Summary
The preservation of mammalian freeze-dried (FD) spermatozoa is commonly performed using small glass ampules; however, they are bulky and breakable. In this study, we present a protocol to prepare and preserve mouse FD sperm using thin plastic sheets. This approach allows storing thousands of mouse strains in a card folder. We can also send the FD sperm domestically using a postcard without any extra equipment.
For complete details on the use and execution of this protocol, please refer to Ito et al. (2021).
Subject areas: Biotechnology and bioengineering, Developmental biology, Model Organisms
Graphical abstract
Highlights
-
•
Protocol to preserve mouse FD sperm in plastic sheets instead of glass ampules
-
•
FD sperm can be preserved for at least three months at −30°C
-
•
Viable mouse sperm can be transported via postcards without any extra protection
-
•
Several mouse strains can be preserved in a single card folder
The preservation of mammalian freeze-dried (FD) spermatozoa is commonly performed using small glass ampules; however, they are bulky and breakable. In this study, we present a protocol to prepare and preserve mouse FD sperm using thin plastic sheets. This approach allows storing thousands of mouse strains in a card folder. We can also send the FD sperm domestically using a postcard without any extra equipment.
Before you begin
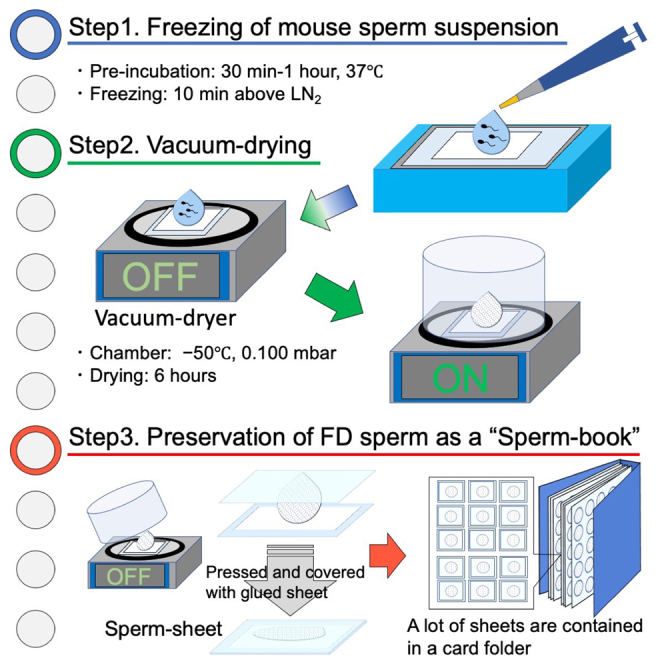
We need mammalian sperm preservation technology in many fields, for example, maintenance of a huge mouse strains that increase every day. Freeze-drying technique is one of the methods to preserve the mammalian sperm (Wakayama and Yanagimachi, 1998) and do not use liquid nitrogen (LN2) or ultra-deep freezer during preservation (Kamada et al., 2018; Wakayama et al., 2021). However, the conventional method using ampoules was not suitable for storing or transporting because the ampoules were made with glass and they were breakable, expensive, and bulky.
This is a workflow to prepare and store mouse freeze-dried (FD) sperm in thin plastic sheets instead of glass ampoules.
As demonstration, in this protocol, Institute of cancer Research (ICR) male mice (10–12 weeks of age), and C57BL/6NCrSlc × C3H/HeSlc (B6C3F1) male mice (8–10 weeks of age) were obtained from SLC Inc. (Hamamatsu, Japan). All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Committee of Laboratory Animal Experimentation of the University of Yamanashi, which is followed in the ARRIVE guideline everything.
The rough estimates of the time required for the entire process are listed below.
Preparation of weighing paper and plastic sheet
Cut the weighing paper and plastic sheet in appropriate sizes (please see below).
Preparation of FD sperm in plastic sheets
Collect sperm from mature mice (after 3 months of age). The freezing takes only 10 min, but the vacuum-drying process lasts for 6 h. The FD sperm placed on the weighing paper are then sealed in glued plastic sheets.
Preservation
FD sperm of many different mouse strains can be preserved in a card folder for more than three months at −30°C.
Transport
Attach the sheet containing FD sperm on the postcard in the same way you would put a stamp on it.
Rehydration of FD sperm to prepare sperm injections for oocytes
FD sperm pellet are picked up from inside the sheet and added into 50 μL of distilled water at ambient temperature.
