Abstract
Long-standing research in animal models and humans with stroke or incomplete spinal cord injury (iSCI) indicate that specific physical training variables, such as the specificity and amount of practice, may influence neurologic recovery and locomotor function. More recent data highlight the contributions of exercise intensity, as estimated indirectly by cardiovascular exertion, as potentially more important than previously considered. The effects of exercise intensity are well described in neurologically intact individuals, although confusion regarding the definitions of intensity and safety concerns have limited its implementation during physical rehabilitation of patients with neurologic injury. The purpose of this review is to delineate some of the evidence regarding the effects of exercise intensity during locomotor training in patients with stroke and iSCI. We provide specific definitions of exercise intensity used within the literature, describe methods used to ensure appropriate levels of exertion, and discuss potential adverse events and safety concerns during its application. Further details on the effects of locomotor training intensity on clinical outcomes, and on neuromuscular and cardiovascular function will be addressed as available. Existing literature across multiple studies and meta-analyses reveals that exercise training intensity is likely a major factor that can influence locomotor function after neurologic injury. To extend these findings, we describe previous attempts to implement moderate to high intensity interventions during physical rehabilitation of patients with neurologic injury, including the utility of specific strategies to facilitate implementation, and to navigate potential barriers that may arise during implementation efforts.
Keywords: Exercise, Locomotion, Rehabilitation
The number of acute-onset neurologic injuries has increased steadily over recent decades, with the incidence of stroke in the US reaching almost 800,000,1 and approximately 17,000 new cases of spinal cord injury (SCI) each year.2 Although these injuries result in multiple impairments, many patients regain some volitional lower extremity function and may have the potential to recover independent walking function.3,4 However, the initial injury and secondary inflammatory processes result in profound impairments in volitional control, including reduced strength, coordination, and postural stability, all of which lead to reduced locomotor capacity, such as decreased gait speed, and poor endurance.5,6 Nonetheless, locomotor recovery may be possible and is often a primary goal of these patients and the rehabilitation professionals who treat them.
Benefits and limitations of task-specific practice
During rehabilitation sessions, physical therapists use multiple strategies to ameliorate patients’ impairments to maximize function.7–9 Although interventions vary substantially, observational studies7–10 suggest that patients typically perform a variety of strengthening, balance, and coordination exercises, in addition to some walking practice. However, the efficacy of these multifaceted strategies to improve walking is uncertain.10,11 For example, a number of investigations in the past 20 years using animal models have suggested that task-specific (stepping) training may facilitate plastic changes in neural networks that can improve stepping capacity.12–14 Initial efforts to translate these findings to clinical populations consisted of trials of locomotor training in patients with motor incomplete SCI (iSCI) and stroke using body weight support (BWS) systems with manual assistance to facilitate stepping.15,16 These studies revealed greater improvements in walking function compared with conventional therapy, which consisted mostly of nonstepping practice. These initial data spurred many additional clinical trials, with evidence suggesting that improvements in locomotor outcomes may be related to the amount of walking practice provided.10,11,17,18 Various strategies have since been developed to increase delivery of stepping practice in patients with walking dysfunction after neurologic injury, including a number of robotic devices which vary in the types or amounts of assistance.19–25
Although these data have been promising, results from specific studies suggest that stepping practice alone is insufficient to maximize walking outcomes. For example, the Locomotor Experience Applied Post-Stroke (LEAPS) trial26,27 compared locomotor outcomes after treadmill training with substantial therapist assistance to approximate “normal” kinematics to nonwalking interventions (ie, balance and therapeutic exercise). Despite likely large differences in amounts of stepping practice between groups, no differences in walking speed were observed. In separate studies, evaluation of the efficacy of robotic-assisted stepping training28,29 indicated smaller gains in walking function when compared with interventions that focused on task-specific (ie, stepping) tasks over ground or treadmill with minimal therapist assistance. Despite similar amounts of walking practice in these studies, greater improvements with nonrobotic training suggest that training parameters other than simply amount of stepping practice are important.
One hypothesis to account for these results was that the magnitude of cardiovascular exertion during stepping practice may have influenced locomotor outcomes. More specifically, therapist-assisted treadmill training provided in the LEAPS trial26,27 and robotic-assisted strategies28,29 may have resulted in limited gains due to the reduced cardiovascular exertion. In the LEAPS trial, midtraining heart rates (HR) achieved during walking training approximated 90 beats per minute, which is consistent with HRs during conventional strategies30–33 but below cardiac exertion levels achieved during moderate-intensity training interventions or even standardized assessments of walking distance (ie, 6-min walk test).34 The reduced cardiovascular exertion is likely due to provision of substantial assistance from therapists to facilitate normal kinematics, which likely reduced patient exertion and accounted for limited HR increases. Similarly, with robotic-assisted stepping, reduced HRs and oxygen consumption are consistently lower than providing walking training without assistance or with assistance only as needed.21,35,36 These reduced neuromuscular demands and associated cardiovascular exertion during robotic or therapist-assisted training have been speculated to contribute to limited gains in walking function.
Definitions of intensity during gait rehabilitation interventions
The influences of cardiovascular exertion in these studies may underscore the important contributions of exercise intensity during training of patients with neurologic injury. In the rehabilitation literature, however, the term “intensity” has varied extensively, often describing training parameters such as duration or frequency,37–39 which contribute to the total amount of practice. Consistent with the field of exercise physiology,40–42 however, intensity is defined here as the rate of work or power output and can be manipulated during locomotor training by increasing walking speeds or altering the loads carried. Exercise intensity is often estimated by the rate of oxygen consumption (V̇O2), and measured indirectly using measures of HRs, the latter of which is commonly used during the treatment of patients with neurologic injury.
To assess exercise intensity, therapists often use a percentage of the maximum HR that could be achieved. Specific calculations include the percentage of age-predicted maximum HR achieved during exercise, or percentage of HR reserve, which accounts for difference between maximum and resting HRs. In experimental studies with initial graded exercise tests, maximal HRs achieved during testing are often used instead of age-predicted maximum HR (HRmax), although the latter is used more often in the clinical setting. However, the strategy used to determine maximal HR is not trivial in neurologic rehabilitation, as both strategies present with limitations. For graded exercise testing, patients with lower extremity weakness are limited by the inability to generate sufficient neuromuscular power, and associated cardiovascular responses may also be limited. Hence, the maximal HRs achieved during this test may be lower than the actual HRmax, which may result in therapists overestimating a patient’s exercise workload.43 Conversely, age-predicted HRmax can be inaccurate due to inter-subject variability44 or use of medications (ie, β-blockers).45 Regardless, it is important to note that estimates of cardiovascular intensity reflect changes in neuromuscular activity required to accomplish locomotor activities in both intact46,47 and impaired populations.21,35
Regardless of the methods used for determining HRmax, the level of intensity is often a major determinant of outcomes after different training paradigms, and with specific thresholds and definitions derived from organizations that specialize in exercise physiology and prescription. Table 1 provides an overview of specific guidelines over the past 20 years and some of their alterations, including American College of Sports Medicine guidelines from previous48 and recent versions49 and the Exercise and Sports Science Australia.50 In general, very light or sedentary activities involve little to no movement, with minimal cardiovascular stress (<35%–57% HRmax). “Light” intensities involve little physical exertion (eg, washing dishes, cooking, working at a desk) in which participants typically work at 20% to 40% of their HR reserve or less than 35% to 63% of HRmax. This level of intensity is consistent with most rehabilitation paradigms assessed in observational studies.30,31 Conversely, “moderate” exercise intensities are associated with an increased level of physical activity, such as walking or cycling, although at paces at which conversation is still possible (40%–60% HR reserve or 55%–76% HRmax). In the past 2 decades, rehabilitation researchers have focused on attempts to achieve higher levels of intensities, including those defined as “vigorous” (eg, “hard’) to “high” (eg, “very hard”). “Vigorous” exercise typically involves achieving HR reserves from 60% to 85% or 70% to 95% HRmax, depending on the reference criteria used (see table 1). In contrast, “high” or “very hard” intensity aims to achieve greater than 85% HR reserve and greater than 90% to 95% HRmax. Of particular interest for clinicians is the changing threshold of HRs at the higher levels of intensity particularly measures of %HRmax and ratings of perceived exertion (RPE) needed to achieve “hard” or “vigorous” intensity (see table 1). For the purposes of this review, we consider high-intensity training as these 2 highest levels of intensity.
