Abstract
Extra-virgin olive oil (EVOO), a popular functional food and major source of fat in the Mediterranean diet, possesses a variety of healthful components, including monounsaturated fatty acids and bioactive phenolic compounds that, individually and collectively, exert beneficial effects on cardiometabolic markers of health and act as neuroprotective agents through their anti-inflammatory and antioxidant activities. The gut microbiota and health of the intestinal environment are now considered important factors in the development of obesity, metabolic disease, and even certain neurodegenerative conditions via the gut-brain axis. Recently, data are emerging which demonstrate that the health-promoting benefits of EVOO may also extend to the gut microbiota. In this review, we aimed to examine findings from recent studies regarding the impact of EVOO on gut microbiota and intestinal health and explore how modulations in composition of gut microbiota, production of microbially produced products, and activity and functioning of the mucosal immune system may lead to favorable outcomes in cardiovascular, metabolic, and cognitive health.
Keywords: Alzheimer disease, extra-virgin olive oil, gut microbiota, metabolic health, mucosal immunity
INTRODUCTION
The Mediterranean diet, rich in plant-based foods and incorporating moderate intake of fish and wine and low intake of red meat and processed foods, has been associated with a reduced risk of development of chronic diseases and increased life expectancy, as evidenced from a number of randomized clinical trials.1–3 An integral component of the Mediterranean diet, extra-virgin olive oil (EVOO), obtained via the mechanical extraction of the olive fruit, without the use of heat or solvents, and required to possess no greater that 0.8% free acidity, is well known for its organoleptic and nutritional properties as well as its beneficial effects on a number of markers of cardiometabolic health and chronic disease risk.4 The health-promoting properties of EVOO have been largely attributed to the high concentration of monounsaturated fatty acids, as well as the presence of a variety of phenolic compounds.4,5 The mechanisms by which EVOO exerts positive outcomes on cardiovascular and metabolic health are numerous and include lipid- and blood glucose–lowering-effects as well as polyphenol-influenced anti-inflammatory and antioxidant effects via inhibition of cyclooxygenase-2, suppression of nitric oxide synthase expression and activation of nuclear factor erythroid-2–related factor.6–10
Extra-virgin olive oil and its phenolic compounds are promising for prevention and treatment of certain neurodegenerative diseases, such as Alzheimer disease (AD).11,12 Studies in humans have indicated that an EVOO-rich Mediterranean diet can significantly reduce the risk of AD.13,14 Rodent studies also support these findings, revealing that oral administration of EVOO and specific EVOO phenolic compounds, in particular, can reduce the accumulation of β-amyloid (Aβ) deposits and tau neuropathologies in mouse models of AD, resulting in improved memory and cognition.15–19
Accumulating evidence supports the notion that consumption of an EVOO-rich Mediterranean diet can also promote favorable outcomes on gut microbiota and associated microbial metabolites, supporting the health of the intestinal environment.20–23 In parallel, positive effects on gut microbiota and intestinal immunity have been observed with consumption of EVOO in animals and humans.24–31 Extra-virgin olive oil affects the gut microbiota by reducing the abundance of pathogenic bacteria, stimulating the growth of beneficial bacteria, and increasing the production of microbially produced short-chain fatty acids (SCFAs), which exert a wide range of anti-inflammatory effects and can modulate the expression of a variety of genes via epigenetic mechanisms.32–34 Administration of EVOO influences the health of the intestinal mucosa and supports gut microbiota homeostasis by encouraging intestinal immunoglobulin A (IgA) production, as quantified by enzyme-linked immunosorbent assays using fecal homogenates; dampening down inflammatory cytokine production35; supporting production and expression of cytokines and transcription factors involved in reducing inflammation and promoting immune tolerance in the intestine27; and protecting against intestinal oxidative injury through the potent antioxidant activity of EVOO phenolic extracts.36
In this review, we highlight recent scientific progress in humans and rodents in which the impact of EVOO consumption on gut microbiota was explored, as was the possible role EVOO-modulated gut microbiota play in regulating intestinal immunity and preventing cardiovascular, metabolic, and cognitive dysfunction and disease. Most reviews to date summarize the health benefits of EVOO on a particular aspect of health, namely cardiovascular, metabolic, and cognitive health (with EVOO included as part of the Mediterranean diet). However, few reviews have been devoted to exploring the impact of EVOO and its constituents on the gut microbiota. Therefore, unlike other recent review publications, in this review, we focused on the impact of EVOO, as well as its constituents, on the gut microbiota and intestinal immunity, with a special emphasis on the role of the gut-brain axis, and explored the potential mechanisms of how EVOO-induced modulations in gut microbiota and intestinal health can lead to therapeutic outcomes in cardiovascular, metabolic, and cognitive health.
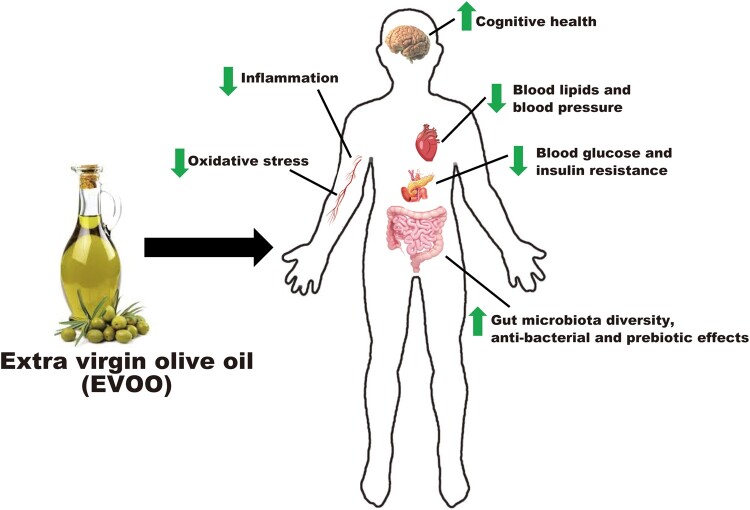
We searched the PubMed database for articles in English, using the following terms: extra-virgin olive oil, polyphenols, gut microbiota, immune system, cardiovascular health, metabolic health and cognition (from 2010 to April 2020). Figure 1 outlines a summary of the widespread health benefits of EVOO.
Figure 1.
Schematic overview of the numerous health benefits of extra-virgin olive oil.
NUTRITIONAL PROPERTIES OF EVOO
Extra-virgin olive oil mainly comprises fatty acids (98%–99% by weight)4 with monounsaturated fatty acids such as oleic acid (18:1 ω9) representing between 55% and 83% of the total fatty acids.33 Other minor, yet valuable, components like phytosterols, tocopherols, squalene, and phenolics make up the remaining (1%–2%) weight of EVOO.4 There are > 100 different phenolic compounds in olive-derived products; the main phenolics are found in EVOO, including the simple phenols, hydroxytyrosol and tyrosol, and the secoiridoids, oleuropein, oleocanthal, and ligstroside, all of which possess potent antioxidant activity and prolong the shelf life of EVOO.5,37 The concentration of phenolic compounds in EVOO can range from 50 to 940 mg/kg, with concentration dependent on a variety of factors, including olive cultivar, growing conditions, and oil extraction processes and techniques.37Tables 1 and 2 display the major fatty acid and major phenolic compositions, respectively, of selected olive varieties used in EVOO. Italian olive cultivars such as Coratina and Leccino have moderate amounts of oleic acid (78.8% ± 3.9% and 76.7% ± 4.1%, respectively) (expressed as mean ± SD), as well as total phenolic concentration (116 mg/kg and 125 mg/kg, respectively).38 Interestingly, the Spanish Picual and Greek Koroneiki cultivars possess higher amounts of oleic acid (80.7% ± 2.0% and 79.2% ± 0.1%, respectively) in comparison with other Spanish commercial varieties such as Arbequina (67.3% ± 4.3%) and Kalamata (68.7% ± 0.1%) (expressed as mean ± SD).39,40 In contrast, Kalamata olives contain greater amounts of individual phenolic compounds as well as total concentration of phenolics (371.0 mg/kg) compared with varieties such as the Arbequina (85.0 mg/kg).38,39 Although there are no data to date, to our knowledge, comparing the different varietals of olives used in EVOO and their associated impact on the gut microbiota, given the intimate relationship between polyphenols and the microbiota, EVOOs with higher concentrations of phenolic compounds would likely exert the most striking effects on the gut microbiota.
