Abstract
Background
Serum sickness-like reaction (SSLR) is an acute inflammatory condition affecting predominantly children. The pathophysiology remains unclear, but drugs are considered the main trigger.
Objective
The aim of this study was to describe the clinical and laboratory features, triggers, and treatment modalities in children diagnosed with SSLR.
Methods
We conducted a 10-year retrospective cohort study including all paediatric patients (0 to 18 years old) with query SSLR referred to the Adverse Drug Reactions Clinic at the Children’s Hospital of Western Ontario. Diagnostic criteria included acute skin rash plus joint inflammation with or without fever.
Results
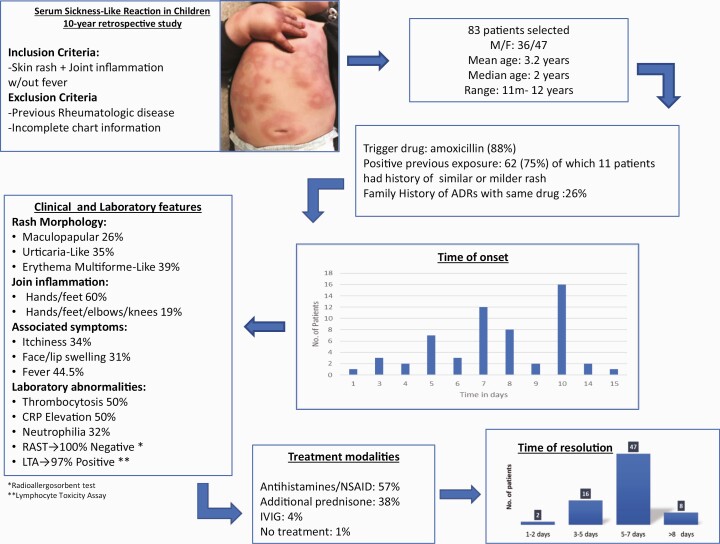
We included 83 patients (47 females). Age ranged from 11 months to 12 years (mean 3.2 years). Amoxicillin was the trigger in 82.7% of patients. The mean time between the exposure to the triggering drug and the development of the symptoms was 8.5 days. Urticaria-like and Erythema multiforme-like lesions were present in 35% and 38.5% of the cases, respectively. Joint inflammation affecting hands/feet was present in 60%. Pruritus, lip/eye swelling, and fever were reported in 33, 31, and 45% of patients, respectively. The lymphocyte toxicity assay (LTA) showed incremental T-cell toxicity in 32 of 34 patients. Children that received treatment with antihistamines/nonsteroidal anti-inflammatory drugs (NSAIDs) plus oral steroids had a mean recovery time shorter than those treated only with antihistamines/NSAIDs (6 versus 8 days; P=0.09).
Conclusions
In our study, SSLR was mostly triggered by amoxicillin and had a mean time presentation of 8.5 days. Further prospective and well-conducted studies are needed.
Keywords: Adverse drug reaction, Children, Serum Sickness-like Reaction
Graphical Abstract
Graphical Abstract.
Serum sickness-like reaction (SSLR) is an immunological condition characterized by sudden development of skin rash and joint inflammation with or without fever, usually preceded by exposure to a drug. It can present in both adult and paediatric populations although it is seen in children more frequently (1). SSLR was named after its resemblance to classic serum sickness (SS), a Type III immune hypersensitivity reaction that was described in patients who had received heterologous serum as antitoxin to treat diphtheria (2,3). Unlike classic SS, SSLR is mainly triggered by drugs (beta-lactam antibiotics) but vaccines and infectious agents have been also implicated in its etiology. The precise pathophysiology of SSLR has not been elucidated but it seems to be different from the classic SS, as SSLR is not associated with antigen–antibody complex formation and the blood concentrations of complement in SSLR are usually normal (4). Some theories consider the possibility that drugs or their metabolites may act as haptens and bind plasma proteins which subsequently induce an abnormal immunologic response, while another theory suggests that drug metabolites by themselves have a direct toxic effect on the lymphocytes of affected patients (5,6).
While beta-lactam antibiotics, especially cefaclor and amoxicillin, are the most common drugs associated with this condition, a great variety of other drugs have been reported to also trigger SSLR, including sulfonamide antibiotics, anti-cancer agents, anticonvulsants, anti-inflammatory agents, griseofulvin, metronidazole, bupropion, and more recently biologic agents such as rituximab, infliximab, and efalizumab among others (7–11).
