Summary
The degeneration of retinal ganglion cells (RGCs) leads to irreversible vision loss in a variety of pathological states. Here, we describe a protocol to evaluate the role of a gene in protecting mouse RGCs when they sustain injuries from excitotoxicity or axonal damage. This protocol includes the procedures for gene transfer through AAV intravitreal injection, induction of RGC injuries by NMDA-induced excitotoxicity or optic nerve crush, and retina immunohistochemistry to assess RGC survival.
For complete details on the use and execution of this protocol, please refer to Guo et al. (2021).
Subject areas: Cell Biology, Cell-based Assays, Microscopy, Model Organisms, Gene Expression, Neuroscience
Graphical abstract

Highlights
-
•
AAV intravitreal injection for gene transfer into retinal ganglion cells (RGCs)
-
•
NMDA-induced excitotoxicity or optic nerve crush to induce RGC death
-
•
Retinal whole-mount immunohistochemistry for RGC labeling
-
•
Confocal microscopy and quantification of RGC survival
The degeneration of retinal ganglion cells (RGCs) leads to irreversible vision loss in a variety of pathological states. Here, we describe a protocol to evaluate the role of a gene in protecting mouse RGCs when they sustain injuries from excitotoxicity or axonal damage. This protocol includes the procedures for gene transfer through AAV intravitreal injection, induction of RGC injuries by NMDA-induced excitotoxicity or optic nerve crush, and retina immunohistochemistry to assess RGC survival.
Before you begin
Experimental design considerations
Retinal ganglion cells (RGCs), the projection neurons of the retina that transmit visual information to the brain, are prone to degeneration in a variety of diseases and conditions, leading to irreversible visual impairment and blindness (Levin and Gordon, 2002). To develop effective neuroprotective approaches to treat retinal degenerative diseases typically characterized by RGC loss, extensive basic research is required to identify genes that can protect RGCs from diverse insults in laboratory animals.
Here, we describe a protocol to evaluate the role of a gene in protecting mouse RGCs from injuries, including our methods to manipulate genes via AAV (adeno-associated virus)-mediated gene transfer in RGCs, to challenge RGCs with injuries, and to examine the effect of gene manipulation on RGC survival. To manipulate genes in RGCs, we detail the procedure for intravitreal injection of AAVs to deliver genes in RGCs. While we give an example vector to overexpress genes in RGCs in this protocol, intravitreal injection of AAVs could achieve various purposes to manipulate a gene when combined with molecular and genetic tools such as site-directed mutagenesis (Guo et al., 2016), conditional knockout or knockin mouse models (Park et al., 2008), and CRISPR-Cas9 system (Wang et al., 2020a). To injure RGCs, we detail the procedure of two inducible models of RGC degeneration: excitotoxicity and optic nerve crush. To examine the effect of gene manipulation on RGC survival, we detail the procedure of Tuj1 immunohistochemistry for retinal whole-mounts as well as how to image and quantify RGCs.
Our primary goal in this protocol is to provide technical details of eye-related methods for prospective users to investigate the role of a gene in protecting mouse RGCs from two well-defined injuries. All studies adhered to the procedures consistent with animal protocols approved by the IACUC at the Icahn School of Medicine at Mount Sinai. 8-week-old C57BL/6 mice of either sex were randomly assigned for experiments.
AAV preparation
Timing: 2–3 weeks
-
1.To prepare AAV for intravitreal injections, we used three types of vectors.
-
a.AAV expression vectorThe main elements in the AAV expression vector comprise a promoter, cDNA of a gene of interest, a regulatory signal, and two inverted terminal repeats (ITRs) surrounding the gene expression cassette (Figure 1A).We usually use a ubiquitous CAG promoter to express genes into RGCs. In addition, the mouse γ–synuclein promoter (mSncg) is also highly efficient in driving gene expression in retinal ganglion cells (Wang et al., 2020b).
 CRITICAL: ITRs are essential for AAV packaging. However, ITRs are inherently unstable and could be lost during plasmid preparation. The integrity of ITRs should be confirmed before AAV production.
CRITICAL: ITRs are essential for AAV packaging. However, ITRs are inherently unstable and could be lost during plasmid preparation. The integrity of ITRs should be confirmed before AAV production. -
b.Serotype-specific AAV Rep-Cap vectorDifferent serotypes of AAV Rep-Cap have distinct transduction efficiency in RGCs. Using AAV2 Rep-Cap, we were able to transfect about 95% of RGCs (Figure 1B).
-
c.AAV Helper vectorAAV Helper vector is required for AAV packaging.
-
a.
-
2.Cell culture, transfection and AAV purification
-
a.Cell cultureTo produce AAV, we co-transfect the above three vectors into AAVpro 293T Cell Line (Takara Bio, 632273). AAVpro 293T cells may be passaged up to 20 times for quality AAV production. AAVpro 293T cells are cultured in DMEM supplemented with 10% FBS and 1X penicillin-streptomycin at 37°C in a 5% CO2 humidified incubator.20–24 h before transfection, split 150 mm plates of confluent AAVpro 293T at the ratio of 1:3. For each virus preparation, we usually transfect ten 150 mm plates with 80%–90% cell confluency. 3 h before transfection, change medium to 22 mL fresh culture medium.