Table 1.
Major fatty acid composition of selected varieties of extra-virgin olive oil
| Olive cultivara |
||||||
|---|---|---|---|---|---|---|
| Fuentes et al. (2018)38 |
Montaño et al. (2016)39 |
Di Lecce et al. (2020)40 |
||||
| Reference | Coratina | Leccino | Arbequina | Picual | Kalamata | Koroneiki |
| Fatty acid methyl ester composition (%) | ||||||
|
C16:0 Palmitic acid |
13.60 ± 0.6 | 13.7 ± 0.7 | 15.3 ± 1.4 | 11.6 ± 1.2 | 15.6±0.1 | 10.6±0.0 |
|
C16:1 Palmitoleic acid |
1.2 ± 0.3 | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.0 ± 0.2 | 2.2 ±0.1 | 0.6±0.0 |
|
C18:0 Stearic acid |
1.91 ± 0.30 | 1.7 ± 0.2 | 1.3 ± 0.5 | 2.0 ± 0.9 | 2.2 ±0.1 | 2.5 ±0.00 |
|
C18:1 Oleic acid |
78.8 ± 3.9 | 76.7 ± 4.1 | 67.3 ± 4.3 | 80.7 ± 2.0 | 68.7 ±0.1 | 79.2±0.1 |
|
C18:2 Linoleic acid |
4.8 ± 0.3 | 6.0 ± 0.4 | 12.7 ± 2.6 | 3.1 ± 0.5 | 10.0±0.1 | 5.3±0.0 |
|
C18:3 α-Linolenic acid |
0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.10 ±0.1 | Not detected |
Data are expressed as mean ± SD.
Table 2.
Major classes of phenolic compounds and total phenolic concentration in selected varieties of Extra virgin olive oil
| Olive cultivar |
||||||
|---|---|---|---|---|---|---|
| Arbequina | Picual | Coratina | Leccino | Kalamata | Koroneiki | |
| Reference | Fuentes et al. (2018)38 | |||||
| Concentration (mg/kg)a | ||||||
| Simple phenols | 62.0 | 64.0 | 62.2 | 42.8 | 102.6 | 97.0 |
| Oleuropein ligstroside derivatives | 8.5 | 156.4 | 40.0 | 66.5 | 216.7 | 87.0 |
| Lignans | 0.0 | 5.9 | 6.9 | 3.1 | 14.4 | 9.1 |
| Flavanoids | 14.1 | 13.4 | 5.06 | 12.8 | 32.1 | 16.6 |
| Total phenols | 85.0 | 240.0 | 116.0 | 125.0 | 371.0 | 210.0 |
Quantification and sum of phenolic compounds was determined by high-performance liquid chromatography.
Accumulating evidence regarding the health benefits of EVOO has also influenced a number of health and nutrition policies, particularly in Europe.5 Specifically, the European Food Safety Authority recommends healthy unsaturated fats like EVOO should be used in place of saturated and trans fats, and the organization also recognizes the particularly beneficial effects of EVOO phenolic compounds on blood low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein (HDL) cholesterol and associated cardiovascular risk.41,42
METABOLIC HEALTH AND CARDIOVASCULAR DISEASE
Greater than one-third of the world’s adult population is now classified as overweight or obese and approximately 25% meets criteria for the metabolic syndrome—a clustering of different metabolic abnormalities including abdominal obesity, dyslipidemia, hyperglycemia, and hypertension.43,44 Obesity and the metabolic syndrome are associated with an increased risk of cardiovascular disease, diabetes mellitus, and all-cause mortality and, worldwide, represent a major public health burden, with safe and effective therapies urgently required.43
The therapeutic effects of EVOO consumption on cardiovascular outcomes and cardiometabolic risk factors have been demonstrated in a number of randomized trials and epidemiological studies.45–47 Most notable of these was the Prevención con Dieta Mediterránea (PREDIMED) trial, a large randomized trial consisting of 7447 participants at high cardiovascular risk with type 2 diabetes mellitus or ≥ 3 major risk factors, including smoking, hypertension, elevated LDL-C level, low HDL cholesterol level, overweight or obesity, or a family history of premature coronary heart disease. Results from the trial showed that consumption of a Mediterranean diet plus either EVOO (participants were provided 1 L/week per household and advised to consume ≥ 4 tablespoons/day per person) or nuts (30 g/day) was associated with a 30% lower risk of development of major cardiovascular events, including myocardial infarction, stroke, or death from cardiovascular causes, compared with a group eating a low-fat diet (participants were provided with professional dietary advice to reduce dietary fat) after a median of 4.8 years’ follow-up.1 In a subset of the PREDIMED study, participants allocated to a Mediterranean diet supplemented with EVOO (as defined in the previous sentence) or nuts (30 g/day) had significantly reduced 24-hour ambulatory blood pressure as well as a total cholesterol and fasting blood glucose levels compared with the control participants after 1 year.45
In another subset of the PREDIMED study, involving participants (n = 3541) with the risk factors listed in the previous paragraph, researchers observed rates of 16.0, 18.7, and 23.6 of new-onset diabetes cases per 1000 person-years for the Mediterranean diet group supplemented with EVOO, Mediterranean diet group plus nuts, and the control diet group, respectively.46 A 40% significant relative risk reduction in diabetes risk for the Mediterranean diet group supplemented with EVOO and a nonsignificant 18% risk reduction in diabetes risk in the Mediterranean diet group supplemented with mixed nuts were observed in comparison with the reduced-fat control diet.46 In addition, in the ATTICA study, a large, population-based study consisting of 3042 Greek men and women with no history of cardiovascular disease over 1 year, overweight/obese participants with a greater adherence (as measured by a validated food-frequency questionnaire) to the Mediterranean diet, including EVOO had significantly greater insulin sensitivity, as assessed by the homeostasis model assessment, 3% lower total cholesterol level, and a 3 mmHg reduction in systolic blood pressure vs overweight/obese participants with low adherence to the Mediterranean diet.47
Acute consumption of EVOO specifically has also proven beneficial in terms of several cardiometabolic markers of health. Findings from a randomized control trial of 30 participants with impaired fasting glucose levels showed that 10 g of EVOO added to meals was associated with a reduction in blood glucose, triglyceride, apolipoprotein B-48, and dipeptidyl peptidase-4 activity levels and a significant increase in insulin and glucagon-like peptide 1 (GLP-1) levels in the peripheral blood.7