Intracytoplasmic sperm injection (ICSI) and embryo transfer (ET)
ICSI and ET techniques are well established and require skilled execution. Please refer to other sources for their protocols.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| (Below for HTF medium) | ||
| Albumine, Bovine Serum, Fraction V, Crystalline | Millipore Corp. | 12657-5GM |
| CaCl2•2H2O | Nacalai Tesque, Inc. | 06731-05 |
| D (+) -Glucose | Wako Ltd | 049-31165 |
| Hypotaurine | Sigma-Aldrich | H1384-1G |
| KCl | Wako Ltd | 163-03545 |
| KH2PO4 | Wako Ltd | 169-04245 |
| MgSO4•7H2O | Nacalai Tesque, Inc. | 21003-75 |
| NaCl | Wako Ltd | 191-01665 |
| NaHCO3 | Wako Ltd | 191-01305 |
| Sodium DL-lactate | Sigma-Aldrich | L7900-100ML |
| Penicillin G Potassium salt | Millipore Corp. | 194536 |
| Phenol red sodium salt | Sigma-Aldrich | P4758-5G |
| Pyruvic acid sodium salt | Wako Ltd | 194734 |
| Experimental models: Organisms/strains | ||
| B6C3F1 male mice, 10–12 weeks of age | SLC Inc. | B6C3F1/Slc |
| ICR male mice, 10–12 weeks of age | SLC Inc. | Slc:ICR |
| Other | ||
| Aluminum foil | MITSUBISHI ALUMINUM CO., LTD. | 4902109301032 |
| Liquid nitrogen | CHIYODA CORPORATION | N/A |
| Plastic sheet | ACCO Brands Japan K. K. | SLMBCZ |
| Styrofarm box | No specification | N/A |
| Vacuum dryer | Labconco | FreeZone2.5® |
| Weighing paper | Sogo Laboratory Glass Works co., Ltd | 4084-03 |
Materials and equipment
The weighing paper was purchased from SOGO Laboratories (Glass works co., Ltd.). Plastic sheets were purchased from Acco brands Japan K.K.. This protocol uses FreeZone® 2.5 L Benchtop Freeze Dryer (Labconco, MO, USA) for vacuum drying. The quality of liquid nitrogen is that can be used for sample storage or medical. We used the Styrofarm box, which size was (w) 275 × (d) 195 × (h) 210 mm, respectively. Ordinary Styrofoam can be used as an alternative according to the preferred size of the experimenter. HTF medium was also used for sperm suspension and it was compounded in our laboratory. These materials and equipment are listed on Key resources table. Make sure the required materials and equipment are ready to use.
Step-by-step method details
Preparing the weighing paper and plastic sheet
Timing: 10 min
-
1.
Turn on the manual mode of refrigeration of the vacuum dryer and set it at a low temperature.
-
2.Prepare the weighing paper and thin plastic sheet.
-
a.Make an aluminum foil boat that can float in the Styrofoam box and act as the vehicle for the weighing paper and plastic sheet (Figure 1A).
- b.
-
c.Float the aluminum foil boat on LN2 poured into the Styrofoam and place a plastic sheet in it. Finally, place the weighing paper on the plastic sheet (Figures 1D and 1E).
-
a.
Note: Pour LN2 into the Styrofoam right before freeze-drying to conserve LN2.
Note: In areas with high humidity, we recommend using a lidded Styrofoam as an LN2 container.
Note: Avoid adding too much LN2, as it might cause improper freezing of the sperm drops, which will later melt prematurely.
CRITICAL: LN2 must be handled with great care, as there are the risks of suffocation caused by saturation of nitrogen gas in the room and cold burns. It is recommended to wear specialized gloves and glasses when pouring LN2 into Styrofoam. Please ventilate and avoid closed spaces.
Figure 1.
Preparation of the weighing paper and plastic sheet
(A) Aluminum foil boat.
(B and C) (B) Weighing paper and (C) plastic sheet are cut into appropriate sizes.
(D) Cut weighing paper and plastic sheet are put in the aluminum boat over liquid nitrogen (LN2).
(E) Setup: (a) Weighing paper, (b) Plastic sheet, (c) Aluminum boat, (d) LN2.
Preparing the sperm
Timing: 30 min–1 h
-
3.Sperm collection
-
a.Euthanize mature mice (> three months old) by cervical dislocation and collect the epididymis (Figures 2A and 2B).