SSLR is considered as an uncommon condition, however, its current prevalence is unknown, as it is rarely reported and usually unrecognized or easily mistaken by other cutaneous entities with annular/polycyclic morphology, such as urticaria, urticaria multiforme (UM), erythema multiforme (EM), infectious rashes, or other drug reactions (12). Laboratory assessment in patients with SSLR is nonspecific showing elevation of inflammatory markers. Diagnostic confirmation in SSLR patients is controversial especially because the oral challenge tests which are considered the ‘gold standard’ may represent a risk to patients. Other options include in vitro testing such as the lymphocyte toxicity assay (LTA), a safer test which is based on the observation that lymphocytes of patients with a history of T-cell-mediated drug hypersensitivity reactions show an increased mortality when they are re-exposed to the trigger drug or their metabolites. Although this test has not been fully validated for clinical use yet, its usefulness as a diagnostic tool in patients with SSLR and other delayed drug hypersensitivity reactions has been demonstrated (13–15).
SSLR is a self-resolving condition with no systemic involvement for which there is no standardized treatment and controversy remains regarding the use of antihistamines, nonsteroidal anti-inflammatory drugs (NSAIDs), and oral corticosteroids due to the lack of studies that specifically evaluate the safety and efficacy of these treatments. The objectives of our study were the following: (a) to describe the epidemiology, clinical, and laboratory features of SSLR in children attending the Adverse Drug Reactions Clinic at our institution, (b) to identify the most common triggers associated with the development of SSLR in our population, and (c) to determine the different treatment strategies used and their efficacy assessed by clinical response.
METHODS
After approval by the Research Ethics Board of Western University, we performed a retrospective cohort study from January 2008 to October 2018 including all paediatric patients with a suspected diagnosis of SSLR referred to the Adverse Drug Reactions Clinic at the Children’s Hospital at London Health Sciences Centre, a tertiary care referral centre in London, Ontario, Canada.
We included children from 0 to 18 years old with skin rash plus joint inflammation with or without fever. We excluded patients with any pre-existent rheumatologic condition (e.g., lupus, dermatomyositis, rheumatoid arthritis), incomplete information or if the diagnosis of SSLR was ruled out afterwards.
Detailed data including age, gender, associated trigger drug, co-morbidities, personal or family history of allergies, time of presentation, location of joint inflammation, additional symptoms, laboratory results including LTA, treatment received and prognosis, were collected. Morphology of skin lesions was divided in three groups (as described in the medical records): Papulo-macular, Urticaria-like (hives), and EM-like (targetoid lesions). Recovery time was defined as resolution of all cutaneous, articular, and/or systemic symptoms.
Descriptive statistics were used to describe baseline characteristics. Students’ t tests were used to compare continuous variables with two-sided P-values reported at a 95% level of confidence.
RESULTS
Results are summarized in Table 1. During the study period, we identified 111 cases referred as potential SSLR, of which 83 met our inclusion criteria. Excluded cases had incomplete clinical features or unavailable information. Forty-seven cases were female (56.6%) and mean age at the time of diagnosis was 3.2 years old. Amoxicillin was the drug most frequently associated with SSLR in this cohort (87%). In 62 cases, there was a history of one or more previous exposures to the suspected trigger drug. Of these, 18% had a similar or milder rash during the previous exposure to the same drug. The mean time between the exposure to the drug and the development of symptoms was 8.5 days (median 7 days). The morphology of the skin rash was described as maculo-papular, urticaria-like, and EM-like in 26%, 35%, and 38% of the patients, respectively. Lip and/or eye swelling was reported in 26 (31%) patients. Regarding joint inflammation, 60% of the patients had edema and pain of both hands and feet while fever was present in 37 patients (44.5%).
Table 1.