Table 1.
Differences in definitions and threshold for intensity from different exercise organizations over the past 20 years
| ACSM v6 (2000) | Exercise and Sports Science Australia (2010) | ACSM v10 (2018) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Definitions | %HRR | %HRmax | RPE | %HRR | %HRmax | RPE | %HRR | %HRmax | RPE |
| Very light/sedentary | <20 | <35 | <10 | <20 | <40 | <8 | <30 | <57 | <9 |
| Light | 20–39 | 35–54 | 10–11 | 20–39 | 40–55 | 8–10 | 30–39 | 57–63 | 9–11 |
| Moderate | 40–59 | 55–69 | 12–13 | 40–59 | 55–70 | 11–13 | 40–59 | 64–76 | 12–13 |
| Hard/vigorous | 60–84 | 70–89 | 14–16 | 60–84 | 70–90 | 14–16 | 60–84 | 77–95 | 14–17 |
| Very hard to maximal | 85–100 | 90–100 | 17–20 | 85–100 | 90–100 | 17–20 | 85–100 | 96–100 | 18–20 |
Abbreviations: ACSM, American College of Sports Medicine; HRR, heart rate reserve.
Evidence and mechanisms underlying high-intensity training
Despite the established role of cardiovascular intensity in the field of exercise physiology,40 its role in physical rehabilitation of patients with neurologic injury has emerged only in the past 15 to 20 years. Macko et al51–54 were among the first to assess the effect of locomotor intensity in individuals post stroke, specifically using a modified cardiac rehabilitation program applied to patients with ambulatory deficits. After a graded exercise test, participants in the chronic stages post stroke engaged in treadmill exercise at speeds necessary to achieve 60% to 80% of their HR reserve (ie, “vigorous intensity”), calculated as the percentage difference between their resting and maximum HRs during baseline exercise testing. The comparison groups varied in the types of activities performed, including massage, stretching, balance, or walking at 30% to 40% HR reserve, or a combination of these activities. Gains in locomotor function after such training often include improvements in timed walking distance, as well as measures of VO2peak or efficiency (reduced V̇O2 at matched workload).51–54 A number of assessor-blinded, randomized controlled trials have replicated these protocols in patients with chronic or subacute stroke,10,55–57 resulting in relatively consistent findings compared with alternative strategies.
Although these data are promising, concerns related to their interpretation have focused on comparisons of aerobic training paradigms to comparison interventions that provided little walking practice. A specific concern of these studies was that provision of walking practice was sufficient to improve locomotor function, rather than focused attention toward achieving higher intensities. Five recent studies have subsequently attempted to examine differences between higher vs lower-intensity stepping training.34,56,58–60 Two studies compared higher vs lower intensity training on walking function in individuals post stroke while trying to match the total work performed or amount of stepping practice. Ivey et al56 randomized participants post stroke to an experimental group that performed treadmill training at faster speeds to achieve higher training intensities (80%–85% HR reserve) and a control group that performed treadmill training at lower speeds at lower HR reserve (<50%), but for longer durations to ensure nearly equivalent total work or energy expenditure. The experimental intervention resulted in significantly greater gains in VO2peak but no differences in clinical measures of walking function. Conversely, in the crossover randomized controlled trial by Holleran et al,34 treadmill and over ground walking activities at high (70%–80% HR reserve) vs low-to-moderate intensities (30%–40% HR reserve) were compared by matching training speeds consistent with low intensity training. To achieve higher exercise intensities at relatively slow treadmill speeds, the authors manipulated the biomechanical demands of walking by altering loads or applying resistance to the trunk and limbs. For example, patients in the experimental group wore a weighted vest (10% body weight) to increase the neuromuscular and metabolic demands of carrying loads.61,62 In addition, leg weights (5–10 lb) were applied to the paretic limb to increase the neuromuscular and metabolic during of limb swing,47 with additional elastic resistance applied at the trunk to increase propulsive demands.46 Using this design, the authors were able to match the amount of stepping practice between training paradigms to isolate the effects of intensity on outcomes. Significantly greater gains in the 6-minute walk test and peak treadmill speeds were observed with higher vs lower intensity training, with differences in gains between groups related to exercise intensity achieved (fig 1A).
Fig 1.
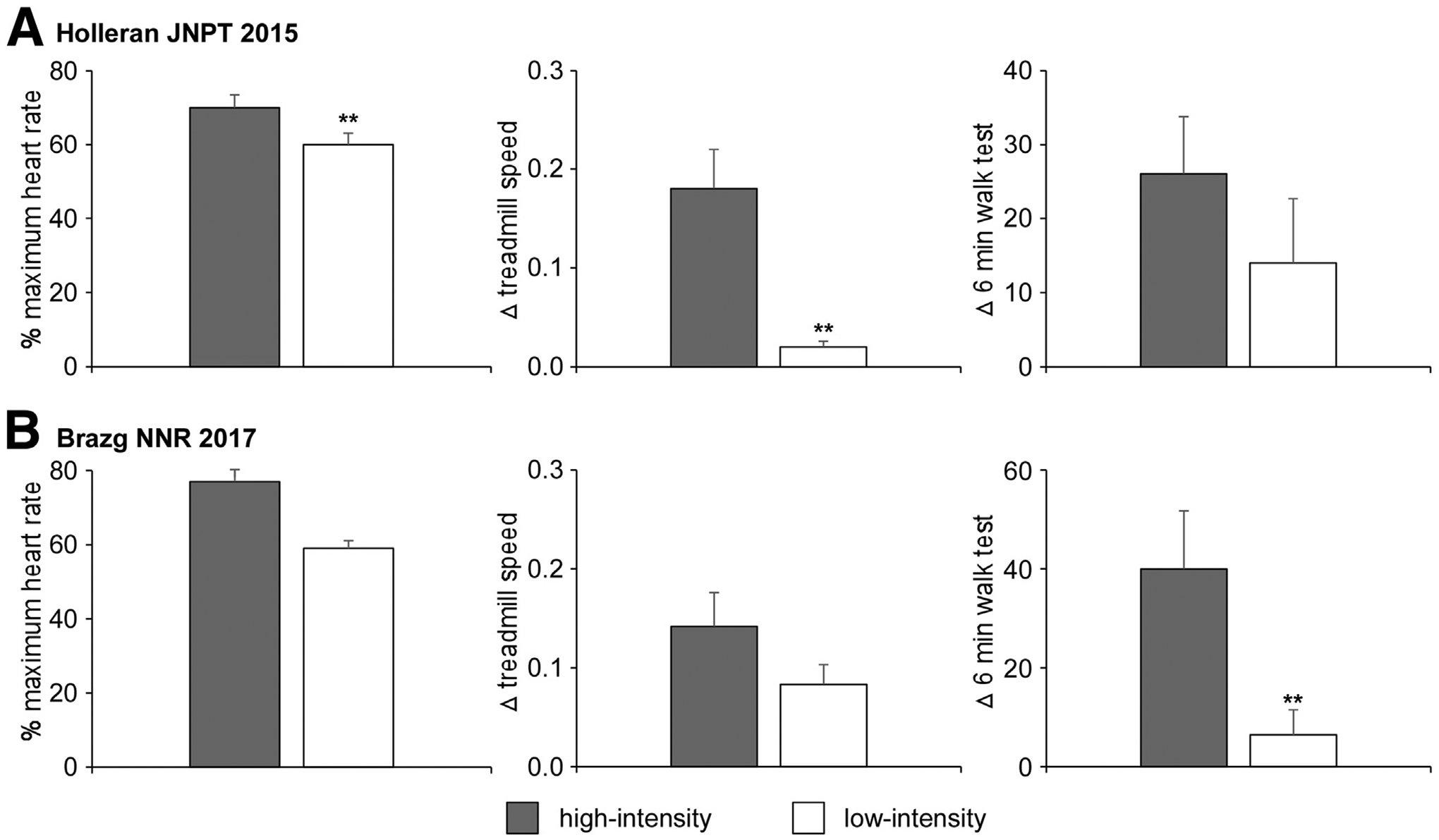
A, Relation between differences in 6-minute walk improvements and percent of predicted maximum HR during 4 weeks of high- vs low-intensity training in participants post stroke during a randomized crossover study. Adapted from Holleran et al.34 B, Relation between differences in 6-minute walk improvements and percent of predicted maximum HR during 4 weeks of high- vs low-intensity training in participants post iSCI during a randomized crossover study. Adapted from Brazg et al.63
A more recent study in ambulatory individuals with chronic motor iSCI also compared high (70%–85% HRmax) vs low intensity training (50%–65% HRmax) by altering the biomechanical demands of walking, with equivalent total stepping practice.63 Consistent with the results above, gains in the 6-minute walk test were significantly greater after high vs low or moderate-intensity training, with significant correlations between differences in gains in walking function and differences in exercise intensity between groups (fig 1B). Additional studies that emphasize the role of high- vs low-to-moderate–intensity training include the use of high-intensity interval training, in which brief intermittent bouts of walking at higher speeds or HRs are inter-leaved with lower intensity or resting activities.58,60 Both of these studies observed significantly greater improvements in walking speed or distance, even with relatively small sample sizes, emphasizing the potential effect size of this intervention.