-
b.Remove the fat and blood and cut the cauda epididymides using scissors (Figure 2C). Push out the condensed sperm mass with your fingers (Figure 2D) and collect and deposit it into 200–500 μL of HTF medium (Quinn et al., 1995) using tweezers (Figures 2D and 2E). Pre-incubate the sperm mass for 30 min to 1 h in the incubator at 37°C and 5% CO2.
-
a.
Note: The volume of the HTF medium can be adjusted according to the amount of collected sperm mass.
Optional: Count the number of sperm and adjust the concentration of the sperm suspension (>1 × 106 sperm/ mL) by adding/removing HTF medium.
Note: Before freeze-drying the sperm, it is better to check their quality and motility using inverted microscope. However, the sperm concentration is inconsequential as this method collects enough sperm for ICSI.
Note: If the collected sperm are too poor to use in experiments (<1 × 106 sperm/ mL), please centrifuge the sperm suspension and reduce the HTF volume to increase the sperm concentration.
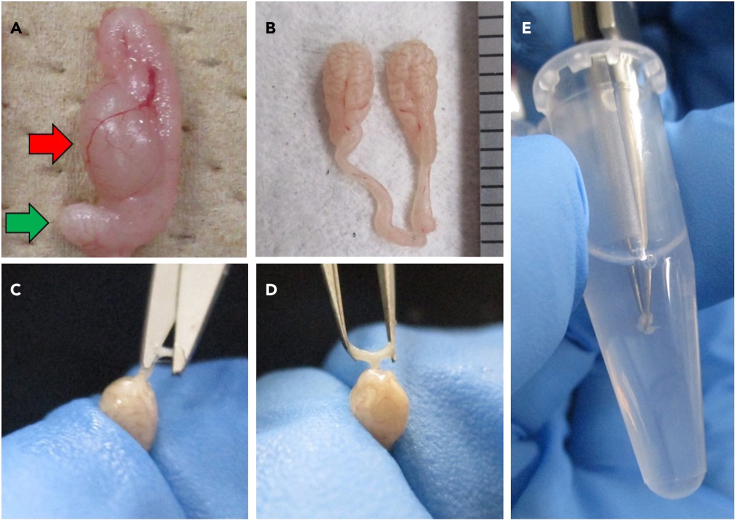
Figure 2.
Sperm collection from male mouse and pre-incubation
(A) Reproductive organs of a male mouse. To collect condensed sperm mass, the cauda epididymides are separated from them. The red arrow indicates the testis and the green arrow indicates the cauda epididymis.
(B) Collected cauda epididymis. Scale bar = 1 mm.
(C) A small incision is made with a pair of fine scissors in the cauda epididymis after removing the adipose tissue and blood.
(D and E) (D) Sperm mass is pushed out with fingers, picked up with tweezers, and (E) transferred into the HTF medium.
Freeze-drying the sperm
Timing: 7 h
-
4.Freezing the sperm
-
a.Drop 50 μL of pre-incubated sperm suspension onto the weighing paper floating in LN2 using a pipette (Figure 3).
-
b.Leave the sperm suspension to freeze inside the Styrofoam for 10 min.
-
a.
Note: Before dropping the sperm suspension, ensure that the weighing paper is cold enough to freeze the suspension.
Note: If the lid of Styrofoam containing LN2 are opened for long time, the moisture in the air cools and condenses on weighing paper and plastic sheet but the inside of Styrofoam gets warm and the sperm drop does not freeze well, which disturb your operation. Therefore, it is recommended not to open the lid of Styrofoam for more than a minute.
Note: Drop the sperm suspension on the weighing paper quickly; otherwise, it will freeze inside the pipet tip.
-
5.Drying the frozen sperm
- a.
-
b.Cover the boat with vacuum-proof lids (Figure 4C).
- c.
- d.
CRITICAL: Transfer the frozen sperm drops into the FreeZone® quickly to keep them from melting. Check the position and height of the lid, the contents inside the vacuum dryer, and vacuum dry the sheets within a few seconds.
-
6.Sealing the FD sperm
-
a.After drying is complete, set the vacuum dryer to ambient pressure by removing the rubber stopper.
-
b.Take out the plastic sheet with the weighing paper and FD sperm from the dryer (Figure 5A).
-
c.Cover each pellet of FD sperm with another weighing paper (cut in 15-mm squares) using tweezers (Figure 5B).
-
d.Cover the FD sperm with a glued plastic sheet and press the sheet using fingers (Figures 5C and 5D).
-
a.
Note: Be careful while sticking the plastic sheets together as the weighing paper and the FD sperm might move due to static electricity.