Moreover, in another randomized, cross-over study of 25 participants, the addition of 10 g of EVOO to a Mediterranean-type meal significantly reduced 2-hour postprandial glucose, LDL-C, and dipeptidyl peptidase-4 levels, significantly increased GLP-1 and gastric inhibitory peptide (GIP) levels in circulating blood.6 Although high-fat diet (HDF)–induced secretion of GIP increases lipoprotein lipase activity in adipocytes, leading to potential fat accumulation and obesity,48 it is unlikely that GIP secretion induced by EVOO consumption would cause any significant changes in adiposity. First, increases in GLP-1, which assist in body weight reduction and fat accumulation as well as in delaying gastric emptying and increasing satiety in humans,48–50 have been proposed to increase the therapeutic efficacy of GIP.51 Interestingly, a feedback loop has been established between GLP-1 and GIP, with GIP promoting an increase GLP-1 levels and dipeptidyl peptidase-4 inhibitors increasing both GLP-1 and GIP levels.52 In support of this, mice fed an HFD and administered both GIP and GLP-1 had greater reductions in appetite and body weight gain than did mice that received individual administration of these incretins.53 In addition, it has been proposed that GIP may exert some of its beneficial effects on metabolic health via the central nervous system; increased expression of GIP in the hypothalamus in mice resulted in regulation of food intake, and played an important role in memory, with GIP-receptor–deficient mice exhibiting impairments in learning, synaptic plasticity, and hippocampal neurogenesis.54,55 Unfortunately, little is known about the impact of long-term EVOO consumption on GIP levels in humans or animals. However, in a couple of studies in rodents, elevations in GLP-1 and associated therapeutic effects on glucose homeostasis and adiposity were observed with long-term EVOO consumption.56,57
A variety of molecular mechanisms have been implicated regarding EVOO’s positive effects on cardiovascular and metabolic health. In particular, some of EVOO’s lipid-lowering effects can be ascribed to the ability of EVOO to increase the capacity of HDL and promote cholesterol efflux as well as increase expression of the ATP-binding cassette transporters ABCA1 and ABCG1 in macrophages.58,59 In addition, olive oil phenolic compounds demonstrate cholesterol binding activity and can impair cholesterol absorption in the intestine, as well as reduce oxidation of LDL via their antioxidant effects.2,60,61 Interestingly, these effects were demonstrated in a randomized crossover, controlled trail with polyphenol-rich olive oil exerting superior effects on LDL oxidation and proatherogenic CD40L gene expression in comparison to olive oil with low polyphenol content.61
The major fatty acid in EVOO, oleic acid, assists in maintaining glucose homeostasis by activating the G protein–coupled receptors GPR-120 and GPR-40, leading to intestinal GLP-1 release and widespread ant-inflammatory effects in a variety of tissues, including liver and adipose tissue.62 Also, oleic acid derived from olive oil can result in increased mRNA expression of fatty acid transport protein 4 receptor and promote subsequent GLP-1 secretion from intestinal L cells.63 Moreover, SCFAs, produced by a number of bacteria, such as Bacteroides and Lactobacillus, which are often elevated with EVOO consumption, are also potent activators for GPR43, playing an important role in blood glucose regulation, with GPR43 knock-out mice exhibiting reduced levels of GLP-1, GIP, and peptide YY secretion.64
COGNITIVE HEALTH
Alzheimer disease (AD), the most common form of dementia and neurodegenerative disorder, is estimated to affect between 40 million and 50 million people worldwide.65 Alzheimer disease is characterized by progressive memory loss and cognitive decline and, pathologically, by the deposition of Aβ plaques and accumulation of hyperphosphorylated tau proteins.66 To date, there are no effective disease-modifying treatments for AD. However, evidence suggests that early lifestyle modifications, such healthy dietary changes, can potentially prevent and reduce the number of people living with dementia and AD.65
In the PREDIMED-NAVARRA randomized trial (n = 522 participants) intake of the Mediterranean diet supplemented with either EVOO (as defined in the previous section) or nuts (30 g/day) resulted in improved cognition in comparison to a low-fat diet (as defined in the previous section) after 6.5 years of the intervention.67 Specifically, participants allocated to a Mediterranean diet supplemented with EVOO or nuts had significantly higher mean Mini-Mental State Examination scores (+0.62; 95%CI, +0.18 to +1.05) and Clock Drawing Test (+0.51; 95%CI, +0.20 to +0.82) scores compared with participants eating the low-fat control diet.67 Also, in another PREDIMED substudy in which several neuropsychological tests were used to assess cognitive health, researchers found that after 4.1-years of follow-up, participants who consumed a Mediterranean diet supplemented with either EVOO or nuts had improved cognitive function compared with those that consumed a low-fat diet.68 In addition, in the Three-City study, a French multicenter cohort study, researchers followed 6947 participants and monitored their olive oil intake and cognitive health using the Mini-Mental State Examination, Benton Visual Retention Test, and Isaacs Set Tests. They found that participants with moderate or intensive olive oil consumption had reduced odds of cognitive deficit for verbal fluency (odds ratio = 0.88; 95%CI, 0.72–1.08) and visual memory (odds ratio = 0.82; 95%CI, 0.68–0.99) vs those who never used olive oil.69
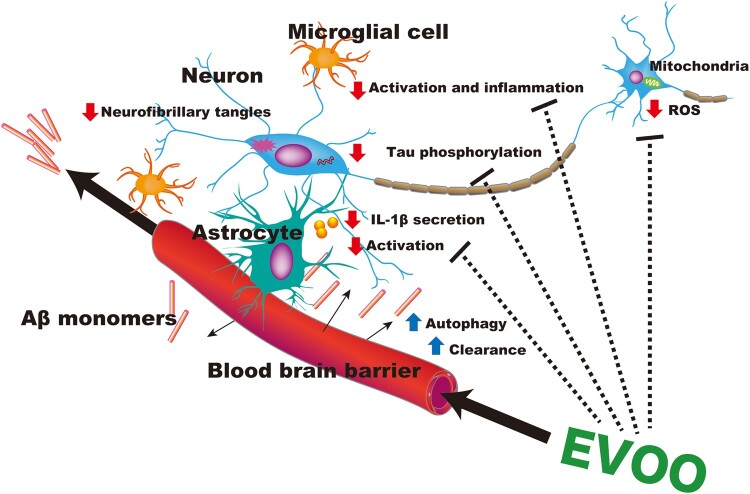
In several rodent studies, EVOO, especially the EVOO secoiridoid oleocanthal, aided the prevention and halted the progression of AD.15–19 Researchers assessing the effect of chronic supplementation of EVOO on tau metabolism and synaptic function in transgenic mice overexpressing human tau found an increase in complexin 1, a presynaptic neuronal protein, and a notable decrease in tau oligomers in mice fed EVOO, resulting in enhanced short-term plasticity and memory.17 In an earlier study by the same authors, a 6-month EVOO diet administered to triple transgenic mice, which possess 3 mutant disease genes associated with AD (PS1M146V, tauP301L, and APPSwe) and in which amyloid plaques and neurofibrillary tangles develop, resulted in a significant reduction in Aβ peptides and a decrease in tau neuropathology as a result of activation of autophagy mechanisms.18 In a study of the effect of 4-week intraperitoneal injection of oleocanthal in TgSwDI mice (transgenic mice that express the human amyloid-β precursor protein gene under the mouse Thy-1 promoter, with Swedish (K670N/M671L), Dutch (E693Q), and Iowa (D694N) mutations that enhance Aβ peptide deposition)70 researchers found a significant reduction of Aβ in the hippocampal parenchyma and microvessels, higher expression of Aβ clearance proteins across a human model of the blood-brain barrier, and decreased astrocyte activation (likely as a result of reduced Aβ burden and inflammatory cytokine production) and IL-1β levels in the brain.16 The same authors showed in a previous study that 6-month consumption of EVOO by TgSwDI mice resulted in lower levels of Aβ, due to beneficial changes in Aβ precursor protein processing.15 Furthermore, oleocanthal-rich EVOO given to TgSwDI mice at an advanced stage of AD resulted in significantly reduced NLRP3 inflammasome activation and increased Aβ clearance by stimulating autophagy through the AMPK− Unc-51-like kinase 1 (ULK1) pathway.19Figure 2 mechanistically outlines the potential therapeutic effects of EVOO in relation to AD.