Despite the changes observed, the effect of high intensity training on comfortable walking speed assessed in a laboratory during over ground walking or community mobility (eg, steps/day) is inconsistent in both stroke10,34 and SCI.59 These findings may be related to the training environment in which many of the studies were implemented. Specifically, most studies used treadmill training to achieve the desired intensities, likely due in part to the convenience of providing safe practice, particularly with safety harness systems. However, despite other studies reporting similarities of treadmill and over ground walking,64,65 the context of treadmill exercise varies substantially from over ground and community mobility.17,66,67 In addition to differences in visual and vestibular feedback between treadmill and over ground conditions, locomotor tasks performed in unconstrained environments require negotiation of various barriers, obstacles, or surfaces and ambient conditions that are difficult to simulate with treadmill walking. Furthermore, individuals with neurologic injury often use assistive devices during over ground walking, whereas treadmill walking often allows use of handrails, and the biomechanical demands of these types of assistance differ substantially. Recent studies suggest that higher intensity training activities can be practiced over ground in contexts that more closely simulate conditions encountered during community mobility, which can elicit substantial gains in walking function.11,17 Collectively, these findings suggest that greater benefits of high intensity training may be realized with practice of tasks more specific to community mobility demands.
Mechanisms underlying gains after high-intensity training
Possible mechanisms underlying observed changes in locomotor performance after high-intensity training have been delineated across multiple physiological systems, albeit primarily for individuals without neurologic injury. Nonetheless, for both neurologically intact and impaired populations, the primary mechanism traditionally believed to contribute to improved locomotor function is enhanced cardiopulmonary function (VO2peak).68 Previous meta-analyses have suggested 10% to 30% improvements in VO2peak in patients with chronic or subacute stroke after high-intensity training.68,69 These gains were related to increased power output (eg, higher treadmill speeds),18,34 indicating that improved aerobic capacity underlies these changes.
Interestingly, however, some high-intensity training paradigms result in minimal gains in VO2peak,10 and associations between clinical walking outcomes and VO2peak are often inconsistent across studies.70 The variability of results may be due to a number of factors, including differences in the specificity or intensity of training or the similarity of the tasks performed during graded exercise testing to clinical measures. However, differences between studies may also be due in part to the observation that improved gait efficiency, or reduced V̇O2 for a given workload, may contribute to improved locomotor performance in selected participants.10,51 For example, although some patients improve VO2peak at higher walking speeds (fig 2A), gains in locomotor efficiency may allow faster walking speeds at lower energetic requirements, thereby masking potential gains in VO2peak if evaluated during graded treadmill testing. Improvements in efficiency can be quantified by decreases in V̇O2 at treadmill speeds matched to pretraining speeds (fig 2B). Recent studies in patients with stroke or iSCI have used a combined measure of changes in VO2peak (Δ VO2peak) and decreases in V̇O2 at matched pretraining peak training speeds (Δ matched V̇O2), to capture changes in both aerobic capacity and efficiency (V̇O2 gain = Δ VO2peak − Δ matched V̇O2).71 Using this calculation, walking improvements are more strongly correlated with improvements in V̇O2 gain than in either Δ VO2peak or Δ matched V̇O2 alone.
Fig 2.
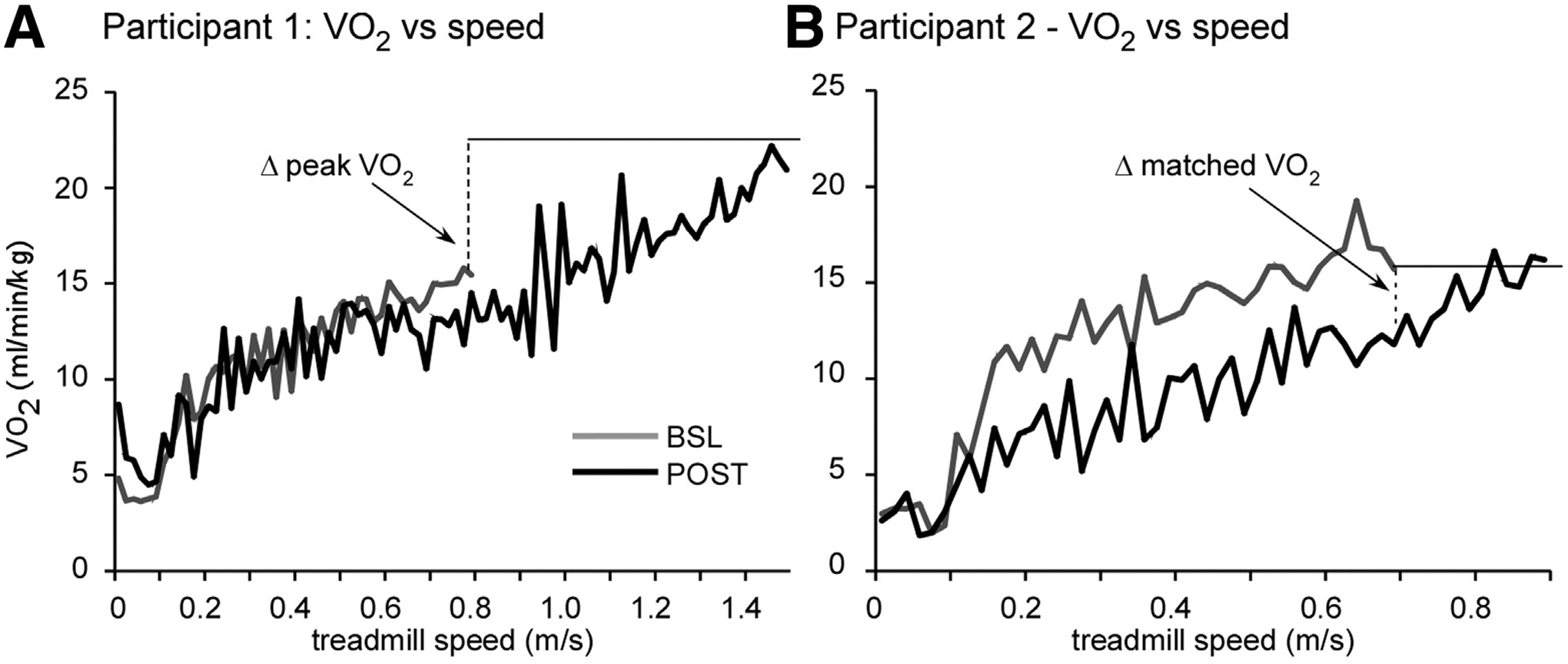
Changes in V̇O2 from baseline (BSL) to posttraining (POST) in 2 participants post stroke after high-intensity locomotor training, demonstrating differential changes in peak aerobic capacity (Δ VO2peak) vs gait efficiency (Δ matched V̇O2). A, Participant demonstrated increased Δ VO2peak with little changes in Δ matched V̇O2. B, Participant demonstrated large changes in Δ matched V̇O2 with no change in (Δ VO2peak). To account for differences, Δ V̇O2 gain is calculated as Δ VO2peak and Δ matched V̇O2. Adapted from Leddy et al.71
Mechanisms underlying changes in peak aerobic capacity and efficiency are likely multifaceted, although details of specific physiological alterations in patients with neurologic injury are not clear. Selected studies indicate that the improved V̇O2 is due to improved cardiovascular function, including some evidence for reduced HR,71 improved atrial emptying during exercise,72 and improved vascular flow at rest and during exercise.73,74 Other changes, such as improvements in blood flow distribution, cardiac output, or stroke volume, commonly seen in neurologically intact individuals40 may be inferred by the gains in VO2peak, but have not been delineated in specific patient populations. Additional respiratory alterations can facilitate observed changes and include increased pulmonary capacity (higher peak ventilation rates), improved ventilatory efficiency (reduced ventilation at matched speeds), and reduced ratio of CO2 production and O2 consumption, which is an indicator of substrate utilization and greater efficiency of O2 utilization.71
Consistent with data from intact individuals, high-intensity training is also associated with changes in neuromuscular function. Indeed, improved neuromuscular control and coordination are primary mechanisms underlying gains in walking performance (ie, speed) and likely account for gains in aerobic capacity and efficiency. Improvements in peak locomotor function appear to be associated with gains in bilateral lower extremity force generating capacity,17,75 secondary to enhanced muscular muscle mass76 or increased volitional (central) activation.76,77 In addition, changes in gait efficiency may be due to changes in neuromuscular coordination, such as altered neuromuscular coactivation or other biomechanical (kinematic or kinetic) alterations78–80 to minimize energy consumption. Exact mechanisms that contribute to improved locomotor function are likely multifaceted and may also vary with individual patients’ neuromuscular or cardiopulmonary responses.