Note: Remove as much air as possible while sealing the plastic sheets (Figure 5C).
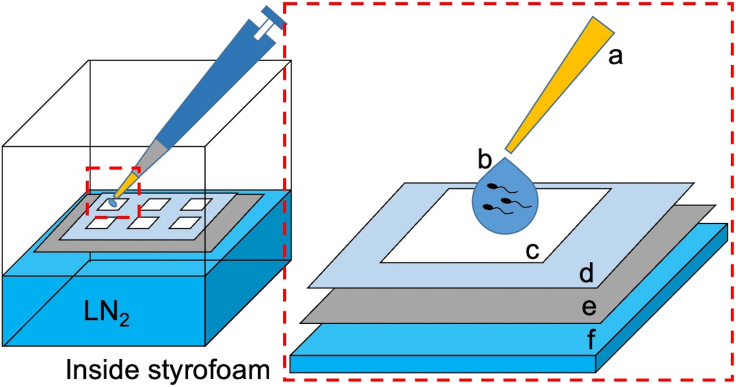
Figure 3.
Freezing process of the sperm suspension
After pre-incubation for 30 min to 1 h in the incubator at 37°C and 5% CO2, sperm suspension in HTF medium are dropped on the weighing paper floating on LN2 and incubated for 10 min. (a) 200 μL yellow pipette tip. (b) 50 μL aliquot of the sperm suspension. (c) Weighing paper (cut into 15-mm squares). (d) Plastic sheet (Cut into 100-mm squares). (e) Aluminum boat. (f) LN2.
Figure 4.
Drying process using vacuum dryer
(A) FreeZone® (Labconco).
(B) The weighing paper and plastic sheet in the aluminum boat are transferred the frozen sperm drop into the vacuum dryer.
(C) A vacuum-proof lid covers the top of the dryer.
(D–F) (D) During vacuum drying process of the frozen sperm drops for 6 h, the display and lamp are maintained as shown. The pressure is maintained at 0.100 mbar, and the temperature is maintained at −50°C. The sperm drop is shiny after freezing (E) and looks powder-like after drying (F).
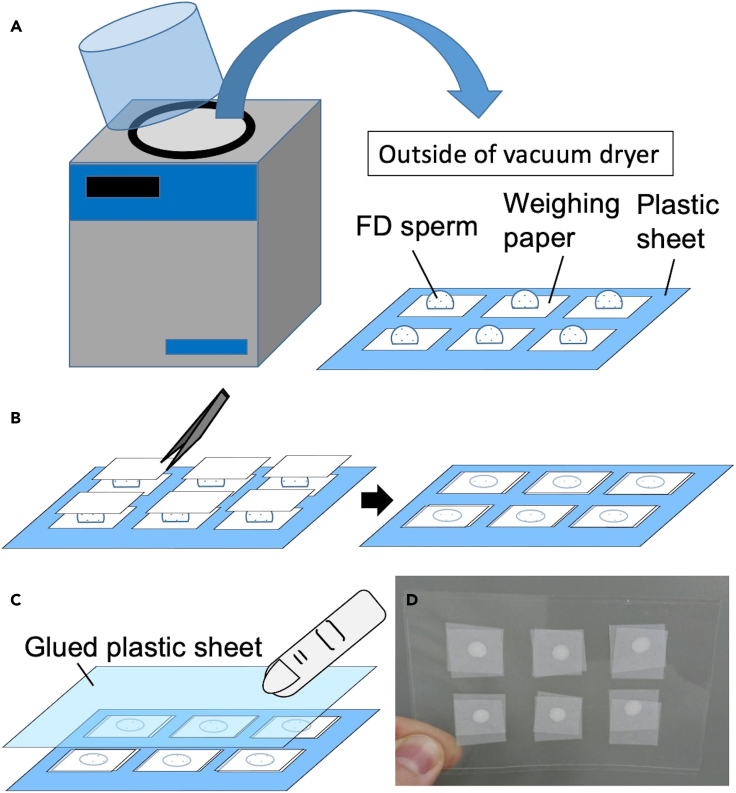
Figure 5.
Inserting FD sperm between the plastic sheets
(A) FD sperm placed on the plastic sheet are removed from the vacuum dryer at ambient pressure.
(B) Another weighing paper (15-mm squares) is put on each FD sperm drop and pressed with tweezers.
(C) Sealing the glued sheet with fingers to release excess air.
(D) FD sperm are stored between the plastic sheets.