Figure 2.
Potential therapeutic effects of extra-virgin olive oil (EVOO) on Alzheimer disease. Extra-virgin olive oil may help prevent and even halt the progression of Alzheimer disease by reducing β-amyloid (Aβ) deposits and tau neuropathologies by enhancing autophagy and clearance mechanisms across the blood-brain barrier. Extra-virgin olive oil reduces astrocyte and microglial cell activation by reducing inflammatory cytokine production. Extra-virgin olive oil’s widespread antioxidant effects may also lead to enhancement of brain antioxidant enzymes and a reduction in reactive oxygen species (ROS).
GUT MICROBIOTA AND MUCOSAL IMMUNITY
To analyze the composition and structure of the gut microbiota, its associated functions, and, in turn, its relationship with diet, culture-independent methods, using high-throughput and rapid computation, are becoming increasingly popular and widely available. Briefly, some of the most common approaches for studying the microbiota include polymerase chain reaction–based DNA profiling techniques, DNA microarray, and next-generation DNA sequencing using 16S rRNA genes as the target.71 In addition, the omics technologies, including metagenomics, metatranscriptomics, metaproteomics, and metabolomics, use analysis of DNA, messenger RNA, proteins, and metabolites to ascertain biological functions of the gut microbiota.71
The crucial role the gut microbiota play in shaping the mucosal immune system and its influence on overall inflammatory status and cardiovascular, metabolic, and brain health are becoming increasingly apparent. Western-style diets, low in dietary fiber and high in high pro-inflammatory fats, are associated with gut dysbiosis, increased gut permeability, systemic inflammation, and a variety of chronic diseases.72 Conversely, healthier dietary patterns like the Mediterranean diet, which is rich in dietary fiber, beneficial fatty acids, and polyphenolic compounds, are associated with greater gut microbial diversity, increased abundance of beneficial bacteria, and reduced levels of inflammation and risk of chronic diseases.20–23 Notably, Ghosh et al23 investigated the effect of a Mediterranean diet on gut microbiota in 612 elderly European people over 12 months and found that adherence to the diet caused an increased abundance in several bacteria associated with improved cognition and was inversely associated with pro-inflammatory markers including C-reactive protein and IL-17.23 Interestingly, using 16S recombinant DNA operational taxonomic unit baseline data to generate principle coordinate analysis plots, a specific pattern of clustering was seen across different ethnicities, with Italian people possessing a distinct microbiota composition from that of people from the United Kingdom and France and from Poland and the Netherlands, which showed more similarities.23 Moreover, Procrustes analyses also showed country-specific patterns in dietary habits, which were reflected in the microbiota composition of the people studied.23
Consumption of EVOO has exhibited positive effects on gut microbiota and intestinal health in human and rodent studies (Table 3). Specifically, in humans, EVOO has prebiotic effects, promoting the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium. Specifically, a Mediterranean diet supplemented with 40 g/day EVOO for 3 months administered to 18 overweight/obese participants resulted in significantly increased levels of lactic acid bacteria compared to baseline.25 Moreover, a study of 32 patients with HIV, aged ≥50 years with undetectable viral load, who consumed 50 g/day EVOO for 12 weeks indicated a significant elevation in abundance of Bifidobacteriaceae species in patients without antiretroviral treatment.26 In addition, in a randomized, controlled, double-blind, cross-over trial including 12 patients with hypercholesterolemia, researchers found that 25 mL/day virgin olive oil for 3 weeks containing 500 mg of phenolic compounds/kg (from a mixture of virgin olive oil and thyme) significantly increased levels of Bifidobacteria compared to levels in study participants who consumed only 80 mg phenolic compounds/kg from virgin olive oil.31
Table 3.
Effect of extra-virgin olive oil on gut microbiota
| Reference | Study characteristics | Methodology | Effect of EVOO gut microbiota |
|---|---|---|---|
| Zhao et al (2019)24 |
Male, SD rats (n = 48) Normal chow diet with ordinary drinking water, high-fat diet with fructose drinking water, diet high in oleic peanut oil with fructose drinking water or EVOO diet with fructose drinking water for 12 weeks |
16S rRNA sequencing |
↑ β-diversity index (compared to high-fat diet with fructose drinking water) ↑ Bifidobacterium (compared to high-fat diet with fructose drinking water) ↓ Blautia (compared to high-fat diet with fructose drinking water) |
| Luisi et al (2019)25 |
Overweight and obese adults (n = 18) and normal-weight control participants (n = 18) Mediterranean diet enriched with 40 g/day EVOO for 3 months |
qPCR | ↑ in lactic acid bacteria (compared to baseline) |
| Olalla et al (2019)26 |
Patients with HIV, aged ≥50 years with undetectable viral load (n = 32) 50 g/day EVOO for 12 weeks |
16S rRNA sequencing |
↑ α-diversity (males) ↑ Lachnospiraceae, Ruminococcus, and Akkermansia (females) ↑ Bifidobacteriaceae (in patients without antiretroviral treatment) ↑ Gardnerella (compared to baseline) ↓ Dethiosulfovibrionaceae (compared to baseline) |
| Millman et al (2020)27 |
Male, C57BL/6 J mice (n = 20) Low-fat purified diet, lard purified diet, high-fat EVOO diet or high-fat flaxseed oil diet for 10 weeks |
16S rRNA sequencing |
↑ α-diversity (compared to lard, purified diet) ↑ Mucispirillum, ↑ Lachnospiraceae, ↑ Bacteroides (compared with low-fat, purified diet) |
| Martinez et al (2019)28 |
Male, Swiss Webster ICR (CD-1) mice (n = 35) Standard diet, high-fat diet enriched with EVOO, high-fat diet enriched with refined olive oil, or high-fat diet enriched with butter for 12 weeks |
16S rRNA sequencing |
↑ Erysipelotrichaeae, ↑ Sutterellaceae (compared to standard diet) ↓ Desulfovibrionaceae (compared to high-fat diet enriched with refined olive oil) |
| Hidalgo et al (2018)29 |
Male, spontaneously hypertensive rats (n = 16) Standard diet or diet enriched with EVOO for 12 weeks |
PCR-denaturing gradient gel electrophoresis analysis | ↑ Lactobacillus, ↑ Clostridium in cluster XIV (compared to standard diet) |
| Prieto et al (2018)30 |
Male, Swiss Webster ICR (CD-1) mice (n = 26) Standard diet, high-fat diet enriched with butter or high-fat diet enriched with EVOO for 12 weeks |
16S rRNA sequencing |
↑ Erysipelotrichaeae, ↑ Sutterellaceae (compared to standard diet) ↓ Desulfovibrionaceae (compared to high-fat diet enriched with butter) |
| Martin-Pelaez (2017)31 |
Randomized, controlled, double-blind, cross-over trial with adults with hypercholesterolemia (n = 12) 25 mL/day virgin olive oil containing either 80 mg PCs/kg, or 500 mg PCs/kg from virgin olive oil and thyme for 3 weeks |
Fluorescence in situ hybridization combined with flow cytometry | ↑ Bifidobacterium (virgin olive oil containing 500 mg PCs/kg compared to 80 mg PCs/kg from virgin olive oil and thyme) |
Abbreviations: EVOO, extra-virgin olive oil; PC, polyphenolic compound; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; SD, Sprague-Dawley.
