Abstract
Observational and biologic studies suggest that aspirin is a promising prevention therapy for breast cancer. However, clinical trials to date have not corroborated this evidence, potentially due to study design. We evaluated the effect of aspirin on mammographic density (MD), an established modifiable risk factor for breast cancer. Electronic medical records from the University of Pennsylvania were evaluated for women who underwent screening mammography, saw their primary care provider and had a confirmed list of medications during 2012-2013. Logistic regression was performed to test for associations between clinically-recorded MD and aspirin use, after adjusting for age, body mass index (BMI) and ethnicity. We identified 26,000 eligible women. Mean age was 57.3, mean BMI was 28.9 kg/m2, 41% were African American and 19.7% reported current aspirin use. Aspirin users were significantly older and had higher BMI. There was an independent, inverse association between aspirin use and MD (Ptrend<0.001). Women with extremely dense breasts were less likely to be aspirin users than women with scattered fibroglandular density (OR=0.73; 95% CI: 0.57–0.93). This association was stronger for younger women (p=0.0002) and for African Americans (p=0.011). The likelihood of having dense breasts decreased with aspirin dose (Ptrend=0.007), suggesting a dose response. We demonstrate an independent association between aspirin use and lower MD in a large, diverse screening cohort. This association was stronger for younger and African American women: two groups at greater risk for Er- breast cancer. These results and others highlight the value of a randomized controlled trial of aspirin for prevention in premenopausal high-risk women.
Keywords: Breast cancer, mammographic breast density, aspirin, biomarkers, risk
Introduction:
Several agents have been shown to prevent breast cancer. Selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) have been shown to reduce the incidence of breast cancer in women by 49% and 65% respectively [1–3]. However, these agents only reduce rates of estrogen receptor positive (ER+) breast cancer [1] and both classes of drugs have side effects and toxicities that are barriers to use, especially for women without cancer [4, 5]. Given the limited prevention choices for premenopausal women, substantial side effects of existing agents, and lack of current options for the prevention of estrogen receptor-negative (ER-) cancers, investigation of additional options for chemoprevention is warranted.
Aspirin may be an ideal breast cancer preventive agent given that it is safe, well tolerated, and because there are strong biologic and epidemiologic data to support its prevention effect [6–10]. In vitro studies have demonstrated that breast cancer cells produce larger amounts of prostaglandins than normal cells and aspirin can inhibit the growth of breast cancer cells [11] [12]. Animal models using COX-2 knockout mice or wild-type mice demonstrate reduced tumor growth when treated with a non-steroidal anti-inflammatory drug [13]. Observational studies have demonstrated a reduction in breast cancer risk for aspirin users [10] [14] [15] [9], protection from both ER- and ER+ breast cancer [16] [17], and greater effects for higher doses [6] [18] [19]. A meta-analysis of randomized trials of aspirin for cardiovascular disease has shown a decrease in cancer incidence, but only a trend toward fewer breast cancers (p=0.07) [20]. Additionally, the Women’s Health Study (WHS) reported no reduction in breast cancer incidence with 100 mg alternate-day aspirin over a median of 9 years’ follow-up [21] [22]. The discrepancy between the strong biologic and epidemiologic data and findings from randomized controlled trials may be related to dosage choice, exposure/treatment time, or differences in the populations studied in these trials. The meta-analysis of cardiovascular trials included trials with a duration of at least 4 years of daily aspirin but doses varied (generally >75mg/day) among these trials. The WHS study examined an alternate-day dosing, which was lower than the effective dose in observational studies. Lastly, the randomized controlled trials performed to date have all been done using populations at average risk for breast cancer. At least one epidemiologic study found a >50% reduction in breast cancer risk associated with aspirin use in a high-risk population (women with benign breast disease) [23], suggesting that women at higher risk for breast cancer may benefit more from aspirin.
Given the strong biological and epidemiologic data supporting a prevention effect for aspirin, additional prospective information is warranted. While the strongest study design would be a trial of aspirin effects on breast cancer incidence, such a trial would require many years and would also be quite costly to complete. The evaluation of aspirin effects on breast cancer biomarkers would provide the necessary data to support moving forward with a large, randomized controlled trial of aspirin on breast cancer prevention. Several biomarkers for breast cancer risk exist, of which breast density is one of the most broadly accepted. High mammographic breast density (MD) is associated with a 4-6 fold increase in breast cancer [24–33]. MD can be modified by both hormonal and non-hormonal agents [25, 34, 35] and studies have shown that a reduction in MD is associated with a reduction in breast cancer risk [36] [37–39]. Importantly, high MD is associated with risk of both ER+ and ER− breast cancers [7, 17, 40].