Results summary
| Sex | |
| Female | 47/83 (56.6%) |
| Age | |
| Mean | 3.2 y/o |
| Median | 2 y/o |
| Range | 11 mo–12 y/o |
| Trigger drug | |
| Antibiotics | 81 (97.5%) |
| Amoxicillin | 72 (87%) |
| Cephalosporin | 7 (8.4%) |
| TMP/SMX | 1 (1.2%) |
| Meropenem | 1 (1.2%) |
| Non-antibiotics | 2 (2.5%) |
| NSAID | 1 (1.2%) |
| Carbamazepine | 1 (1.2%) |
| Reason for drug prescription | |
| Otitis Media | 33 (41%) |
| Upper Respiratory infection | 16 (20%) |
| Chest infection | 15 (18%) |
| Pharyngitis | 14 (17%) |
| Urinary tract infection | 2 (2%) |
| Dental abscess | 1 (1%) |
| Epilepsy | 1 (1%) |
| Time to develop symptoms after drug exposure | (days) |
| Mean | 8.5 |
| Median | 7 |
| Range | 1–90* |
| *(carbamazepine-associated SSLR) | |
| History of previous exposure to the trigger drug | |
| Negative | 6 (7%) |
| Unknown | 15 (18%) |
| Positive | 62 (75%) |
| History of rash during previous exposure to the trigger drug | 11/62 (18%) |
| Positive family history of drug reaction | 26 (31%) |
| Penicillin | 24/26 (92%) |
| TMP/SMX | 2/26 (8%) |
| Positive personal history of other allergies | 5 (6%) |
| Environmental | 1 |
| Peanut | 2 |
| Lactose/milk | 2 |
| Positive history of eczema or asthma | 6 (7%) |
| Eczema | 4 |
| Asthma | 2 |
| Skin Rash Morphology description | |
| Maculopapular | 22 (26.5%) |
| Urticaria-like | 29 (35%) |
| EM-like | 32 (38.5%) |
| Joint involvement | |
| Hands/Feet | 50 (60%) |
| Only feet | 7 (8.4%) |
| Only Hands | 2 (2.4%) |
| Elbows and/or knee | 3 (3.6%) |
| >3 Joint areas | 16 (19.2%) |
| No specified | 5 (6%) |
| Other clinical features | |
| Fever | 37 (44.5%) |
| Face/lip swelling | 26 (31%) |
| Pruritus | 28 (34%) |
| Malaise/irritability | 83 (100%) |
| Laboratory abnormalities (N=24) | |
| Leukocytosis | 5 (22%) |
| Thrombocytosis | 12 (50%) |
| Neutrophilia | 7 (31.8%) |
| Lymphopenia | 5 (22%) |
| CRP elevation | 12 (50%) |
| Penicillin RAST (N=17) | |
| Negative | 17 (100%) |
| LTA (N=34) | |
| Positive | 32 (94%) |
| Overall duration of skin rash (days) | |
| Mean | 7 |
| Median | 5 |
| Range | 2–45 |
| Treatment received | |
| Antihistamines/NSAIDs plus Oral Corticosteroids | 32 (38.5) |
| Antihistamines/NSAIDs only | 48 (57.8) |
| Other (IVIG) | 3 (3.6) |
Urticaria-like: Erythematous annular and edematous plaques with central clearing; Erythema multiforme-like: Erythematous annular lesions with central purplish discoloration; LTA Lymphocyte toxicity assay; N = Total number of patients with available information; NSAID Nonsteroidal anti-inflammatory drugs; RAST Radioallergosorbent test; SSLR Serum sickness-like reaction; TMP/SMX Trimetropin/Sulphametoxasol.
Laboratory data were available in 24 cases. The most common abnormalities included leukocytosis (20%), thrombocytosis (50%), and CRP elevation (50%). Only two patients had complement C3-C4 measured and both cases reported normal levels. A radioallergosorbent test (RAST) was performed in 17 patients, all with negative results. Results of LTA were available for 34 patients, of which 32 (94%) were reported as positive. Due to the potential risk of symptom recurrence, oral challenge tests were not done in any of the patients.
Treatment modalities included antihistamines with or without NSAIDs (57.8%), additional systemic corticosteroids (methylprednisolone or prednisone) (38.5%), and intravenous immunoglobulin in three patients (3.6%). The overall recovery time ranged from 1 to 45 days with a mean of 7.1 (SD 6). Overall, patients who received additional treatment with systemic corticosteroids showed a trend of shorter recovery time (mean 6 days, SD 2.8) when compared with those treated with NSAIDs/antihistamines alone (mean 8 days, SD 7.4). All patients recovered without complications or sequelae and there were no fatalities.