Safety of high-intensity training
Despite the demonstrated efficacy of high-intensity training in stroke and iSCI populations, concerns regarding safety and feasibility commonly arise during attempted clinical implementation. A primary concern is the ability to safely reach the targeted HR ranges during training, particularly in patients with elevated risks of additional cardio- or cerebrovascular events. Indeed, most high-intensity protocols use a pretraining exercise test with metabolic and electrocardiographic (ECG) assessments supervised by trained personnel to ensure safety. Using guidelines for exercise termination,81 the peak HR responses are often used to guide HR ranges for exercise prescription and the potential risks appear to be minimal with subsequent training. A recent meta-analysis suggesting positive outcomes for aerobic training paradigms indicated limited adverse consequences across more than 20 independent investigations.68 Nonetheless, graded exercise testing in patients with neurologic injury may not be feasible given the specialized equipment and personnel required. Additional guidelines have been articulated to minimize these concerns, with specific recommendations to limit exercise training intensity to RPE up to 11–14 (range, 6–20) and HRs up to 55% to 80% HRmax.82,83 If graded exercise testing is performed without recommended monitoring, previous guidelines suggest maximum HRs reach up to 85% of age-predicted HRmax. More recent guidelines suggest the potential benefits of higher intensity activities may be warranted given the limited risk, although HRs and other vitals should be monitored continuously.84
With these guidelines in mind, 2 investigations in the past 5 years have attempted to implement moderate- to high-intensity training as standard of care during inpatient stroke rehabilitation.18 In one study from a larger US rehabilitation clinic, therapists were encouraged to maximize stepping practice in their patients at HRs of 70% or greater of age-predicated HRmax, with higher RPE scores allowed (RPEs≥14), with goals to achieve up to 85%HRmax or 17 to 18 RPEs for as much time during the session as possible. Such training was performed in the context of their scheduled physical sessions (45–60min) over the patient’s length of stay (median, 28d). Gains in locomotor function in 201 participants were substantial and correlated with both the amount and intensity of locomotor training, without increased rate of adverse events above conventional interventions. However, targeted intensities were sometimes difficult to achieve, with only 38% of the total sessions reaching RPEs of 14 or more in documented training sessions., although this patient population was severely impaired at the start of training (median Berg Balance Scores, 5 of 56 points). Indeed, achieving high intensities in those with greater mobility deficits tends to be more difficult than in controlled studies with participants who are typically ambulatory.
In a separate study conducted in Oslo, Norway,85 a historical comparative efficacy trial of more than 100 patients post stroke monitored training activities and outcomes for approximately 12 months before and after attempts to implement moderate- to high-intensity training during inpatient rehabilitation. As above, therapy sessions were typically 1 hour per day and higher intensity exercise activities were performed during scheduled rehabilitation sessions, with goals to achieve 70% to 85% of HRmax over the length of stay. The results indicated substantially greater outcomes in all locomotor measures at discharge and when groups were matched for total duration of inpatient rehabilitation (ie, length of stay). Importantly, the gains in walking outcomes at the first week with high-intensity training postimplementation were comparable to improvements in walking function after 3 weeks of conventional physical therapy interventions prior to implementation. In both cohorts, patients were much higher functioning (average Berg scores, ~30 points) than inpatient rehabilitation populations in the US, as described previously. In this population, however, HRs and RPEs achieved during training were similar to or slightly higher than those achieved in the US population, with no significant adverse events.
Does high-intensity training reinforce abnormal motor behaviors?
Additional concerns of high-intensity training include the risk of increasing maladaptive musculoskeletal or neuromuscular responses with training. In particular, long-standing observations86 and previous research suggest increased involuntary neuromuscular activity of paretic upper87 and lower extremities88 during performance of activities at higher intensities or increased difficulty. These responses manifest as increased reflex excitability, patterns of involuntary limb posturing in characteristic patterns, or synergies, such as hip and knee extension, hip adduction, with ankle plantarflexion and inversion.89 Such abnormal postures could theoretically increase the risk of musculoskeletal (ligamentous) injury (eg, ankle inversion or knee hyperextension). To mitigate risks of injury, bracing or taping of paretic ankle or knee joints can allow continued stepping without compromising joint integrity. To date, there are limited data describing the potential associations between biomechanical gait abnormalities and orthopedic injuries in patients with neurologic injury, particularly as related to high-intensity training.
A related issue is the development of maladaptive neuromuscular responses with high-intensity training. Long-standing theories in neurologic rehabilitation86 suggest that allowing practice of abnormal movement strategies can lead to persistent aberrant movement patterns that are more difficult to rectify in the chronic stages. Mitigating these strategies is therefore a common goal of conventional physical therapy interventions,90,91 and therapists often attempt to normalize walking patterns during training. However, there are no research findings to indicate that allowing abnormal stepping practice reinforces abnormal kinematics strategies. Similarly, there is no data to indicate that normalizing stepping performance during training results in more normal kinematic patterns compared with alternative strategies. Rather, data from neurologically intact individuals suggest that aberrant motor patterns may be overcome with training.92,93 Previous work suggests that training at higher aerobic intensities (ie, speeds) appears to result in selected improvements, and not degradation, of movement quality as measured by kinematic assessments.78–80,94 With repeated training, improvements in measures of interlimb (spatiotemporal symmetry) and intralimb (inter-joint consistency)95 coordination are observed. In addition, the incidence of abnormal compensatory behaviors (measured as swing-phase hip abduction) were revealed only in a subpopulation (ie, 20%–25%) of participants at the highest treadmill speeds post training. Interestingly, these participants also presented with lower Fugl-Meyer scores prior to training, and both changes in speed and baseline Fugl-Meyer scores were associated with increased hip abduction.95 A potential hypothesis is that these individuals did not possess the ability to individuate lower extremity movements to increase knee flexion or ankle dorsiflexion for limb clearance and therefore required greater hip abduction (ie, circumduction) to successfully to advance the limb at higher speeds. Importantly, when patients walked at post-training speeds matched to their peak pretraining velocity, there was no increase in hip abduction. These latter data suggest that specific “maladaptive” kinematic patterns were not reinforced with training, but rather utilized only to ensure limb clearance at higher speeds.68 In general, there may be limited negative consequences of performing high-intensity training, particularly considering the potential functional and cardiovascular benefits observed.
Implementation of high-intensity training in the clinical setting
Given the benefits of high-intensity training and potentially minimal adverse events, implementation of such training in the clinical setting has been an area of recent interest.18 Endeavors to implement evidence-based strategies may benefit from an understanding of the potential barriers that can impede clinical adoption and the type of specific strategies that have been used to overcome these obstacles. Similarly, recognition of potential facilitators, or factors that enable implementation, could result in strategies that simplify adoption and utilization.96 For high-intensity training, a number of facilitators and barriers may contribute to the success of implementation strategies, with selected factors described below.