The effect of aspirin on MD has been evaluated, but with conflicting results [41–44]. This may be due to several reasons: insufficient duration of aspirin exposure, small change in MD expected with such a short duration, and the small numbers of women enrolled on prior trials. We sought to evaluate the association between aspirin and MD in a large, diverse cohort utilizing more current digital imaging technologies.
Materials and Methods:
Eligibility:
Individuals were selected from patients of 36 Primary Care/OBGyn practices associated with the University of Pennsylvania. Institutional Review Board approval (#815757) was granted for review of the electronic health records (EHR).
Women were included in this retrospective study if they had both a screening mammogram between 01/01/2012-12/31/2013 and an ambulatory visit within the year prior to their screening mammogram. Women were excluded if they had a prior history of in situ or invasive breast cancer; were under the age of 40; or did not have information in the EHR regarding age, body mass index (BMI), aspirin use, or Breast Imaging-Reporting and Data System (BI-RADS) density. Using these criteria, we identified a total of 111,410 women seen by the core set of affiliated Primary Care/ObGyn practices, from which 80,776 were excluded as they did not have a screening digital mammogram on record. From this population, an additional 1394 were excluded because they either had no ambulatory visit in the year prior to their screening mammogram (n=1,325), or had a prior history of invasive or in-situ breast cancer (n=69: Figure 1). From the remaining population, a total of 29,240 had a confirmed current list of active medications. Eleven percent of these women were under the age of 40, or had missing information on age, BMI, race, or BI-RADS breast density (N=3,240 excluded). The remaining 26,000 women were used as our study population.
Figure 1:
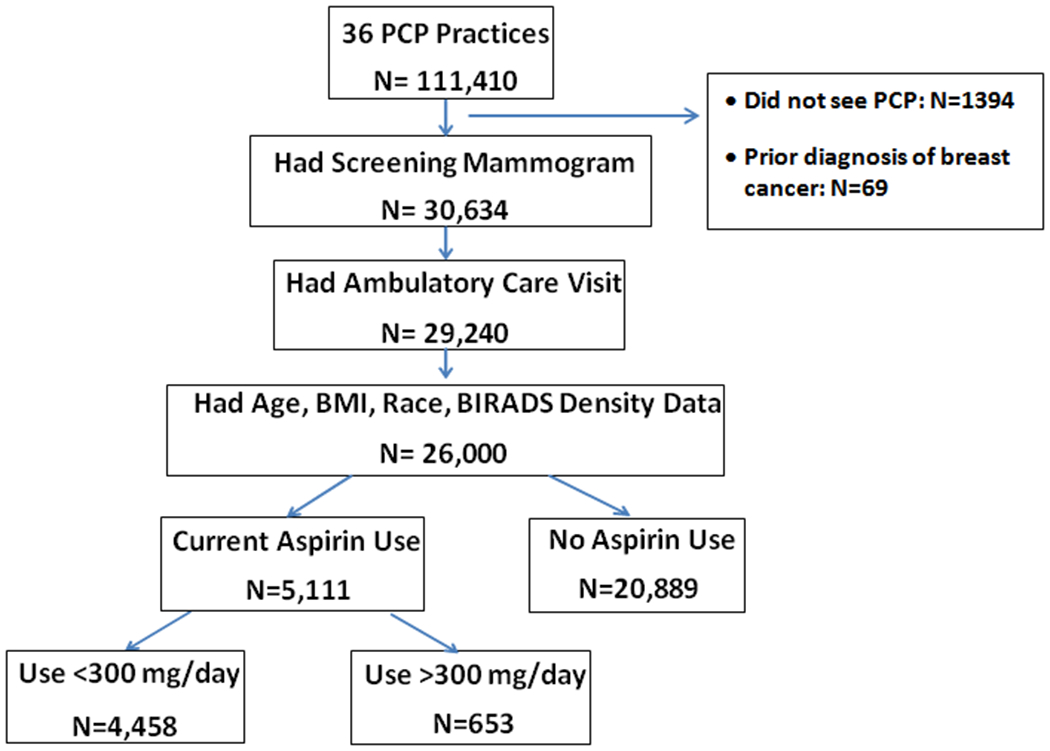
Schema of cohort: Outlines the patient population after application of inclusions/exclusion criteria.