In rodents, consumption of EVOO increased α-diversity, a measure of species richness, as well as β-diversity, a measure of the differences in composition between samples, compared with controls.24,27 These results are associated with favorable effects on metabolic health, because reduced microbial diversity is associated with increased chronic inflammation and subsequent development of metabolic diseases.73 Extra-virgin olive oil ingestion in rodents also can promote the growth of certain types of beneficial bacteria including species of Lactobacillus, Bifidobacterium, Bacteroides, and Clostridium, and members of the Erysipelotrichaeae.24–31 In additionally, EVOO has demonstrated antibacterial and bacteriostatic effects against bacteria such as those in the Desulfovibrionaceae,26,28,30 a family of opportunistic pathogens associated with obesity and inflammation,74 and Blautia spp.,24 which positively correlates with visceral fat accumulation in Japanese men and women.75
The ability of EVOO to act as both a prebiotic, stimulating the growth of beneficial bacteria, and an antibacterial, suppressing the growth of pathogenic bacteria, is likely attributable to the array of phenolic compounds EVOO contains. Approximately 90%–95% of ingested phenolic compounds escape digestion in the small intestine and reach the colon, where they are catabolized into bioactive secondary structures—metabolites of their respective parent phenolic compounds that have been transformed by resident microbiota and can modify the composition of the gut microbiota.76 Specifically, Martinez et al28 showed that administration of a diet high in EVOO (vs refined olive oil, which is devoid of polyphenols) to Swiss Webster mice resulted in reduced levels of species in the Desulfovibrionaceae. The authors concluded the observed antibacterial effects against these pathogenic species likely were involvement of phenolic compounds in the EVOO. In support of this, C57BL6/J mice that were administered a gavage of hydroxytyrosol at 50 mg/kg/day were rescued from HFD-induced gut microbiota dysbiosis, showing reduced levels of pathogenic Proteobacteria and Ferribacter as well as restored bacterial diversity and intestinal barrier integrity, as exhibited by the reduction in lipopolysaccharide-binding protein in the plasma.77 In another study examining the bactericidal effects of selected Turkish EVOOs on cultured bacterial strains, notable antibacterial activity was found against the foodborne pathogens Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella enteritidis, with refined olive, canola, and hazelnut oils (all with similar fatty acid composition), by comparison, unable to significantly reduce microbial growth.78 Moreover, in the same study, individual phenolic compounds in the EVOO, including tyrosol, vanillin, vanillic, and cinnamic acids, also exhibited slight antimicrobial activity. Authors Karaosmanoglu et al78 pointed out a synergistic interaction between the phenolic compounds and, in turn, an enhanced antimicrobial effect when they were combined. Importantly, it should be highlighted that in humans, phenolic compounds in EVOO are not consumed in isolation; they are eaten as part of a food matrix and can synergistically function together, as well as with other components in EVOO, such as fatty acids, which can enhance the availability of a variety of polyphenols.79
In addition to phenolic compounds and their well-established interaction and influence on the microbiota, other minor components in EVOO such as sterols and tocopherols may influence gut microbiota composition, although studies are limited. In a study of dietary intake of 28 nutrients, assessed by food frequency questionnaires completed by 60 pregnant women in the Norwegian Microbiota Study cohort, dietary intake of the tocopherol, vitamin E, was associated with a decrease in pathogenic Proteobacteria.80 Moreover, high-dose phytosterol esters intragastrically administered to rats daily for 12 weeks restored HFD-induced dysbiosis, modulating levels of Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia similar to levels of the control group.81 In addition, the study also showed that administration of high-dose phytosterol esters significantly increased mRNA expression level of Claudin-1 and Occludin in the colon, in comparison to the HFD group, likely enhancing intestinal barrier integrity.81
Regarding the possible impact on gut microbiotia of oleic acid in EVOO, results of several rodent studies point to a positive effect. A study comparing the effects of diets enriched with peanut oil high in oleic acid, EVOO, and chow supplemented with drinking water containing high levels of fructose drinking water in Swiss Webster rats, researchers found that both the peanut oil and EVOO significantly increased the relative abundance of Bifidobacterium and reduced Blautia levels and also had favorable effects on body weight, cholesterol levels, and insulin sensitivity.24 Moreover, when researchers exposed ICR outbred mice to HFD or a HFD supplemented with either an oleic acid–derived compound (1500 mg/kg/day) or a combination of n-3 fatty acids (EPA and DHA, 3000 mg/kg/day), they found that mice supplemented with the oleic acid compound had greater gut microbial diversity as well as lower body weight compared with HFD-fed mice.82 Moreover, a similar study showed that diets high in oleic acid–rich olive oil fed to C57BL6/J mice were associated with significantly greater abundance of members of the Bacteroidaceae in comparison to mice fed diets high in palm oil, flaxseed or fish oil, and high levels of sucrose.83
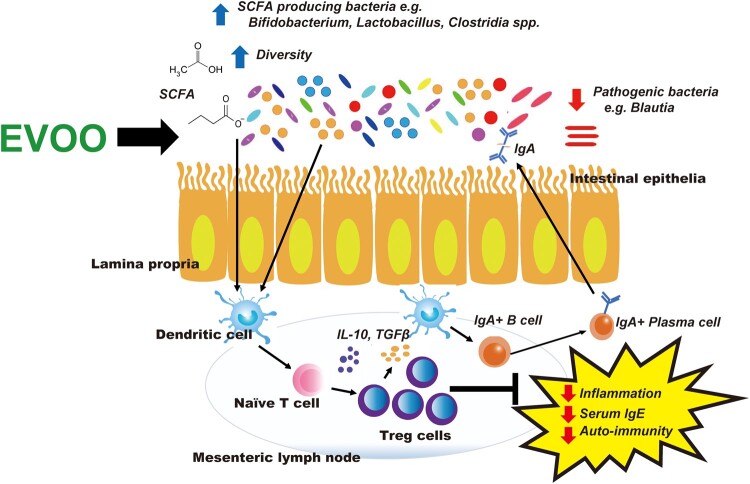
Short-chain fatty acids such as acetate, butyrate, and propionate, produced by bacteria, not only exert therapeutic effects on colonic cells and are an important source of energy but also have a profound impact and influence on the mucosal immune system.84,85 Short-chain fatty acids can increase anti-inflammatory and immunotolerogenic T-regulatory cells (Tregs) via induction of dendritic cells, activating innate lymphoid cells to produce Treg-inducing cytokines such as IL-10 and TGF-β, and can promote the development of Tregs by inhibiting histone deacetylases at the FoxP3 locus.86,87 Moreover, SCFAs can assist in strengthening the gut barrier and preventing bacterial and lipopolysaccharide (LPS) infiltration and subsequent inflammation by upregulating the expression of tight-junction proteins.88,89 Extra-virgin olive oil phenolics can also provide barrier protection and prevent pathogen invasion via effects on mucosal immunity. In a randomized control trial including 10 patients with hypercholesterolemia (total cholesterol, > 200 mg/dL), ingestion of a 500 mg/kg polyphenol-rich EVOO increased intestinal IgA, which has an important role in the homeostasis of the microbiota community and provides protection against pathogenic bacteria.35 In addition, in another study of the effects of EVOO and EVOO phenolics for 30 days in mice with dextran sodium sulphate–induced colitis, EVOO and EVOO polyphenols significantly reduced inflammation by lowering MCP-1, TNF-α, cyclooxygenase-2, and nitric oxide synthase expression levels.90Figure 3 summarizes the impact of EVOO on microbiota observed in studies and the potential effects on the mucosal immune system.
Figure 3.
Impact of extra-virgin olive oil (EVOO) on gut microbiota and mucosal immunity. Extra-virgin olive oil modulates the gut microbiota by acting as both a prebiotic (encouraging the growth of beneficial bacteria) and antibacterial (reducing the growth of pathogenic bacteria). Extra-virgin olive oil promotes the growth of certain bacteria that are capable of producing microbial metabolites such as short-chain fatty acids (SCFAs). Short-chain fatty acids and other gut microbiota-generated metabolites can positively influence the mucosal immune system by increasing T-regulatory (Treg) cells as well as the production of anti-inflammatory cytokines such as IL-10 and TGF-β, which assist in reducing local inflammation and promoting immune tolerance to commensals and other harmless dietary antigens. Extra-virgin olive oil may also promote an increase in intestinal IgA, providing further protection against pathogenic bacteria and promoting homeostasis of the gut microbiota.
