noticed that mammary tumors from MMTV–CDC37/c-myc animals contained significantly higher levels of c-myc than tumors from MMTV–c-myc animals independent of changes in mitotic index. This increase did not reflect effects of Cdc37 expression on MMTV-driven c-myc mRNA nor did it reflect an ability of Cdc37 to augment the number of cells expressing c-myc. Recent studies suggested that activation of the ras pathway stabilizes c-myc (51). It is therefore possible that Cdc37, through its interaction with kinases in the ras pathway, indirectly stabilizes c-myc. Since Cdc37 does not appear to have an effect on c-myc levels in phenotypically normal tissues, its effects on c-myc levels in tumors may require additional events. Further studies are required to determine whether increased levels of c-myc via CDC37 expression are an important component of the collaborative effects seen in vivo.
In summary, our results suggest that the presence of Cdc37 may be rate limiting for the establishment of oncogenic signaling pathways that promote transformation. Although the effects observed here are in response to Cdc37 expression, recent studies provide correlative data indicative of a role for Cdc37 in human cancer. Normal human prostate epithelium has low to undetectable levels of Cdc37 (58a). However, Cdc37 is highly expressed in human prostatic cancer and is also expressed in preneoplastic lesions in the prostate, a finding consistent with its induction at an early stage of prostate cancer (58a). Similarly, our results indicate that Cdc37 induction occurs during transformation by c-myc. The mechanisms responsible for Cdc37 induction during the transformation process remain to be determined. We also note that the role of CDC37 in transformation suggested by this work may explain the sensitivity of various tumor types to clinically relevant anzamycin derivatives (23, 39, 67, 68), which are known to bind Hsp90 and disrupt ras- and cyclin D-dependent signaling pathways.
ACKNOWLEDGMENTS
We thank Phil Leder for providing MMTV-ras mice, Norman Greenberg for access to microscope facilities, Dan Medina for advice on whole-mount analysis, and Charles Sherr for enlightening discussions.
This work was supported by NIH grants GM54137 and by the Welch Foundation (J.W.H.) and by the Baylor SPORE in Prostate Cancer (F.D.). L.S. is supported by a predoctoral training grant from the Department of Defense.
REFERENCES
- 1.Aktas H, Cai H, Cooper G M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the Cdk inhibitor p27KIP1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 3.Bortner D M, Rosenberg M P. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol Cell Biol. 1997;17:453–459. doi: 10.1128/mcb.17.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breter H J, Ferguson J, Peterson T A, Reed S I. Isolation and transcriptional characterization of three genes which function at start, the controlling event of the Saccharomyces cerevisiae cell division cycle: CDC36, CDC37, and CDC39. Mol Cell Biol. 1983;3:881–891. doi: 10.1128/mcb.3.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brugge J S. Interaction of the Rous sarcoma virus protein pp60src with cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- 6.Brugge J S. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981;25:363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- 7.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng M, Sexl V, Sherr C J, Roussel M F. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutforth T, Rubin G M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 11.Dai K, Kobayashi R, Beach D. Physical interaction of mammalian CDC37 with CDK4. J Biol Chem. 1996;271:22030–22034. doi: 10.1074/jbc.271.36.22030. [DOI] [PubMed] [Google Scholar]
- 12.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 13.Fliss A E, Fang Y, Boschelli F, Caplan A J. Differential in vivo regulation of steroid hormone receptor activation by cdc37p. Mol Biol Cell. 1997;8:2501–2509. doi: 10.1091/mbc.8.12.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber M R, Farrell A, Deshaies R J, Herskowitz I, Morgan D O. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grammatikakis N, Grammatikakis A, Yoneda M, Yu Q, Banerjee S D, Toole B P. A novel glycosaminoglycan-binding protein is the vertebrate homologue of the cell cycle controlprotein, Cdc37. J Biol Chem. 1995;270:16198–16205. doi: 10.1074/jbc.270.27.16198. [DOI] [PubMed] [Google Scholar]