Current aspirin use and dose were recorded based on the primary care encounter closest to the time of the screening mammogram. Dose was calculated based on medication name and tablets used and were categorized as above/below 300mg/day. Categorical breast density BI-RADS assessment was recorded from the clinical screening evaluation report on record per the American College of Radiology (ACR) BI-RADS four-category density scale (1 = almost entirely fatty, 2 = scattered fibroglandular densities, 3 = heterogeneously dense, and 4 = extremely dense) [45].
Data Analysis:
Descriptive statistics were determined to assess patient characteristics according to aspirin use status. Student’s t-tests (for continuous variables) and chi-squared tests (for categorical variables) were used to assess differences in the distribution of patient characteristics according to aspirin use. All tests were two-sided, with p<0.05 considered statistically significant.
A series of multivariable logistic regression models were used to examine the associations between aspirin use and mammographic breast density. All regression models were adjusted for potentially confounding variables, selected a priori based on prior evidence and data availability. These included age (as a continuous variable), body mass index (BMI) (treated as a continuous measure), and ethnicity (categorized into 3 groups: white, African American and other). The first set of models evaluated aspirin use as a function of mammographic breast density and the other covariates. We used two models: one with all four categories of density, and one with breast density as a dichotomous variable (non-dense (almost entirely fat or scattered fibroglandular) breasts) vs. dense (heterogeneously or extremely dense)). Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated and a test of trend in odds of aspirin use with increasing (ordinal) density categories was performed.
To examine covariate interactions with breast density, we used a second set of logistic regression models with dichotomous breast density as the outcome variable. Cross-product interaction terms were included to evaluate potential effect modification by age and ethnicity. We also examined breast density in relation to multiple aspirin use categories defined by dose (non-users, <300 mg, ≥300 mg), while adjusting for other covariates. To examine a trend in odds of dense breasts with increasing aspirin dose, we included aspirin dose as an ordinal categorical variable.
Results:
The characteristics of the 26,000 women who fit the study criteria and are the subject of this analysis are included in Table 1. The mean age was 57.3 (range 40 - 89), 19.6% were under the age of 50 and 13.9% were over the age of 70 years old. The mean BMI was 28.9 kg/m2 (range 14 - 84), with 1.5% being underweight and 64.8% overweight (of which 56.8% were obese). The majority of the population reported Caucasian ethnicity (52%) and 41% were African American.
Table 1.
Patient Characteristics
| Characteristic | Aspirin Non-Users N = 20889 |
Aspirin Users N = 5111 |
P * |
|---|---|---|---|
| Age, mean (SD) | 55.3 (10.2) | 65.3 (9.8) | <0.0001 |
| Age categories, no. (%) | |||
| 40-49 | 7425 (35.6) | 359 (7.0) | |
| 50-59 | 6824 (32.7) | 1120 (21.9) | <0.0001 |
| 60-69 | 4718 (22.6) | 1940 (38.0) | |
| ≥70 | 1922 (9.2) | 1692 (33.11) | |
| BMI, mean (SD) | 28.5 (7.2) | 30.4 (7.6) | <0.0001 |
| BMI, categories, no. (%) | |||
| <18.5 | 351 (1.7) | 48 (0.9) | |
| 18.5-24 | 7462 (35.7) | 1282 (25.1) | |
| 25-29 | 5774 (27.6) | 1509 (29.5) | <0.0001 |
| 30-34 | 3745 (17.9) | 1076 (21.1) | |
| ≥35 | 3557 (17.0) | 1196 (23.4) | |
| Race | |||
| White | 11125 (53.3) | 2394 (46.8) | |
| African American | 8158 (39.1) | 2504 (49.0) | <0.0001 |
| Other | 1606 (7.7) | 213 (4.2) | |
| Breast density, no. (%) | |||
| BI-RADS 1 | 2006 (9.6) | 861 (16.9) | |
| BI-RADS 2 | 9346 (44.7) | 2859 (55.9) | <0.0001 |
| BI-RADS 3 | 8480 (40.6) | 1312 (25.7) | |
| BI-RADS 4 | 1057 (5.1) | 79 (1.6) |
Student’s t-test was used for continuous variables, and the chi-squared test for categorical variables. All tests were two-sided.