-
b.TransfectionTransfection is performed using polyethylenimine (PEI). Dissolve 50 mg PEI into 50 mL DPBS (pH 4.5) by heating in 55°C water bath and vortexing. Sterilize using 0.22 μm filter, make 5 mL aliquots, and store at −20°C.To transfect ten 150 mm plates, add 60 μg AAV expression plasmid, 60 μg AAV Rep-Cap plasmid, 180 μg Helper plasmid, and 2.4 mL PEI solution in 30 mL serum free DMEM, mix by vortexing, and incubate 10–15 min at room temperature (20°C–25°C). Add 3 mL transfection mixture to each plate.
-
c.Cell lysis48–72 h after transfection, scrape cells off the plates, transfer the cells and medium to 50 mL tubes, pellet cells by centrifuging at 200 × g for 10 min at 4°C and discard the supernatant.Note: Dispose of the supernatant by adding 10% bleach in volume.Resuspend the cell pellet in 12 mL lysis buffer (150 mM NaCl, 20 mM Tris-HCl, pH 8.0). Lyse cells by freezing in −80°C dry ice/ethanol bath and thawing in - 37°C water bath, and repeat it twice. Then add MgCl2 (final concentration: 1 mM) and Benzonase (final concentration: 50 U/mL) to the cell lysate and incubate for 60 min in 37°C water bath. Centrifuge the lysate at 3,700 × g for 45 min at 4°C and collect supernatant containing AAVs.
 Pause point: Supernatant can be stored at −20°C overnight (16–20 h) at this point.
Pause point: Supernatant can be stored at −20°C overnight (16–20 h) at this point. -
d.AAV purification
-
a.
Figure 1.
Preparation of experiments
(A) Main elements of the AAV expression cassette. The total size limit of AAV expression cassette is about 4.7kb.
(B) AAV2-mediated gene transfer of GFP in RGCs labeled by Tuj1 immunoreactivity.
Scale bar, 40 μm. Original images were recently published (Guo et al., 2021).
(C) Device for intravitreal injection including the syringe, tubing, and micropipette. Mineral oil is filled in the device.
(D) Leica microscopes for surgeries and taking surgical images.
(E) Surgical tools. From top to bottom: Bulldog clamp, forceps for dissection and surgery, dissection scissors, forceps designated for optic nerve crush, and mouse head holder.
Make discontinuous iodixanol gradients (from top to bottom, 9 mL 17%, 6 mL 25%, 5 mL 40%, 4.2 mL 60% of iodixanol medium) in a Beckman OptiSeal centrifuge tube. Mark the interface of 40% and 60% layers. Gently load the supernatant over the 17% layer and fill the tube with lysis buffer to its full capacity. Place the centrifuge tubes into an ultracentrifuge rotor (we use Beckman VTi 50) and centrifuge at 242,000 × g for 2 h at 18°C in a Beckman Ultracentrifuge (We use Beckman Optima L-90K).
After centrifugation, mount the tubes on a lab stand, and put a glass beaker under the tube for waste collection. Pierce the tube near the top to allow airflow. Insert a 21-gauge needle at the interface of 40% and 60% layers, and slowly extract 3 mL solution containing AAV. Use 15mL Amicon centrifugal filters to change the buffer to DPBS and to further concentrate. AAV titer is assessed by quantitative PCR. We usually make 5 serial dilutions of AAV expression plasmid to generate a standard curve for AAV titering. For more technical details, we refer prospective users to the published protocol for AAV production and characterization (Grieger et al., 2006; Khan et al., 2011).
Note: AAV solution can be further concentrated by 0.5 mL Amicon centrifugal filters if necessary.
Pause point: AAV solution can be aliquoted and stored at −80°C for later use.
-
3.
AAV solution for intravitreal injection
AAV titers, in the range of 1–4 × 1013 genome copies per milliliter, are good for efficient RGC transduction. Right before intravitreal injection, we recommend adding 1% Fast Green into the AAV solution. Adding the dye is not mandatory but very helpful to visualize AAV injection into the vitreous body.
CRITICAL: Freezing AAV solution with Fast Green may generate precipitates. Therefore, we recommend preparing the needed volume of AAV solution with Fast Green right before injections.
Preparation of surgical instruments, equipment, and tools
Timing: 20 min
-
4.Assemble the device for intravitreal injection (Figure 1C).
-
a.Squeeze the needle of Hamilton syringe (50 μL, 22s gauge) into PE tubing.
-
b.Filling mineral oil into the syringe and tubingFor the initial filling with a large volume of mineral oil, pull out the syringe plunger, immerse the free end of the tubing into mineral oil, aspirate from the syringe barrel end until mineral oil filling up the syringe, and insert the plunger back into the syringe without introducing air bubbles. For small volume filling after injections, simply draw more oil from the free tubing end.
-
c.Micropipettes pulling and openingPull glass capillaries (Sutter Instrument) with a micropipette puller (Sutter Instrument) to make micropipettes according to manufacturer’s instructions (https://www.sutter.com/manuals/P-1000_OpMan.pdf). Cut open the closed end of pulled micropipettes with scissors or a blade.
-
d.Connect the micropipette with the free end of the tubing. Push the plunger and fill the mineral oil into the micropipette, leave an air segment at the tip of the micropipette. Now the device is ready for intravitreal injection.
 CRITICAL: All the connecting junctions should be airtight.
CRITICAL: All the connecting junctions should be airtight.
-
a.
-
5.