Preserving the FD sperm
Timing: At least3months in a freezer
Note: Files with multiple pages are useful for storing several samples (Figures 6A and 6B). We call this the Sperm Book.
CRITICAL: FD sperm can be stored at 15°C–25°C only for three days.
Figure 6.
Storing and transporting of FD sperm preserved in plastic sheets
(A) Sperm book.
(B) A card file can compactly store several sheets containing FD sperm.
(C–E) (C) FD sperm in plastic sheets can be stored for three months at −30°C. Before mailing, the sheets storing FD sperm are (D) put in an envelope or (E) attached to a postcard.
Domestically mailing the FD sperm
Timing: 1–2 days
-
8.
Store the plastic sheet containing FD sperm at −30°C until mailing.
-
9.
Place the sheets in an envelope (Figure 6D) or tightly attach them to a postcard using sticky tape to prevent them from peeling off (Figure 6E).
-
10.
Remove the sheets from the freezer on the day of mailing and post the envelope or postcards containing the sheets.
-
11.
For domestic mail, the sheets will be delivered within a few days. Once received, store the sheets again at −30°C until further use.
Note: To protect the sheets from light and scratches, wrap them in aluminum foil (Figure 6E).
Note: In the current study, the sheet preservation at 15°C–25°C is limited to three days; thus, the transport should not take a longer time.
Collecting and rehydrating the FD sperm preserved in plastic sheets
Timing: 3 min
-
12.
Separate the plastic sheets preserving FD sperm by cutting them with scissors (Figure 7A).
-
13.
Cut the sheet (Figure 7B) and peel off the weighing paper to expose the FD sperm (Figure 7C).
-
14.
Pick the FD sperm pellets with tweezers and transfer them into 1.5 mL tubes (Figure 7D). Add 50 μL of sterile distilled water and mix it with a pipette.
Note: FD sperm can be scraped with tweezers if they are attached to the weighing paper.
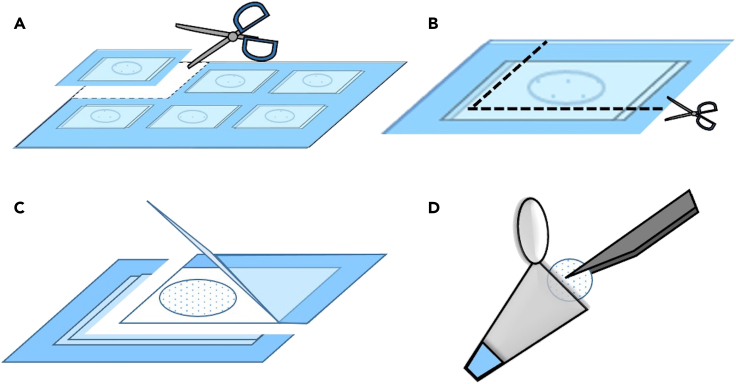
Figure 7.
Collection and rehydration of FD sperm
(A) Sheets are taken out from the freezer and cut into squares.
(B) The plastic sheet and weighing paper are cut along the dotted lines.
(C) Sheets are peeled off to expose the FD sperm.
(D) FD sperm are transferred to 50 μL of distilled water with tweezers and rehydrated.
Expected outcomes
The DNA integrity of FD sperm preserved using this protocol is comparable to that of the conventional method using a glass ampoule. The FD sperm sealed in the plastic sheets remain the developmental ability after three months preservation at −30°C. FD sperm transported using a postcard domestically within 3 days can also be used for ICSI. The offspring rate was not compromised after mailing, and healthy pups were obtained after performing ET at 2-cell stage. These results have been published in iScience (Ito et al., 2021).
Limitations
Even though FD sperm stored in plastic sheets at −30°C remain viable for more than three months, the storage at 15°C–25°C was limited to three days.
ICSI and ET into recipient female are required to generate offspring from FD sperm but they are very common method. The outlines of the ICSI and ET are listed below.
Before ICSI, 1–2 μL of the rehydrated sperm suspension was directly added into the drop of polyvinylpyrrolidone in the microinjection chamber immediately for microinjection. Apply piezo pulses to separate the sperm head from the tail and then inject the head into the oocyte. The next day of ICSI, ET was performed at the 2-cell stage to 0.5-day pseudo-pregnant ICR female mice mated with a vasectomized male the night before the transfer. The offspring can be delivered naturally or by cesarean section on 18.5 days after gestation and allowed to mature (Ito et al., 2019).