Equipment
Many clinicians have voiced concerns related to the equipment described in research studies used for high-intensity training. One specific concern has been the costs of motorized treadmill systems with BWS systems. These systems can be extremely helpful for more impaired patients to reduce limb loading to facilitate stepping and increase the safety of training. However, most high-intensity training studies use treadmill systems with only a safety harness and lower-cost, fall-arrest systems in case of loss of balance.34,60 For clinical settings that treat highly impaired patients, some of the motorized BWS devices that limit vertical center of mass movement may be sufficient, as there is no evidence to suggest that the more advanced, more expensive unweighting systems are necessary. In addition, however, many clinical settings may have recumbent stepping or cycling equipment that can facilitate high-intensity training, which can be a reasonable alternative.97,98 However, a recent meta-analysis suggests smaller locomotor gains with cycling vs walking exercises,69 reemphasizing the potential importance of task-specific training. Indeed, recent studies99 and practice guidelines100 have suggested more limited gains in locomotor function when higher intensity, nonwalking activities (ie, strengthening, cycling, circuit training) are performed compared with those attained with walking.
For over ground training, selected studies have demonstrated that high-intensity training performed under different conditions, including stair-climbing, is feasible and may facilitate substantial gains in walking function as well as aerobic capacity and efficiency in individuals with both stroke and iSCI.11,17,18,63 If harness systems are required during over ground training, specific overhead railing or wheeled systems (eg, Hoyer lifts) typically used for transfers may be modified to allow the safe performance of stepping practice. Such modifications would require supervision from trained personnel, but have been used in research11,17 and during clinical practice18 to facilitate more task-specific practice for return to independent walking.
Personnel
A related concern is the lack of available physical assistance from therapists or aides to provide high-intensity training. This specific barrier may arise from the traditional belief that attempts to “normalize” gait patterns during stepping training in impaired patients,15,101,102 which requires substantial physical effort and more personnel. However, most aerobic training studies report minimal, if any, assistance provided. In patients with substantial lower extremity or trunk weakness, previous studies have used assistance only as needed to ensure continuous stepping and to maximize volitional activity to achieve higher intensities.29 If additional assistance to normalize kinematics is provided, patients may reduce volitional activity, as indicated by reduced cardiovascular exertion during training, which has not resulted in greater improvements than nonwalking paradigms.26,27 Uses of robotic devices to facilitate “normal” gait patterns has faced similar limitations with reduced training intensity21,35 and leads to smaller gains than higher intensity stepping activities provided with minimal physical assistance.28,29 If therapist assistance is needed but limited, others have recently used elastic assistance at the limbs to assist limb swing.78
Measurement of training intensity
Additional concerns revolve around appropriate measures of training intensity, as patients often present with altered autonomic responses to exercise, increased risk of cardiovascular disease, or use of medications that alter HRs and blood pressure responses. Of primary concern may be the lack of exercise testing with trained personnel and assessment of cardiac function (ie, ECG) before the initiation of training, as discussed previously. As the available literature and current recommendations suggest that higher intensity activities may still be possible using specific training parameters,84 certain caveats related to attaining these intensities could be considered.
In general, the primary measure of cardiovascular intensity is HR, with available guidelines with other studies targeting up to 80% to 85% of age-predicted HRmax.84 As documented in the literature, however, cardiovascular responses in both neurologic intact and impaired populations can vary substantially from age-predicted responses44,71 due to β-blockers45,103 or to interindividual variability.44 As such, the use of RPEs is often recommended as a surrogate measure of intensity. The initial scale varies proportionally with HR responses, with scores of 6 to 20, roughly equivalent to HR responses of younger neurologically intact individuals during exercise assessments.104,105 Previous guidelines suggest use of RPEs between 11 and 14,83 although recent studies have used higher ranges14–17 without significant adverse effects, whereas ranges from 11 to 14 are considered moderate intensities.50 The discrepancies between these ranges is an area of further research and may not be trivial, as differences in training outcomes while targeting these different RPE levels can be substantial.34,63
A further concern with use of RPEs in patients with neurologic injury is its potential use as a measure of task difficulty vs intensity, with the latter intended to estimate subjective workload or power output and speed. Although subjective measures of task difficulty and intensity may be similar, selected tasks that require greater skill or accuracy (eg, stepping on balance beams, negotiating uneven, compliant terrains) may not require substantial cardiovascular exertion due to slower walking speeds, although they may require increased volitional coordination to successfully complete the intended tasks. Recent evidence in those with stroke suggest that it may be challenging for individuals post stroke to separate the perceived difficulty of the task from the perceived exertion of the task when using the RPE scale. Using a 0 to 10 scale of perceived difficulty, a significant and moderate relationship was observed with the RPE scale during lower extremity tasks, including walking.106 Furthermore, scores on the RPE scale and HR were not related during the tasks. Such findings are consistent with previous literature demonstrating no correlation between RPE and HR during the 6- and 12-minute walk test in patients post stroke.107 As such, measures of RPE may be useful, although recognition of these potential issues may facilitate better understanding of a specific patient’s workload.
Given the potential limitations of HR and RPE, an additional measure of intensity for individuals with stroke or iSCI may be the rate of stepping activities. In neurologically intact individuals,108–110 ranges of stepping rates that achieve specific exercise intensities have been identified, with selected recommendations of approximately 100 steps per minute as an indicator of moderate intensity activities. In patients with neurologic injury,111,112 identification of similar thresholds have been estimated with various methods, although their clinical utility across patient populations is not certain.
We recently attempted to delineate ranges of stepping rates consistent with prespecified thresholds of exercise intensities in 35 individuals more than 6 months after stroke, and 13 individuals more than 6 months after iSCI (recruited from previous trials43,63). During graded treadmill exercise testing, we simultaneously measured stepping rates, HRs, and V̇O2 and categorized HRs and V̇O2 as light or low (40%–55% predicted HRmax, <3 metabolic equivalents [MET]=3.5 kg/ml O2/min), moderate (55%–70% predicted HRmax, 3–6 METs), and high or vigorous (>70% predicted HRmax, >6 METs) exercise intensities. For those with medications that reduce HRs, ranges were reduced by 10 beats per minute. During treadmill tests, speed was increased by 0.1 m/s increments every minute until the individual requested to stop or met American College of Sports Medicine criteria for termination of a graded exercise test (with simultaneous ECG recordings). Participants were also stratified by their self-selected walking velocity (<0.4 m/s, 0.4–0.8 m/s, and >0.8 m/s) to evaluate the extent to which stepping intensities within each HR or V̇O2 range varied with gait dysfunction.
Stepping intensity ranges for participants within each gait speed category were established by determining average stepping cadence during treadmill walking when HRs or V̇O2 were within the specific exercise intensities described above. Table 2 details the mean and 95% confidence intervals of stepping rates in each intensity category, stratified according to participants’ self-selected velocity. In addition, Figure 3 depicts changes in HRs vs changes in treadmill speed (fig 3A) or vs changes in stepping rates (ie, cadence) (fig 3B) in patients with stroke stratified by self-selected walking speeds. Although there was significant variability, stepping rates using HRs were more consistent within each gait speed classification, as the level of neurologic impairments often limited inferences from the analyses using METs. More directly, most participants within any category of gait impairments could not reach higher intensities using MET levels, although HRs may be elevated within or beyond these ranges during similar walking speeds. With HRs as the primary intensity measure, individuals with self-selected gait speeds less than 0.4 m/s could rarely achieve low exercise intensities, and typically the slowest treadmill speeds resulted in % predicted HRmax within moderate to high or vigorous intensity ranges. Furthermore, there was no separation between these last 2 ranges. For individuals walking at 0.4 to 0.8 m/s, all ranges of intensity could be achieved, with stepping rates less than 70steps per minute indicative of walking at lower or light intensities, rates between 70 and 90 steps per minute were moderate intensities, and more than 90 steps per minute were typically higher or vigorous activities. For individuals walking at speeds greater than 0.8 m/s, stepping ranges below approximately 80 steps per minute are considered low intensity, whereas 80 to 100 steps per minute are moderate intensity, and more than 100 steps per minute are considered high intensity. Using these data, participants with neurologic injury and available activity monitors may be able to quantify relative intensities during specific training durations when other measures of HR are not available. Using these ranges for prescription of walking-based exercise program for patients with neurologic injury, walking bouts of 30 minutes at moderate to higher intensities (ie, American College of Sports Medicine recommended guidelines), approximately 2000 to 3000 steps or more could be targeted. These stepping amounts are consistent with previous studies targeting 30 to 40 minutes of high-intensity training in patients with stroke or iSCI.10,11,17,34,63
Table 2.