A total of 5,111 (19.7%) women had an “active or current use of aspirin medication” confirmed in their EHR, while 20,889 had no indication of aspirin use. Of women reporting aspirin use, 12.8% used more than 300 mg/day. Aspirin users were significantly older and had higher BMI (Table 1). There was a greater percentage of African American women among aspirin users (39.1 vs 49.0%, p<0.0001). A greater proportion of aspirin users had BI-RADS 1 and 2 densities than non-users (72.8% vs 54.3%, p<0.0001).
After adjusting for age, BMI and ethnicity, there was an independent, inverse association between aspirin use and mammographic density (ptrend<0.001). Compared with women with scattered fibroglandular tissue, , women with either heterogeneously (OR=0.84, CI: 0.78-0.92) or extremely dense (OR=0.73; CI: 0.57-0.93) breasts were less likely to be aspirin users, while women with entirely fat breasts were more likely to use aspirin (OR=1.15, CI: 1.04-1.27) (Table 2). This effect was also seen when density was analyzed as a dichotomized variable (dense=BI-RADS 3+4 and non-dense=BI-RADS 1+2: see Table 2). Women with dense breasts were less likely to be aspirin users than those with non-dense breasts (OR=0.82, CI: 0.76-0.89).
Table 2.
Relationship between Breast Density and Aspirin Use
| Aspirin Non-Users N = 20889 | Aspirin Users N = 5111 | OR (95% CI)* | |
|---|---|---|---|
| Mammographic breast density | |||
| Entirely fat | 2006 (9.6) | 861 (16.9) | 1.15 (1.04 – 1.27) |
| Scattered fibroglandular | 9346 (44.7) | 2859 (55.9) | 1.00 (Reference) |
| Heterogeneously dense | 8480 (40.6) | 1312 (25.7) | 0.84 (0.78 – 0.92) |
| Extremely dense | 1057 (5.1) | 79 (1.6) | 0.73 (0.57 – 0.93) |
| Ptrend<0.001 | |||
| Dichotomized breast density | |||
| Non-dense | 11352 (54.3) | 3720 (72.8) | 1.00 (Reference) |
| Dense | 9537 (45.7) | 1391 (27.2) | 0.82 (0.76 −0.89) |
Logistic regression results for model with aspirin use as the outcome and BI-RADS density as a categorical variable of interest, adjusted for age (continuous, 1st and 2nd order terms), bmi, and race.
In separate models with dichotomous density as the outcome variable, the association between aspirin use and density varied with age (pinteraction<0.001) and ethnicity (pinteraction =0.011). This association was strongest among younger women (OR=0.48, CI: 0.37-0.63, for ages 40-49) and African American women (OR=0.70, CI: 0.62-0.79: Table 3).
Table 3.
Relationship between aspirin use and dichotomized breast density as the outcome, including interactions between aspirin use and age and race.
| Effect Modification by Age | |
|---|---|
| OR (95% CI)* for non-dense vs. dense | |
| Aspirin use at age 40-49 (n=7784) | 0.48 (0.37 – 0.63) |
| Aspirin use at age 50-59 (n=7944) | 0.77 (0.66 – 0.90) |
| Aspirin use at age 60-69 (n=6658) | 0.87 (0.76 – 0.98) |
| Aspirin use at age ≥70 (n=3614) | 0.93 (0.79 – 1.09) |
| Pinteraction = 0.0002** | |
| Effect Modification by Race | |
| OR (95% CI)* for non-dense vs. dense | |
| Aspirin use for race = white (n=13519) | 0.89 (0.80 – 0.98) |
| Aspirin use for race = black (n=10662) | 0.70 (0.62 – 0.79) |
| Aspirin use for race = other (n=1819) | 0.78 (0.57 – 1.08) |
| Pinteraction = 0.011*** | |
Logistic regression results for a models with dichotomized density (dense=BI-RADS 3,4 and non-dense=BI-RADS 1,2), adjusted for age, race, and BMI.
Chi-square likelihood ratio test (df. = 3) comparing the logistic regression model with and without interaction between aspirin use and age.
Chi-square likelihood ratio test (df. 2) comparing the logistic regression model with and without interaction between aspirin use and race.
We also evaluated the effect of aspirin dose on MD (Table 4), and identified a lower likelihood of having dense breasts (BI-RADS 3+4) with increasing aspirin dose (OR=0.62, CI: (0.50-0.76) for >300 mg compared to non-users; ptrend=0.007).