- 16.Grammatikakis N, Lin J H, Grammatikakis A, Tsichlis P N, Cochran B H. p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas K, Staller P, Geisen C, Bartek J, Eilers M, Moroy T. Mutual requirement of CDK4 and Myc in malignant transformation: evidence for cyclin D1/CDK4 and p16INK4A as upstream regulators of Myc. Oncogene. 1997;15:179–192. doi: 10.1038/sj.onc.1201171. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison K A, Brott B K, De Leon J H, Perdew G H, Jove R, Pratt W B. Reconstitution of the multiprotein complex of pp60src, hsp90, and p50 in a cell-free system. J Biol Chem. 1992;267:2902–2908. [PubMed] [Google Scholar]
- 19.Kato J, Matsuoka M, Polyak K, Massague J, Sherr C J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 20.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 21.Kerkhoff E, Rapp U R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kratochwil K. Experimental analysis of the prenatal development of the mammary gland. Modern Problems Paediatr. 1975;15:1–15. [Google Scholar]
- 23.Kwon H J, Yoshida M, Muroya K, Hattori S, Nishida E, Fukui Y, Beppu T, Horinouchi S. Morphology of ras-transformed cells becomes apparently normal again with tyrosine kinase inhibitors without a decrease in the ras-GTP complex. J Biochem. 1995;118:221–228. doi: 10.1093/oxfordjournals.jbchem.a124882. [DOI] [PubMed] [Google Scholar]
- 24.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 25.Lamphere L, Fiore F, Xu X, Brizuela L, Keezer S, Sardet C, Draetta G F, Gyuris J. Interaction between Cdc37 and Cdk4 in human cells. Oncogene. 1997;14:1999–2004. doi: 10.1038/sj.onc.1201036. [DOI] [PubMed] [Google Scholar]
- 26.Land H, Parada L F, Weinberg R A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 27.Lavoie J N, L'Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 28.Leone G, DeGregori J, Sears R, Jakoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 29.Lin A W, Barradas M, Stone J C, van L. Aelst, Serrano M, Lowe S W. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangues R, Seidman I, Pellicer A, Gordon J W. Tumorigenesis and male sterility in transgenic mice expressing a MMTV/N-ras oncogene. Oncogene. 1990;5:1491–1497. [PubMed] [Google Scholar]
- 32.Mangues R, Seidman I, Gordon J W, Pellicer A. Overexpression of the N-ras proto-oncogene, not somatic mutational activation associated with malignant tumors in transgenic mice. Oncogene. 1992;7:2073–2076. [PubMed] [Google Scholar]
- 33.Mateyak M K, Obaya A J, Sedivy J M. c-Myc regulates cyclin D-cdk4 and -cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–4683. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol 1994. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McConnell B B, Gregory F J, Stott F J, Hara E, Peters G. Induced expression of p16(INK4a) inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittnacht S, Paterson H, Olson M F, Marshall C J. Ras signalling is required for inactivation of the tumour suppressor pRb cell-cycle control protein. Curr Biol. 1997;7:219–221. doi: 10.1016/s0960-9822(97)70094-0. [DOI] [PubMed] [Google Scholar]
- 38.Munoz M J, Jimenez J. Genetic interactions between Hsp90 and the Cdc2 mitotic machinery in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1999;261:242–250. doi: 10.1007/s004380050963. [DOI] [PubMed] [Google Scholar]
- 39.Murakami Y, Mizuno S, Hori M, Uehara Y. Reversal of transformed phenotypes by herbimycin A in src oncogene expressed rat fibroblasts. Cancer Res. 1988;48:1587–1590. [PubMed] [Google Scholar]
- 40.Parry D, Mahony D, Wills K, Lees E. Cyclin D-cdk subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol Cell Biol. 1999;19:1775–1783. doi: 10.1128/mcb.19.3.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peeper D S, Upton T M, Ladha M H, Neuman E, Zalvide J, Bernards R, DeCaprio J A, Ewen M E. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- 42.Perdew G H, Wiegand H, Vanden Heuvel J P, Mitchell C, Singh S S. A 50 kilodalton protein associated with raf and pp60(v-src) protein kinases is a mammalian homolog of the cell cycle control protein cdc37. Biochemistry. 1997;36:3600–3607. doi: 10.1021/bi9612529. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Roger I, Solomon D L, Sewing A, Land H. Myc activation of cyclin E/Cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27(Kip1) binding to newly formed complexes. Oncogene. 1997;14:2373–2381. doi: 10.1038/sj.onc.1201197. [DOI] [PubMed] [Google Scholar]