IMPACT ON GUT-BRAIN AXIS AND MODULATION OF METABOLIC HEALTH
A number of EVOO’s favorable effects on cardiometabolic and cognitive health as well as mucosal immunity can be linked to the gut microbiota. Presence of the genus Bacteroides, which has been found to be elevated with EVOO consumption,27 correlates negatively with plasma triglyceride levels in mice and humans.27,91 In addition, presence of members of the Lachnospiraceae, known to increase with EVOO consumption,26,27 positively correlated with plasma HDL cholesterol in humans.91Lactobacillus, also elevated with EVOO consumption25,29 when administered in probiotic form, promoted a significant reduction in plasma total cholesterol levels in mice as well as human patients with hypercholesterolemia (LDL-C, 3.35–4.91mmol/L and total cholesterol, 5.16–7.64mmol/L).92–94 Demonstrated abilities of Lactobacillus to lower plasma cholesterol levels may be due partly to the enhanced bile salt hydrolase activity this genus exhibits, as well as the ability to convert cholesterol to coprostanol, both leading to an impairment of cholesterol absorption in the intestine.95,96
Short-chain fatty acids, produced by a number of bacteria whose populations are often elevated with EVOO consumption, can exert therapeutic effects on blood glucose homeostasis. Specifically, the SCFA acetate activates GPR43, promoting GLP-1 release from colonic L cells.97 In addition, activation of GPR41 by propionate or butyrate can induce both GLP-1 and peptide YY secretion from L cells in the small intestine.98
In regards to gut microbiota–mediated effects on cognitive health via EVOO, certain commensals, including Lactobacilli and Bifidobacterium are often greatly reduced in patients with AD.99,100 Both of these bacterial types, populations of which are elevated with EVOO consumption, can produce γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter, likely influencing GABAergic firing in the brain via the enteric and vagal nervous systems.99 Specifically, oral administration of a strain of Lactobacillus to mice modulated GABAAα2, GABAAα1, and GABAB1b receptor mRNA expression in the brain of mice, reducing anxiety and depressive‐like symptoms and behaviors, with the same effects not observed in vagotomized mice.101 However, the exact mechanisms of how “gut GABA” may modulate the central nervous system and elicit specific regulatory effects in the brain remain largely unknown.102 In addition, EVOO may exert protective effects on cognitive health via its antibacterial activity toward certain pathogenic species of bacteria that have been considered a risk factor for AD in the pathogens interaction hypothesis.103 This hypothesis suggests that Aβ originally functioned as an antimicrobial peptide, controlling the population of pathogenic microbes.104 However, chronic infection by microbial pathogens may act as a trigger for development of sporadic AD by causing increased and prolonged levels of inflammation, microglial senescence, and microbial biofilm formation, all of which may potentially aggregate with Aβ, leading to plaque formation.104,105
Short-chain fatty acids may also assist in reducing inflammation in the intestine by upregulating tight-junction protein expression in the intestine, reinforcing barrier integrity, and preventing infiltration of bacterial products like lipopolysaccharide and subsequent inflammatory signaling. In a study of the impact of butyrate on barrier integrity using an lipopolysaccharide-treated IPEC-J2 cell model, from porcine jejunal cells, researchers found that butyrate significantly restored the impairment of intestinal barrier integrity and increased expression of tight-junction proteins such as claudins-3 and 4, and activated the Akt/mTOR pathway, promoting the synthesis of tight-junction proteins.106
Extra-virgin olive oil–induced microbiota may possibly exert beneficial effects on the mucosal immune system, in addition to SCFA-induced Treg induction, and IgA production, through the ability of phenolics and other gut microbiota–generated compounds to act as aryl hydrocarbon receptor ligands. Aryl hydrocarbon receptor is expressed by a number of immune cells and involves transcription of several genes.107 Aryl hydrocarbon receptor activation induces Foxp3+ Treg differentiation, possibly via epigenetic mechanisms at the Foxp3 locus.108,109 Aryl hydrocarbon receptor signaling in dendritic cells can also promote an increase in Foxp3+ Tregs by increasing the expression of enzymes involved in retinoic acid production, an enhancer of Foxp3+ Treg induction.109 Furthermore, aryl hydrocarbon receptor activation in IL-22–producing innate lymphoid cells is required for defense against pathogens and modulation of the composition of gut microbiota by antimicrobial peptide expression and production.110,111 In addition, the population of certain bacterial species (eg, the Lactobacilli) increase with EVOO consumption and produce aryl hydrocarbon receptor ligands from tryptophan, thus affecting mucosal immunity status.112
From all the experimental evidence regarding recommendations of the optimal amount of EVOO to be consumed to reap health benefits on cardiovascular and brain health from large scale epidemiological studies such as the PREDIMED and associated substudies, researchers have observed that consumption of at least 4 tablespoons (59 mL) per day is associated with a 30% lower risk of occurrence of cardiovascular events and improved cognitive function compared with a low-fat diet.1,67,68 In addition to using EVOO in replace of saturated fats, the European Food Safety Authority also suggests a minimum daily intake of 20 g of EVOO to assist in obtaining optimal cholesterol levels and protection of LDL from oxidative damage.41,42 Although evidence in relation to the optimal amount of EVOO required to modulate the gut microbiota is still in its infancy, beneficial effects on the microbiota have been observed in 2 intervention studies at doses of 40 and 50 g/day, (∼3 and 4 tablespoons, respectively).25,26 It is likely that a daily dose of EVOO, rich in phenolic compounds, is required to obtain sufficient levels in body tissues, including the brain, because plasma half-life and the level of excretion via the kidneys vary greatly depending on the type of phenolic compound.79
CONCLUSION
Consumption of EVOO clearly demonstrates widespread beneficial effects on a variety of aspects of health and disease and also shows great therapeutic potential in positively modifying the gut microbiota as well as the activity and functioning of the mucosal immune system. Investigation of the mechanisms whereby EVOO-induced modulations in gut microbiota and microbially produced products influence cardiovascular, metabolic, mucosal, and cognitive health outcomes still remains a relatively unexplored area of research with exciting prospects.
Acknowledgments
The authors thank Yuko Murayama, Chie Horiguchi, Ikumi Nomura, and Tomoko Ikematsu for technical assistance. They also thank Mamiko Hirata and Chikako Noguchi for secretarial assistance.
Author contributions. J.F.M. wrote the manuscript under the assistance and supervision of S.O. T.U., S.I., M.S., and H.M. read and provided comments about the manuscript. All authors approved the final manuscript.