Note: Refer to Behringer et al. (2014) for the detailed protocol for ICSI and ET (Behringer et al., 2014).
Troubleshooting
Problem 1
The sperm suspension spread on the weighing paper when you start freezing it and do not freeze properly (step 4a).
Potential solution
It is recommended that the surface of LN2 should be shallow in the Styrofoam; otherwise, the sperm suspension is not frozen well due to the outer air.
Problem 2
Formation of frost around the frozen sperm drops (steps 4b and 5).
Potential solution
The frost will sublimate, leaving the FD sperm and thus will not create any problems.
Problem 3
FD sperm fall off from the weighing paper during vacuum drying (steps 5c and 5d).
Potential solution
Place a tube stand under the aluminum sheet to decrease the impact of the vibration during drying.
Problem 4
The FD sperm dislocate due to wind pressure when removed from the vacuum dryer (step 6b).
Potential solution
Place another weighing paper on the FD sperm and press it lightly with tweezers before removing the sheets slowly.
Problem 5
The FD sperm are too sticky to inject into oocytes after rehydration, or the zygotes derived from FD sperm do not develop into pronuclei or 2-cell stages after ICSI.
Potential solution
This could happen if the sheets are not sealed well and excess air remains between them, or the FD sperm are moist before they are placed in the plastic sheets as they are not sealed in plastic sheets immediately after drying. Please remove excess air while sealing the FD sperm (step 6d) or shorten the time between the end of vacuum drying process and the placing of the sperm between the sheets (step 6).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Daiyu Ito (g20dib01@yamanashi.ac.jp).
Materials availability
All materials and reagents used in this protocol will be made available upon request.
Acknowledgments
We thank Drs. S. Kishigami, S. Wakayama, M. Ooga, Y. Fujimoto, S. Funaya, Mr. M. Nakamura, Miss C. Yamaguchi, R. Emura, Li Yang, and Mr. Natsuki Ushigome for assistance in preparing this manuscript. This work was partially funded by the Naito Foundation to S.W.; Asada Science Foundation, Canon Foundation (M20-0006), and the Takeda Science Foundation to T.W.; and Research Fellowships of Japan Society for the Promotion of Science for Young Scientists to D.I. (JSPS KAKENHI grant number JP20J23364). The authors would like to thank Editage for the English language review.
Author contributions
D.I. and T.W. obtained funding and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Daiyu Ito, Email: g20dib01@yamanashi.ac.jp.
Teruhiko Wakayama, Email: twakayama@yamanashi.ac.jp.
Data and code availability
Original data for expected outcomes of this study are available from Ito et al. (2021).
References
- Behringer R., Gertsenstein M., Nagy K.V.a.A.N. 4th Edition. Cold Spring Harbor Laboratory Press; 2014. Manipulating the Mouse Embryo: A Laboratory Manual. [Google Scholar]
- Ito D., Wakayama S., Kamada Y., Shibasaki I., Kamimura S., Ooga M., Wakayama T. Effect of trehalose on the preservation of freeze-dried mice spermatozoa at room temperature. J. Reprod. Dev. 2019;65:353–359. doi: 10.1262/jrd.2019-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D., Wakayama S., Emura R., Ooga M., Wakayama T. Mailing viable mouse freeze-dried spermatozoa on postcards. iScience. 2021;24:102815. doi: 10.1016/j.isci.2021.102815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Wakayama S., Shibasaki I., Ito D., Kamimura S., Ooga M., Wakayama T. Assessing the tolerance to room temperature and viability of freeze-dried mice spermatozoa over long-term storage at room temperature under vacuum. Sci. Rep. 2018;8:10602. doi: 10.1038/s41598-018-28896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P., Moinipanah R., Steinberg J.M., Weathersbee P.S. Successful human in vitro fertilization using a modified human tubal fluid medium lacking glucose and phosphate ions. Fertil. Steril. 1995;63:922–924. doi: 10.1016/s0015-0282(16)57504-9. [DOI] [PubMed] [Google Scholar]
- Wakayama T., Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat. Biotechnol. 1998;16:639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- Wakayama S., Ito D., Kamada Y., Shimazu T., Suzuki T., Nagamatsu A., Araki R., Ishikawa T., Kamimura S., Hirose N., et al. Evaluating the long-term effect of space radiation on the reproductive normality of mammalian sperm preserved on the International Space Station. Sci. Adv. 2021;7:eabg5554. doi: 10.1126/sciadv.abg5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data for expected outcomes of this study are available from Ito et al. (2021).