- 44.Rane S G, Dubus P, Mettus R V, Galbreath E J, Boden G, Reddy E P, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat Genet. 1999;22:44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 45.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 46.Reynisdottir I, Massague J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 47.Robbins D J, Cheng M, Zhen E, Vanderbilt C A, Feig L A, Cobb M H. Evidence for a Ras-dependent extracellular signal-regulated protein kinase (ERK) cascade. Proc Natl Acad Sci USA. 1992;89:6924–6928. doi: 10.1073/pnas.89.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roussel M F, Theodoras A M, Pagano M, Sherr C J. Rescue of defective mitogenic signaling by D-type cyclins. Proc Natl Acad Sci USA. 1995;92:6837–6841. doi: 10.1073/pnas.92.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudolph B, Saffrich R, Zwicker J, Henglein B, Muller R, Ansorge W, Eilers M. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 1996;15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- 50.Sakakura T. Mammary embryogenesis. In: Neville M C, Daniel C W, editors. The mammary gland: development, regulation, and function. New York, N.Y: Plenum Press; 1987. pp. 37–66. [Google Scholar]
- 51.Sears R, Leone G, DeGregori J, Nevins J R. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–179. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 52.Serrano M, Gomez-Lahoz E, DePinho R A, Beach D, Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995;267:249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- 53.Serrano M, Lin A W, McCurrach M E, Beach D, Lowe S W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 54.Sherr C J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 1998;12:2984–2991. doi: 10.1101/gad.12.19.2984. [DOI] [PubMed] [Google Scholar]
- 55.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 56.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49:465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 57.Stancato L F, Chow Y-H, Hutchison K A, Perdew G H, Jove R, Pratt W B. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- 58.Stepanova L, Leng X, Parker S, Harper J W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 58a.Stepanova, L., G. Yang, F. DeMayo, T. M. Wheeler, M. Finegold, T. C. Thompson, and J. W. Harper. Induction of human Cdc37 in prostate cancer correlates with the ability of targeted Cdc37 expression to promote prostatic hyperplasia. Oncogene, in press. [DOI] [PubMed]
- 59.Stewart T A, Pattengale P K, Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MMTV/myc fusion genes. Cell. 1984;38:627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- 60.Thomas S M, DeMarco M, D'Arcangelo G, Halegoua S, Brugge J S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992;68:1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- 61.Tremblay P J, Pothier F, Hoang T, Tremblay G, Brownstein S, Liszauer A, Jolicaeur P. Transgenic mice carrying the mouse mammary tumor virus ras fusion gene: distinct effects in various tissues. Mol Cell Biol. 1989;9:854–869. doi: 10.1128/mcb.9.2.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Straten A, Rommel C, Dickson B, Hafen E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J. 1997;16:1961–1969. doi: 10.1093/emboj/16.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 64.Wang T C, Cardiff R D, Zukerberg L, Lees E, Arnold A, Schmidt E V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 65.Warne P H, Viciana P R, Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 66.Whitelaw M L, Hutchison K, Perdew G H. A 50-kDa cytoxolic protein complexed with the 90-kDa Heat shock protein (hsp90) is the same protein complexed with pp60v-src hsp90 in cells transformed by the Rous sarcoma virus. J Biol Chem. 1991;266:16436–16440. [PubMed] [Google Scholar]
- 67.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;117:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whitesell L, Shifrin S D, Schwab G, Neckers L M. Benzoquinoid ansamycins possess selective tumoricidal activity unrelated to src kinase inhibition. Cancer Res. 1992;52:1721–1728. [PubMed] [Google Scholar]
- 69.Winston J T, Coats S R, Wang Y-Z, Pledger W J. Regulation of the cell cycle machinery by oncogenic ras. Oncogene. 1996;12:127–134. [PubMed] [Google Scholar]
- 70.Wood K W, Sarnecki C, Roberts J M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 71.Xu Y, Singer M A, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X-F, Settleman J, Kyriakis J M, Takeuchi-Suzuki E, Elledge S J, Marshall M S, Bruder J T, Rapp U R, Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993;364:308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- 73.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]