Stepping cadence by different intensity thresholds
| Walking Speed | Low Intensity | Moderate Intensity | High Intensity |
|---|---|---|---|
| >0.8 m/s | 62±13 (53–72) | 88±16 (78–98) | 106±11 (99–113) |
| 0.4–0.8 m/s | 55±19 (43–67) | 81±21 (71–92) | 95±15 (88–102) |
| <0.4 m/s | 69±2.8 (44–57) | 76±33 (50–102) | 78±30 (58–98) |
NOTE. Data are presented as mean ± SD (95% confidence interval).
Fig 3.
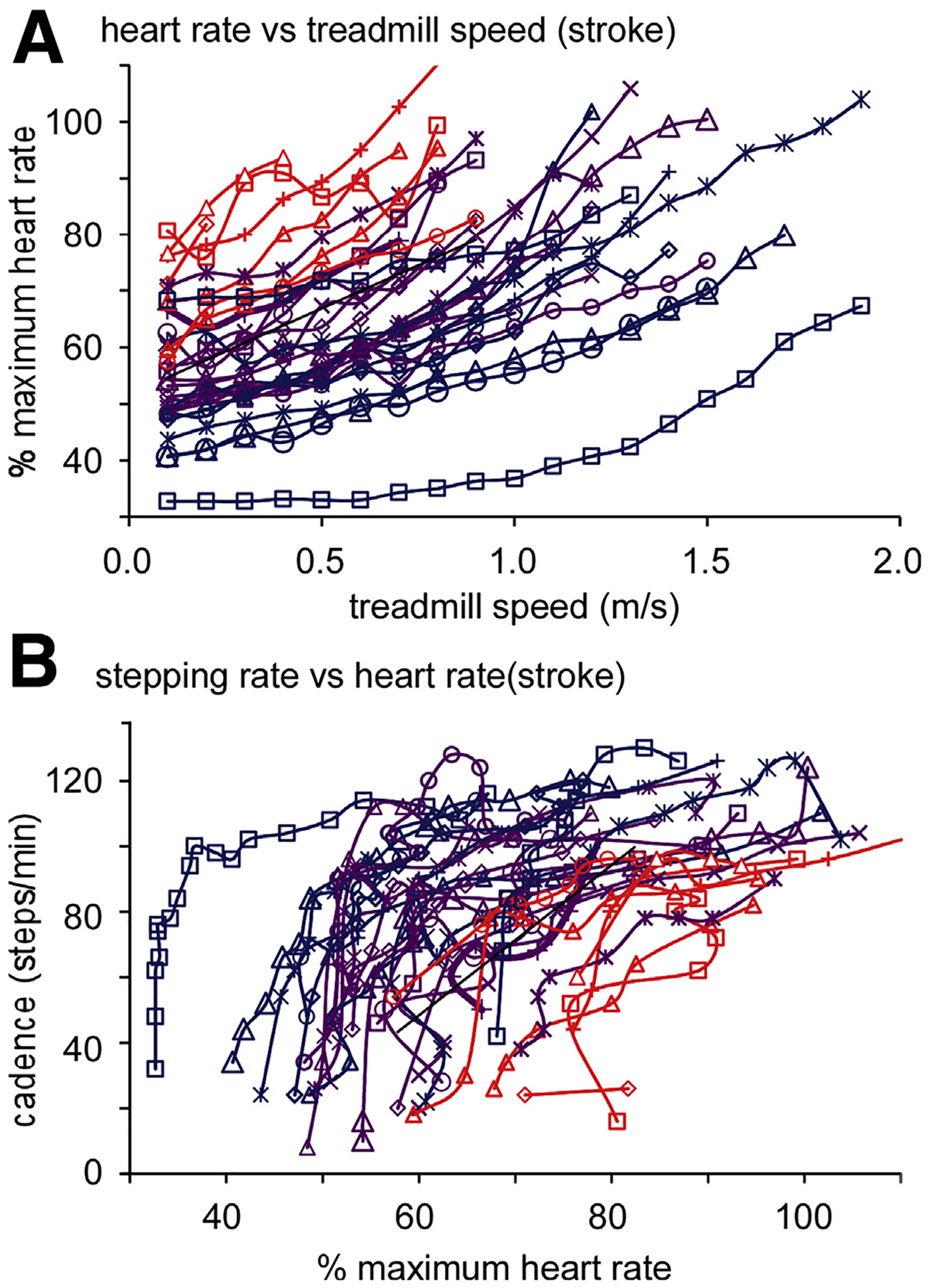
A, Changes in HRs in patients with stroke (stratified by self-selected walking speeds of <0.4 m/s, 04–0.8 m/s, and >0.8 m/s during graded treadmill testing). B, Cadence (stepping rates) of patients at different HRs. Using HR thresholds for light or low, moderate, and high or vigorous intensities, stepping data were used to identify approximate ranges of cadences at different intensities (ie, table 2).
Conclusions
In summary, the available data suggest that training of walking tasks at moderate to higher intensity, using definitions initially described in the field of exercise physiology, result in significant improvements in walking function in neurologically impaired patients. Greater changes are typically observed compared with conventional rehabilitation strategies or even compared with low-intensity walking exercise. Concerns regarding safety of such training may be mitigated by previous data demonstrating the relative low risk of higher intensity interventions. Furthermore, strategies to facilitate application in the clinical setting should allow therapists to more rapidly implement such training to improve walking function in the patients they treat.
Acknowledgments
Supported by the National Institute on Disability and Rehabilitation Research (grant nos. H133B031127 and H133B140012) and the Bullock Foundation.
List of abbreviations:
- BWS
body weight support
- ECG
electrocardiographic
- HR
heart rate
- HRmax
maximum heart rate
- iSCI
incomplete spinal cord injury
- LEAPS
Locomotor Experience Applied Post-Stroke trial
- MET
metabolic equivalent
- RPE
ratings of perceived exertion
- SCI
spinal cord injury
- V̇O2
rate of oxygen consumption
- VO2peak
peak oxygen consumption
References
- 1.American Heart Association. Heart disease and stroke statistics. Available at: http://www.americanheart.org/downloadable/heart/1200078608862HS_Stats%202008.final.pdf. Accessed September 20, 2017.