DISCUSSION
To our knowledge, this is the largest cohort of paediatric patients with a diagnosis of SSLR reported in the literature and the only one where all patients had both main clinical features, namely skin rash and joint inflammation. Previous studies have reported that SSLR secondary to cefaclor in children affects an estimated 0.4 to 0.5% of all antibiotic courses and represents 4% of all adverse drug reactions associated with amoxicillin (2,8). In our study, SSLR represented 15.4% of all patients with cutaneous adverse drug reaction referred to our clinic over the 10-year study period. In addition, SSLR represented 0.02% of all causes of consult and 0.9% of all sudden skin rashes seen in the paediatric emergency department of our institution between January 2014 and October 2018, for which such data were available. The mean age of presentation of our patients (3.2 years old) was similar to other reports (Supplementary Appendix 1), with children 4 years and younger being the most commonly affected. This could be explained by the fact that worldwide, the highest percentage of antibiotic prescriptions are for children younger than 5 years of age (16,17). However, we cannot rule out the possibility that immune mechanisms, abnormal drug metabolism, infectious agents, or other factors inherent to this age group have a role in the development of this condition.
The most common drugs associated with SSLR in our patients were amoxicillin (87%) followed by cephalosporins (8.5%) as might be expected since beta-lactam antibiotics have the highest rate of prescription among children (18). Other drugs included NSAIDs and anticonvulsants. Regarding SSLR causality, we were unable to rule out the potential role of infectious agents due to the lack of appropriate serological testing; however, none of the cases developed symptoms without a history of drug exposure. Although SSLR has been previously associated with vaccines and some infectious agents as potential triggers, there are no publications that accurately assess the role of these agents in the etiology of this condition in children (19), although several case reports and case series in adults have suggested an association between H1N1 influenza vaccination and SSLR. In our study, there were no cases of SSLR associated with vaccines.
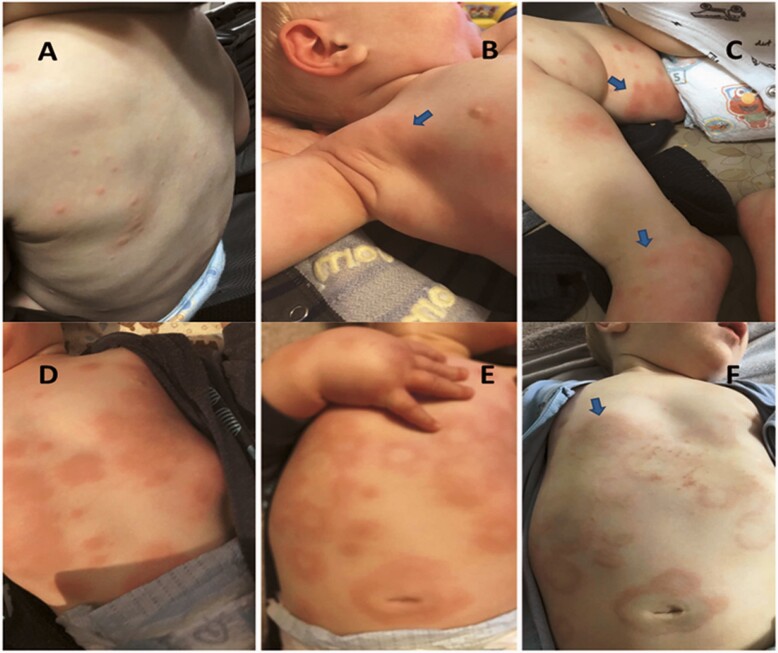
The diagnosis of SSLR is made clinically even though there are no validated diagnostic criteria. The main cutaneous manifestations of SSLR include skin rash and joint inflammation with or without fever. In our study, urticaria and EM-like were the most common rash descriptions, which is consistent with other series (20). However, unlike previous reports, we do consider that true EM or urticaria are not part of the clinical manifestations of SSLR (6,21) but instead are completely different conditions, as many other authors also believe (Table 2). Based on our experience, we have noticed that skin rash in patients with SSLR evolves during different stages of the disease. Initially, skin lesions start on the trunk as inflammatory papules, described often by parents as ‘mosquito bites’. These lesions rapidly spread centrifugally, flatten and enlarge, forming annular lesions with central discoloration that simulate a ‘target’ lesion or converge in a polycyclic arrangement, similar to urticaria or urticaria multiforme but unlike the latter, SSLR lesions remain in the same location (fixed) for several days (Figure 1). Pruritus does not present in all patients and when it does it is mostly mild with poor response to antihistamines. Many patients rather complain of a ‘burning’ sensation instead. Joint inflammation is another important feature of SSLR and is usually characterized by symmetric joint involvement manifested by difficulty walking and severe pain. In our study, 60% of children developed joint inflammation in both hands and feet, while the rest were affected in only one or more than three joints bilaterally. Similar findings have been reported by other authors (22,23). The true nature of joint inflammation in patients with SSLR remains very controversial as many consider that the pain and increment of volume of hands and feet are only secondary to skin edema; however, the clinical features that these patients present are consistent with true inflammation (i.e., erythema, edema, pain, increment of temperature, and decrease of function) affecting different joints. Whether the inflammation is peri/intra-articular or from the overlying tissue is unclear and there are no studies that have addressed this question yet.