Funding. This work was supported in part by Grants-in-Aid from Japan Society for the Promotion of Science (KAKENHI grant 20K08912); Council for Science, Technology and Innovation, Cross-ministerial Strategic Innovation Promotion Program, “Technologies for Creating Next-generation Agriculture, Forestry and Fisheries,” Uehara Memorial Foundation, Japan Agency for Medical Research and Development, and Rotary Yoneyama Memorial Foundation Fellowships.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:E34. [DOI] [PubMed] [Google Scholar]
- 2. Tosti V, Bertozzi B, Fontana L.. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. 2018;73:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. [DOI] [PubMed] [Google Scholar]
- 4. Romani A, Ieri F, Urciuoli S, et al. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients. 2019;11:1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piroddi M, Albini A, Fabiani R, et al. Nutrigenomics of extra-virgin olive oil: a review. Biofactors. 2017;43:17–41. [DOI] [PubMed] [Google Scholar]
- 6. Violi F, Loffredo L, Pignatelli P, et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr Diabetes. 2015;5:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carnevale R, Loffredo L, Del Ben M, et al. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clin Nutr. 2017;36:782–787. [DOI] [PubMed] [Google Scholar]
- 8. Takashima T, Sakata Y, Iwakiri R, et al. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J Nutr Biochem. 2014;25:186–192. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez-Fidalgo S, Villegas I, Cardeno A, et al. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clin Nutr. 2010;29:663–673. [DOI] [PubMed] [Google Scholar]
- 10. Martinez-Huelamo M, Rodriguez-Morato J, Boronat A, et al. Modulation of Nrf2 by olive oil and wine polyphenols and neuroprotection. Antioxidants (Basel). 2017;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roman GC, Jackson RE, Reis J, et al. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev Neurol (Paris). 2019;175:705–723. [DOI] [PubMed] [Google Scholar]
- 12. Dinu M, Pagliai G, Casini A, et al. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72:30–43. [DOI] [PubMed] [Google Scholar]
- 13. Walters MJ, Sterling J, Quinn C, et al. Associations of lifestyle and vascular risk factors with Alzheimer's brain biomarker changes during middle age: a 3-year longitudinal study in the broader New York City area. BMJ Open. 2018;8:E023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berti V, Walters M, Sterling J, et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90:e1789–e1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qosa H, Mohamed LA, Batarseh YS, et al. Extra-virgin olive oil attenuates amyloid-beta and tau pathologies in the brains of TgSwDI mice. J Nutr Biochem. 2015;26:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qosa H, Batarseh YS, Mohyeldin MM, et al. Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem Neurosci. 2015;6:1849–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lauretti E, Nenov M, Dincer O, et al. Extra virgin olive oil improves synaptic activity, short-term plasticity, memory, and neuropathology in a tauopathy model. Aging Cell. 2020;19:E13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lauretti E, Iuliano L, Pratico D.. Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: role of autophagy. Ann Clin Transl Neurol. 2017;4:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al Rihani SB, Darakjian LI, Kaddoumi A.. Oleocanthal-rich extra-virgin olive oil restores the blood-brain barrier function through NLRP3 inflammasome inhibition simultaneously with autophagy induction in TgSwDI mice. ACS Chem Neurosci. 2019;10:3543–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 21. Garcia-Mantrana I, Selma-Royo M, Alcantara C, et al. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. 2018;9:890 Doi: 10.3389/fmicb.2018.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagpal R, Shively CA, Appt SA, et al. Gut microbiome composition in non-human primates consuming a Western or Mediterranean diet. Front Nutr. 2018;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao Z, Shi A, Wang Q, et al. High oleic acid peanut oil and extra virgin olive oil supplementation attenuate metabolic syndrome in rats by modulating the gut microbiota. Nutrients. 2019;11:3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luisi MLE, Lucarini L, Biffi B, et al. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front Pharmacol. 2019;10:1366 Doi: 10.3389/fphar.2019.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olalla JG, de Lomas JM, Chueca N, et al. Effect of daily consumption of extra virgin olive oil on the lipid profile and microbiota of HIV-infected patients over 50 years of age. Medicine (Baltimore). 2019;98:E17528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Millman J, Okamoto S, Kimura A, et al. Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur J Nutr. 2020;59:2411–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martinez N, Prieto I, Hidalgo M, et al. Refined versus extra virgin olive oil high-fat diet impact on intestinal microbiota of mice and its relation to different physiological variables. Microorganisms. 2019;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hidalgo M, Prieto I, Abriouel H, et al. Changes in gut microbiota linked to a reduction in systolic blood pressure in spontaneously hypertensive rats fed an extra virgin olive oil-enriched diet. Plant Foods Hum Nutr. 2018;73:1–6. [DOI] [PubMed] [Google Scholar]
- 30. Prieto I, Hidalgo M, Segarra AB, et al. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS One. 2018;13:E0190368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin-Pelaez S, Mosele JI, Pizarro N, et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur J Nutr. 2017;56:119–131. [DOI] [PubMed] [Google Scholar]
- 32. Gavahian M, Mousavi Khaneghah A, Lorenzo JM, et al. Health benefits of olive oil and its components: impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci Technol. 2019;88:220–227. [Google Scholar]
- 33. Marcelino G, Hiane PA, Freitas KC, et al. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients. 2019;11:1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Žugčić T, Abdelkebir R, Alcantara C, et al. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci Technol. 2019;83:63–77. [Google Scholar]
- 35. Martin-Pelaez S, Castaner O, Sola R, et al. Influence of phenol-enriched olive oils on human intestinal immune function. Nutrients. 2016;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Incani A, Serra G, Atzeri A, et al. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem Toxicol. 2016;90:171–180. [DOI] [PubMed] [Google Scholar]
- 37. Servili M, Sordini B, Esposto S, et al. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants (Basel). 2013;3:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fuentes E, Paucar F, Tapia F, et al. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem. 2018;243:285–294. [DOI] [PubMed] [Google Scholar]
- 39. Montaño A, Hernández M, Garrido I, et al. Fatty acid and phenolic compound concentrations in eight different monovarietal virgin olive oils from Extremadura and the relationship with oxidative stability. Int J Mol Sci. 2016;17:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Lecce G, Piochi M, Pacetti D, et al. Eleven monovarietal extra virgin olive oils from olives grown and processed under the same conditions: effect of the cultivar on the chemical composition and sensory traits. Foods. 2020;9:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to oleic acid intended to replace saturated fatty acids (SFAs) in foods or diets and maintenance of normal blood LDL-cholesterol concentrations (ID 673, 728, 729, 1302, 4334) and maintenance. EFSA J. 2011;9:2043. [Google Scholar]
- 42.EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639). EFSA J. 2011;9:2033–2058. [Google Scholar]
- 43. Chooi YC, Ding C, Magkos F.. The epidemiology of obesity. Metabolism. 2019;92:6–10. [DOI] [PubMed] [Google Scholar]
- 44. Nolan PB, Carrick-Ranson G, Stinear JW, et al. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Prev Med Rep. 2017;7:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Domenech M, Roman P, Lapetra J, et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: one-year randomized, clinical trial. Hypertension. 2014;64:69–76. [DOI] [PubMed] [Google Scholar]
- 46. Salas-Salvado J, Bullo M, Estruch R, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014;160:1–10. [DOI] [PubMed] [Google Scholar]
- 47. Tzima N, Pitsavos C, Panagiotakos DB, et al. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis. 2007;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seino Y, Yabe D.. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J Diabetes Invest. 2013;4:108–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shah M, Vella A.. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Samms RJ, Coghlan MP, Sloop KW.. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metab. 2020;31:410–421. [DOI] [PubMed] [Google Scholar]
- 52. Farr OM, Tsoukas MA, Triantafyllou G, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: a randomized, placebo-controlled, crossover study. Metabolism. 2016;65:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Finan B, Ma T, Ottaway N, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5:209ra151–209ra151.. [DOI] [PubMed] [Google Scholar]