Microscopes
We use Leica M60 Stereomicroscope for intravitreal injection and retinal dissection (Figure 1D, left). For optic nerve crush, a Leica M60 stereomicroscope was mounted to a PROMICRA PRO-MLS motorized stand to adjust focus with a foot pedal. A beam splitter, a HAYEAR Camera and adaptor, a second observation tube and adaptor were mounted for imaging of the surgery (Figure 1D, right).
-
6.Tools (Figure 1E)
-
a.Bulldog clamp
-
b.Forceps
-
c.Scissors
-
d.Mouse head holder
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-Tubulin β3 (Tuj1) (1:250) | Biolegend | Cat# ab801202; RRID: AB_10063408 |
| Alexa Fluor® 488 AffiniPure Donkey Anti-Mouse IgG (H+L) (1:500) | Jackson ImmunoResearch Labs | Cat# 715-545-151; RRID:AB_2341099 |
| Alexa Fluor® 594 AffiniPure Donkey Anti-Mouse IgG (H+L) (1:500) | Jackson ImmunoResearch Labs | Cat# 715-585-151; RRID:AB_2340855 |
| Chemicals, peptides, and recombinant proteins | ||
| DMEM | Fisher Scientific | Cat# 11995-040 |
| DPBS | Fisher Scientific | Cat# 14190144 |
| NaCl (5 M) | Fisher Scientific | Cat# 24740011 |
| Tris-HCl (1 M, pH 8.0) | Fisher Scientific | Cat# 15568025 |
| Ketamine | Vedco Inc. | Cat# NDC 50989 |
| Xylazine | Akorn Inc. | Cat# NDC 59399 |
| FBS | Fisher Scientific | Cat# MT35010CV |
| Penicillin-streptomycin | Fisher Scientific | Cat# BW17602E |
| Polyethylenimine (PEI) | Polysciences | Cat# 23966-1 |
| Benzonase | Sigma | Cat# E1014_25kU |
| Iodixanol | Fisher Scientific | Cat# NC1059560 |
| Fast Green | Fisher Scientific | Cat# S25692 |
| Mineral oil | Fisher Scientific | Cat# BP26291 |
| N-Methyl-D-aspartic Acid (NMDA) | Millipore Sigma | Cat# 454575 |
| Fluoromount-G Slide Mounting Medium | Fisher Scientific | Cat# OB100-01 |
| Experimental models: Cell lines | ||
| AAVpro 293T Cell Line | Takara Bio | Cat# 632273 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J (8-week-old, male or female) | The Jackson Laboratory | Cat# JAX:000664, RRID: IMSR_JAX:000664 |
| Recombinant DNA | ||
| pAAV-GFP | Obtained from Dr. Kevin Park | N/A |
| pAAV-EBFP | Guo et al. (2021) | N/A |
| pAAV-CaMKIIα T286D | Guo et al. (2021) | N/A |
| pRep-Cap | Addgene | Cat# 104963 |
| pHelper | Addgene | Cat# 112867 |
| Software and algorithms | ||
| GraphPad Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ | Schindelin et al. (2012) | https://imagej.net/Fiji |
| Other | ||
| Confocal microscope | Zeiss | LSM 800 |
| Stereomicroscope | Leica | M60 |
| Motorized microscope stand | Promicra | Promicra PRO-MLS |
| Beam splitter | Leica | Wild 319449 |
| Microscope camera | HAYEAR | HY-3307 |
| Camera adaptor | Leica | Leica 10445319 |
| Observation tube | Leica-Wild | Wild 411576 |
| Observation tube adaptor | Leica | Wild 10411576 |
| Micropipette puller | Sutter Instruments | Model P-1000 |
| Ultracentrifuge | Beckman | Optima L-90K |
| Rotor | Beckman | VTi 50 |
| Syringe | Hamilton | Cat# 80965 |
| Bulldog clamp | Fine Scientific Tools | Cat# 18051-28 |
| Mouse head holder | KOPF | Model 921-E |
| Dumont #55 Forceps | Fine Science Tools | Cat# 11255-20 |
| Dumont #5 Fine Forceps | Fine Science Tools | Cat# 11254-20 |
| Dumont #5 Fine Forceps, only for optic nerve crush | Fine Science Tools | Cat# 11254-20 |
| Scissors | Crimson International Eye instruments | Cat# 6004A-105 |
| Heating pad | ConductScience | Cat# RWD-800-00179-00 |
| OptiSeal centrifuge tube | Beckman | Cat# 362183 |
| 15 mL Amicon centrifugal filters | Fisher Scientific | Cat# UFC901024 |
| 0.5 mL Amicon centrifugal filters | Fisher Scientific | Cat# UFC501024 |
| PE tubing | Warner Instruments | Cat# 64-0752 |
| Glass capillaries | Sutter Instrument | Cat# BF150-75-10 |
| Underpads | Fisher Scientific | Cat# 22-037-950 |
| Slides | Fisher Scientific | Cat# 22-034979 |
| Cover glass | Fisher Scientific | Cat# 12-542A |
Materials and equipment
Lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl (5 M) | 150 mM | 3 mL |
| Tris-HCl (1 M, pH 8.0) | 20 mM | 2 mL |
| H2O | n/a | 95 mL |
| Total | n/a | 100 mL |
Sterile filter and store at room temperature (20°C–25°C).
Alternatives: Other brands of reagents are also suitable.
Step-by-step method details
Intravitreal injection
Timing: 2 h
This section describes the procedure of intravitreal injection.
-
1.Mouse anesthesia
-
a.Weigh each adult mouse and inject the appropriate amount of anesthetic solution (ketamine-100 mg/kg and xylazine-10 mg/kg) intraperitoneally.