Table 2.
| Rash | Facial edema/ itchiness | Fever | Joint involvement | Mucous membrane involvement/ ulceration | Other systemic features | |
|---|---|---|---|---|---|---|
| SSLR | Fixed erythematous annular/polycyclic skin lesions with central clearing and or purplish discoloration (lesions resolve 5–10 days) | Occasional | Common |
Always Edema, redness, severe joint pain and inability to walk |
No | Malaise and irritability. Poor response to antihistamines |
| Urticaria | Papular, pruritic patches (hives) Migratory (hives resolve < 24 h) |
Common | Seldom (related to underlying viral illness) | No | No | Mild malaise associated with underlying viral illness. Improves with antihistamines |
| Erythema Multiforme | Symmetric, involving palms, soles, face, oral mucosa. Typical target lesions. Non-migratory. (lesions resolve after several days and may leave postinflammatory hyperpigmentation) |
Uncommon | Occasional | Uncommon |
Yes | Malaise +/- Cough, respiratory symptoms |
| Urticaria Multiforme | Transient onset of annular and polycyclic lesions with dusky/purplish center (lesions resolve 24–48 h) | Common | Occasional low grade | Acral edema without joint inflammation | Oral edema but no erosions or blisters | Minimal systemic affection Improves with antihistamines |
Figure 1.
(A) Initial SSLR lesions (inflammatory papules). (B, C, F) Erythematous/annular lesions with purplish central discoloration simulating EM’s target lesions (blue arrows). (E) Severe joint inflammation accompanied by overlaying erythema. (D, E, F) Erythematous lesions that converge in plaques and later evolve in annular lesions with central clearing. Notice how skin lesions remain in the same location for several days unlike true urticaria.
Although fever is also considered a feature of SSLR, it is not always present and its presence in the literature ranges from 33% to 100%. In our study, it was reported in 47% of all children. Other symptoms reported were malaise and irritability, and there was no evidence of systemic involvement (12). Until now, there are no laboratory hallmarks that confirm the diagnosis of SSLR, but in some cases, it helps to rule out other conditions. SSLR patients usually present with elevated inflammatory markers. Shiari et al. reported a series of cases of 29 children with SSLR, 46% of which showed leukocytosis, 76% had high erythrocyte sedimentation rate (ESR), and 20 of 24 had low levels of Complement (C3, C4, and CH50) (6). However, in other series, levels of complement have been reported as normal or slightly elevated. In our cohort, laboratory studies were not always performed but the most common abnormalities included leukocytosis, thrombocytosis, and CRP elevation. RASTs performed in 17 of our patients were reported as negative, which supports the theory that SSRL is not IgE-mediated but rather is the result of a T-cell hypersensitivity reaction (23). To asses drug causality, some authors consider that an oral provocation test is a safe diagnostic tool to evaluate SSLR patients (24). However, recurrence of symptoms could present which could be upsetting and uncomfortable especially for young children. Because of this reason in our clinic we routinely use LTA instead. Our laboratory has extensive experience using this test to evaluate other T-cell-mediated drug hypersensitivity reactions, such as Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis and Drug Hypersensitivity Syndrome (13). Although LTA is not commercially available and has not been validated yet for the diagnosis of SSLR specifically, this test has been proven to have a clinical sensitivity between 80% and 90% to identify patients at risk of a drug hypersensitivity reaction. These tests can be used as an aid in the diagnosis of suspected hypersensitivity reactions to a wide variety of commonly used drugs including sulfonamides, aromatic anticonvulsants, antibiotics, and NSAIDs. The usefulness of LTA in SSLR patients is based on the observations from previous studies that have found that T-cells from affected patients show a higher degree of cellular toxicity when exposed to the drug in question compared to T-cells of healthy controls. Additionally, LTA could also be of help in mechanistic studies of SSLR (14,15). In this study, 34 LTA were done, of which 32 (94%) had a marked decrease in cell viability observed when exposed to different concentrations of the suspected drug.