- 2.National Spinal Cord Injury Statistical Center: facts and figures at a glance. Birmingham: University of Alabama at Birmingham; 2018. [Google Scholar]
- 3.van Middendorp JJ, Hosman AJ, Donders AR, et al. A clinical prediction rule for ambulation outcomes after traumatic spinal cord injury: a longitudinal cohort study. Lancet 2011;377:1004–10. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:e2–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saraf P, Rafferty MR, Moore JL, et al. Daily stepping in individuals with motor incomplete spinal cord injury. Phys Ther 2010;90:224–35. [DOI] [PubMed] [Google Scholar]
- 6.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil 2005;86:1552–6. [DOI] [PubMed] [Google Scholar]
- 7.Lang C, Macdonald J, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Pys Ther 2007;31:3–11. [DOI] [PubMed] [Google Scholar]
- 8.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil 2009;90:1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zbogar D, Eng JJ, Miller WC, Krassioukov AV, Verrier MC. Movement repetitions in physical and occupational therapy during spinal cord injury rehabilitation. Spinal Cord 2017;55:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke 2010;41:129–35. [DOI] [PubMed] [Google Scholar]
- 11.Hornby TG, Holleran CL, Hennessy PW, et al. Variable intensive early walking poststroke (VIEWS): a randomized controlled trial. Neurorehabil Neural Repair 2016;30:440–50. [DOI] [PubMed] [Google Scholar]
- 12.Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Philos Trans R Soc Lond B Biol Sci 2006;361:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 1998;79:1329–40. [DOI] [PubMed] [Google Scholar]
- 14.Edgerton VR, Courtine G, Gerasimenko YP, et al. Training locomotor networks. Brain Res Rev 2008;57:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci 1995;7:823–9. [DOI] [PubMed] [Google Scholar]
- 16.Hesse S, Bertelt C, Jahnke MT, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke 1995;26:976–81. [DOI] [PubMed] [Google Scholar]
- 17.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabil Neural Repair 2014;28:643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornby TG, Holleran CL, Leddy AL, et al. Feasibility of focused stepping practice during inpatient rehabilitation poststroke and potential contributions to mobility outcomes. Neurorehabil Neural Repair 2015;29:923–32. [DOI] [PubMed] [Google Scholar]
- 19.Colombo G, Wirz M, Dietz V. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord 2001;39: 252–5. [DOI] [PubMed] [Google Scholar]
- 20.Hesse S, Werner C, Uhlenbrock D, von Frankenberg S, Bardeleben A, Brandl-Hesse B. An electromechanical gait trainer for restoration of gait in hemiparetic stroke patients: preliminary results. Neurorehabil Neural Repair 2001;15:39–50. [DOI] [PubMed] [Google Scholar]
- 21.Hornby TG, Kinnaird CR, Holleran CL, Rafferty MR, Rodriguez KS, Cain JB. Kinematic, muscular, and metabolic responses during exoskeletal-, elliptical-, or therapist-assisted stepping in people with incomplete spinal cord injury. Phys Ther 2012;92:1278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinkensmeyer DJ, Aoyagi D, Emken JL, et al. Tools for understanding and optimizing robotic gait training. J Rehabil Res Dev 2006;43:657–70. [DOI] [PubMed] [Google Scholar]
- 23.Banala SK, Kim SH, Agrawal SK, Scholz JP. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Trans Neural Syst Rehabil Eng 2009;17:2–8. [DOI] [PubMed] [Google Scholar]
- 24.Hurt CP, Wang J, Capo-Lugo CE, Brown DA. Effect of progressive horizontal resistive force on the comfortable walking speed of individuals post-stroke. J Neuroeng Rehabil 2015;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M, Hornby TG, Landry JM, Roth H, Schmit BD. A cable-driven locomotor training system for restoration of gait in human. SCI. Gait Posture 2011;33:256–60. [DOI] [PubMed] [Google Scholar]
- 26.Duncan PW, Sullivan KJ, Behrman AL, Rose DK, Tilson JK. Locomotor Experience Applied Post-Stroke (LEAPS): what does the outcome of the LEAPS RCT mean to the physical therapist? Paper presented at: American Physical Therapy Association Combined Sections Meeting. February 9–12, 2011; New Orleans, LA. [Google Scholar]
- 27.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med 2011;364: 2026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair 2009;23:5–13. [DOI] [PubMed] [Google Scholar]
- 29.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 2008;39:1786–92. [DOI] [PubMed] [Google Scholar]
- 30.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Arch Phys Med Rehabil 2002;83:1378–83. [DOI] [PubMed] [Google Scholar]
- 31.Kuys S, Brauer S, Ada L. Routine physiotherapy does not induce a cardiorespiratory training effect post-stroke, regardless of walking ability. Physiother Res Int 2006;11:219–27. [DOI] [PubMed] [Google Scholar]
- 32.Zbogar D, Eng JJ, Noble JW, Miller WC, Krassioukov AV, Verrier MC. Cardiovascular stress during inpatient spinal cord injury rehabilitation. Arch Phys Med Rehabil 2017;98:2449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prajapati SK, Mansfield A, Gage WH, Brooks D, McIlroy WE. Cardiovascular responses associated with daily walking in subacute stroke. Stroke Res Treat 2013;2013:612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J Neurol Phys Ther 2015; 39:95–102. [DOI] [PubMed] [Google Scholar]
- 35.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther 2006;86:1466–78. [DOI] [PubMed] [Google Scholar]
- 36.Lefeber N, De Keersmaecker E, Henderix S, Michielsen M, Kerckhofs E, Swinnen E. Physiological responses and perceived exertion during robot-assisted and body weight-supported gait after stroke. Neurorehabil Neural Repair 2018;32:1043–54. [DOI] [PubMed] [Google Scholar]
- 37.Winstein CJ, Miller JP, Blanton S, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair 2003;17:137–52. [DOI] [PubMed] [Google Scholar]
- 38.Page SJ. Intensity versus task-specificity after stroke: how important is intensity? Am J Phys Med Rehabil 2003;82:730–2. [DOI] [PubMed] [Google Scholar]
- 39.Page SJ. The role of timing and intensity of rehabilitation therapies. Top Stroke Rehabil 2006;13:viii–ix. author reply ix-x. [PubMed] [Google Scholar]
- 40.Katch VL, McArdle WD, Katch FI. Essentials of exericse physiology. Baltimore: Lippincott Williams & Williams; 2011. [Google Scholar]
- 41.Hornby TG, Moore JL, Lovell L, Roth EJ. Influence of skill and exercise training parameters on locomotor recovery during stroke rehabilitation. Curr Opin Neurol 2016;29:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornby TG, Straube DS, Kinnaird CR, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil 2011; 18:293–307. [DOI] [PubMed] [Google Scholar]
- 43.Hornby TG, Henderson CE, Plawecki A, et al. Contributions of stepping intensity and variability to mobility in individuals post-stroke. Stroke 2019;50:2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arena R, Myers J, Kaminsky LA. Revisiting age-predicted maximal heart rate: can it be used as a valid measure of effort? Am Heart J 2016;173:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joyner MJ, Freund BJ, Jilka SM, et al. Effects of beta-blockade on exercise capacity of trained and untrained men: a hemodynamic comparison. J Appl Physiol 1986;60:1429–34. [DOI] [PubMed] [Google Scholar]
- 46.Gottschall J, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol 2003;94:1766–72. [DOI] [PubMed] [Google Scholar]
- 47.Gottschall J, Kram R. Energy cost and muscular activity required for leg swing during walking. J Appl Physiol 2005;99:23–30. [DOI] [PubMed] [Google Scholar]
- 48.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 6th ed. Baltimore: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 49.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 10th ed. Philadelphia: Wolters Kluwer; 2018. [Google Scholar]
- 50.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 2010;13: 496–502. [DOI] [PubMed] [Google Scholar]
- 51.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke 1997;28:326–30. [DOI] [PubMed] [Google Scholar]
- 52.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke 2005;36:2206–11. [DOI] [PubMed] [Google Scholar]
- 53.Macko RF, Katzel LI, Yataco A, et al. Low-velocity graded treadmill stress testing in hemiparetic stroke patients. Stroke 1997;28:988–92. [DOI] [PubMed] [Google Scholar]
- 54.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil 2001;82:879–84. [DOI] [PubMed] [Google Scholar]
- 55.Globas C, Becker C, Cerny J, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabil Neural Repair 2012;26:85–95. [DOI] [PubMed] [Google Scholar]
- 56.Ivey FM, Stookey AD, Hafer-Macko CE, Ryan AS, Macko RF. Higher treadmill training intensity to address functional aerobic impairment after stroke. J Stroke Cerebrovasc Dis 2015;24:2539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luft AR, Macko RF, Forrester LW, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke 2008;39:3341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munari D, Pedrinolla A, Smania N, et al. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med 2016;54:408–18. [DOI] [PubMed] [Google Scholar]
- 59.Yang JF, Musselman KE, Livingstone D, et al. Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair 2014;28:314–24. [DOI] [PubMed] [Google Scholar]
- 60.Boyne P, Dunning K, Carl D, et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther 2016;96: 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol 2005;98:579–83. [DOI] [PubMed] [Google Scholar]