Table 4.
Dose-response relationship between aspirin use and breast density
| Non-dense N = 15072 | Dense N = 10928 | OR (95% CI) for dense breasts | |
|---|---|---|---|
| Aspirin use | |||
| Users (>300) | 512 (3.4) | 141 (1.3) | 0.62 (0.50 – 0.76) |
| Users (≤300) | 3208 (21.3) | 1250 (11.4) | 0.84 (0.77 – 0.91) |
| Non-users | 11352 (75.3) | 9537 (87.3) | 1.00 (Reference) |
| Ptrend=0.007 |
Test for trend in odds ratios for aspirin use: p-value = 0.007.
Discussion:
In this large and diverse cohort, we have demonstrated an independent association between aspirin use and lower MD. Aspirin users were 27% less likely to have extremely dense breast tissue and 18% less likely to have dense breasts (BI-RADS 3 and 4), after accounting for age, BMI and race. This association between MD and aspirin use was stronger for both younger women and African American women: two groups at greater risk for Er- breast cancer. The strength of this association increased with decreasing age. Aspirin users age 40-49 were 52% less likely to have higher MD, but there was little evidence for an association between aspirin and density among women over age 70. Among African American women, aspirin users were 30% less likely to have higher MD than non-users. We also identified a dose response to aspirin, with lower MD for higher daily dose (ptrend=0.007). Women using <300mg/day were 16% less likely to have higher MD and women using >300 mg/day of aspirin were 38% less likely to have higher MD than non-users.
Several prior studies have investigated the link between aspirin use and MD. Maskarinec et al. examine the effect of all NSAIDs on MD among 1474 pre- and post-menopausal women [41]. While the overall study was negative, they did find that MD was slightly lower among pre-menopausal women with long-term NSAID use and observed a marginally significant trend of increasing MD with length of aspirin use among pre-menopausal women. Stone et al studied 3286 women and found no effect of either aspirin or NSAIDs in general on MD. They evaluated both total dense area and percent density, and examined dose and duration of aspirin use [42]. Both of these investigators combined prior different cohorts and evaluated all NSAID together, which may have contributed to their generally negative findings.
The largest cohort study was performed by Terry et al [43]. In a cohort of 29,284 post-menopausal women, no overall change in MD was associated with NSAID use. They did report that NSAID users were more likely to have lower MD (BI-RADS 1 or 2) and that aspirin use was associated with an 11-40% chance of staying not dense (compared to staying dense) [43]. Our study was of similar size, but included both pre- and post-menopausal women, as well as a more ethnically diverse population, and only evaluated the effect of aspirin.
In the only prospective study performed to date, McTiernan et al. treated 143 post-menopausal women with 325 mg aspirin or placebo for 6 months and found no change in MD [44]. However, this study was quite small, including only post-menopausal women and evaluating a very short aspirin exposure.
In a recent study of women with a family history of breast cancer, pre-menopausal women were shown to have a reduction in breast cancer risk associated with aspirin use (HR 0.57, CI 0.33-0.98) [46]. This study supports our finding of an effect in younger women and highlights the importance of investigating the effects of aspirin in higher risk populations.
Our study has several strengths; it was based on a large study population (26,000 women) with diverse ethnicity (41% African American) and broad age range (including both pre- and post-menopausal women). Additionally, we evaluated the effect of MD using newer screening techniques (digital mammography). Our study also demonstrates the value of EHRs in research. There are however, limitations of our study which must be considered. We did not evaluate the effect of aspirin on cancer incidence. Other studies have done so and shown that a change in MD is associated with a change in breast cancer risk [36–39]. As this is a retrospective, observational study, selection bias and confounding must be considered. Women using aspirin in our study tended to be older and have elevated BMI. We used statistical methods to adjust for these differences, including multivariable logistic regression and stratified analyses. Future prospective, controlled studies are needed to definitively assess the effect of aspirin on breast density, particularly in relation to dose and duration.
In conclusion we have demonstrated an independent association between aspirin use and lower mammographic density in a large and diverse breast cancer screening cohort. Our results suggest that this association is stronger for younger and African American women: two groups at greater risk for Er- breast cancer. These results, in combination with prior laboratory and epidemiologic evidence, highlight the potential value and need for a randomized, controlled trial of aspirin as a preventive agent for breast cancer.
Acknowledgements:
This work was supported in part by grants U54CA163313 and U54CA163303 from the National Cancer Institute.