-
b.Keep the animal in a container till there is no response to a toe pinch.
-
a.
-
2.
Eyeball immobilization
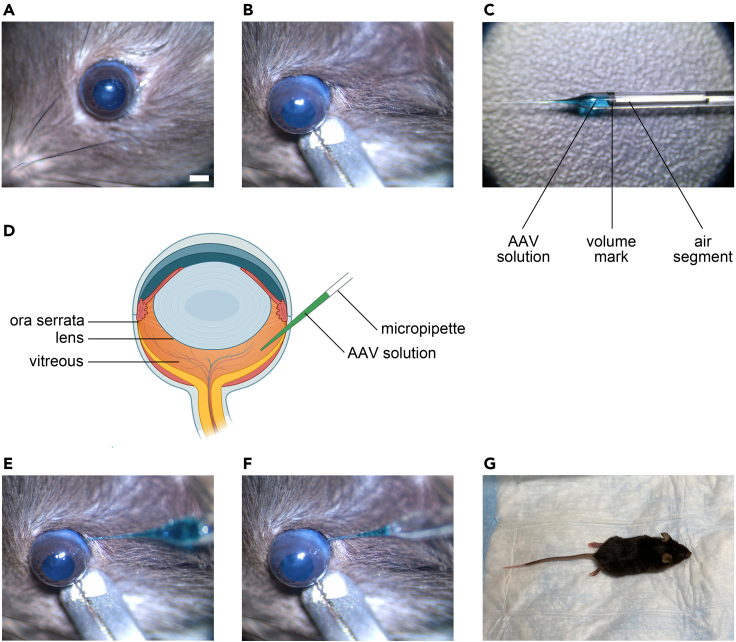
Align the eyeball in the viewing field center of the stereomicroscope (Figure 2A). Clip the lower eyelid with a bulldog clamp and secure the eyeball to a steady position (Figure 2B).
-
3.Intravitreal injection
-
a.Load a set volume of AAV solution into micropipette. A good strategy is to draw a line on the micropipette to mark the injection volume (Figure 2C). This will help keep the injection volume consistent and reduce variations among animals.
-
b.The lens of the mouse eyeball is relatively big. So the angle and the inserting depth of the micropipette should be well adjusted to avoid touching the lens or the retina (Figure 2D).
-
c.Insert the micropipette just behind the ora serrata (Figure 2E). (Troubleshooting 1)
-
d.Hold the micropipette steady, and slowly push the plunger to inject the virus solution into the vitreous with the other hand. If the virus solution contains fast green, the operator will notice the dye spreading inside the vitreous. Control the speed of injection (10–15 s/μL) and leave a small portion of the virus solution in the micropipette to avoid air or oil into the vitreous (Figure 2F). (Troubleshooting 2)
-
e.Hold the micropipette in position for a few more seconds and gently pull it out of the eyeball. (Troubleshooting 3)
-
f.After intravitreal injection, release the bulldog clamp, and let the eyeball stand for 30 s. Gently move the eyelids with forceps to help the eyeball back into orbit.
-
a.
CRITICAL: The whole process of intravitreal injection should be performed smoothly and quickly, as too long or too slow manipulations may result in eyeball dryness.
-
4.Postoperative care
-
a.Apply antibiotic ointment onto the eye to prevent infection and dryness.
-
b.Put the mouse on underpads warmed by a heating pad (37 degrees Celsius) in a container (Figure 2G).
 CRITICAL: Use new underpads for every cage of mice to avoid possible animal fighting.
CRITICAL: Use new underpads for every cage of mice to avoid possible animal fighting. -
c.Monitor the condition of the mouse periodically until it wakes up, then put it back into the cage and return it to the animal facility.
-
a.
Pause point: Wait two weeks after intravitreal AAV injection before proceeding to the next step.
Figure 2.
Procedure of intravitreal injection
(A) Eyeball alignment in the viewing field center of microscope. Scale bar, 1 mm.
(B) Eyeball immobilization by a bulldog clamp clipping the lower eyelid.
(C) Virus loading in the micropipette. Note the mark of injection volume and air segment separating the AAV solution and mineral oil.
(D) Illustration of mouse eyeball and intravitreal injection. The lens of mouse eyeball is relatively big. The micropipette should not touch the lens or the retina. Created with BioRender.com.
(E) Insertion of the micropipette behind the ora serrata.
(F) Injection of virus into the vitreous. Leave a small portion of the virus solution in the micropipette to avoid air or oil into the vitreous.
(G) Postoperative care. Pre-warm the heating pad and underpads when starting the experiment.
Inducing injuries to RGCs
Timing: 2 h
This section describes the procedure of two methods to injure RGCs: NMDA-induced excitotoxicity and optic nerve crush.
-
5.
Mouse anesthesia
Use the anesthesia method described in step 1. Then proceed to either step 6 or step 7.
-
6.