- 62.Griffin TM, Roberts TJ, Kram R. Metabolic cost of generating muscular force in human walking: insights from load-carrying and speed experiments. J Appl Physiol 2003;95:172–83. [DOI] [PubMed] [Google Scholar]
- 63.Brazg G, Fahey M, Holleran CL, et al. Effects of training intensity on locomotor performance in individuals with chronic spinal cord injury: a randomized crossover study. Neurorehabil Neural Repair 2017. Oct 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J Appl Physiol 2008;104:747–55. [DOI] [PubMed] [Google Scholar]
- 65.Riley PO, Paolini G, Della Croce U, Paylo KW, Kerrigan DC. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait Posture 2007;26:17–24. [DOI] [PubMed] [Google Scholar]
- 66.Shumway-Cook A, Patla A, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental components of mobility disability in community-living older persons. J Am Geriatr Soc 2003;51: 393–8. [DOI] [PubMed] [Google Scholar]
- 67.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Phys Ther 2002;82:670–81. [PubMed] [Google Scholar]
- 68.Pang MY, Charlesworth SA, Lau RW, Chung RC. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovasc Dis 2013;35:7–22. [DOI] [PubMed] [Google Scholar]
- 69.Boyne P, Welge J, Kissela B, Dunning K. factors influencing the efficacy of aerobic exercise for improving fitness and walking capacity after stroke: a meta-analysis with meta-regression. Arch Phys Med Rehabi 2017;98:581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Outermans J, van de Port I, Wittink H, de Groot J, Kwakkel G. How strongly is aerobic capacity correlated with walking speed and distance after stroke? Systematic review and meta-analysis. Phys Ther 2015;95:835–53. [DOI] [PubMed] [Google Scholar]
- 71.Leddy AL, Connolly M, Holleran CL, et al. Alterations in aerobic exercise performance and gait economy following high-intensity dynamic stepping training in persons with subacute stroke. J Neurol Phys Ther 2016;40:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang A, Eng JJ, Krassioukov AV, et al. Exercise-induced changes in cardiovascular function after stroke: a randomized controlled trial. Int J Stroke 2014;9:883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Billinger SA, Gajewski BJ, Guo LX, Kluding PM. Single limb exercise induces femoral artery remodeling and improves blood flow in the hemiparetic leg poststroke. Stroke 2009;40:3086–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Billinger SA, Kluding PM. Use of Doppler ultrasound to assess femoral artery adaptations in the hemiparetic limb in people with stroke. Cerebrovasc Dis 2009;27:552–8. [DOI] [PubMed] [Google Scholar]
- 75.Straube DD, Holleran CL, Kinnaird CR, Leddy AL, Hennessy PW, Hornby TG. Effects of dynamic stepping training on nonlocomotor tasks in individuals poststroke. Phys Ther 2014;94:921–33. [DOI] [PubMed] [Google Scholar]
- 76.Gregory CM, Bowden MG, Jayaraman A, et al. Resistance training and locomotor recovery after incomplete spinal cord injury: a case series. Spinal Cord 2007;45:522–30. [DOI] [PubMed] [Google Scholar]
- 77.Hornby TG, Lewek MD, Thompson CK, Heitz R. Repeated maximal volitional effort contractions in human spinal cord injury: initial torque increases and reduced fatigue. Neurorehabil Neural Repair 2009;23:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leech KA, Kinnaird CR, Holleran CL, Kahn J, Hornby TG. Effects of locomotor exercise intensity on gait performance in individuals with incomplete spinal cord injury. Phys Ther 2016;96:1919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ardestani MM, Henderson CE, Salehi SH, Mahtani GB, Schmit BD, Hornby TG. Kinematic and neuromuscular adaptations in incomplete spinal cord injury after high- versus low-intensity locomotor training. J Neurotrauma 2019;36:2036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ardestani MM, Kinnaird CR, Henderson CE, Hornby TG. Compensation or recovery? altered kinetics and neuromuscular synergies following high-intensity stepping training poststroke. Neurorehabil Neural Repair 2019;33:47–58. [DOI] [PubMed] [Google Scholar]
- 81.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 2004;36:533–53. [DOI] [PubMed] [Google Scholar]
- 82.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/A-merican Stroke Association. Stroke 2014;45:2532–53. [DOI] [PubMed] [Google Scholar]
- 83.Gordon NF, Gulanick M, Costa F, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Stroke 2004;35:1230–40. [DOI] [PubMed] [Google Scholar]
- 84.MacKay-Lyons M, Billinger SA, Eng JJ, et al. Aerobic exercise recommendations to optimize best practices in care after stroke: AEROBICS 2019 update. Phys Ther 2020;100:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore JL, Nordvik JE, Erichsen A, et al. Implementation of high-intensity stepping training during inpatient stroke rehabilitation improves functional outcomes. Stroke 2020;51:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bobath B Adult hemiplegia: evaluation and treatment. 3rd ed. Oxford: Butterworth-Heinemann; 1990. [Google Scholar]
- 87.Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain 2007;130:159–69. [DOI] [PubMed] [Google Scholar]
- 88.Neckel N, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. J Neuroeng Rehabil 2006;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 2010; 103:844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umphred DA, Lazaro RT, Roller M, Burton G. Neurological rehabilitation. 6th ed. Maryland Heights, MO: Mosby; 2012. [Google Scholar]
- 91.O’Sullivan SB, Schmitz TJ, Fulk G. Physical rehabilitation. 6th ed. Philadelphia: F.A. Davis; 2013. [Google Scholar]
- 92.Byl NN. Focal hand dystonia may result from aberrant neuro-plasticity. Adv Neurol 2004;94:19–28. [PubMed] [Google Scholar]
- 93.Blake DT, Byl NN, Cheung S, et al. Sensory representation abnormalities that parallel focal hand dystonia in a primate model. Somatosens Mot Res 2002;19:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyrell CM, Roos MA, Rudolph KS, Reisman DS. Influence of systematic increases in treadmill walking speed on gait kinematics after stroke. Phys Ther 2011;91:392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahtani GB, Kinnaird CR, Connolly M, et al. Altered sagittal- and frontal-plane kinematics following high-intensity stepping training versus conventional interventions in subacute stroke. Phys Ther 2017;97:320–9. [DOI] [PubMed] [Google Scholar]
- 96.Straus SE, Tetroe J, Graham I. Defining knowledge translation. CMAJ 2009;181:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin H, Jiang Y, Wei Q, Wang B, Ma G. Intensive aerobic cycling training with lower limb weights in Chinese patients with chronic stroke: discordance between improved cardiovascular fitness and walking ability. Disabil Rehabil 2012;34:1665–71. [DOI] [PubMed] [Google Scholar]
- 98.Jin H, Jiang Y, Wei Q, Chen L, Ma G. Effects of aerobic cycling training on cardiovascular fitness and heart rate recovery in patients with chronic stroke. NeuroRehabilitation 2013;32:327–35. [DOI] [PubMed] [Google Scholar]
- 99.Lotter JK, Henderson CE, Plawecki A, et al. Task-specific versus impairment-based training on locomotor performance in individuals with chronic spinal cord injury: a randomized crossover study. Neurorehabil Neural Repair 2020;34:627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hornby TG, Reisman DS, Ward IG, et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J Neurol Phys Ther 2020;44:49–100. [DOI] [PubMed] [Google Scholar]
- 101.Behrman A, Harkema S. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 2000;80:688–700. [PubMed] [Google Scholar]
- 102.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair 2007;21: 137–51. [DOI] [PubMed] [Google Scholar]
- 103.Mier CM, Domenick MA, Wilmore JH. Changes in stroke volume with beta-blockade before and after 10 days of exercise training in men and women. J Appl Physiol 1997;83:1660–5. [DOI] [PubMed] [Google Scholar]
- 104.Borg G Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 1982;3:153–8. [DOI] [PubMed] [Google Scholar]
- 105.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. [PubMed] [Google Scholar]
- 106.Wood J, Reisman D, Drake M, Morton S. The overlap between perceptions of cardiovascular demand and task difficulty in individuals with chronic stroke performing moderate intensity exercise. New Orleans, LA: Paper presented at: APTA Combined Sections Meeting 2018; February 22–24; 2018. [Google Scholar]
- 107.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke 2002;33:756–61. [DOI] [PubMed] [Google Scholar]
- 108.Abel M, Hannon J, Mullineaux D, Beighle A. Determination of step rate thresholds corresponding to physical activity intensity classifications in adults. J Phys Act Health 2011;8:45–51. [DOI] [PubMed] [Google Scholar]
- 109.Marshall SJ, Levy SS, Tudor-Locke CE, et al. Translating physical activity recommendations into a pedometer-based step goal: 3000 steps in 30 minutes. Am J Prev Med 2009;36:410–5. [DOI] [PubMed] [Google Scholar]
- 110.Wang H, Zhang YF, Xu LL, Jiang CM. Step rate-determined walking intensity and walking recommendation in Chinese young adults: a cross-sectional study. BMJ Open 2013;3:e001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sandroff BM, Riskin BJ, Agiovlasitis S, Motl RW. Accelerometer cut-points derived during over-ground walking in persons with mild, moderate, and severe multiple sclerosis. J Neurol Sci 2014;340:50–7. [DOI] [PubMed] [Google Scholar]
- 112.Baque E, Sakzewski L, Trost SG, Boyd RN, Barber L. Validity of accelerometry to measure physical activity intensity in children with an acquired brain injury. Pediatr Phys Ther 2017;29:322–9. [DOI] [PubMed] [Google Scholar]