Footnotes
Conflict of Interest: The authors have no potential, perceived or aactual conflict of interest to disclose
Contributor Information
Marie E. Wood, Department of Medicine, University of Vermont School of Medicine
Brian L. Sprague, Department of Surgery, University of Vermont School of Medicine
Andrew Oustimov, Perelman School of Medicine, University of Pennsylvania.
Marie B. Synnstvedt, Department of Medicine, Perelman School of Medicine, University of Pennsylvania
Melissa Cuke, Department of Medicine, University of Vermont School of Medicine.
Emily F. Conant, Department of Radiology, Perelman School of Medicine, University of Pennsylvania
Despina Kontos, Department of Radiology, Perelman School of Medicine, University of Pennsylvania.
REFERENCES:
- 1.Fisher B, et al. , Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst, 1998. 90(18): p. 1371–88. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, et al. , Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med, 2011. 364(25): p. 2381–91. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, et al. , Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet, 2014. 383(9922): p. 1041–8. [DOI] [PubMed] [Google Scholar]
- 4.Dent SF, et al. , Aromatase inhibitor therapy: toxicities and management strategies in the treatment of postmenopausal women with hormone-sensitive early breast cancer. Breast Cancer Res Treat, 2011. 126(2): p. 295–310. [DOI] [PubMed] [Google Scholar]
- 5.Makubate B, et al. , Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer, 2013. 108(7): p. 1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ready A, et al. , NSAID use and breast cancer risk in the VITAL cohort. Breast Cancer Res Treat, 2008. 109(3): p. 533–43. [DOI] [PubMed] [Google Scholar]
- 7.Kirsh VA, et al. , Nonsteroidal antiinflammatory drug use and breast cancer risk: subgroup findings. Am J Epidemiol, 2007. 166(6): p. 709–16. [DOI] [PubMed] [Google Scholar]
- 8.Thun MJ, Jacobs EJ, and Patrono C, The role of aspirin in cancer prevention. Nat Rev Clin Oncol, 2012. 9(5): p. 259–67. [DOI] [PubMed] [Google Scholar]
- 9.Bosetti C, et al. , Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol, 2012. 23(6): p. 1403–15. [DOI] [PubMed] [Google Scholar]
- 10.Algra AM and Rothwell PM, Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol, 2012. 13(5): p. 518–27. [DOI] [PubMed] [Google Scholar]
- 11.Bennett A, et al. , Prostaglandins and breast cancer. Lancet, 1977. 2(8039): p. 624–6. [DOI] [PubMed] [Google Scholar]
- 12.Sotiriou C, et al. , The aspirin metabolite salicylate inhibits breast cancer cells growth and their synthesis of the osteolytic cytokines interleukins-6 and −11. Anticancer Res, 1999. 19(4B): p. 2997–3006. [PubMed] [Google Scholar]
- 13.Kundu N, et al. , Increased cyclooxygenase-2 (cox-2) expression and activity in a murine model of metastatic breast cancer. Int J Cancer, 2001. 93(5): p. 681–6. [DOI] [PubMed] [Google Scholar]
- 14.Mangiapane S, Blettner M, and Schlattmann P, Aspirin use and breast cancer risk: a meta-analysis and meta-regression of observational studies from 2001 to 2005. Pharmacoepidemiol Drug Saf, 2008. 17(2): p. 115–24. [DOI] [PubMed] [Google Scholar]
- 15.Luo T, et al. , Aspirin use and breast cancer risk: a meta-analysis. Breast Cancer Res Treat, 2012. 131(2): p. 581–7. [DOI] [PubMed] [Google Scholar]
- 16.Bardia A, et al. , Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat, 2011. 126(1): p. 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasky TM, et al. , Non-steroidal anti-inflammatory drugs (NSAIDs) and breast cancer risk: differences by molecular subtype. Cancer Causes Control, 2011. 22(7): p. 965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahme E, et al. , Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer, 2005. 5: p. 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris RE, et al. , Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res, 2003. 63(18): p. 6096–101. [PubMed] [Google Scholar]
- 20.Rothwell PM, et al. , Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet, 2012. 379(9826): p. 1602–12. [DOI] [PubMed] [Google Scholar]
- 21.Cook NR, et al. , Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med, 2013. 159(2): p. 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang SM, et al. , Low-dose aspirin and breast cancer risk: results by tumour characteristics from a randomised trial. Br J Cancer, 2008. 98(5): p. 989–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallicchio L, et al. , Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer, 2006. 106(7): p. 1443–52. [DOI] [PubMed] [Google Scholar]