NMDA-induced excitotoxicity
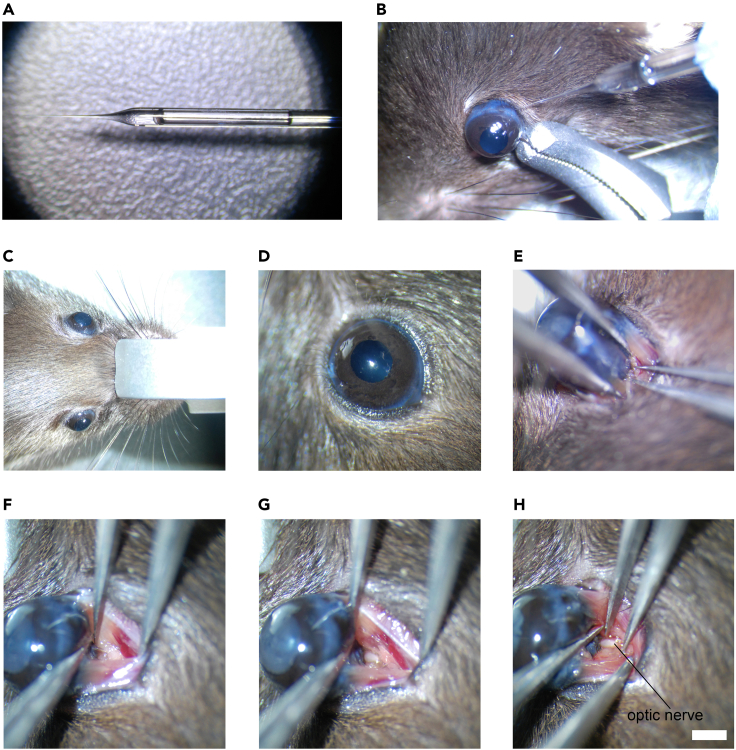
Intravitreal injection of a high dose of NMDA (20 mM, 1.5 μL) will induce severe excitotoxic damage to RGCs (Figures 3A and 3B).
-
7.Optic nerve crush
-
a.After anesthesia, secure the mouse head position using the head holder (Figure 3C).
 CRITICAL: Unexpected head movement during surgery may cause the failure of the crush.
CRITICAL: Unexpected head movement during surgery may cause the failure of the crush. -
b.Turn the head holder to make the right eye face upward for a right-handed operator (or make the left eye face upward for a left-handed operator), and align the eyeball in the viewing field center of the surgical microscope (Figure 3D).
-
c.Use a pair of forceps to expose the eyeball from the ventral/temporal side (Figure 3E). Use another pair of forceps to open the connective tissues covering the optic nerve (Figure 3F). Adjust microscope focal plane to focus on the optic nerve (Figure 3G). Use a designated pair of forceps to crush the optic nerve for 5 s at approximately 1 mm behind the globe (Figure 3H). (Troubleshooting 4)
 CRITICAL: Before performing surgery on each mouse, clean all surgical instruments with 75% ethanol.
CRITICAL: Before performing surgery on each mouse, clean all surgical instruments with 75% ethanol. CRITICAL: The tips of a pair of forceps will have a high chance of abrasion and deformation if they are being used for multiple purposes, resulting in failures of the crush, such as incomplete nerve crush and bleeding. Use a designated pair of forceps for crush will help keep consistency between operations. Keeping the crush time and site consistent will also help get more objective conclusions.Note: If bleeding happens, euthanize the animal and exclude it from the study.
CRITICAL: The tips of a pair of forceps will have a high chance of abrasion and deformation if they are being used for multiple purposes, resulting in failures of the crush, such as incomplete nerve crush and bleeding. Use a designated pair of forceps for crush will help keep consistency between operations. Keeping the crush time and site consistent will also help get more objective conclusions.Note: If bleeding happens, euthanize the animal and exclude it from the study. -
d.After optic nerve crush, restore the eyeball into the orbit of the eye socket and release the mouse head.
-
a.
-
8.
Postoperative care
Figure 3.
Methods to induce RGC injuries
(A and B) Excitotoxicity induced by intravitreal injection of NMDA. (A) NMDA loading in the micropipette. (B) Injection of NMDA into the vitreous.
(C–H) Procedures of optic nerve crush. (C) Mouse head immobilization on the head holder. (D) Eyeball alignment in the viewing field center of microscope. (E) Exposure the back of eyeball. (F) Opening the connective tissues covering the optic nerve. (G) Lowering the focal plane of microscope to focus on the optic nerve. (H) Crushing the optic nerve with a designated pair of forceps. Scale bar, 1 mm.
Perform all the postoperative care procedures described in step 4.
Pause point: Wait one week after NMDA injection or two weeks after optic nerve crush before proceeding to the next step.
Retina collection and immunohistochemistry
Timing: 2 days
Clear and definitive labeling of RGCs is a prerequisite for RGC quantification. Immunohistochemistry using antibodies recognizing neuron-specific class III beta-tubulin (Tuj1) is a well-established method to label and count RGCs after injuries (Park et al., 2008). Compared to RBPMS (another reliable RGC marker that predominantly labels RGC somas), Tuj1 immunohistochemistry also provides information on the morphology of RGC dendrites and nerve fiber conditions. This section describes the procedures of retinal sample collection and Tuj1 immunohistochemistry for retinal whole-mounts.
-
9.
Transcardial perfusion of mice
We routinely perfuse the mouse before tissue collection, as it facilitates immunohistochemistry with primary antibodies of mouse origin and helps preserve morphology of the retina.-
a.Use the anesthesia method described in step 1.
-
b.Perform rapid transcardial perfusion with 4% PFA according to general procedures, which we will not detail here.