In our study, no skin biopsy was performed. Usually, due to its invasive nature, skin biopsy is rarely requested but it could be useful when ruling out other inflammatory conditions. Histopathology is characterized by perivascular and mid-dermal inflammatory infiltrate with admixed neutrophils, eosinophils, and lymphocytes, usually without leukocytoclastic vasculitis (12,25).
Regarding personal history, the data obtained did not show any relevant association with SSLR; however, it was interesting to observe that 25% of all cases had at least one family member with a history of previous reactions associated with the same trigger drug. Unfortunately, detailed information about those reactions was not available but this observation certainly warrants further study.
Currently, there are no guidelines for the management of SSLR in children and the use of antihistamines, NSAIDs, and oral corticosteroids remains controversial. In our study, 99% of the children received at least one dose of oral antihistamines and/or NSAIDs; 58% of them received these as only treatments, while 38% had additional treatment with oral corticosteroids. Three patients were treated with IVIG as they were initially diagnosed as Kawasaki disease. The mean time to symptom resolution was shorter in children who received additional oral steroids compared to children that only received antihistamines/NSAIDs, although the difference was not statistically significant (6 versus 8 days; P=0.09). However, this finding should be taken with caution given the limitations of our work and further studies are certainly required. Nevertheless, it is reasonable to assume that an inflammatory condition might respond better to a potent anti-inflammatory drug than to antihistamines or NSAIDs alone.
Even with the limitations of its retrospective design, we believe that the information collected in this large cohort of patients is valuable. Further prospective, collaborative, and well conducted studies are needed to gain a better understanding of the pathophysiology of this condition, develop accurate diagnostic criteria, and assess safer and more effective treatment options.
Author Contributions: BRDP-M conceptualized and designed the study, drafted the initial manuscript, designed the data collection instruments, collected data, and revised the manuscript. AA was involved in the acquisition, analysis, and interpretation of data. BM was involved in the acquisition, analysis, and interpretation of data. AL-L contributed to the concept and design of the study, carried out the statistical analyses, and added valuable intellectual content to the manuscript. MR conceptualized and designed the study, coordinated, and supervised data collection including in vitro toxicity data, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supplementary Material
Funding: No external funding for this manuscript.