- 24.Boyd NF, et al. , Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst, 1992. 84(15): p. 1170–9. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe M, et al. , Breast cancer risk and measured mammographic density. . European Journal of Cancer Prevention, 1998. 7 (Suppl 1): p. S47–S55. [DOI] [PubMed] [Google Scholar]
- 26.Boyd NF, et al. , Mammographic densities and risk of breast cancer among subjects with a family history of this disease. J Natl Cancer Inst, 1999. 91(16): p. 1404–8. [DOI] [PubMed] [Google Scholar]
- 27.Boyd NF, et al. , Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep, 2001. 3(4): p. 314–21. [DOI] [PubMed] [Google Scholar]
- 28.Lam PB, et al. , The association of increased weight, body mass index, and tissue density with the risk of breast carcinoma in Vermont. Cancer, 2000. 89(2): p. 369–75. [DOI] [PubMed] [Google Scholar]
- 29.Heine JJ and Malhotra P, Mammographic tissue, breast cancer risk, serial image analysis, and digital mammography. Part 1. Tissue and related risk factors. Acad Radiol, 2002. 9(3): p. 298–316. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson C, et al. , Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev, 1999. 8(10): p. 863–6. [PubMed] [Google Scholar]
- 31.Boyd NF, et al. , Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst, 1995. 87(9): p. 670–5. [DOI] [PubMed] [Google Scholar]
- 32.Harvey JA and Bovbjerg VE, Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology, 2004. 230(1): p. 29–41. [DOI] [PubMed] [Google Scholar]
- 33.Warwick J, et al. , Breast density and breast cancer risk factors in a high-risk population. Breast, 2003. 12(1): p. 10–6. [DOI] [PubMed] [Google Scholar]
- 34.Sala E, et al. , High risk mammographic parenchymal patterns and diet: a case-control study. Br J Cancer, 2000. 83(1): p. 121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konez O, Goyal M, and Reaven RE, Can tamoxifen cause a significant mammographic density change in breast parenchyma? Clin Imaging, 2001. 25(5): p. 303–8. [DOI] [PubMed] [Google Scholar]
- 36.Cuzick J, et al. , Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst, 2011. 103(9): p. 744–52. [DOI] [PubMed] [Google Scholar]
- 37.van Gils CH, et al. , Changes in mammographic breast density and concomitant changes in breast cancer risk. Eur J Cancer Prev, 1999. 8(6): p. 509–15. [DOI] [PubMed] [Google Scholar]
- 38.Nyante SJ, et al. , Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer. J Natl Cancer Inst, 2015. 107(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Work ME, et al. , Changes in mammographic density over time in breast cancer cases and women at high risk for breast cancer. Int J Cancer, 2014. 135(7): p. 1740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziv E, et al. , Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomarkers Prev, 2004. 13(12): p. 2090–5. [PubMed] [Google Scholar]
- 41.Maskarinec G, et al. , Nonsteroidal anti-inflammatory drugs (NSAIDs) and mammographic density. Breast Cancer Res Treat, 2008. 112(1): p. 133–9. [DOI] [PubMed] [Google Scholar]
- 42.Stone J, et al. , The association between mammographic density measures and aspirin or other NSAID use. Breast Cancer Res Treat, 2012. 132(1): p. 259–66. [DOI] [PubMed] [Google Scholar]
- 43.Terry MB, et al. , Nonsteroidal anti-inflammatory drugs and change in mammographic density: a cohort study using pharmacy records on over 29,000 postmenopausal women. Cancer Epidemiol Biomarkers Prev, 2008. 17(5): p. 1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McTiernan A, et al. , No effect of aspirin on mammographic density in a randomized controlled clinical trial. Cancer Epidemiol Biomarkers Prev, 2009. 18(5): p. 1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balleyguier C, et al. , BIRADS classification in mammography. Eur J Radiol, 2007. 61(2): p. 192–4. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, et al. , Lifetime use of nonsteroidal anti-inflammatory drugs and breast cancer risk: results from a prospective study of women with a sister with breast cancer. BMC Cancer, 2015. 15: p. 960. [DOI] [PMC free article] [PubMed] [Google Scholar]