-
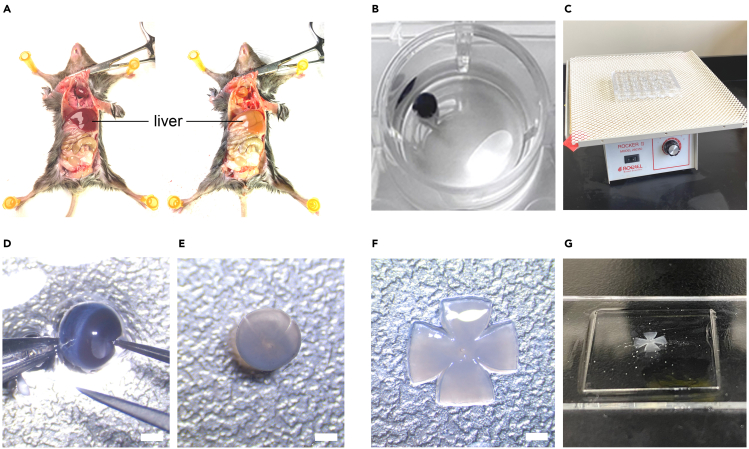
c.One sign of a good perfusion is the paling and hardening of the liver (Figure 4A).
-
a.
-
10.Enucleation and post-fixation of the eyeball
-
a.Right after perfusion, enucleate the eyeball using a pair of forceps.
-
b.Clean up the eyeball in PBS and make a small opening on the cornea.
 CRITICAL: A small opening on the cornea will help preserve the shape of the eyeball during fixation.
CRITICAL: A small opening on the cornea will help preserve the shape of the eyeball during fixation. - c.
-
a.
-
11.Retina dissection
-
a.After fixation, dissect the retina out of the eyeball (Figure 4D).
-
b.Gently and thoroughly clean the inner surface of the retina with forceps or a synthetic sable paint brush.
 CRITICAL: Some non-retinal structures/tissues near the ganglion cell layer may interfere with RGC staining.
CRITICAL: Some non-retinal structures/tissues near the ganglion cell layer may interfere with RGC staining. -
c.Cut the retinal cup into a cross shape (Figure 4E).
-
a.
Pause point: Once dissected out, retinas can be stored in PBS at 4 degrees for a few days.
-
12.Tuj1 immunohistochemistry in retina whole-mounts
-
a.Transfer the retina into a 1.5 mL tube, and incubate in 1.2 mL blocking buffer for 1 h.
-
b.Incubate the retina in 200 μL primary antibody (Tuj1) solution (1:250) at room temperature overnight (16–20 h).
-
c.Wash the retina in 1.2 mL PBS 3 times for 15 min each.
-
d.Incubate the retina in 200 μL secondary antibody solution (1:500) at room temperature for 2 h.
-
e.Wash the retina in 1.2 mL PBS 3 times for 15 min each.
 CRITICAL: For antibody incubation, make sure that the retina always stays at the bottom of the tube and is fully immersed in the solution. All incubation and washing steps should be carried out on a shaker.
CRITICAL: For antibody incubation, make sure that the retina always stays at the bottom of the tube and is fully immersed in the solution. All incubation and washing steps should be carried out on a shaker. - f.
-
a.
CRITICAL: Level the cover glass horizontally will help make the subsequent imaging steps easier and faster.
Pause point: Leave the slide in dark for 1–2 days to let the mounting medium semi-dry before proceeding to the next step.
Figure 4.
Procedure of retina collection and immunohistochemistry
(A) Transcardial perfusion of mice. Note the difference of liver color before (left) and after (right) perfusion.
(B) Post-fixation of the eyeball in 4% PFA.
(C) Putting plate on a shaker during fixation.
(D) Dissection of the retina. Care should be taken to keep the retina intact. Scale bar, 1 mm.
(E) Cutting the retina into a cross shape. Scale bar, 1 mm.
(F) Transferring and spreading the retina cross on a slide, with the RGC side facing up. Scale bar, 1 mm.
(G) Mounting of the retina with mounting medium and cover glass.
Confocal microscopy and RGC quantification
Timing: 2 days
The RGC density in the mouse retina varies in different quadrants as well as different distances from the optic disc. To achieve objective assessment of RGC numbers among animals in different treatment groups, it is critical to sample multiple regions in retinal flat-mounts from all quadrants at a fixed radius. This section describes the procedures of confocal imaging and RGC quantification.
-
13.Image acquisition
-
a.We take confocal images of retinal whole-mounts using a Zeiss LSM 800 microscope with a 20× objective lens to image a 320 × 320 μm square area.Note: The size of the imaging area may vary due to specific objective lens as well as the equipment brand and settings.
-
b.The center of the sampling square is located at ∼500 μm from the edge of the retinal flat-mounts leading to the optic disc. Tuj1 immunostaining labels both RGC somas and axons. Based on our experience, there are less dense RGC axons and discernible RGC somas for counting in these regions.