- 54. Kim SJ, Nian C, Karunakaran S, et al. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7:E40156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Faivre E, Gault VA, Thorens B, et al. Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J Neurophysiol. 2011;105:1574–1580. [DOI] [PubMed] [Google Scholar]
- 56. Prieto PG, Cancelas J, Villanueva-Peñacarrillo ML, et al. Effects of an olive oil-enriched diet on plasma GLP-1 concentration and intestinal content, plasma insulin concentration, and glucose tolerance in normal rats. Endocrine. 2005;26:107–115. [DOI] [PubMed] [Google Scholar]
- 57. Cancelas J, Prieto PG, Villanueva-Peñacarrillo ML, et al. Effects of an olive oil-enriched diet on glucagon-like peptide 1 release and intestinal content, plasma insulin concentration, glucose tolerance and pancreatic insulin content in an animal model of type 2 diabetes. Horm Metab Res. 2006;38:98–105. [DOI] [PubMed] [Google Scholar]
- 58. Helal O, Berrougui H, Loued S, et al. Extra-virgin olive oil consumption improves the capacity of HDL to mediate cholesterol efflux and increases ABCA1 and ABCG1 expression in human macrophages. Br J Nutr. 2013;109:1844–1855. [DOI] [PubMed] [Google Scholar]
- 59. Rosenblat M, Volkova N, Coleman R, et al. Antiatherogenicity of extra virgin olive oil and its enrichment with green tea polyphenols in the atherosclerotic apolipoprotein-E-deficient mice: anhanced macrophage cholesterol efflux. J Nutr Biochem. 2008;19:514–523. [DOI] [PubMed] [Google Scholar]
- 60. Amiot MJ, Riva C, Vinet A.. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17:573–586. [DOI] [PubMed] [Google Scholar]
- 61. Castaner O, Covas MI, Khymenets O, et al. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am J Clin Nutr. 2012;95:1238–1244. [DOI] [PubMed] [Google Scholar]
- 62. Oliveira V, Marinho R, Vitorino D, et al. Diets containing α-linolenic (ω3) or oleic (ω9) fatty acids rescues obese mice from insulin resistance. Endocrinology. 2015;156:4033–4046. [DOI] [PubMed] [Google Scholar]
- 63. Poreba MA, Dong CX, Li SK, et al. Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am J Physiol Endocrinol Metab. 2012;303:E899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McNelis JC, Lee YS, Mayoral R, et al. GPR43 potentiates beta-cell function in obesity. Diabetes. 2015;64:3203–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nichols E, Szoeke CEI, Vollset SE, et al. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. DeTure MA, Dickson DW.. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. 2019;14:32 Doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318–1325. [DOI] [PubMed] [Google Scholar]
- 68. Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175:1094–1103. [DOI] [PubMed] [Google Scholar]
- 69. Berr C, Portet F, Carriere I, et al. Olive oil and cognition: results from the Three-City Study. Dement Geriatr Cogn Disord. 2009;28:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Vickle GD, Esh CL, Daugs ID, et al. Tg-SwDI transgenic mice exhibit novel alterations in AβPP processing, Aβ degradation, and resilient amyloid angiopathy. Am J Pathol. 2008;173:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gong J, Yang C.. Advances in the methods for studying gut microbiota and their relevance to the research of dietary fiber functions. Food Res Int. 2012;48:916–929. [Google Scholar]
- 72. Martinez KB, Leone V, Chang EB.. Western diets, gut dysbiosis, and metabolic diseases: are they linked? Gut Microbes. 2017;8:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van den Munckhof ICL, Kurilshikov A, Ter Horst R, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. 2018;19:1719–1734. [DOI] [PubMed] [Google Scholar]
- 74. Rowan F, Docherty NG, Murphy M, et al. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis Colon Rectum. 2010;53:1530–1536. [DOI] [PubMed] [Google Scholar]
- 75. Ozato N, Saito S, Yamaguchi T, et al. Blautia genus associated with visceral fat accumulation in adults 20–76 years of age. Npj Biofilms Microbiomes. 2019;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ozdal T, Sela DA, Xiao J, et al. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu Z, Wang N, Ma Y, et al. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front Microbiol. 2019;10:390 Doi: 10.3389/fmicb.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Karaosmanoglu H, Soyer F, Ozen B, et al. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J Agric Food Chem. 2010;58:8238–8245. [DOI] [PubMed] [Google Scholar]
- 79. Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev. 2014;72:429–452. [DOI] [PubMed] [Google Scholar]
- 80. Mandal S, Godfrey KM, McDonald D, et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. 2016;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Song L, Li Y, Qu D, et al. The regulatory effects of phytosterol esters (PSEs) on gut flora and faecal metabolites in rats with NAFLD. Food Funct. 2020;11:977–991. [DOI] [PubMed] [Google Scholar]
- 82. Mujico JR, Baccan GC, Gheorghe A, et al. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110:711–720. [DOI] [PubMed] [Google Scholar]
- 83. Patterson E, O' Doherty RM, Murphy EF, et al. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br J Nutr. 2014;111:1905–1917. [DOI] [PubMed] [Google Scholar]
- 84. Silva YP, Bernardi A, Frozza RL.. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). 2020;11:25 Doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tanoue T, Atarashi K, Honda K.. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016;16:295–309. [DOI] [PubMed] [Google Scholar]
- 86. Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. [DOI] [PubMed] [Google Scholar]
- 87. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 88. Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hsieh CY, Osaka T, Moriyama E, et al. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. 2015;3:E12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sanchez-Fidalgo S, Cardeno A, Sanchez-Hidalgo M, et al. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24:1401–1413. [DOI] [PubMed] [Google Scholar]
- 91. Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Michael DR, Davies TS, Moss JWE, et al. The anti-cholesterolaemic effect of a consortium of probiotics: an acute study in C57BL/6J mice. Sci Rep. 2017;7:2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fuentes MC, Lajo T, Carrion JM, Cune J.. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br J Nutr. 2013;109:1866–1872. [DOI] [PubMed] [Google Scholar]
- 94. Jones ML, Martoni CJ, Prakash S.. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr. 2012;66:1234–1241. [DOI] [PubMed] [Google Scholar]
- 95. Molinero N, Ruiz L, Sanchez B, et al. Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol. 2019;10:185 Doi: 10.3389/fphys.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kriaa A, Bourgin M, Potiron A, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. 2019;60:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kimura I, Ozawa K, Inoue D, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Greiner TU, Backhed F.. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5:753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Boonstra E, de Kleijn R, Colzato LS, et al. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front Psychol. 2015;6:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li Z, Zhu H, Zhang L, et al. The intestinal microbiome and Alzheimer's disease: a review. Anim Models Exp Med. 2018;1:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bauer KC, Huus KE, Finlay BB.. Microbes and the mind: emerging hallmarks of the gut microbiota-brain axis. Cell Microbiol. 2016;18:632–644. [DOI] [PubMed] [Google Scholar]
- 103. Alkasir R, Li J, Li X, et al. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fulop T, Witkowski JM, Bourgade K, et al. Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer's sisease? Front Aging Neurosci. 2018;10:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Frolich L. Alzheimer's disease - the ‘microbial hypothesis’ from a clinical and neuroimaging perspective. Psychiatry Res Neuroimaging. 2020;306:111181. [DOI] [PubMed] [Google Scholar]
- 106. Yan H, Ajuwon KM.. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 2017;12:E0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gutierrez-Vazquez C, Quintana FJ.. regulation of the immune response by the aryl 99hydrocarbon receptor. Immunity. 2018;48:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Quintana FJ. The aryl hydrocarbon receptor: a molecular pathway for the environmental control of the immune response. Immunology. 2013;138:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Singh NP, Singh UP, Singh B, et al. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:E23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. [DOI] [PubMed] [Google Scholar]
- 111. Koper JEB, Loonen LMP, Wells JM, et al. Polyphenols and tryptophan metabolites activate the aryl hydrocarbon receptor in an in vitro model of colonic fermentation. Mol Nutr Food Res. 2019;63:e1800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lazar V, Ditu LM, Pircalabioru GG, et al. Gut microbiota, host organism, and diet trialogue in diabetes and obesity. Front Nutr. 2019;6:21 Doi: 10.3389/fnut.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]