Potential Conflicts of Interest: All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. De Schryver S NE, Ben-Shoshan M. Severe serum sickness-like reaction: Challenges in diagnosis and management. J C Exp Dermatol Res 2015;6(3):1000279. [Google Scholar]
- 2. Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: Three case reports. Ann Allergy Asthma Immunol 2001;86(3):330–4. [DOI] [PubMed] [Google Scholar]
- 3. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: The role of cefaclor in serum sickness-like reactions. J Paediatr Child Health 2003;39(9):677–81. [DOI] [PubMed] [Google Scholar]
- 4. Yerushalmi J, Zvulunov A, Halevy S. Serum sickness-like reactions. Cutis 2002;69(5):395–7. [PubMed] [Google Scholar]
- 5. Yorulmaz A, Akın F, Sert A, Ağır MA, Yılmaz R, Arslan Ş. Demographic and clinical characteristics of patients with serum sickness-like reaction. Clin Rheumatol 2018;37(5):1389–94. [DOI] [PubMed] [Google Scholar]
- 6. Shiari R, Eshgh FA, Rowshanzamir E, Derakhshanfar H. Clinical and laboratory profile of serum sickness-like reaction in children. Indian J Rheumatol. 2011;6(4):173–7. [Google Scholar]
- 7. Slama TG. Serum sickness-like illness associated with ciprofloxacin. Antimicrob Agents Chemother 1990;34(5):904–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Isaacs D. Serum sickness-like reaction to cefaclor. J Paediatr Child Health 2001;37(3):298–9. [DOI] [PubMed] [Google Scholar]
- 9. Colton RL, Amir J, Mimouni M, Zeharia A. Serum sickness-like reaction associated with griseofulvin. Ann Pharmacother 2004;38(4):609–11. [DOI] [PubMed] [Google Scholar]
- 10. VanCleave HZ, Sanchez AC, Lieberman JA, Ellenburg JT, Mabry WA. Probable metronidazole induced serum sickness-like reaction in a paediatric patient. J Clin Pharm Ther 2016;41(6):736–8. [DOI] [PubMed] [Google Scholar]
- 11. Waibel KH, Katial RK. Serum sickness-like reaction and bupropion. J Am Acad Child Adolesc Psychiatry 2004;43(5):509. [DOI] [PubMed] [Google Scholar]
- 12. Mathur AN, Mathes EF. Urticaria mimickers in children. Dermatol Ther 2013;26(6):467–75. [DOI] [PubMed] [Google Scholar]
- 13. Elzagallaai AA, Jahedmotlagh Z, Del Pozzo-Magaña BR, et al. Predictive value of the lymphocyte toxicity assay in the diagnosis of drug hypersensitivity syndrome. Mol Diagn Ther 2010;14(5):317–22. [DOI] [PubMed] [Google Scholar]
- 14. Elzagallaai AA, Rieder MJ. In vitro testing for diagnosis of idiosyncratic adverse drug reactions: Implications for pathophysiology. Br J Clin Pharmacol 2015;80(4):889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elzagallaai AA, Loubani E, Del Pozzo-Magaña BR, Rieder MJ. Pathogenesis of Beta-lactam-induced serum sickness-like reaction: The potential role of reactive drug metabolites. Canadian Society of Pharmacology and Therapeutics Conference; 2020 June 10-12, 2020; Virtual. [Google Scholar]
- 16. Bergene EH, Nordeng H, Rø TB, Steinsbekk A. Requests for new oral antibiotic prescriptions in children within 2 days: A Norwegian population-based study. Fam Pract 2018;35(6):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aabenhus R, Hansen MP, Siersma V, Bjerrum L. Clinical indications for antibiotic use in Danish general practice: Results from a nationwide electronic prescription database. Scand J Prim Health Care 2017;35(2):162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaz LE, Kleinman KP, Raebel MA, et al. Recent trends in outpatient antibiotic use in children. Pediatrics 2014;133(3):375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milstien JB, Gross TP, Kuritsky JN. Adverse reactions reported following receipt of Haemophilus influenzae type b vaccine: An analysis after 1 year of marketing. Pediatrics 1987;80(2):270–4. [PubMed] [Google Scholar]
- 20. Patel S, Mancini AJ. Serum sickness-like reaction in children: A retrospective review. J Am Acad Dermatol 2009;60(3):AB6. [Google Scholar]
- 21. Heckbert SR, Stryker WS, Coltin KL, Manson JE, Platt R. Serum sickness in children after antibiotic exposure: Estimates of occurrence and morbidity in a health maintenance organization population. Am J Epidemiol 1990;132(2):336–42. [DOI] [PubMed] [Google Scholar]
- 22. Kunnamo I, Kallio P, Pelkonen P, Viander M. Serum-sickness-like disease is a common cause of acute arthritis in children. Acta Paediatr Scand 1986;75(6):964–9. [DOI] [PubMed] [Google Scholar]
- 23. Kearns GL, Wheeler JG, Rieder MJ, Reid J. Serum sickness-like reaction to cefaclor: Lack of in vitro cross-reactivity with loracarbef. Clin Pharmacol Ther 1998;63(6):686–93. [DOI] [PubMed] [Google Scholar]
- 24. Mill C, Primeau MN, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in Children. JAMA Pediatr 2016;170(6):e160033. [DOI] [PubMed] [Google Scholar]
- 25. Tolpinrud WL, Bunick CG, King BA. Serum sickness-like reaction: Histopathology and case report. J Am Acad Dermatol 2011;65(3):e83–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.