-
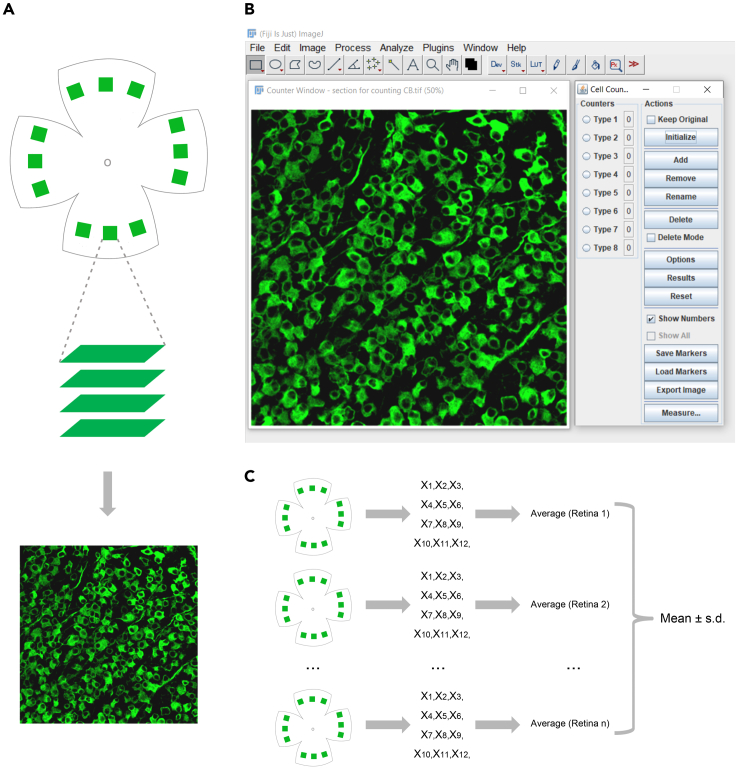
c.For the above imaging size and position, we sample 3 squares in each petal of the retinal whole-mount, and a total of 12 squares for one retinal whole-mount are sampled (Figure 5A top).
-
d.For each square, take Z-stack confocal images focusing on RGC somas and do maximum intensity Z-projection to generate a composite image for RGC counting (Figure 5A middle and bottom).
-
a.
Pause point: Collected and stored images can be analyzed later at any time.
-
14.RGC counting
-
a.Download and install Fiji ImageJ (https://imagej.net/software/fiji/) (Schindelin et al., 2012).
-
b.To count RGC numbers in each image, open the image in ImageJ and click “Plugins>Analyze>Cell Counter” to open the control panel.
-
c.Click “Initialize” in the “Actions” region, the image will be in Counter Window (Figure 5B).
-
d.Choose one “Type number” in the “Counter” region, a dot will appear in the circle on the left of the type number.Note: The software allows different colors for each type. Usually, one type is enough for simple RGC counting. More types are used to count multiple sub-classes of cells.
-
e.Click each RGC soma once to count RGCs. A dot will mark the cell after a click. The number of RGCs in an image will appear in the rectangle on the right of the type number.
-
a.
-
15.Quantification and statistical analysis
-
a.Average RGC numbers from all 12 squares in a retina.
-
b.For each experimental group, collect multiple retinas for quantification. 3-6 retinas are reasonable numbers.
-
c.Calculate “mean” and “standard deviation (s.d.)” for comparison among groups (Figure 5C).
-
d.Make graphs using GraphPad or other statistical software. In addition to absolute numbers of RGCs, the percentages of RGCs relative to those in the uninjured retina can be easily calculated. Both the absolute numbers and percentages of RGCs relative to the uninjured retina are helpful information to evaluate RGC survival after experimental manipulations/treatments (Figures 6B and 6D).
-
a.
Figure 5.
Retinal imaging and RGC quantification
(A) Image acquisition using confocal microscopy. Top: Illustration of sampling squares around the whole retina. Created with BioRender.com. Middle: Taking Z-stack confocal images to include all RGC somas. Bottom: Maximum intensity Z-projection to generate a composite image for RGC counting.
(B) RGC counting using ImageJ. The retinal whole-mount image here was recently published (Guo et al., 2021).
(C) Schematic diagram of data collection and analysis to obtain RGC survival results. Created with BioRender.com.
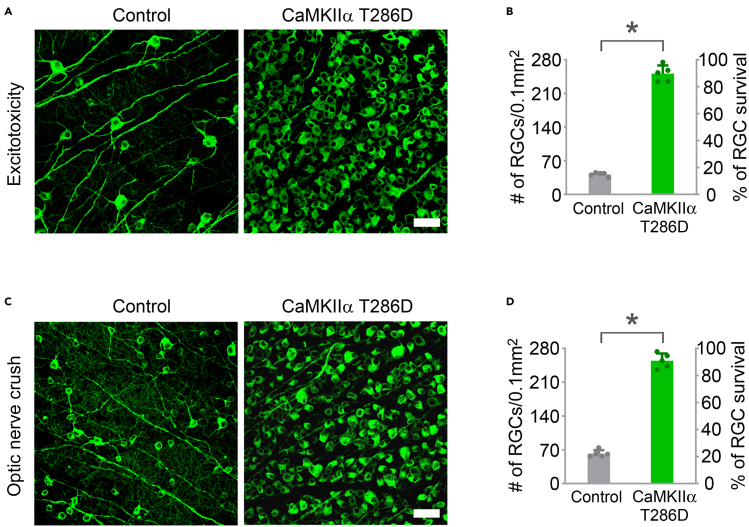
Figure 6.
Experimental images and quantification of RGC survival after CaMKII-mediated protection from excitotoxicity or optic nerve crush
(A) Confocal images of retinal whole-mounts showing surviving RGCs labeled by Tuj1 immunoreactivity at 7 days after NMDA injection in control (AAV-EBFP) and AAV-CaMKIIα T286D treated eyes. Scale bar, 40 μm.
(B) Quantification of RGC survival at 7 days post NMDA injection, expressed as numbers of RGCs (left Y-axis), and percentages of RGCs relative to those in the uninjured retina (right Y-axis). Data are presented as mean ± s.d., n=5 retinas per group. Unpaired t-test, ∗p < 0.0001.
(C) Confocal images of retinal whole-mounts showing surviving RGCs labeled by Tuj1 immunoreactivity at two weeks after optic nerve crush in control (AAV-EBFP) and AAV-CaMKIIα T286D treated eyes. Scale bar, 40 μm.
(D) Quantification of RGC survival at two weeks after optic nerve crush. Data are presented as mean ± s.d., n=5 retinas per group. Unpaired t-test, ∗p < 0.0001.
Original images and data were recently published (Guo et al., 2021).
Expected outcomes
AAV2-mediated gene delivery can transduce more than 95% of RGCs (Figure 1B). (Troubleshooting 5)
NMDA-induced excitotoxicity results in more than 80% RGC loss at one week after NMDA injection (Figures 6A and 6B). Optic nerve crush leads to about 75% RGC loss two weeks after injury (Figures 6C and 6D). AAV-mediated gene transfer of CaMKIIα T286D (as an example of a neuroprotective gene) protects the vast majority of RGCs (∼90%) at 1 week after NMDA injection or two weeks after optic nerve injury (Figures 6A–6D) (Guo et al., 2021).
Limitations
High titer and high quality of AAV preparations are essential for RGC transduction and objective evaluation of the gene function. Furthermore, there is a packaging size limit of the AAV genome. Multiple vectors may be required for co-delivering genes longer than the AAV packaging size (Tornabene et al., 2019).
Troubleshooting
Problem 1
Difficulties to insert the micropipette into the eyeball due to too much eyeball swaying (step 3c).
Potential solution
Redo the clipping using the bulldog clamp to stabilize the eyeball.
Aim at the center axis of the eyeball for micropipette insertion.
Problem 2
Injection failures, for example, virus solution going too slowly into the vitreous, and injecting air bubbles or even mineral oil into the vitreous (step 3d).
Potential solution
Check the tightness of the injection device. Too many air bubbles in the device or leakage of mineral oil will harm the injection results.
Adjust the tip opening size of the micropipette.
Problem 3
Backflow of AAV solution (step 3e).
Potential solution
Reduce the puncture size of the micropipette.
Do not put pressure on the eyeball after injection.
Problem 4
Bleeding during optic nerve crush (step 7c).
Potential solution
Avoid damaging the extraocular tissues by the tips of the forceps.
Access the optic nerve through the tissue space between extraocular muscles.
Straightly pull back the crushing forceps before releasing the eyeball from the holding forceps.
Avoid unnecessary maneuvers of the mouse head or the operator’s hands.
Problem 5
Low AAV2 transfection efficiency (expected outcomes).
Potential solution
Check AAV2 titer and quality.
Adjust the depth of micropipette insertion to increase AAV2 solution spreading in the vitreous.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Bo Chen (bo.chen@mssm.edu)
Materials availability
Reagents generated in this study are available upon reasonable request from the lead contact.
Acknowledgments
This work was supported by National Institutes of Health grants R01 EY028921 and R01 EY024986, an unrestricted challenge grant from Research to Prevent Blindness, and The Harold W. McGraw, Jr. Family Foundation for Vision Research.
Author contributions
X.G. conceived the project, designed and performed experiments, analyzed data, and wrote the paper. C.S. designed and performed experiments. J.Z. designed and performed experiments. B.C. conceived the project, designed experiments, analyzed data, supervised the project, and wrote the paper. All authors have read and approved the paper.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Xinzheng Guo, Email: xinzheng.guo1@mssm.edu.
Bo Chen, Email: bo.chen@mssm.edu.
Data and code availability
This study did not generate datasets or code.
References
- Grieger J.C., Choi V.W., Samulski R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Guo X., Snider W.D., Chen B. GSK3beta regulates AKT-induced central nervous system axon regeneration via an eIF2Bepsilon-dependent, mTORC1-independent pathway. Elife. 2016;5:e11903. doi: 10.7554/eLife.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zhou J., Starr C., Mohns E.J., Li Y., Chen E.P., Yoon Y., Kellner C.P., Tanaka K., Wang H., et al. Preservation of vision after CaMKII-mediated protection of retinal ganglion cells. Cell. 2021;184:4299–4314.e12. doi: 10.1016/j.cell.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.F., Hirata R.K., Russell D.W. AAV-mediated gene targeting methods for human cells. Nat. Protoc. 2011;6:482–501. doi: 10.1038/nprot.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin L.A., Gordon L.K. Retinal ganglion cell disorders: types and treatments. Prog. Retin. Eye Res. 2002;21:465–484. doi: 10.1016/s1350-9462(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B., Xu B., Connolly L., Kramvis I., Sahin M., et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornabene P., Trapani I., Minopoli R., Centrulo M., Lupo M., de Simone S., Tiberi P., Dell'Aquila F., Marrocco E., Iodice C., et al. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci. Transl. Med. 2019;11:eaav4523. doi: 10.1126/scitranslmed.aav4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhang F., Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–150. doi: 10.1016/j.cell.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhuang P., Huang H., Li L., Liu L., Webber H.C., Dalal R., Siew L., Fligor C.M., Chang K.C., et al. Mouse gamma-synuclein promoter-mediated gene expression and editing in mammalian retinal ganglion cells. J. Neurosci. 2020;40:3896–3914. doi: 10.1523/JNEUROSCI.0102-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate datasets or code.